Abstract
This study was aimed to determine the profiles of serum cytokines (IL‐1β, TNF‐α, IL‐4, IL‐5) and chemokines (MCP‐1: monocyte chemoattract protein‐1 and RANTES: regulated on activation normal T cell expressed and secreted) in individuals with an asthmatic versus a non‐asthmatic background with bacterial, viral or mixed acute respiratory infection. Asthmatic (n = 14) and non‐asthmatic (n = 29) patients with acute viral, bacterial or mixed (bacterial and viruses) respiratory infection were studied. Patients were also analysed as individuals with pneumonia or bronchitis. Healthy individuals with similar age and sex (n = 10) were used as controls. Cytokine/chemokine content in serum was determined by ELISA. Increased cytokine/chemokine concentration in asthmatic and non‐asthmatic patients was observed. However, higher concentrations of chemokines (MCP‐1 and RANTES) in asthmatic patients infected by viruses, bacteria or bacteria and viruses (mixed) than in non‐asthmatic patients were observed. In general, viral and mixed infections were better cytokine/chemokine inducers than bacterial infection. Cytokine/chemokine expression was similarly increased in both asthmatic and non‐asthmatic patients with pneumonia or bronchitis, except that RANTES remained at normal levels in bronchitis. Circulating cytokine profiles induced by acute viral, bacterial or mixed lung infection were not related to asthmatic background, except for chemokines that were increased in asthmatic status.
Introduction
Respiratory viral or bacterial infections can have profound effects on asthma 1. Viral respiratory infections are found in association with asthma exacerbations 1, 2. The infections associated with these wheezing events are multiple and include respiratory syncytial virus (RSV), human rhinovirus, metapneumovirus, parainfluenza, coronavirus and other viruses as well as typical and atypical bacteria 3. Previous studies have shown differences in the bronchoalveolar cytokine profiles between non‐asthmatic and asthmatic patients 4. Several cytokines have been described to play an important role in the pathogenesis of asthma, such as IL‐1β, TNF‐α, IL‐4, IL‐5 and chemokines (MCP‐1: monocyte chemoattract protein‐1; RANTES: regulated on activation normal T cell expressed and secreted) 2. In this regard, TNF‐α induces pro‐inflammatory events, apoptosis and emphysema in lung tissues 5, 6, 7, 8, 9. IL‐1 induces releases other cytokines and chemokines and eosinophil accumulation producing neutrophilic inflammation in respiratory disorders 10, 11, 12, 13. In addition, IL‐1β can induce increased production of IL‐4 and IL‐5 that increase IgE production 14, 15 involved in allergic airway inflammation 16, 17, 18, 19. MCP‐1 (monocyte chemoattract protein‐1) and RANTES (regulated on activation normal T cell expressed and secreted) are important in leucocyte recruitment to lung tissues during lung inflammatory events 1, 2, 20, 21. However, circulating cytokine response after viral or bacterial respiratory infections in asthmatic and non‐asthmatic status has been little studied. Increased circulating blood levels of several cytokines during viral respiratory tract infection have been reported 2. These cytokines may represent inflammatory markers with different profiles in asthmatic and non‐asthmatic patients. In addition to the asthmatic background, the infection type (viral or bacterial) and the site of respiratory infection (upper or lower) could be important to define the circulating cytokine profile. Therefore, the aim of this study was to describe changes in the level of several inflammatory (IL‐1β, TNF‐α) and Th2 (IL‐4, IL‐5) cytokines and chemokines (MCP‐1 and RANTES) in the systemic circulation during acute viral or bacterial infections in patients with asthmatic or non‐asthmatic background and their relationship with the respiratory infection site (upper and lower).
Material and methods
Patients
The study population was drawn from outpatient clinics. Male and female patients (n = 43) presenting clinical diagnosis of acute respiratory infection (ARI) were studied (Table 1). The inclusion criteria were those individuals who had at least one respiratory symptom, such as cough, wheeze, running nose or sneeze, and who were suspected by a professional physician to have respiratory infection. Patients with previous diagnosis of asthma according to the criteria of the Global Initiative for Asthma (GINA) Program 22, but without asthma manifestation (only asthma background), were selected and classified as asthmatic patients in this study (n = 14). Patients without antecedents of asthma (no asthmatic background) were classified as non‐asthmatic patients (n = 29). No patients were treated with antibiotics, anti‐allergic or steroid drugs when blood samples were obtained. Patients with asthmatic background did not present asthma manifestations at least one month previous to the moment of blood sample obtaining. Suggestive clinical and X‐rays findings were used for the diagnosis of pneumonia (asthmatic: n = 8; non‐asthmatic: n = 9) or bronchitis (asthmatic: n = 6; non‐asthmatic: n = 20). Pneumonia criteria were as follows: temperature >38 °C, cough with or without sputum, hemoptysis, pleuritic chest pain, myalgia, dyspnoea, fatigue, rales, rhonchi, wheezing, bronchial breath sounds, dullness to percussion and gastrointestinal symptoms. Regarding chest radiography, the presence of pleural effusion, lobar consolidation or diffuse infiltrates was observed. The physical examination of patients presenting acute bronchitis showed the presence or absence of fever and tachypnea, cough with or without sputum, wheezing, rhonchi and prolonged expiration. Evidence of lung consolidation was absent. Viral (only the presence of studied viruses: asthmatic: n = 5; non‐asthmatic: n = 16), bacterial (only the presence of studied bacteria: asthmatic: n = 4; non‐asthmatic: n = 7) and mixed (the presence of both studied bacteria and viruses: asthmatic: n = 5; non‐asthmatic: n = 6) infection was confirmed by the presence of the agent in specimens from nasopharynx and bronchoalveolar lavage. This study was performed in 43 patients who were obtained from a patient screen (161 patients). Only patients with confirmed presence of studied micro‐organisms in both types of samples were selected. Healthy individuals with similar age (15–72 years) and sex (n = 10) were studied as controls. Controls were individuals without respiratory symptoms, evidence of lung radiography alterations and negative micro‐organism results in bronchoscopy and nasal samples. We excluded individuals who had cardiac disease, immunodeficiency and chronic inflammatory disease. In addition, they were negative for studied micro‐organisms and had normal levels of IgE, normal chest radiography and non‐asthmatic background. Blood samples were obtained from patients at the time when specimens from nasopharynx and bronchoalveolar were obtained. Serum from controls and patients was stored at −70 °C until use. This work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The Ethics Committee of Instituto de Investigaciones Clínicas Dr. Américo Negrette and FONACIT (Caracas, Venezuela) approved this study, and written informed consent was obtained from all patients and controls prior to blood collection.
Table 1.
Characteristics and infection type of asthmatic and non‐asthmatic patients with acute respiratory infection and controls
| Parameters | Asthmatic | Non‐asthmatic | Controls |
|---|---|---|---|
| n | 14 | 29 | 10 |
| Age (years): range; mean: median | 17–70; 37.7; 34 | 18–77; 41.9; 43 | 15–72; 36.2; 27 |
| Gender (m/f) | 12/2 | 18/11 | 6/4 |
| Infection type (n) | |||
| Viral | 5 | 16 | |
| Bacterial | 4 | 7 | |
| Mixed | 5 | 6 | |
| Type of micro‐organisms (%)a | |||
| RSV | 42.86 | 34.48 | |
| Adenovirus | 14.29 | 31.03 | |
| Parainfluenza | 7.14 | 20.69 | |
| Influenza | 3.45 | ||
| SP | 21.43 | 20.69 | |
| KP | 14.21 | ||
| PA | 7.14 | ||
| EC | 7.14 | 6.90 | |
| HBS | 7.14 | 3.45 | |
| LP | 7.14 | ||
| SM | 3.45 | ||
| PA | 3.45 | ||
RSV, Respiratory syncytial virus;SP, Streptococcus pneumoniae; EC, Escherichia coli; KP, Klebsiella pneumoniae; PA, Pseudomona aeruginosa; SA, Staphylococcus aureus; HBS, Hemolytic group A beta Streptococcus; SM, Serratia marcenses; LP, Legionella pneumophila.
Values were obtained in asthmatic (n = 10) and non‐asthmatic (n = 29) including viral, bacterial and mixed infections.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Respiratory virus identification
Viral replication was demonstrated in HEp‐2 cell cultures (protocol ‐520‐I; National Institute for Health, USA) 23. Cells were grown to 50% confluence in Eagle's minimum essential medium (MEM) containing 7% FBS and 1% antibiotic/antimycotic. Nasopharynx and bronchoalveolar samples from patients were sonicated and centrifuged, and supernatants (200 μl) were added to HEp‐2 cell cultures. Cultures were incubated for 1 h, and then, 300 μl of MEM containing 10% FBS and 1% antibiotic/antimycotic were added and incubated at 37 °C in 5% CO2 for 96–120 h. Viral cell infection (respiratory syncytial virus, parainfluenza 1, 2 and 3, influenza A and B and adenovirus) was determined by direct immunofluorescence using a commercial kit (Light Diagnostics SimulFluor Respiratory Screen Kit; Chemicon Internacional, Temecula, CA, USA). Infected and uninfected culture (negative control) slides were incubated with FITC‐conjugated antibodies against viral antigens for 1 h; after washing, positive fluorescence in nuclei or nuclei/cytoplasm represented viral infection. Positive antigen control slides provided by commercial assay were used as a positive control. Samples were analysed one time to determine the presence of viruses.
Bacterial identification
Nasopharynx and bronchoalveolar samples from patients were incubated on McConkey agar, sheep blood agar with kanamycin or chocolate agar and incubated for 14–48 h at 37 °C under aerobic, microaerobic or anaerobic conditions for the bacterial presence. Bacterial species were identified using conventional bacteriological techniques 24, 25. The presence of serum IgG anti‐atypical bacteria (Legionella pneumophilla, Coxiella burnetti, Chlamydia trachomatis, Chlamydophila pneumoniae y Micoplasma pneumoniae) was determined using an available commercial kit (Pneumoslide IgG; Vircell, SL, Granada, Spain).
Quantification of serum IgE and IL‐1 β, TNF‐α, IL‐4, IL‐5, MCP‐1 and RANTES
IL‐1 β, TNF‐α, IL‐4, IL‐5, MCP‐1 and RANTES contents were measured using commercial available ELISA kits (TNF‐α, IL‐4 and IL‐5; Diaclone, Fleming, France; MCP‐1; Endogen, Rockford, IL, USA; IL‐1 β and RANTES; Alpco Diagnostics, Salem, NH, USA), and the results were expressed as pg/ml. Serum IgE content was determined by ELISA (Calbiochem Inc., San Diego, CA, USA). ELISA analyses were performed by triplicate.
Statistical analysis
Values were expressed as mean ±standard deviation. Data analysis was performed using a nonparametric ANOVA (Kruskal–Wallis test) and Dunn's multiple comparisons test. Two‐tailed P values <0.05 were considered statistically significant.
Results
Increased levels of IgE in asthmatic patients (n = 14) compared with non‐asthmatic patients (n = 29) and controls (n = 10) were observed (asthmatic: 380.50 ± 13.22*; non‐asthmatic: 56.80 ± 9.29; controls: 18.49 ± 5.51 IU/ml; *P < 0.01 versus control and non‐asthmatic patients). Respiratory syncytial virus, adenovirus, parainfluenza and influenza in studied patients were identified (asthmatic: n = 5; non‐asthmatic: n = 16). Several bacteria were found: Streptococcus pneumoniae, Escherichia coli, Klebsiella pneumoniae, Pseudomona aeruginosa, Staphylococcus aureus, Hemolytic group A beta Streptococcus, Serratia marcenses and Legionella pneumophila (asthmatic: n = 9; non‐asthmatic: n = 13). Percentage of infected patient according to asthmatic and non‐asthmatic status is shown in Table 1.
In general, the serum levels of IL‐1β, TNF‐α, IL‐4, IL‐5, MCP‐1 and RANTES in patients with acute respiratory infections were found increased when compared with healthy controls. Cytokine expression was similar in asthmatic and non‐asthmatic patients, and only chemokines (MCP‐1 and RANTES) in asthmatic patients (n = 5) compared with control (n = 10) and non‐asthmatic patients (n = 16) were significantly increased (P < 0.05) (Fig. 1). When subgroups were studied according to microorganism type infection, patients infected by virus (asthmatic: n = 5; non‐asthmatic: n = 16) and with mixed infection (bacterium and virus: asthmatic: n = 5; non‐asthmatic: n = 6) showed increased production of cytokines in asthmatic and non‐asthmatic patients compared to controls, but only asthmatic patients showed elevated values of chemokines (Figs 2 and 3). Bacterial infection induced systemic increment of cytokines and chemokines in asthmatic (n = 4) and non‐asthmatic (n = 7) patients compared with healthy controls; however, TNF‐α and RANTES were found increased only in asthmatic patients (Fig. 4).
Figure 1.
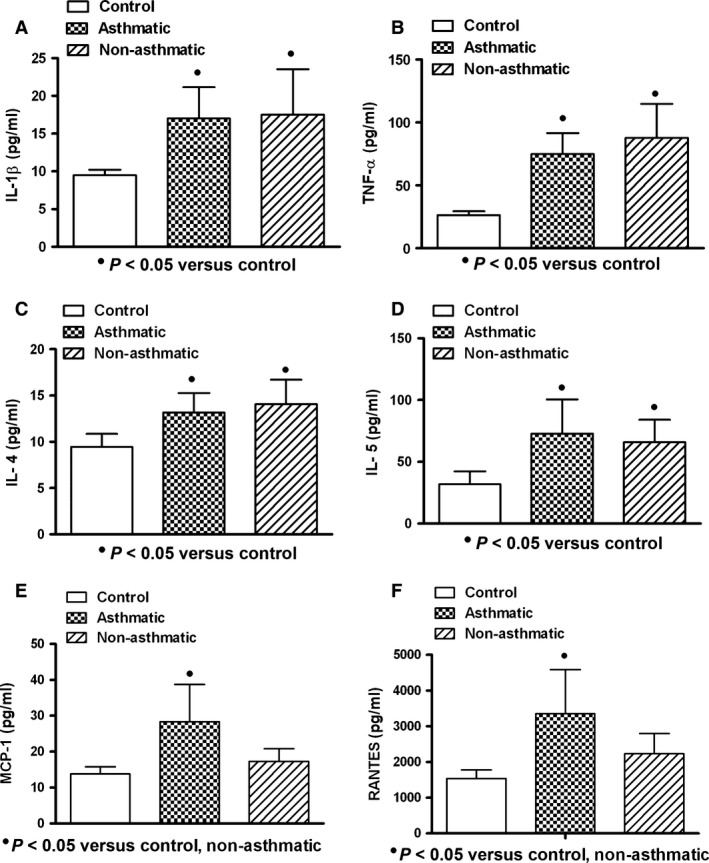
Expression of cytokine/chemokine in serum of patients with acute respiratory infection. Similar increased levels of interleukin‐1 beta (IL‐1β), tumour necrosis factor‐alpha (TNF‐α) and interleukins 4 and 5 (IL‐4, IL‐5) in asthmatic and non‐asthmatic patients were observed (A, B, C, D). Higher levels of monocyte chemoattractant protein‐1 (MCP‐1) and regulated on activation normal T cell expressed and secreted factor (RANTES) in asthmatic patients were found (E, F). Asthmatic (n = 14); non‐asthmatic (n = 29); control (n = 10). Data were analysed using a nonparametric ANOVA (Kruskal–Wallis test) and Dunn's multiple comparisons test. Values were expressed as mean ± standard deviation.
Figure 2.
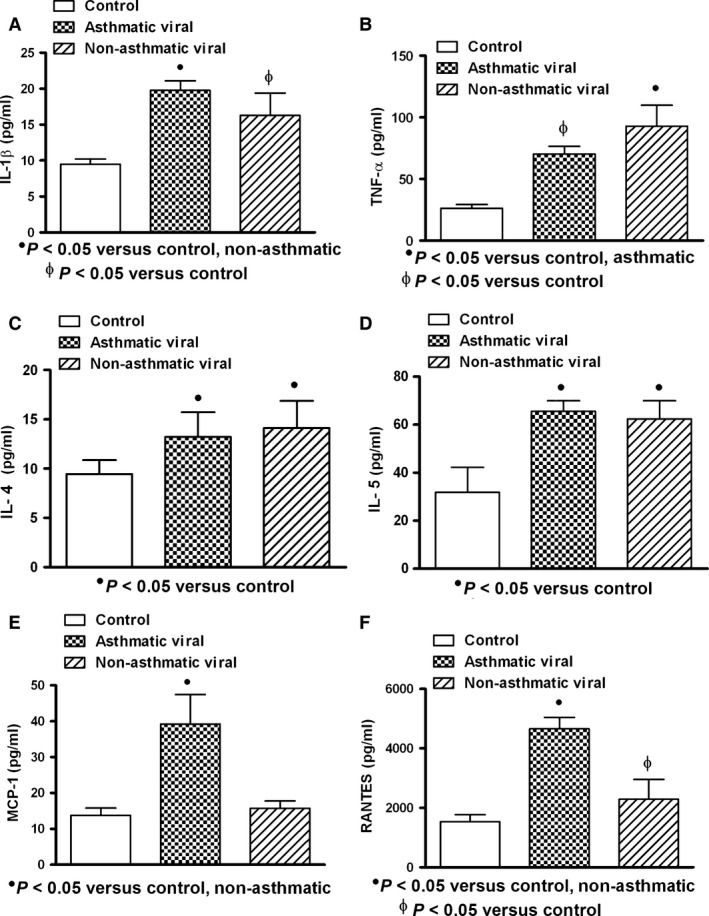
Expression of cytokine/chemokine in serum of patients with acute viral respiratory infection. Increased levels of interleukin‐1 beta (IL‐1β), tumour necrosis factor‐alpha (TNF‐α) and interleukins 4 and 5 (IL‐4 and IL‐5) in asthmatic and non‐asthmatic patients were observed (A, B, C, D). Higher levels of TNF‐α in non‐asthmatic patients than those observed in asthmatic patients were found (B). Monocyte chemoattractant protein‐1 (MCP‐1) and regulated on activation normal T cell expressed and secreted factor (RANTES) in asthmatic patients were observed increased (E, F). Asthmatic (n = 5); non‐asthmatic (n = 16); control (n = 10). Data were analysed using a nonparametric ANOVA (Kruskal–Wallis Test) and Dunn's multiple comparisons test. Values were expressed as mean ± standard deviation.
Figure 3.
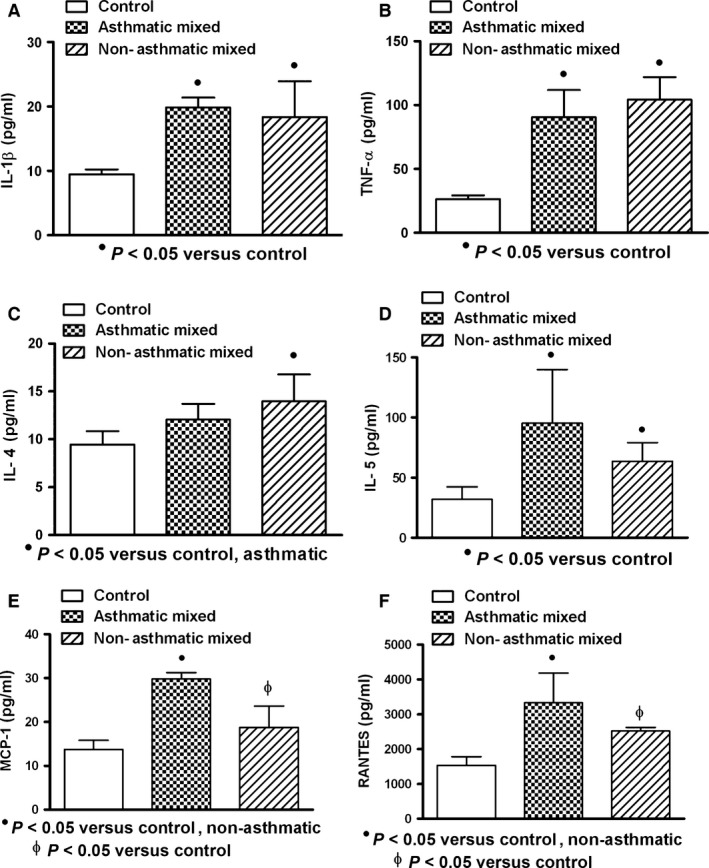
Expression of cytokine/chemokine in serum of patients with acute mixed (viral and bacterial) respiratory infection. Increased levels of interleukin‐1 beta (IL‐1β), tumour necrosis factor‐alpha (TNF‐α), interleukin 5 (IL‐5), monocyte chemoattractant protein‐1 (MCP‐1) and regulated on activation normal T cell expressed and secreted factor (RANTES) in asthmatic and non‐asthmatic patients were observed (A, B, D, E, F). Higher levels of IL‐4 in non‐asthmatic patients than those observed in asthmatic patients were found (C). Asthmatic (n = 5); non‐asthmatic (n = 6); control (n = 10). Data were analysed using a nonparametric ANOVA (Kruskal–Wallis Test) and Dunn's multiple comparisons test. Values were expressed as mean ± standard deviation.
Figure 4.
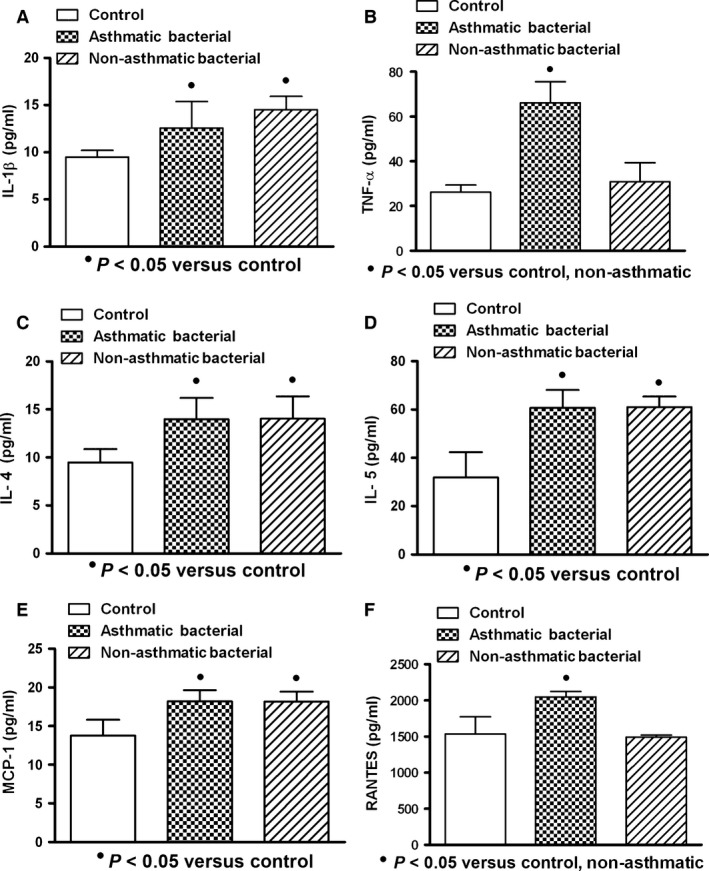
Expression of cytokine/chemokine in serum of patients with acute respiratory bacterial infection. High levels of interleukin‐1 beta (IL‐1β), interleukins 4 and 5 (IL‐4 and IL‐5) and monocyte chemoattractant protein‐1 (MCP‐1) in asthmatic and non‐asthmatic patients were observed (A, C, D, E). Tumour necrosis factor‐alpha (TNF‐α) and regulated on activation normal T cell expressed and secreted factor (RANTES) in asthmatic patients were observed incremented (B, F). Higher levels of TNF‐α in non‐asthmatic patients than those observed in asthmatic patients were found (B). Asthmatic (n = 4); non‐asthmatic (n = 7); control (n = 10). Data were analysed using a nonparametric ANOVA (Kruskal–Wallis test) and Dunn's multiple comparisons test. Values were expressed as mean ± standard deviation.
In general (values from asthmatic and non‐asthmatic patients with ARI), viral and mixed infections were better cytokine/chemokine inducers than bacterial infection (Fig. 5); however, similar inducer effect on IL‐4 and IL‐5 by all studied infections was observed (Fig. 5C,D). Asthmatic and non‐asthmatics patients with pneumonia (asthmatic: n = 8; non‐asthmatic: n = 9) (Fig. 6) or bronchitis (asthmatic: n = 6; non‐asthmatic: n = 20) (Fig. 7) showed increment on studied cytokine/chemokine when compared with healthy controls. However, patients with asthmatic background and pneumonia showed MCP‐1 and RANTES levels increased compared with control and non‐asthmatic patients (Fig. 6E,F), and only asthmatic patients with bronchitis had elevated levels of MCP‐1 and RANTES (Fig. 7E,F).
Figure 5.
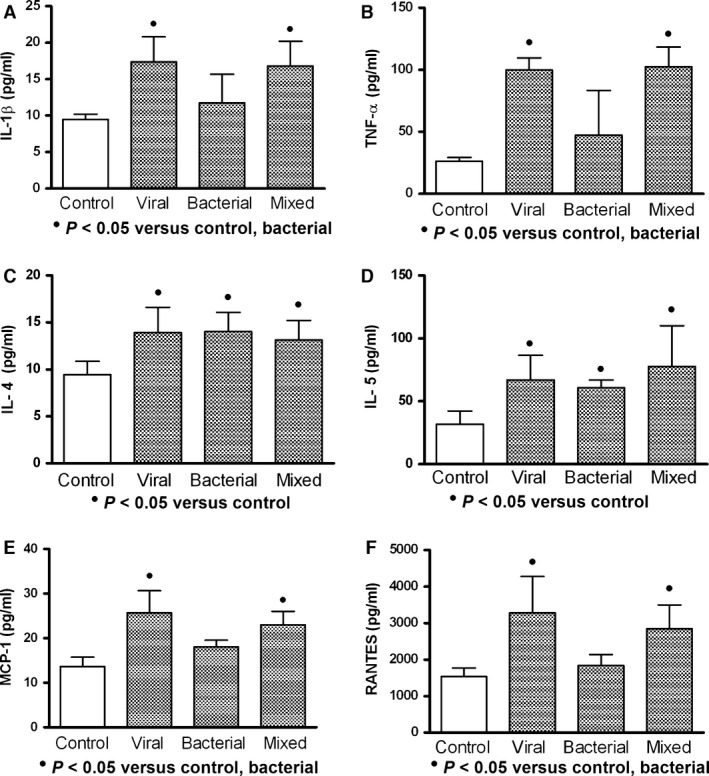
Effect of different acute respiratory infections in the cytokine/chemokine production. Viral and mixed (viral and bacterial) infections induced higher production of interleukin‐1 beta (IL‐1β), tumour necrosis factor‐alpha (TNF‐α), monocyte chemoattractant protein‐1 (MCP‐1) and regulated on activation normal T cell expressed and secreted factor (RANTES) than those observed in bacterial infection (A, B, E, F). High values of interleukins 4 and 5 (IL‐4 and IL‐5) were observed, but they remained similar in all types of infections (C, D). Viral (n = 21); bacterial (n = 11); mixed (n = 11); control (n = 10). These data were obtained from asthmatic and non‐asthmatic patients. Data were analysed using a nonparametric ANOVA (Kruskal–Wallis test) and Dunn's multiple comparisons test. Values were expressed as mean ± standard deviation.
Figure 6.
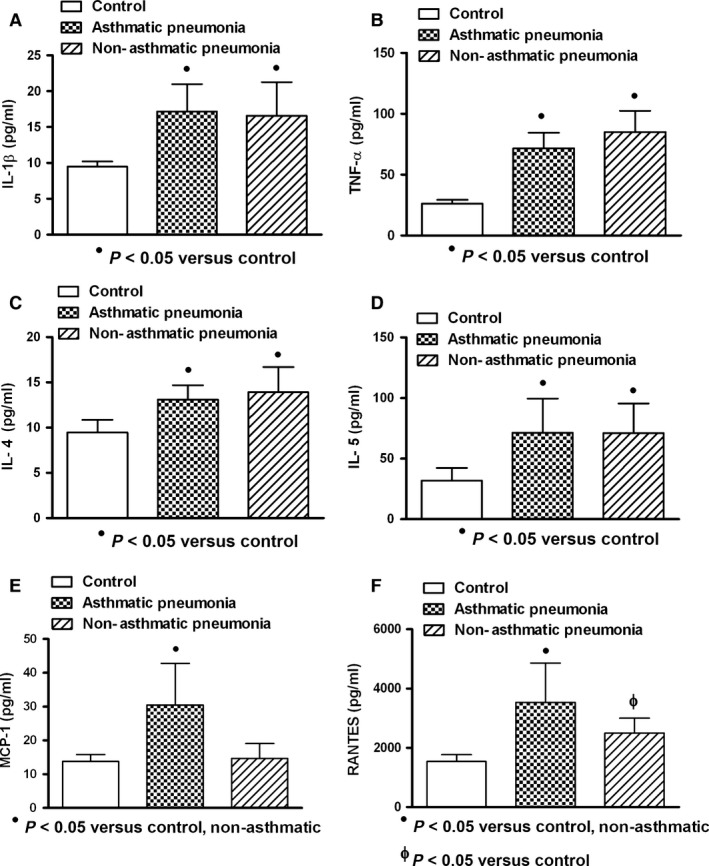
Cytokine/chemokine production in asthmatic and non‐asthmatic patients with pneumonia. Similar increased levels of interleukin‐1 beta (IL‐1β), tumour necrosis factor‐alpha (TNF‐α) and interleukins 4 and 5 (IL‐4 and IL‐5) in asthmatic and non‐asthmatic patients were observed (A, B, C, D). Higher levels of monocyte chemoattractant protein‐1 (MCP‐1) and regulated on activation normal T cell expressed and secreted factor (RANTES) in asthmatic patients were found (E, F). Asthmatic (n = 8); non‐asthmatic (n = 9); control (n = 10). Data were analysed using a nonparametric ANOVA (Kruskal–Wallis test) and Dunn's multiple comparisons test. Values were expressed as mean ± standard deviation.
Figure 7.
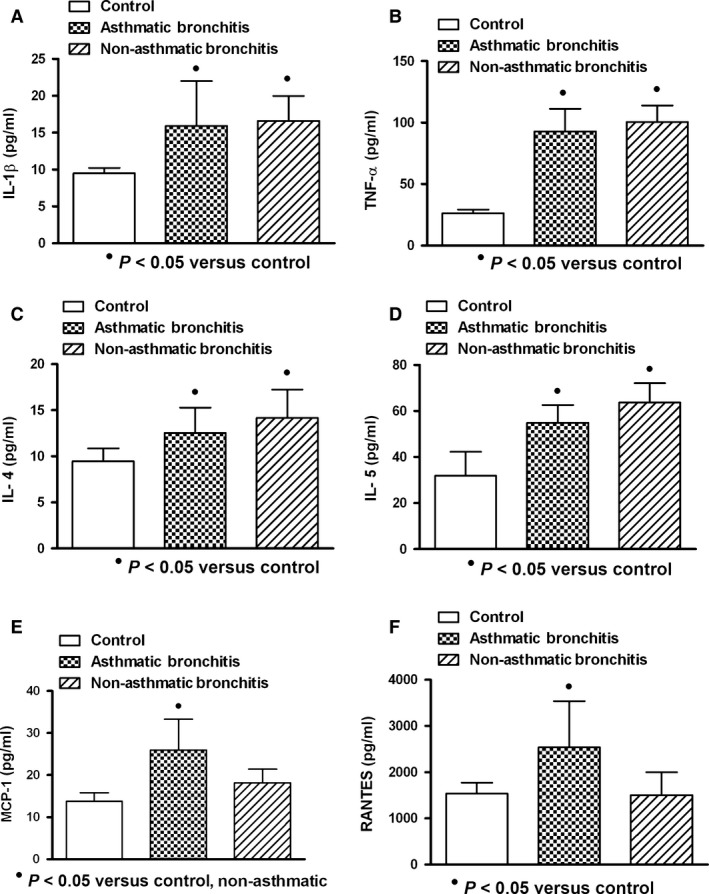
Cytokine/chemokine production in asthmatic and non‐asthmatic patients with bronchitis. Increased levels of interleukin‐1 beta (IL‐1β), tumour necrosis factor‐alpha (TNF‐α) and interleukins 4 and 5 (IL‐4 and IL‐5) were observed. They remained similar in asthmatic and non‐asthmatic patients (A, B, C, D). Monocyte chemoattractant protein‐1 (MCP‐1) in asthmatic patients was found increased. Regulated on activation normal T cell expressed and secreted factor (RANTES) was found similar in patients compared to control values (E, F). Asthmatic (n = 6); non‐asthmatic (n = 20); control (n = 10). Data were analysed using a nonparametric ANOVA (Kruskal–Wallis test) and Dunn's multiple comparison test. Values were expressed as mean ± standard deviation.
Discussion
Asthma is a complex chronic disease of the lung with an incidence growing at all ages. The complexity of this disease is partly due to the environmental insults such as allergens and microbial infections that play differential roles in the pathogenesis. Microbes may play important roles in the exacerbation of asthma 3. To determine differential circulating cytokine profiles that could be related to the interaction of asthma–microbes, asthmatic and non‐asthmatic patients with microbial acute respiratory infection were studied. Patient with and without asthmatic background had similar systemic cytokine response during microbial acute respiratory infection; however, only asthmatic patients had significant increment of chemokines, suggesting a role of asthmatic background in this differential response. As chemokines are important for the recruitment and tissue localization of both immune and structural cells in the lung, they can be relevant in asthma exacerbation 26. During this study, patients with asthmatic background had no clinical manifestation of asthma, suggesting that increased level of chemokines could be an early manifestation of asthma exacerbation.
Asthma is characterized by airway inflammation, airway wall remodelling and bronchial hyper‐responsiveness. Activated T helper type 2 (Th2) lymphocytes, eosinophils and activated mast cells are the characteristic features to make the difference between atopic versus non‐atopic asthma 27, 28, 29. However, the pro‐inflammatory and Th2 cytokine responses in asthmatic and non‐asthmatic patients after lung microbial infection were similar during this study, suggesting that systemic Th2 and pro‐inflammatory responses are common aspects of lung infection. The source of increased levels of cytokine/chemokines in the patient's circulation after lung infection could involve lung tissues and/or systemic tissues. The respiratory tract is a frequent primary site of microbial infections resulting in the development of acute respiratory distress syndrome. During the lung inflammatory events, the alveolar compartmentalization could be lost, allowing the passage of released cytokines to the bloodstream by any other organ to the pulmonary endothelium or inversely from the lung to the bloodstream. Cytokines such as IL‐1, TNF‐α and IL‐8 have important roles in the lung dysfunction 30. In this study, patients with microbial lung infection had increased serum levels of pro‐inflammatory cytokines (IL‐1β and TNF‐α), Th2 cytokines (IL‐4 and IL‐5) and chemokines (MCP‐1 and RANTES), suggesting a complex immune interaction during microbial respiratory infection. The source of those cytokines may be cells localized in lung tissue. In this regard, epithelial cells, mast cells, basophils, monocyte/macrophages and lymphocytes could secrete diverse types of cytokines and chemokines (IL‐1, TNF, IL‐4, IL‐5, IL8, MCP‐1, RANTES) during lung tissue–microbes interactions 21, 31, 32. However, it cannot rule out the role of other cells in other tissues.
Potential viral pathogens in acute respiratory infection include respiratory syncytial virus (RSV), human rhinovirus (HRV), human metapneumovirus, human parainfluenza virus (HPIV), human bocavirus, influenza viruses, adenoviruses and enterovirus. Both RSV and HRV have been most commonly linked to the exacerbation of asthma as well as to the pathogenesis 32. Infection by parainfluenza and influenza viruses has generally been detected in asthma exacerbations 33. The presence of some of those pathogens (RSV, adenovirus, HPIV and influenza) was found in this study; however, they were not associated with clinical manifestations of asthma. Patients with ARI were mainly infected by RSV. This virus is involved in serious lower respiratory tract disease in children and can induce the produce of several cytokines (IL‐6, IL‐1, TNF‐α, IL‐8 and GM‐CSF), chemokines (IL‐8, IP‐10, MCP‐1 and RANTES) and chemokine receptor expressions in vivo and in vitro 34, 35, 36. Accordingly, in this study, serum from RSV‐infected patients had increased levels of IL‐1β, TNF‐α, IL‐4, IL‐5, MCP‐1 and RANTES. As similar serum cytokine/chemokine profile was observed in adenovirus and parainfluencia virus infections, this viral cytokine/chemokine inducer effect probably is a common feature of respiratory virus infection. In addition, infection with RSV could be a risk factor for further lung diseases, as the persistence of cytokines and chemokines in fully recovered RSV‐infected patients can provide a substratum for the development of subsequent asthma 37.
Recent studies have begun to introduce typical bacteria such as Streptococcus pneumonia, as well as atypical bacteria such as Chlamydia pneumonia as being responsible for acute wheezing in children 38. Streptococcus pneumonia was the main pathogen found in bacterial infected patients. This bacterium is the most common cause of pneumonia in the world 39, 40, that when risk factors weaken the protective immunity, pneumococcus can cause serious diseases 40. Growing evidence indicates that the inflammasome plays a key role in the pathogenesis of acute and chronic respiratory diseases. The inflammasome can be activated by Streptococcus pneumonia, inducing the chronic inflammation of the airways of patients with asthma and chronic obstructive pulmonary disease 41. This could be a mechanism to induce the bacterial cytokine/chemokine production observed in this study. However, cytokines induced by bacterial respiratory infection and type I interferon induced by virus (mixed infection) can be protective for Streptococcus pneumonia infection 42, 43.
The cytokine/chemokine response pattern observed in this study was not related to the microbial infection type (viral, bacterial or mixed); however, bacterial infection had lower cytokine/chemokine inducer effect. We have no clear explanation for this finding; however, it has been reported that bacteria exert differential effects on eosinophils (important cell in respiratory pathology), resulting in the exacerbation or the inhibition of inflammation in lung infection 44. As patients with ARI were infected by different bacteria, the serum values observed in bacterial infected patients could represent the result of those responses. Values found in mixed‐infected patients (viral and bacterial) showed similar values than those observed in viral infection, suggesting that the higher viral cytokine inducer effect was involved in cytokine/chemokine production in mixed infection.
Cytokine/chemokines can have harmful effects on the lung regardless of cytokine production sources. In this study, increased expression of IL‐1β, TNF‐α, IL‐4, IL‐5, MCP‐1 and RANTES in the serum of patients with viral and bacterial respiratory infection could be involved in the clinical manifestations observed in the patients. These cytokines could have deleterious effect in the lung and other tissues. TNF‐α can induce the activation of several pro‐inflammatory events 5, 6, 7, 8 and apoptosis in lung tissues resulting in emphysema 9. IL‐1 induces neutrophilic inflammatory responses in respiratory disorders 10 and releases other cytokines and chemokines 11, 12. In addition, IL‐1β induces eosinophil accumulation, and it is a Th2 and B cell growth factor 13, properties that could be related to the activation of Th2 profile observed in this study, as shown by the increased production of IL‐4 and IL‐5. These cytokines stimulate B‐lymphocytes to produce IgE 14, 15 and increase allergic airway inflammation 16, 17, 18. Increased levels of circulating chemokines found in asthmatic patients could reflect lung chemokine production that may be important in leucocyte recruitment to lung tissues. RANTES is involved in the chemoattraction of eosinophils, monocytes and T‐lymphocytes, and therefore, it has high relevance in asthma 21. MCP‐1 is involved in the recruitment of regulatory and effector leucocytes (monocytes, lymphocytes and basophils) into tissues 20. Therefore, chemokines could play an important role in asthma pathogenesis and in ARI. In addition, the increased systemic concentration of those cytokines/chemokines could activate circulating leucocytes with further deleterious effects in the lung 1, 2.
Increased serum concentration of IgE associated with increased production of circulating IL‐4 and IL‐5 was found in patients with asthmatic background, suggesting a possible role of IgE in the increased levels of those cytokines and in the asthma pathogenesis. In this regard, IgE has a role in the allergic inflammation, involving basophil and mast cell degranulation, activation of monocyte/macrophages and stimulation of Th2 cells, inducing local infiltration of IL‐4 and IL‐5 secreting Th2‐cells and amplifying tissue inflammation 19.
Previous reports have shown differences between asthma and other pulmonary diseases regarding cytokine profiles. Bronchoalveolar lavage cytokine profiles between chronic obstructive pulmonary disease (COPD) and asthma have been reported. The profile of cytokine expression in COPD is different from that observed in asthma. Infiltration of eosinophils and Th2‐cells involving IL‐4, IL‐5 and IL‐13 productions is usually found in asthma. IL‐8, IL‐1 and TNF‐α play more prominent roles in COPD 4. In our study, the bronchoalveolar lavage cytokine profile was not analysed; however, circulating cytokine profiles showed no differences in the expression of pro‐inflammatory and Th2 cytokine profiles in both asthmatic and non‐asthmatic patients, suggesting similar immune response during microbial infection. Only high levels of chemokines in asthmatic patients were observed, suggesting an increased cellular infiltration (monocytes, neutrophils or eosinophils) at tissue level. In this regard, the role of chemokines in microbial‐associated asthma exacerbations has been reported 36.
In conclusion, the present study demonstrates that serum cytokine profiles in asthmatic and non‐asthmatic patients with viral and/or bacterial respiratory infection were similar. Asthmatic status or the anatomic places of infection (bronchitis or pneumonia) were not relevant in the pro‐inflammatory and Th2 cytokine profiles, except that high values of chemokines were observed in asthmatic patients. Bacterial and viral respiratory infections did not induce different circulating cytokine profiles; however, lower cytokine/chemokine inducer effect by bacterial infection was observed. The precise mechanism, regarding the effect of those systemic cytokines in driving microbial respiratory infection processes, remains elusive.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgment
This work was supported by grants from Fondo de Investigación de la Seguridad Social (Spain), Consejería de Educación, Comunidad de Madrid, MITIC‐CM (S‐2010/BMD‐2502), Instituto de Salud Carlos III, MEC (PIO51871, CIBERehd) and CONDES (Maracaibo, Venezuela) and by Instituto de Investigaciones Clínicas “Dr. Américo Negrette”, Facultad de Medicina, Universidad del Zulia, Maracaibo, Venezuela. The funding source had no involvement in any aspect of this manuscript.
References
- 1. Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther 2008;117:313–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heymann PW, Carper HT, Murphy DD et al Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004;114:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brar T, Nagaraj S, Mohapatra S. Microbes and asthma: the missing cellular and molecular links. Curr Opin Pulm Med 2012;18:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J 2001;18:50s–9s. [PubMed] [Google Scholar]
- 5. Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease‐9 and tissue inhibitor of metalloprotease‐1 from alveolar macrophages in cigarette smokers: regulation by interleukin‐10. Am J Respir Crit Care Med 2000;162:1355–60. [DOI] [PubMed] [Google Scholar]
- 6. Riise GC, Larsson S, Lofdahl CG, Andersson BA. Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur Respir J 1994;7:1673–7. [DOI] [PubMed] [Google Scholar]
- 7. Bem RA, Bos AP, Wosten‐van Asperen RM et al Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection. Am J Respir Cell Mol Biol 2010;42:697–705. [DOI] [PubMed] [Google Scholar]
- 8. Pryhuber GS, Bachurski C, Hirsch R, Bacon A, Whitsett JA. Tumor necrosis factor‐alpha decreases surfactant protein B mRNA in murine lung. Am J Physiol 1996;270:L714–21. [DOI] [PubMed] [Google Scholar]
- 9. Morissette MC, Parent J, Milot J. Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know. Int J Chron Obstruct Pulmon Dis 2009;4:19–31. [PMC free article] [PubMed] [Google Scholar]
- 10. Henderson C, Goldbach‐Mansky R. Autoinflammatory diseases: new insights into clinical aspects and pathogenesis. Curr Opin Rheumatol 2010;22:567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hal M. Inflammasome and IL‐1β‐mediated disorders. Curr Allergy Asthma Rep 2010;10:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makó V, Czúcz J, Weiszhár Z. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL‐1β, TNF‐α, and LPS. Cytometry A 2010;77:962–70. [DOI] [PubMed] [Google Scholar]
- 13. Lampinen M, Carlson M, Hakansson LD. Cytokine‐regulated accumulation of eosinophils in inflammatory disease. Allergy 2004;59:793–805. [DOI] [PubMed] [Google Scholar]
- 14. Kumar RK, Hitchins MP, Foster PS. Epigenetic changes in childhood asthma. Dis Model Mech 2009;2:549–53. [DOI] [PubMed] [Google Scholar]
- 15. Madore AM, Laprise C. Immunological and genetic aspects of asthma and allergy. J Asthma Allergy 2010;3:107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levine SJ, Wenzel SE. The role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes: Are we getting closer? Ann Intern Med 2010;152:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luzina IG, Keegan AD, Heller NM, Rook GA, Shea‐Donohue T, Atamas SP. Regulation of inflammation by interleukin‐4: a review of “alternatives”. J Leukoc Biol 2012;92:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koyasu S, Moro K. Role of innate lymphocytes in infection and inflammation. Front Immunol 2012;3:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung DY. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol 1995;96:302–18. [DOI] [PubMed] [Google Scholar]
- 20. Rose CE Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP‐1) in inflammatory disorders of the lung. Microcirculation 2003;10:273–88. [DOI] [PubMed] [Google Scholar]
- 21. Conti P, DiGioacchino M. MCP‐1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc 2001;22:133–7. [DOI] [PubMed] [Google Scholar]
- 22. Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program . The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–78. [DOI] [PubMed] [Google Scholar]
- 23. Falsey AR, Formica MA, Walsh EE. Diagnostic of respiratory syncytial virus infection: comparison of reverse transcription‐PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 2002;40:817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finegold S, Sheperd W, Spaulding E. Manual of Microbiology Diagnostic. México: Manual Moderno, 1998. ISBN: 950060745X 97895000607452 [Google Scholar]
- 25. Koneman E, Allen S, Dowell V. Microbiology Diagnostic. Buenos Aires: Médica Panamericana, 1992. ISBN: 978‐950‐06‐0895‐4 [Google Scholar]
- 26. Palmqvist C, Wardlaw AJ, Bradding P. Chemokines and their receptors as potential targets for the treatment of asthma. Br J Pharmacol 2007;151:725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Humbert M, Durham SR, Ying S et al IL‐4 and IL‐5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against ‘intrinsic’ asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med 1996;154:1497–504. [DOI] [PubMed] [Google Scholar]
- 28. Humbert M, Grant JA, Taborda‐Barata L et al High‐affinity IgE receptor (FcepsilonRI)‐bearing cells in bronchial biopsies from atopic and nonatopic asthma. Am J Respir Crit Care Med 1996;153:1931–7. [DOI] [PubMed] [Google Scholar]
- 29. Ying S, Humbert M, Barkans J et al Expression of IL‐4 and IL‐5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol 1997;158:3539–44. [PubMed] [Google Scholar]
- 30. Costa EL, Schettino IA, Schettino GP. The lung in sepsis: guilty or innocent? Endocr Metab Immune Disord Drug Targets 2006;6:213–6. [DOI] [PubMed] [Google Scholar]
- 31. Tirado R, Ortega A, Sarmiento RE, Gómez B. Interleukin‐8 mRNA synthesis and protein secretion are continuously up‐regulated by respiratory syncytial virus persistently infected cells. Cell Immunol 2005;233:61–71. [DOI] [PubMed] [Google Scholar]
- 32. Fujitsuka A, Tsukagoshi H, Arakawa M et al A molecular epidemiological study of respiratory viruses detected in Japanese children with acute wheezing illness. BMC Infect Dis 2011;11:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McErlean P, Greiman A, Favoreto S, Avila C. Viral diversity in asthma. Immunol Allergy Clin North Am 2010;30:481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morrison PT, Sharland M, Thomas LH et al Chemokine‐receptor upregulation and disease severity in respiratory syncytial virus infection. Clin Immunol 2008;128:85–93. [DOI] [PubMed] [Google Scholar]
- 35. Hackett TL, Singhera GK, Shaheen F et al Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to RSV and air pollution. Am J Respir Cell Mol Biol 2011;45:1090–100. [DOI] [PubMed] [Google Scholar]
- 36. Kallal LE, Lukacs NW. The role of chemokines in virus‐associated asthma exacerbations. Curr Allergy Asthma Rep 2008;8:443–50. [DOI] [PubMed] [Google Scholar]
- 37. Zeng R, Li C, Li N, Wei L, Cui Y. The role of cytokines and chemokines in severe respiratory syncytial virus infection and subsequent asthma. Cytokine 2011;53:1–7. [DOI] [PubMed] [Google Scholar]
- 38. Bisgaard H, Hermansen M, Bønnelykke K et al Association of bacteria and viruses with wheezy episodes in young children: prospective nested birth cohort study. BMJ 2010;341:c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hollingshead SK, Briles DE. Streptococcus pneumoniae: new tools for an old pathogen. Curr Opin Microbiol 2001;4:71–7. [DOI] [PubMed] [Google Scholar]
- 40. van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009;374:1543–56. [DOI] [PubMed] [Google Scholar]
- 41. dos Santos G, Kutuzov MA, Ridge KM. The inflammasome in lung diseases. Am J Physiol Lung Cell Mol Physiol 2012;303:L627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of γδ T cells. J Virol 2012;86:12304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang H, Ko HJ, Yang JY et al Interleukin‐1 promotes coagulation, which is necessary for protective immunity in the lung against Streptococcus pneumoniae infection. J Infect Dis 2013;207:50–60. [DOI] [PubMed] [Google Scholar]
- 44. Hosoki K, Nakamura A, Kainuma K et al Differential activation of eosinophils by bacteria associated with asthma. Int Arch Allergy Immunol 2013;161:16–22. [DOI] [PubMed] [Google Scholar]


