Abstract
Tumour necrosis factor (TNF), an important proinflammatory cytokine, plays a role in the regulation of cell differentiation, proliferation and death, as well as in inflammation, innate and adaptive immune responses, and also implicated in a wide variety of human diseases. The presence of DNA sequence variations in regulatory region might interfere with transcription of TNF gene, influencing the circulating level of TNF and thus increases the susceptibility to human diseases (infectious, cancer, autoimmune, neurodegenerative and other diseases). In this review, we have comprehensively analysed various published case–control studies of different types of human diseases, in which TNF gene polymorphism played a role, and computationally predicted several single nucleotide polymorphisms (SNPs) lie in transcription factor–binding sites (TFBS) of transcription factors (TFs). It has been observed that TNF enhancer polymorphism is implicated in several diseases, and TNF rs1800629 and rs361525 SNPs are the most important in human disease susceptibility as these might influence the transcription of TNF gene. Thirty‐two SNPs lies in TFBS of 20 TFs have been detected in the TNF upstream region. It has been found that TNF enhancer polymorphism influences the serum level of TNF in different human diseases and thus affects the susceptibility to diseases. The presence of DNA sequence variation in TNF gene causes the modification of transcriptional regulation and thus responsible for association of susceptibility/resistance with human diseases.
Introduction
Tumour necrosis factor (TNF) cytokine, produced as the part of host defence against infection. This cytokine is involved in multiple inflammatory and immune responses and plays role in the pathogenesis of many autoimmune and infectious diseases. TNF gene is located on chromosome 6 in the class III region of the major histocompatibility complex (MHC) and is flanked by the lymphotoxin ‘a’ and ‘b’ genes (Fig. 1). A close linkage among HLA class I (HLA‐B), class II (HLA‐DR) and TNF genes has been reported [1]. TNF gene is tightly regulated at the level of transcription [2, 3].
Figure 1.
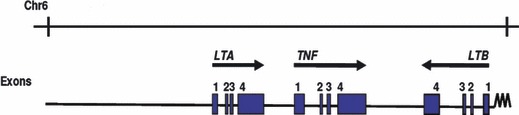
Representation of tumor necrosis factor, lymphotoxin alpha and beta gene.
DNA sequence variation in promoter regions of genes encoding cytokines influences susceptibility to infection and has been associated with a large number of complex human diseases. Reports indicated that polymorphism in the 5′ regulatory region of the gene has been correlated with many infectious and inflammatory diseases [4, 5]. The association of TNF rs1799964 and rs1800630 polymorphisms with advanced‐stage endometriosis in the Korean population have been reported. The TNF rs1800750 polymorphism affects the binding of TF OCT‐1 and alters the DNA–protein interaction. The in vitro study of TNF promoter polymorphism function was stimulated by several case–control studies of the polymorphism in relation to human disease [6]. The polymorphism rs361525 has been associated with insulin resistance syndrome and obesity [7], and rs1800629 has been associated with various inflammatory and autoimmune diseases [8, 9, 10, 11, 12, 13]. Associations between polymorphism (rs1799964, rs1799724, rs1800630) and immune‐mediated diseases such as rheumatoid arthritis and Crohn’s disease (CD) have been reported [14, 15]. Limited reports are available showing that variants (rs1800629 and rs361525) are involved in the regulation of cytokine production [16]. The rs1799964 polymorphism has been associated with extra intestinal manifestations of CD including uveitis, erythema nodosum and large joint arthropathy [17] and Crohn’s disease itself [16]. It is clear that TNF enhancer polymorphism is implicated in several case–control studies. In the present review, the literature regarding the role of TNF‐α polymorphism has been studied with respect to different human diseases and different populations. Several single nucleotide polymorphisms (SNPs) in TFBS of different TFs have been predicted computationally. The purpose of this review is to provide an overview of what is currently known about the role of gene level polymorphism of TNF and susceptibility/resistance to human diseases and to highlight directions that are likely to see major advances.
Infectious diseases
Bacterial diseases
Pulmonary tuberculosis. Mycobacterial tuberculosis is the leading cause of mortality in India as well as in the world. Approximately one‐third of the world’s population is suffering from Mycobacterial diseases [18, 19]. Pulmonary tuberculosis, caused by M. tuberculosis, is a granulomatous disease of the lungs. The host genetic factor plays a significant role in determining susceptibility to developing the active form of the disease [20, 21]. A number of genes have been identified, which are important in tuberculosis [22, 23, 24]. Elevated serum tumour necrosis factor‐α (sTNF‐α) levels have been reported in patients with advanced tuberculosis in comparison with those with mild tuberculosis and healthy controls. Several polymorphisms within the promoter region of TNF‐α and the intron 1 of LT‐α have been associated with altered circulating levels of TNF‐α [25, 26]. Some of these polymorphisms have been determine susceptibility or resistance to tuberculosis in several ethnic groups [27, 28, 29, 30, 31, 32, 33]. Sharma et al. [34] carried out a case–control study, including patients with pulmonary tuberculosis and controls in North India. In this study, five promoter SNPs in TNF‐α gene and one SNP rs909253 in LTα gene were detected in patients with tuberculosis and controls samples collected from North India (Fig. 2). No significant differences in allele frequencies between the patients with tuberculosis and controls were reported. Serum TNF‐α levels showed a significant difference between patients with tuberculosis and controls, and none of the polymorphism affects the serum TNF levels. Ates et al. [27] reported that IL10 and TNF promoter polymorphisms have been associated with altered levels of circulating IL10 and TNF‐α. A significant association was reported between TB and rs1800896 G‐allele. IL10 GCC and ACC haplotypes distribution showed a significant difference between patients with TB and controls. No statistically significant association was detected between rs1800871, rs1800629, rs1800750, rs361525 polymorphisms, functional TNF‐α/IL‐10 genotypes and TB.
Figure 2.
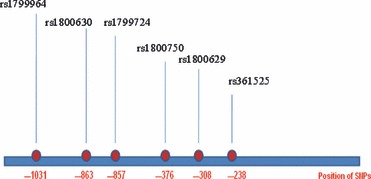
Representation of tumor necrosis factor promoter SNPs along with their positions.
Leprosy. Leprosy is a mycobacterial disease, caused by Mycobacterium leprae that initially affects the peripheral nervous system and patients displaying contrasting clinical, immunological and pathological manifestations. Many factors and metabolic pathways including TLR/LIR‐7, VDR, TNF‐α and TGF‐β have been reported to play role in disease. Goulart and Goulart [35] reviewed the complex molecular interactions in affected individuals influenced by the pathogenetic background. A significant association between the TNF rs1800629 A‐allele and multibacillary leprosy has been reported from India [36]. In Brazil, this allele was associated with resistance against multibacillary leprosy [37, 38]. A significant association of TNF rs1800629 was found in borderline tuberculoid leprosy patients with the magnitude of in vivo delayed type hypersensitivity skin test reactivity to cutaneously injected M. leprae antigens. It has been reported that signalling deficient mutations in certain Toll‐like receptors (TLR2; act upstream of TNF) can be strongly correlated with lepromatous leprosy. TNF rs1800629 regulatory polymorphism plays an important role in patients with leprosy in a Brazilian population [39], and in patients with leprosy, higher frequency of TNF rs1800629, GG genotype, and a decreased frequency of GA/AA genotypes were reported as compared to the control group. The GG genotype was particularly higher in patients with tuberculoid (TT) and borderline (BB) leprosy. A lower frequency of GCC/GCC haplotype of IL‐10 in patients with lepromatous leprosy (LL) than in controls was also reported. TNF‐alpha polymorphism rs361525 and rs1800629, and its association with the outcome of different clinical forms of leprosy have been reported by Vanderborght et al. [39]. TNF polymorphism rs361525 and rs1800629 have shown differences in the frequency of the haplotypes along the ethnic groups, but no statistical differences were observed in haplotype frequencies between patients with multibacillary (MB) and paucibacillary (PB). A lower bacteriological index (BI) among the TNF polymorphism rs1800629 carriers was reported, while higher BI in rs361525 carriers [40].
Recurrent acute otitis media. Acute otitis media (AOM) is caused by bacterial infection in children. Genetic variations in immunoresponse genes are reported to influence susceptibility to infectious diseases [41], and increased expression of TNF‐α, IL‐1β, IL‐6 and IL‐10 was observed during experimental otitis media in animals. Polymorphism in immune response genes such as IL10, IL6 and IL4 has been associated with altered cytokine expression levels [42]. The polymorphism rs361525 was associated with the otitis‐prone condition and lower specific anti‐pneumococcal antibody levels after vaccinations in comparison with carriers of the A‐allele. TNF polymorphism rs1800630 A‐allele was associated with lower specific anti‐pneumococcal IgG levels compared with children carrying C/C genotype of rs1800630.
Typhoid fever. Typhoid fever is caused by Salmonella enterica infection with serotype Typhi and 22 million cases of typhoid fever occur worldwide per year, resulting in 200,000 deaths. Indonesian study suggested a protective role of DRB1*12021 for complicated typhoid fever. Keuter et al. [43] found a lower level of TNF‐α in the patients with acute phase of typhoid fever than in convalescence. The seven polymorphisms have been found within the genes BAT1 (a member of the DEAD‐box protein family encoding an ATP‐dependent RNA helicase and a negative regulator of inflammation), LTA and TNF. All three genes, or haplotypes spanning these genes, have been associated with a variety of infectious and inflammatory diseases. Dunstan et al. [44] genotyped eighty SNPs in a region of 150 kb in Vietnamese individuals. Thirty‐three SNPs with a minor allele frequency of greater than 4.3% were used to construct haplotypes. Fifteen SNPs which tagged the 42 constructed haplotypes were selected. The haplotype‐tagging SNPs (T1–T15) were genotyped, and allelic frequencies of seven SNPs (T1, T2, T3, T5, T6, T7 and T8) have shown a significant difference between typhoid cases and controls. Haplotype‐based analysis of the tag SNPs provided positive evidence of association with typhoid. The analysis detected a low‐risk cluster of haplotypes that each carries the minor allele of T1 or T7, but not both, and otherwise carries the combination of alleles *12122*1111 at T1–T11. Individuals who carry the typhoid fever–protective haplotype *12122*1111 also produce a relatively low TNF‐α response to LPS.
Severe sepsis in trauma patients. In the non‐coronary intensive care unit, sepsis is the prevalent cause of death. A restriction fragment length polymorphism (RFLP) present in TNF gene is correlated with increased level of TNF‐α in plasma and a high mortality rate in patients with severe sepsis. This non‐synonimous polymorphism in the first intron of the TNF‐β gene (1064–1069 position) is responsible for an amino acid change at position 26 (asparagine for the TNFB1 sequence and threonine for the TNFB2 sequence) [45]. Previously, the mortality rate in severe sepsis was found to be significantly increased in patients homozygous for the allele TNFB2 of the Nco1 polymorphism compared with heterozygous patients [46]. A statistically highly significant association was obtained between the genotype of the biallelic Nco1 polymorphism of the TNF β gene and the development of severe sepsis after severe blunt trauma. Subjects homozygous for the allele TNFB2 have a significantly increased risk of the development of severe post‐traumatic sepsis.
Sepsis‐induced organ failure causes death because of activation of a mediator cascade initiated by microbial components [47]. Mira et al. [48] reported the association of TNF2 (rs1800629 SNP with A‐allele) with Septic Shock Susceptibility and Mortality. This polymorphism has been correlated with enhanced spontaneous and stimulated TNF‐alpha production both in vitro and in vivo and has been associated with morbidity and mortality of severe forms of cerebral malaria [49], fulminans purpura [9], and mucocutaneous leishmaniasis (MCL) [10]. Variation in TNF2 allele frequencies between the controls and patients with septic shock was reported. The patients with septic shock had significantly greater TNF2 allele frequency in comparison with those who had died.
NcoI polymorphism. NcoI is a restriction enzyme used in the typing of polymorphism. The presence of A‐allele eliminates the restriction site for the enzyme NcoI, while G‐allele creates restriction site for NcoI restriction enzyme.
Mediterranean spotted fever. Cytokines plays important role in the protective immune response against Rickettsia conorii. A significantly elevated levels of IFN‐γ, TNF‐α, IL‐10 and IL‐6 in serum was observed in patients with acute‐phase Mediterranean spotted fever (MSF) compared with the levels found during the convalescent phase of the disease or in healthy controls. Forte et al. [50] carried out genotyping of the TNF‐alpha (rs1800629), interleukin‐10 (rs1800896, rs1800871 and rs1800872) and IFN‐gamma (rs2430561) in a group of Sicilian patients affected by MSF. No significant differences in TNF‐α rs1800629 G/A genotype frequencies were observed. The rs2430561 TT genotype was associated with an increased production of IFN‐gamma. This study suggested that IL‐10 and IFN‐γ gene interaction might be involved in susceptibility to MSF.
Viral diseases
Clearance of hepatitis B virus infection. Hepatitis B virus (HBV) infection is a global public health problem, and more than 350 million peoples are infected with HBV worldwide. Tumour necrosis factor‐alpha (TNF‐α) plays an important role in host immune response to HBV. Kim et al. [51] carried out a case–control study of hepatitis B‐infected patients and controls and genotyped seven TNF‐α polymorphism in Korean. The results of the study showed that the presence of the rs1800629 A‐allele or the absence of the rs1800630 A‐variant was strongly associated with the resolution of HBV infection. The two TNF‐α haplotypes were significantly associated with HBV clearance, showing protective antibody production and persistent HBV infection. Thus, those variations that affect the level of gene product might influence the outcome of disease. SNP rs1800629 A is common in Iranian population, but has no association with development of chronic HBV infection [52].
SARS‐CoV infection. Severe acute respiratory syndrome (SARS) disease is caused by a novel coronavirus‐SARS‐CoV.
Host genetic factors may play a role in the occurrence and progress of SARS‐Cov infection. Wang et al. [53] conducted case–control study including SARS‐infected patients, health care workers and controls. They found no differences in TNF‐α genotype distribution at the rs1799964, rs1800630, rs1800629 and rs361525 among the three populations. The CT and CC genotypes of rs1799964 were associated with a risk effect on femoral head necrosis. The rs1800630 AC genotype was another risk effect associated with femoral head necrosis in cured SARS‐infected patients compared to CC genotype.
Severe dengue virus infection. Cascade of cytokine produced included TNF and LTA in severe dengue virus (DENV) infections. The TNF rs361525 A polymorphism marking the TNF‐4, LTA‐3 haplotype, was significantly increased in patients with secondary dengue haemorrhagic fever (DHF) compared to those with secondary dengue fever (DF) in Thais [54]. Two extended MHC haplotypes containing TNF‐4 and LTA‐3, together with HLA‐B48, B57 and DPB1*0501, have been reported only in patients with secondary DHF. These observations indicate that polymorphism in functionally distinct MHC‐encoded proteins contributes to the risk of developing severe secondary DENV infection.
Guivier et al. [55] found that two SNPs within the TNF‐alpha promoter (−302GG/GG and −296A/A) were associated with higher TNF‐α gene expression and were more frequent in non‐endemic areas among European populations of bank voles.
Protozoan parasites
Plasmodium falciparum malaria. Malaria is the most common parasitic disease of the tropics caused by the sporozoa of the genus Plasmodium, is endemic in more than 90 countries, and together with HIV and tuberculosis constitutes one of the major causes of death by infectious diseases worldwide. During P. falciparum malarial infection, TNF has been described as both protective and pathogenic, and at low levels, TNF kills the parasite by macrophage activation and subsequent release of cytokines, whereas high TNF level has been associated with severe manifestations like acute respiratory distress and cerebral malaria. It has been reported that SNPs (rs1799964, rs1799724, rs1800750, rs1800629 and rs361525) in the proximal enhancer of the TNF gene have different associations with malaria in different populations [49, 56, 57, 58]. Sinha et al. [59], genotyped these SNPs in patients with P. falciparum malarial infection and controls in Indian population. They found association of the rs1799964 and rs1800630 with increased risk of severe malaria. TNF enhancer haplotype CACGG (rs1799964, rs1800630, rs1799724, rs1800629 and rs361525) correlated with enhanced plasma TNF levels in both patients with falciparum malarial infection and controls and were associated with increased susceptibility to severe malaria. No association between rs1800629 polymorphism and susceptibility to cerebral malaria among central Sudanese children was reported [60].
Mucocutaneous leishmaniasis. Leishmania braziliensis infection is responsible for MCL. It is a severe form of American cutaneous leishmaniasis (ACL). High serum TNF‐α levels have been reported in this disease. So TNF regulatory polymorphism may have some putative role in circulating level of TNF‐α and thus in disease manifestation. In Venezuelan case–control study, homozygotes for allele 2 of a polymorphism in intron 2 of the TNF‐β gene showed a high relative risk of MCL disease, and a significantly higher frequency of allele 2 of rs1800629 polymorphism was predicted in patients with MCL compared with endemic controls. Polymorphism affecting TNF‐α production may be associated with susceptibility to the mucocutaneous disease [10].
Chagas disease. The parasite Trypanosoma cruzi causes chronic Chagas disease cardiomyopathy (CCC), affecting 18 million individuals in Latin America. One‐third of patients with CCC develop heart failure, and their survival is reduced by 50% compared to patients with other cardiomyopathies. Aguiar and Prestes [61] reported the role of TNF polymorphism in this disease. Elevated TNF‐α levels in plasma and heart tissues were observed in patients. The TNF‐α such as TNFa2, TNFa microsatellite allele 2 and the TNF2 rs1800629, TNF promoter polymorphism allele 2 were genotyped. Patients positive for TNF2 or TNFa2 alleles display a significantly shorter survival time compared with those carrying other alleles. No association of TNF‐α polymorphism with Chagas disease in Brazilian patients have been found [62]. The TNFa microsatellite and rs1800629 polymorphism in an association study were detected. The patients with CCC were grouped in three categories according to degree of left ventricular (LV) dysfunction into severe, mild to moderate and absent. No significant differences between either CCC and asymptomatic (ASY) patients or patients with CCC, according to severity of cardiomyopathy with respect to TNFa or rs1800629 TNF promoter polymorphism, were reported.
Chronic beryllium disease and beryllium sensitization. Sato et al. [63] detected the role of TNF‐α polymorphism in development of chronic beryllium disease (CBD). They genotyped five TNF‐α promoter polymorphism in patients with CBD, sensitized subjects and control subjects and measured TNF‐α production in beryllium‐stimulated and beryllium‐unstimulated BAL. A significantly increased TNF‐α production was reported in patients with CBD compared with those only sensitized in beryllium‐stimulated, but not beryllium‐unstimulated, BAL cell. No significant association has been reported between TNF promoter polymorphism or haplotypes and CBD‐sensitized patients, and controls. The rs1799724 T allele has been shown to be associated with BAL cell TNF‐α production.
Human African trypanosomiasis and host inflammatory cytokine response profile. Lean et al. [54] identified two trypanosomiasis with dramatically different disease virulence profiles in Uganda and Malawi. The two disease profiles were associated with markedly different levels of TNF‐α and transforming growth factor β (TGF‐β) in plasma. In Uganda, but not Malawi, early‐stage TNF‐α was elevated, while in Malawi, but not Uganda, early‐stage TGF‐β was elevated. Rapid progression of disease in Uganda is associated with TNF‐α‐mediated inflammatory pathology.
Invasive pulmonary aspergillosis. The role of TNF‐α and lymphotoxin‐alpha (LT‐α) in fungal infection diseases has been reported [64]. The presence of polymorphism in TNF‐α and LT‐α genes or their receptors might increase the susceptibility of haematologic patients to develop invasive pulmonary aspergillosis (IPA). SNPs in TNF‐α, LT‐α and tumour necrosis factor receptor 2 (TNFR2) and a variable number of tandem repeats (VNTRs) in TNFR2 were investigated in haematologic patients and controls. Similar genotype and alleles frequencies were detected between patients and controls. TNF‐α and LT‐α polymorphisms were not associated with the presence of IPA. A strong association of IPA with VNTR in the promoter region of the TNFR2 gene was found.
Cancer
Breast cancer
Cancer is the major health problem and leading cause of death. Several genetic polymorphisms have been reported to associated with disease. The genetic factors play important role in the epidemiology and pathogenesis of cancer. TNF genetic polymorphism can regulate gene expression and have been associated with inflammatory and malignant conditions. Azmy et al. [65] have been detected the role of TNF‐α rs1800630 and rs361525 polymorphisms in breast cancer susceptibility and severity. Breast cancer cases and controls have shown similar allele frequencies for both polymorphisms. No association was found between rs1800629, rs361525 and susceptibility to breast cancer in North European population. Role of TNF rs361525 in breast cancer risk was investigated by Gaudet et al. [66], in breast cancer cases and controls, in European, from 30 studies in the Breast Cancer Association Consortium. Jung et al. [67] have detected 12 SNPs in 11 apoptosis‐related genes in the apoptosis pathway.
Cervical cancer
Human papillomavirus (HPV) 16 infection is an important factor for cervical cancer. Alteration in local levels of TNF in the cervix may affect the immune response of an individual, hence affecting the persistence of HPV. Excess TNF‐α can result in harmful inflammatory responses, whereas too little can contribute to persistent infection. TNF‐α is one of the primary cytokines released after HPV infection and upregulates the expression of antigen‐processing and presentation pathway components for class I HLA. Eleven TNF SNPs were associated with susceptibility to HPV16‐associated cervical cancer. A significant difference in genotype distribution of three SNPs between the cases and controls were reported. Haplotype distribution also showed a significant difference between cases and controls. A new association was reported between several TNF‐SNPs and susceptibility to cervical cancer [68].
Prostate cancer
The associations between six TNF SNPs (rs1799964, rs1800630, rsl799724, rs1800629, rs361525 and rs1800610) and prostate cancer risk were investigated [69]. No TNF SNP was associated with prostate cancer risk in Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), while in the Nutrition Cohort, associations were significant for two highly correlated variants (rs1799724, 1800610). In pooled analyses, no single SNP was associated with prostate cancer risk. No differences in haplotype distribution between case/control status in PLCO, but marginal associations in the Nutrition Cohort and the pooled analysis, were reported. The TNF +488A has been reported to be associated with common variable immunodeficiency in addition to prostate cancer. The association between prostate cancer risk and rs1800629 in 296 patients diagnosed with prostate cancer and in 311 healthy controls was studied.
Bladder cancer
Polymorphism at position TNF−859 shows no disease association. TNF regulatory polymorphism may alter the expression and alter the risk of developing bladder cancer and subsequent tumour behaviour. TNF‐α polymorphism, TNF +488A and TNF−859T are significantly associated with risk of bladder cancer.
Non‐Hodgkin’s lymphoma
Seidemann et al. [70] studied tumour necrosis factor and lymphotoxin‐alpha genetic polymorphism and outcome in paediatric patients with non‐Hodgkin’s lymphoma (NHL). The study examines the association of TNF‐α rs1800629 and LT‐α rs909253 polymorphisms with diagnostic NHL. Patients with Burkitt’s lymphoma (BL) and B cell acute lymphoblastic leukaemia patients carrying at least two variant alleles (high‐producer haplotypes) had an increased risk of events. TNF‐α rs1800629 and LT‐α rs909253 polymorphisms were negative prognostic factors in paediatric BL and in B cell acute lymphoblastic leukaemia (B‐ALL).
Pancreatic, lung cancer and renal cell carcinoma
A case–control study of pancreatic cancer was conducted in the San Francisco Bay area by Duell et al. [71]. No association between pancreatic cancer risk and TNF rs1800629 polymorphism was reported. Pancreatitis was significantly associated with TNF rs1800629 GA + AA among patients with pancreatic cancer. A significant difference in genotype frequencies of rs1800629 and rs361525 was reported between patients with lung cancer and the healthy controls and also between patients with lung cancers of various stages. The study was carried out by Shih et al. [72], in 202 patients, 205 controls in Taiwan. Individuals with rs1800629 AA/GA genotypes against GG genotype had higher odds ratios (ORs) while individuals with rs361525 AA/GA genotypes against GG genotype had lower ORs for lung cancer. The patients carrying AA or GA genotype at rs1800629, or a GG genotype at rs361525, had a tendency to advanced disease. A significant association between TNF‐α rs1800629 and rs361525 polymorphism and the susceptibility to lung cancer was demonstrated. A case–control study of patients with renal cell carcinoma (RCC) and healthy controls was conducted by Basturk et al. [73]. G‐allele frequency of rs1800629 was significantly higher in the patients than in controls. These findings suggest that the TNF‐α rs1800629 G/G genotype may be considered as potential risk factors for RCC, whereas TNF‐α rs1800629 G/A genotypes may play role as a protective factors. Onishi et al. [74], detected the genetic polymorphism of TNF‐α (α1, α2) and TNF‐β (β1, β2). All patients having TNF‐β1/1 homozygote were alive, and a significantly favourable prognosis in the patients with TNF‐β1/1 homozygote compared with other TNF‐β polymorphism was observed. In the Turkish population, rs1800629 polymorphism is associated with an increased risk of hepatocellular carcinoma as this polymorphism plays role in the regulation of expression level. A case–control study was designed by Akkiz et al. [75], and they found that rs1800629 genotype was significantly associated with the risk of HCC. The presence of the high producer allele rs1800629 A in the TNF‐α gene was associated with an increased risk of the development of HCC in Turkish population.
Acute pancreatitis. Tumour necrosis factor α (TNFα) plays important roles in the pathogenesis of acute pancreatitis (AP). Ozhan et al. [76] determined two TNF promoter polymorphisms (rs1800629 and rs361525) in patients with AP and healthy controls. The frequencies of these polymorphisms were similar in both patients with mild or severe pancreatitis and in controls.
Autoimmune diseases
Sarcoidosis
Sarcoidosis is a complex disease with autoimmune basis, a multisystemic granulomatous disorder which occurs in almost all populations. Disease manifestations are localized to lung and skin, but the involvement of other parts such as eyes, lymph nodes, parotid glands, heart, liver and spleen can also occur. Sharma et al. [25] reported for the first time the association of TNF haplotypes and genotypes with sarcoidosis and its prognosis in the Indian population. Five promoter polymorphism in the TNF‐α gene and one in LTα gene (rs909253) were genotyped in North Indian patients. They have measured sTNF‐α and serum angiotensin–converting enzyme (SACE) levels. Serum TNF‐alpha and SACE levels are influenced by rs1800629 and rs361525 polymorphisms. The patients and controls have significant differences in haplotype frequencies. The haplotype GTCCGG was identified as the major risk/susceptibility haplotype and was associated with increased SACE levels in the patients.
Cystic fibrosis conductance regulator, tumour necrosis factor, interferon‐alpha‐10, interferon‐alpha‐17 and interferon‐gamma genotyping as potential risk markers in pulmonary sarcoidosis pathogenesis were detected by Makrythanasis et al. [77], in Greek patients. They have detected a statistically significant increase of CFTR mutation carriers in patients with sarcoidosis than in the control population. A difference was observed within sarcoidosis patients group where patients with CFTR mutations suffered more frequently from dyspnoea than those without.
Multiple sclerosis
Tumour necrosis factor (TNF‐α), a proinflammatory cytokine, plays an important role in multiple sclerosis (MS) pathogenesis. In Turkish population, Akcali et al. [78] reported that rs1800629 and rs361525 polymorphisms are not associated but rs1799724 allele is associated with the multiple sclerosis disease. A statistically significant increase in rs1799724 CC genotype was found in MS patients than in controls, while rs1799724 CT genotype showed a significant negative correlation with patients with MS. No differences in the distribution of rs1800629 and rs361525 alleles were observed. None of the three polymorphisms (rs1800629, rs361525 and rs1799724) showed relation with disease. Significant difference of rs1799724 CC genotype was identified with the low disease index. Thus, rs1799724 CC genotype may cause susceptibility to MS in the Turkish population. TNF‐β and TNF‐α gene (rs1800629 and rs361525) polymorphisms and susceptibility to MS were determined in Caucasian patients with MS, and healthy controls from Norway [79]. TNF‐β genotypes were significantly associated with MS. TNF‐α genotypes were not associated with MS. Huizinga et al. [80], reported TNF‐α promoter polymorphism and susceptibility to multiple sclerosis in different groups of patients. TNF‐α production in whole blood cultures upon stimulation with LPS was determined in individuals from 61 families. Highest TNF production is characterised in three families, and in contrast, the lowest TNF production is characterised in three families. The difference of highest and lowest TNF production could not be attributed to the promoter polymorphism rs1800629, rs361525 or rs1800750, although rs361525 GA donors produced low TNF upon culture with endotoxin compared with TNF rs361525 GG donors. The frequency of the rs361525 GG genotype was increased in patients with MS in a nursing home compared to patients with MS in an outpatient’s clinic or Dutch controls. TNF‐α rs1800629 and rs361525 polymorphisms have no association with MS, but the microsatellite allele a11 is associated with the disease in French patients [81]. In French patients with MS and controls, TNF‐α rs1800629 and rs361525 and a microsatellite polymorphisms were investigated. TNF‐α rs1800629 and rs361525 polymorphisms have shown no significant differences between patients with MS and controls. Very significant association was found between allele frequency for the a11 allele and MS.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a type of systemic autoimmune disease. Rheumatoid arthritis has both environmental and genetic background, with genetic factors contributing 15–30% of the overall risk. The genetic studies have given different associations in different populations. The TNF +488A have been reported to be associated with rheumatoid arthritis [82], while TNF +489 polymorphism does not contribute to susceptibility to rheumatoid arthritis in Europeans. In Caucasian TNF, rs1800629 polymorphism is not associated with response to TNF‐α blockers in patients with rheumatoid arthritis and does not serve as a genetic risk factor for RA susceptibility and severity in Americans. TNF2 allele has been associated with susceptibility and severity to a variety of autoimmune and inflammatory diseases, including RA, systemic lupus erythematosus and ankylosing spondylitis. The presence of TNF2 allele increases the production of TNF‐alpha and thus increases the host’s resistance to infection. Aguillon et al. [82] suggested that RA is favoured by the presence of the rs1800629 polymorphism and is responsible for increased TNF production. Ten European, three Latin American and one Asian studies were analysed by Lee et al. [83], and no association was found between RA and the TNF‐α rs1800629 A‐allele in the overall population.
HLA‐B27‐positive ankylosing spondylitis
The association between TNF‐α promoter polymorphism and ankylosing spondylitis (AS) susceptibility was reported with inconsistent results. Chung et al. [84] conducted a case–control study including six TNF‐alpha promoter polymorphism. They found a significant differences in the allelic and genotypic frequencies at rs1799964, rs1799724 and rs1800750 in patients with HLA‐B27 (+) and AS and random controls, but not in patients with AS and HLA‐B27 (+) healthy individuals. Haplotype (rs1799964 T/rs1799724 C/rs1800630 C/rs1800629 G) increases the risk of susceptibility to AS compared to random controls, whereas haplotype (rs1799964 C/rs1799724 A/rs1800630 C/rs1800629 G) have shown to be associated with decreased susceptibility to AS compared to random controls. One Latin American and seven European studies were analysed by Lee and Song [85]. No association between AS and rs1800629 A‐allele, AA and AA + AG genotypes were reported.
Graves’ disease
In the development of Graves’ disease (GD), a role is played by TNF‐α. Gu et al. [86] investigated the association of TNF‐α polymorphism rs1800629, rs361525 and rs3093661 with GD in Chinese population. A significant difference in distribution of rs361525 and rs3093661 allelic frequencies between Graves’ disease and control individuals was reported. The G‐alleles of rs361525 and rs3093661 SNPs have been associated with higher risk of GD as compared with A‐alleles. No significant difference of rs1800629 allelic frequency was observed. The haplotype GGG was associated with an increased risk of GD, whereas the haplotype GAA was protective.
Type 1 diabetes mellitus
Type 1 diabetes mellitus (TIDM) is an autoimmune disorder, which involves T cell‐mediated destruction of the pancreatic β‐cells [87]. Several reports had shown the association of polymorphism with the disease TIDM [87, 88, 89, 90]. The proinflammatory cytokines are elevated in patients at the onset of diabetes. A significant increase of rs1800629 G/A and A/A genotypes in North Indian patients with T1DM were reported [91]. Das et al. [92] suggested a significant association of rs1800629 A‐allele and G/A genotype with T1DM in North Indians, but no association with rs361525 polymorphism. The same increase in the prevalence of rs1800629 A‐allele in patients with diabetes in the Hungarian population was reported [93]. In Chinese and Caucasian populations, rs1800629 variant was not considered as a genetic factor for susceptibility to T1DM and the associations of the TNF gene are because of a Linkage disequilibrium (LD) between TNF and DR3‐DQB 1*0201 haplotype reported [94]. Initially, rs1800629 and rs361525 variants show association with T1DM, but after adjusting the data for LD with DRB1‐DQB1 and B18‐DR3 haplotypes, the association lost its significance [93]. Boraska et al. [95] studied relation of TNF gene promoter polymorphism (rs1800629 and rs361525) with TIDM in a case–control study from South Croatia. Haplotype (rs1800629 A and rs361525 G) was observed more often in patients with TIDM than in controls. SNP rs1800629 was found to be more frequent in patients with TIDM. The author did not find strong evidence of association of TNF promoter polymorphism with TIDM. Independent association of TNF polymorphism with type 1 diabetes susceptibility have been found in Korean [96]. Seven SNPs in the TNF genes (TNFα and TNFβ) were genotyped in a Korean, along with HLA DRB1, DQB1 and MICA (MHC class I chain–related genes). Three SNPs and two common TNF haplotypes showed significant association with the risk of TIDM.
Type 2 diabetes
In case of type 2 diabetes, high levels of cytokines have been considered as risk factors. Kubaszek et al. [97] investigated TNF‐α and IL‐6 polymorphisms and found that TNF‐α rs1800629 A‐allele was associated with an approximate twofold higher risk of type 2 diabetes compared with the rs1800629 G. The rs1800629 A‐allele of TNF‐α rs1800629 polymorphism is a predictor for the conversion from IGT (impaired glucose tolerance) to type 2 diabetes.
Diabetic nephropathy
In diabetic nephropathy, glucose auto‐oxidation and production of free radicals causes protein glycation that increases the concentrations of proinflammatory cytokines. Myeloperoxidase (MPD) is a heme enzyme, participating in microorganism killing by phagocytes. Patients with chronic renal failure results from diabetic nephropathy show a significant reduction in the intracellular myeloperoxidase level and myeloperoxidase gene promoter polymorphism (−463, G/A) causes a decreased gene expression. In a case–control study, no significant differences in TNF genotype and allele frequencies between the groups and patients with diabetic nephropathy were found. A lower frequency of TNF1/TNF1 genotype has been reported [98]. Significant differences of TNF plasma level in patients with diabetic nephropathy and other renal diseases were reported. A statistically significant difference in MPO genotype frequencies between patients with diabetic nephropathy and patients with other renal diseases was observed. MPO, GG and AA genotypes were significantly more common in patients with diabetic nephropathy. A correlation between the MPO genotype and an earlier onset of the disease was observed while such a relationship was not found for the TNF genotype. It has been found that in patients with diabetic nephropathy, TNF variants were more frequent than in non‐diabetic patients with chronic renal failure.
Inflammatory bowel disease
Crohn’s disease is a chronic inflammatory disease of the intestines. This is closely related to another chronic inflammatory condition that involves only the colon called ulcerative colitis. Together, CD and ulcerative colitis are referred to as inflammatory bowel disease (IBD). The chromosomal region, 6p21, IBD3, has been identified as an IBD‐susceptibility locus [99, 100, 101]. IBD3 region encompasses the tumour necrosis factor α (TNF) gene. TNF‐alpha is considered as a strong candidate gene for IBD. Levels of TNF are elevated in the serum, mucosa and stool of patients with IBD. TNF production is under a strong genetic influence [102]. The positive association of TNF rs1799724 C with UC was reported in Caucasians and is also supported by a small Japanese case–control study. The same study reported an association of TNF rs1799724 T with Japanese CD, although a significant effect of this allele was not observed in a larger patient cohort [103].
TNF‐α and Fc‐gamma receptor polymorphism with infliximab in Crohn’s disease
The associations between TNF‐alpha and Fc‐gamma receptor (Fc‐gammaR) polymorphism with infliximab (IFX) treatment for CD are not well known. Patients with CD were given IFX 5 mg/kg intravenously and followed prospectively for 8 weeks, and the Crohn’s disease activity index (CDAI) was measured before and after 8 weeks of treatment [104]. On the basis of predicted CD activity index, patients were grouped as responders or non‐responders. The TNF‐alpha, Fc‐gammaRIIA and Fc‐gammaRIIIA genotype distribution was not significantly different between responders and non‐responders 8 weeks after treatment. Fc‐gammaRIIIB genotype distribution has shown significant differences between responders and non‐responders after 8 weeks. Fc‐gammaRIIIB polymorphism may be an important factor for clinical response to IFX treatment in CD.
Asthma
Asthma is a complex polygenic disease in which gene–environment interactions have shown to play important role. TNFα gene is one of the important candidate genes involved in pathogenesis of asthma. Several studies have investigated TNFα rs1800629 polymorphism (rs1800629 G designated as TNF1 and rs1800629 A designated as TNF2) and asthma susceptibility in different populations. A positive association between TNF2 and asthma [79, 105, 106, 107, 108, 109, 110, 111, 112] have been reported. Some studies have been reported a negative association [113, 114, 115, 116], and one study reported a positive association between TNF1 and asthma [117]. Gao et al. [118] included 2409 patients with asthma and 3266 controls, in the study. They found that TNF2 allele confers a significant risk of developing asthma.
Risk of primary biliary cirrhosis
Juran et al. [119] recently reported an association between primary biliary cirrhosis (PBC) and (rs231725) polymorphism of the immunoreceptor gene cytotoxic T‐lymphocyte antigen 4 (CTLA4). They have detected its interaction with the rs1800629 polymorphism in which TNF2A allele has been shown to increase the TNF production. The genotyping of polymorphism was carried out in patients with PBC and in controls from US and Canada. Allele frequency for TNF2A was elevated in patients with PBC, but only borderline significance was detected. TNF2A carriage was significantly increased in CTLA4 ‘A/A’ PBC patients compared with CTLA4 ‘A/A’ controls; no apparent increase in TNF2A carriage was shown in CTLA4 (A/G or G/G) individuals. TNF2A amplifies the CTLA4 (rs231725, A/A) genotype risk of PBC.
Behcet’s disease
Behcet’s disease (BD) is a chronic multisystem inflammatory disorder, the hallmarks of which are recurrent oral and genital ulceration, skin lesions, and uveitis. It has been reported that rs1799964 polymorphism has been associated with Behcet’s disease [120].
Dermal diseases
Atopy on irritant contact dermatitis in health care workers
Davis et al. [121] studied the effects of TNF‐alpha G to A rs1800629 polymorphism on chronically damaged skin of healthcare workers. They have genotyped TNF‐alpha rs1800629 polymorphism and measured the epidermal response. Excess hand erythema decreased with hand hygiene exposure and increased during time off for AA/GA genotypes, but had opposite effects for GG. AA/GA had smaller reductions in dryness with lotion treatment and larger reductions in excess erythema than GG. Repeated exposure to water and sodium lauryl sulphate produced higher erythema in normal skin for AA/GA than for GG genotype. The study suggested that the TNF‐alpha rs1800629 polymorphism and an atopic history influence the severity of irritation and recovery from exposure.
Risk of psoriasis vulgaris
Several studies have given different association between TNF‐α polymorphism and psoriasis risk. The rs1800629 and rs361525 polymorphisms have been reported to influence the transcription of the TNF‐α gene and have been implicated in psoriasis risk. Li et al. [122] conducted psoriasis case and control study. The rs361525 GA + AA genotypes had significantly increased risk, compared with the GG genotype, whereas a significantly reduced psoriasis risk was associated with rs1800629 GA + AA genotypes compared with the GG genotype.
Tumour necrosis factor‐α antagonists are effective in the treatment for refractory psoriasis. In many diseases such as rheumatoid arthritis, ankylosing spondylitis, and CD, treatment with this therapy results in induction of psoriasis in some cases. Cohen et al. [123] conducted a systematic analysis of the six cases to investigate anti‐TNF‐α‐induced psoriasis, and they observed among inflammatory patient cohort treated with anti‐TNF‐alpha (infliximab or etanercept). No patient had history of psoriasis. There was great variation in the age of affected patients and in the onset of psoriasis after initiation of TNF‐α antagonists.
Cardiovascular diseases
Cardiovascular disease
Mellick [62] genotyped five SNPs in TNF promoter region in subjects with a history of a single myocardial infarction (MI) and population‐based controls without a history of MI. rs1800630 and rs1800629, the most common haplotypes in the Swedish population, were reported. In this study, an association has been reported between TNF haplotype and plasma levels of plasminogen activator factor inhibitor 1 (PAI‐1). The plasma level of C‐reactive protein and the homoeostasis model assessment (HOMO) also showed no statistically significant relationships. The increased plasma levels of IL‐6 and TNF‐alpha were associated with Left ventricular diastolic dysfunction (LVDD). A link between low‐grade inflammation and the presence of LVDD has been suggested by this study.
Impact of cytokine genotype on cardiovascular surrogate markers in hemodialysis patients
Cytokine gene polymorphism plays important role in the risk of many diseases, including cardiovascular diseases (CVDs). Yilmaz et al. [124] have evaluated the role of cytokine gene polymorphism in carotid intima‐media thickness (CIMT) and left ventricular mass index (LVMI) progression in non‐diabetic haemodialysis (HD) patients. TNF‐α and IL‐10 polymorphisms were determined in the study. Risk factors for cardiovascular diseases have no difference between TNF‐alpha rs1800629 high‐/low‐producer genotype groups. CIMT and LVMI progressions were detected at higher levels in patients with high‐producer genotypes (AA + AG) than in patients with the low‐producer genotype (GG). The rs1800629 polymorphism was strongly associated with C‐reactive protein (CRP). Analysis also showed that the combination of high production of TNF‐α and low production of IL‐10 was associated with higher average IMT, LVMI progression and elevated average CRP levels compared with a combination of low production of TNF‐α and high production of IL‐10.
Spontaneous deep intracerebral haemorrhage
Association of TNF‐α gene with spontaneous deep intracerebral haemorrhage was investigated by Chen et al. [125] in the Taiwan population. Deep parenchymal structure including the basal ganglia, thalamus, brainstem and cerebellum is the most frequently affected site of spontaneous intracerebral haemorrhage (SICH). Rost et al. [126] comprehensively reviewed the candidate genes of SICH reported during 1996–2007. Reported candidate genes that show association with SICH were involved in the pathways of the vessel wall integrity (ACE, APOE, neprilysin, endoglin, TGF‐β1), endothelial dysfunction (ACE), inflammation markers (IL‐6, TNF) and haemostasis (APOE, CD‐14, Factor VII and XIII, VKORC1). Spontaneous deep intracerebral hemorrhage (SDICH) risks were positively associated with TNF (rs1799964 C and rs1800629 A) in men but inversely associated with (rs1800630 A) in females [126]. There were significant interaction effects between gender and SNPs (rs1799964, rs1800630 and rs1800629) on SDICH risks.
TNF‐α polymorphism and homocysteine levels in patients with ischaemic strokes
Kim et al. [127] carried out case–control studies including patients with ischaemic stroke, patients with silent brain infarctions SBIs and controls. Significant differences in the frequency of the TNF‐α rs1800629 polymorphism were found between the patients with ischaemic stroke and the control group. The frequency of the TNF‐α (rs1800629 GA + AA) genotype was higher in the group having highest homocysteine (tHcy) levels than in the group having lowest tHcy levels. The tHcy levels were significantly and inversely correlated with folate levels in the TNF‐α (rs1800629 GG) and TNF‐α (rs361525 GG) genotypes in the ischaemic stroke, SBI and control groups. TNF‐α rs1800629 polymorphism is responsible for susceptibility to ischaemic stroke and is associated with high tHcy levels in Koreans. No relationship between TNF‐α polymorphism and SBI susceptibility was found in this study.
Neurological/behavioural disorders
Alzheimer disease
Alzheimer’s disease (AD) is one of the most common types of chronic neurodegenerative diseases. Vascular dementia, AD and stroke are all associated with inflammation, but they have different initiating factors. Polymorphism in the TNF and apolipo protein E (APOE) was reported to increase AD risk. Laws Simon et al. [128] conducted a case–control study and investigated −850C>T, rs1800629 in TNF and the APOE polymorphism in controls and patients with sporadic AD. The frequency of (−850C/T) genotypes and T allele was significantly different in AD individuals, while the (rs1800629) SNP was not associated with AD. T allele of (−850) polymorphism significantly modified risk associated with possession of the APOE e4 allele only, and (−850) T allele was found to be associated with lower levels of CSF Aβ42. In a Southern China population, patients with sporadic Alzheimer’s disease (SAD) have a significantly increased frequency of rs1800629 A‐allele as compared with controls. The carriers of A‐allele have a significantly increased risk of SAD. Level of TNF‐α in serum of SAD group was much higher than that in control group, and the elevated serum TNF‐alpha level was closely associated with the risk of SAD detected by Yang et al. [129]. Seventeen studies that investigated the association between five TNF‐α polymorphism (−850, rs1800629, rs1800630, rs361525 and rs1799964) and AD were retrieved and analysed [130]. The presence of T allele significantly increased the risk of AD associated with carriage of the apolipoprotein E epsilon 4 allele in Caucasian Australians and Northern Europeans. A significant association of (−850) polymorphism with AD risk and non‐significant difference in genotype distribution of (rs1800629) polymorphism in AD was found. For the (rs361525 and rs1799964) polymorphism, Di Bona et al. [130] did not find an association with AD. Only four studies investigated rs361525 variant, and the results were not significant. Current findings suggested an association between (−850C>T) polymorphism and the risk of developing AD. No positive associations between TNF‐alpha promoter haplotypes and AD disease in Italian population have been reported by Tedde et al. [131].
Event‐related potential indices of attention and mental rotation
Tumour necrosis factor plays an important role in glutamatergic neural transmission [132] and serve essential functions in neural plasticity [133] and cognitive processes like learning and memory [134, 135]. SNPs in TNF have profound impact on this disease. A‐allele of rs1800629 fastens cognitive processing speed in a visual task, compared with G‐allele carriers [136]. Mental rotation describes the cognitive process of imagining an object turning around. Mental rotation is usually examined using objects (e.g. letters) that are rotated by certain degrees clockwise or counter‐clockwise from the vertical upright. This angular displacement allows a parametrical modulation of task difficulty and the demand of processing in working memory. Working memory processes are closely interrelated to attentional processes as attention permits information to be further stored and processed in working memory. Attentional processes are reflected by the visual N1 event‐related potential (ERP)‐component. The visual N1 may reflect effects of attention on sensory processing or an integrated process of perception and attention. The visual N1 is an exogenous potential that is modulated by attentional processes modifying the magnitude of neural responses to incoming information.
Beste et al. [136] examined the association of the TNF‐α rs1800629 polymorphism with attention and mental rotation performance in an event‐related potential (ERP) study in healthy participants. The results show that carriers of rs1800629 A‐allele display elevated attentional processes as compared to the GG genotype group. Carriers of the rs1800629 A allele performed better than the GG genotype group. The finding of enhanced attentional and mental rotation performance in A‐allele carriers supports recent findings that the A‐allele of this SNP enhances cognitive performance on a general measure of cognitive processing speed.
Labile anger
Interferon‐alpha increases the expression of TNF‐α. During interferon‐alpha therapy in psychiatric symptoms, TNF‐α polymorphism played a role in susceptibility to this disorder. Recently role of TNF‐α rs1800629 polymorphism in labile anger and depression was investigated by Lotrich et al. [137]. A‐allele of rs1800629 was associated with worsened labile anger and fatigue during treatment but not with major depression incidence or increased Beck Depression Inventory II. Labile anger was not predicted by the serotonin transporter polymorphism. During treatment with an exogenous cytokine, vulnerability to worsening labile anger distinct from major depression is associated with genetic variability in TNF‐α.
Neuropathic pain
Tumour necrosis factor‐alpha has been reported to play a role in neuropathic pain. Leung and Cahill [138] described the role of TNF‐α in neuropathic pain. Neuropathic pain is pathological pain where nociceptive responses persist beyond the resolution of damage to the nerve or its surrounding tissue. Animal models of neuropathic pain based on various types of nerve injuries have persistently implicated a pivotal role for TNF‐α at both peripheral and central levels of sensitization.
Increased risk of new haemorrhage
Achrol et al. [139] identified SNPs associated with increased risk of new intracranial haemorrhage (ICH) after brain arteriovenous malformation (BAVM). Achrol et al. [125] investigated four promoter SNPs in interleukin‐6 and tumour necrosis factor (rs1800629, rs361525). An association has been found between TNF‐α rs361525 polymorphism and increased risk of new ICH after diagnosis. The patients with TNF‐α rs361525 AG genotype had increased risk of new ICH. No other SNP was found to be associated with new ICH.
Others
Endometriosis
Genetic factors play role in endometriosis [5, 140]. Elevated levels of TNF‐α in peritoneal fluid of women with endometriosis have been reported, suggesting that TNF‐α may be involved in the development of endometriosis. Asghar et al. [5] investigated the possible association between endometriosis and the TNF‐α gene promoter polymorphism rs1799964, rs1799724, rs1800629, rs361525 and rs1800630 in a Japanese population. No significant differences in frequencies between the crude endometriosis cases and controls were reported for the above‐studied polymorphism. Division of endometriosis group in a subgroup of women with stage IV disease only, the frequency of rs1799964 C allele, was significantly lower in this subgroup than controls. Therefore, the study suggested that the TNF‐α rs1799964 polymorphism might be associated with susceptibility to endometriosis.
Muscle phenotypes
During ageing, there is 2‐ to 4‐fold increase in plasma levels of inflammatory mediators such as TNF‐α, IL‐6, interleukin 1 receptor antagonist (IL‐1Ra), soluble TNF‐α receptor (sTNFR), acute‐phase proteins, such as C‐reactive protein (CRP), and neutrophils has been reported. This low‐grade inflammation may play an important role in age‐related diseases such as Alzheimer’s disease, atherosclerosis, type 2 diabetes, osteoporosis, as well as sarcopenia. TNF‐α played role in many age‐related inflammatory changes, whereas other cytokines like IL‐6, IL‐1Ra, sTNFR, as well as acute‐phase proteins (APPs) like CRP, reflect responses to upregulated local or generalized TNF‐α activity [141]. The authors have detected five TNF promoter SNPs, including rs1799964, rs1799724, rs1800629, rs361525 and rs1800630. The rs1799964 and rs1800630, putative high‐expression alleles individually or in the haplotype rs1799964 C‐ rs1800630 A‐ rs1799724 C‐ rs1800629 G‐ rs361525 G, were associated with lower muscle mass in men. Carriers of rs1799964 C, compared with non‐carriers, exhibited lower arm muscle mass also tending to be lower. Similarly, rs1800630 A allele carriers (linked with rs1799964), compared with non‐carriers, exhibited lower arm muscle mass. Carriers of the haplotype rs1799964 C‐ rs1800630 A‐ rs1799724 C‐ rs1800629 G‐ rs361525 G, compared with non‐carriers, exhibited lower arm muscle mass and trunk muscle mass.
Paget’s disease
Interleukin (IL)‐6, a cytokine, plays an important role in the differentiation and activation of osteoclasts and might be involved in osteoblast stimulation in Paget’s disease of bone (PDB). Corral‐Gudinol et al. [142] investigated the association of IL‐6, IL‐8 and TNFα (rs1800629 and rs361525) polymorphism in patients with PDB and healthy controls in Spanish population. No significant association between genotype and allele distribution of any of the cytokines polymorphism and PDB was observed. The study concluded that Paget’s disease of bone is not associated with polymorphism in interleukin‐6, interleukin‐8 and tumour necrosis factor‐alpha genes.
Proliferative vitreoretinopathy disease
Genetic factors have role in proliferative vitreoretinopathy (PVR). Eleven SNPs in the lymphotoxin‐alpha (LTA), TNF α, leucocyte‐specific transcript 1 (LST1) and the activating natural killer receptor p30 (NCR3) were analysed, and a significant association in the non‐synonymous polymorphism rs2229094 (T/C causes cysteine to arginine change in the signal peptide) in the LTA gene has been reported [143]. This marker was also present in all significant haplotypic associations and was not observed in any non‐significant associations. The strong association found in the rs2229094 (T/C) of the LTA gene may indicate an important role of this polymorphism in the development of PVR.
Risk of fracture in older women
Tumour necrosis factor‐α is a proinflammatory cytokine that promotes osteoclastic bone resorption. Moffett et al. [64] detected the association between TNF rs1800629 polymorphism and osteoporosis phenotypes in older women. Women with the A/A genotype had greater subperiosteal width and endocortical diameter than those with the G/G genotype, and there was a greater distribution of bone mass away from the neutral axis of the femoral neck in women with the A/A genotype, resulting in greater indices of bone bending strength. TNF rs1800629 polymorphism was not associated with a reduced risk of other fractures. A potential role has been played by TNF‐α polymorphism in the aetiology of osteoporosis.
Susceptibility to oral lichen planus
Kimkong et al. [144] investigated the association between oral lichen planus (OLP) susceptibility and clinical type in the Thai population and found a higher proportion of TNF‐α, rs1800629 AA genotype (high producer genotype) among patients with PDB when compared to healthy controls. For polymorphism (rs1800630 and rs361525), no significant association with OLP development was reported. Thus, in Thai population, TNF‐alpha rs1800629 AA genotype might play a role in the susceptibility and severity of OLP.
Idiopathic recurrent miscarriage
Reports indicated that approximately, 1–3% of healthy women experienced recurrent miscarriage (RM), defined as three or more consecutive pregnancy losses prior to the twentieth week of gestation. Zammiti et al. [145] reported that high expression of tumour necrosis factor (TNF)‐α and lymphotoxin‐α (LT‐α) was associated with pregnancy complications, including idiopathic recurrent miscarriage (RM). TNF‐α (rs361525, rs1800629) and LT‐α (rs909253) polymorphism were investigated in RM and control women. Higher frequency of rs361525 A, but not the rs1800629 A or the LT‐α rs909253 G, allele was reported in patients. The rs361525 G/G was lower in patients. Association of the rs361525 SNP with idiopathic RM was confirmed by regression analysis. Haplotypes rs1800629 A/rs361525 G/rs909253 G and rs1800629 G/rs361525 A/rs909253 G played a susceptible role in idiopathic RM. Palmirotta et al. [146] reported that TNFA gene promoter polymorphism and susceptibility to recurrent pregnancy loss in Italian women.
Discussion
Tumour necrosis factor a pleiotropic cytokine regulating a broad range of biological activities including inflammation (Fig. 3). The inflammatory effects of TNF are mediated mainly by activation of the transcription factor NF‐κB and mitogen‐activated protein kinases (MAPKs). TNF binds to TNFR1 (receptor subunits, p55–p60) and TNFR2 (receptor subunits, p75‐p80). TNFR1 is constitutively expressed by most cell types, and TNFR2 expression is induced mainly in immune and endothelial cells. Therefore, any factor that affects the serum level of TNF might be important in outcome of disease. The presence of polymorphism in regulatory region may alter regulation expression level and thus affect disease manifestation.
Figure 3.
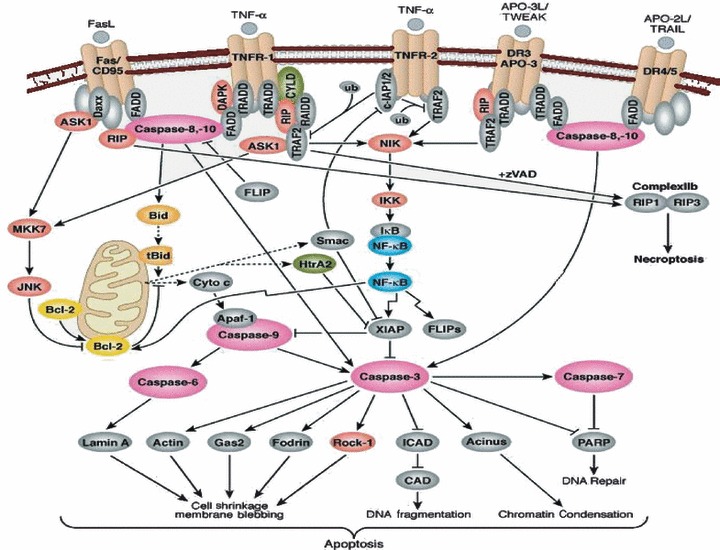
Tumor necrosis factor binding to its receptors eliciting an immune response.
Regulation of gene expression at the level of transcription is the most important. Several mechanisms play important role in gene regulation. Promoter hypermethylation and the presence of polymorphism in regulatory region are the two most important factors that interfere with the gene regulation, and our hypothesis is that during disease conditions, there is upregulation or downregulation of gene (transcriptional dysregulation). For example, infection of Plasmodium falciparum upregulates the expression of TNF‐α. In certain cancers, there is downregulation of genes. Promoter polymorphism lies in transcription factor–binding sites (cis elements) and hypermethylation of CpG (CG dinucleotide) islands can contribute to dysregulation. CpG islands are present in the promoters regions of almost half of mammalian genes [147], leading to gene silencing. DNA methylation could repress transcription by either or both of the following two mechanisms: (1) the methyl group may disrupt the binding sites of the transcription factors (TFs) and result in the failure of transcription [148, 149, 150]. (2) Methylated cytosines may attract methyl‐CpG‐binding domain proteins (MBD) which would bring repressors to silence the gene [151]. Methylation in CpG Island interferes with gene regulation and thus responsible for outcome of disease. There is an inverse correlation of DNA methylation with gene expression. High levels of DNA methylation in CpG‐rich promoters are strongly associated with downregulation of gene expression [151]. The several reports observed a strong anticorrelation of DNA methylation with CpG density and GC content, indicating that DNA methylation declines the more the sequence resembles a CpG island [45, 151, 152]. Methylation levels of 1320 CpG sites in regulatory regions of 416 genes in cells from acute lymphoblastic leukaemia (ALL) children were studied by Milani et al. [153].
A large number of diseases have been reported, in which promoter polymorphism affects the susceptibility to diseases (1, 2, 3). DNA sequence variations (polymorphism) that affect the transcription of genes play important roles in the pathogenesis of many complex diseases [154].While most discovered genetic defects create missense or non‐sense substitutions in protein‐coding sequences, there remain disease‐associated genes for which there is no difference in protein‐coding information between individuals of different phenotypes. Examples of the latter include CD36 type II deficiency [155], phenotypic variances in plasma dopamine‐beta‐hydroxylase [156] and serum angiotensin I converting enzyme levels [157]. The gene for TNF is polymorphic. Several TNF promoter SNPs have been reported to be associated with disease in humans. DNA sequence variations modifying transcriptional regulation of gene [154] play important role in many complex diseases. The first 200 bp of the promoter are highly conserved across a range of species, with the murine, bovine and porcine promoters showing approximately 80% homology with the human promoter; while further upstream, there is far less conservation between species. It has been reported that TNF rs1800630 polymorphism was associated with reduced level of serum TNF‐α, because this polymorphism is strongly influence the binding of nuclear proteins [158].
Table 1.
Details of polymorphism, infectious disease association, P‐values and OR, population type and case studies with references.
| Polymorphism | Disease associations/no association (infectious) | P‐values and OR | Population | Cases/controls (n) | References |
|---|---|---|---|---|---|
| rs1799964, rs1799724, rs1800629,rs361525,rs1800630 and rs909253 | No association with tuberculosis | 0.78 | North Indian | 185 patients and 155 controls | Sharma et al. [34] |
| rs1800629,rs361525, rs1800750 | No association with tuberculosis | ns | Turkey | 128 TB patients and 80 controls | Ates et al. [27] |
| rs1800629 and rs361525 | No statistical differences in haplotypes frequencies between patients with MB and PB | ns | Afro‐and Euro‐Brazilians | 631 patients with leprosy | Vanderborght et al. [39] |
| rs1800629 | rs1800629 higher frequency of GG in healthy controls, along with a decreased frequency of GA/AA genotypes was observed among patients with leprosy as compared to the control group | 0.009 | Brazilian | 240 controls and 167 patients with leprosy | Franceschi et al. [38] |
| rs1800629 | Higher symptom scores of rAOM. | <0.02 | USA | 128 subjects | McCormick et al. [167] |
| rs1800750, rs361525 | rs1800750 GG, rs361525 GG & TLR genotypes were associated with rAOM. TNFA‐376 G/G genotype was associated with the otitis‐prone condition in children | Crude OR: 3.10; P = 0.05; adjusted OR: 3.06; P = 0.07 | Netherlands | 348 patients and 463 controls | Emonts et al. [168] |
| 80 SNPs | Significant differences in allele frequencies between controls and patients with typhoid fever | >0.05 | Vietnam | 380 patients and controls | Dunstan et al. [44] |
| TNFB gene | Severe post‐traumatic sepsis was significantly increased in patients homozygous for the allele TNFB2 | OR of 3.07, P = 0.004 | German | 110 patients with severe blunt trauma | Majetschak et al. [169] |
| rs1800629 | TNF2 allele is strongly associated with susceptibility to Septic Shock Susceptibility and Mortality | 0.002 | France | 89 patients and 87 controls | Mira et al. [48] |
| rs1800629, rs2430561 | No significant differences in rs1800629, G/A genotype frequencies. The rs2430561 TT genotype, associated with an increased production of IFN‐gamma, and significantly less frequent in patients with MSF than in the control group | Odds ratio [OR], 0.18 | Sicilian | 80 patients with MSF and in 288 control | Forte et al. [50] |
| rs1800629 | No association with development of chronic HBV infection | ns, for inter group comparison | Iranian | 100 patients 89 healthy controls | Somi et al. [52] |
| rs1799964, rs1800630, −572(A/C), rs1800629 and rs361525 | No differences in TNF‐α genotype distribution at the rs1799964, rs1800630, −572(A/C), rs1800629 and rs361525 among the three populations. Compared with TT genotype, the CT genotype at the −204 locus was associated with a protective effect on SARS | OR 0.95 (0.90–0.99) | China | 75 SARS patients, 41 health care workers and 92 healthy controls | Wang et al. [53] |
| rs361525 | Haplotype of rs361525 and LTA significantly increased in patients with secondary dengue hemorrhagic fever | 0.0009 | Thais | 435 with Dengu infection | Vejbaesya et al. [170] |
| rs1800629 G (TNF1), rs1800629 A (TNF2) | TNF2 is not associated with predisposition to CM. The rs1800629 genotypes and alleles did not differ significantly between patients with CM and controls | 0.271 | Central Sudan | 109 patients with CM | Mergani et al. [60] |
| rs1799964, rs1799724, rs1800750, rs1800629 and rs361525 | Significant differences in TNF levels were seen between genotypes of both −1031 and −863 in malaria cases and controls | rs1799964 P = 0.0178, rs1800630, P = 0.0481 | Indian | 121 patients and 121 controls | Sinha et al. [59] |
| rs1800629 | A significantly higher frequency of allele 2 of rs1800629 was also observed in patients with MCL | <0.05 | Venezuelan | 49 patients with ACL and 43 control | Cabrera et al. [10] |
| rs1800629 | No association of TNF‐α polymorphisms with Chagas disease | ns | Brazilian | 166 patients and 80 controls | Drigo et al. [171] |
| rs1800629 | No association with toxoplasmic retinochoroiditis (TR). No significant difference in the genotype | χ2 = 0.79, P = 0.67 | Brazilian | 100 patients with TR and 100 control | Cordeiro et al. [172] |
| rs1800629, rs361525 | rs1800629 AA genotype plays a relevant role in the susceptibility and severity of OLP | OR = 10.93 | Thais | 75 patients and 154 controls | Kimkong et al. [173] |
MB, multibacillary; PB, paucibacillary; CM, cerebral malaria; OLP, oral lichen planus; rAOM, recurrent acute otitis media; HBV, hepatitis B virus; MCL, mucocutaneous leishmaniasis; ACL, American cutaneous leishmaniasis; MSF, Mediterranean Spotted fever; DHF, dengue hemorrhagic fever; CM, cerebral malaria; LTA, lymphotoxin alpha; SARS, severe acute respiratory syndrome; TNF‐α, tumor necrosis factor‐alpha.
Table 2.
Details of polymorphism, P‐values and OR, cancer risk association, population type and case studies with references.
| Polymorphism | Disease associations/No associations (cancer risk) | P‐values and OR | Population | Cases and controls (n) | References |
|---|---|---|---|---|---|
| rs1800630, rs361525 | No association with breast cancer | OR = 0.82 [0.64–1.04], P = 0.637 | North European | 709 patients and 498 controls | Azmy et al. [65] |
| rs361525 | Evidence against an overall association between invasive breast cancer risk and TNF rs361525 | OR 1.00 (0.95–1.06) | Europe, USA and Australia | 30,000 cases and 30,000 controls | Gaudet et al. [66] |
| 10 SNPs | Genotype distribution of 3 SNPs differed significantly between case subjects and 1 or both of the control groups | <0.05 | New Mexico | 341 cases and 241 controls | Deshpande et al. [68] |
| rs1799724, rs1800610 | Significant association with prostate cancer risk | Significant | Nutrition Cohort | 2321 cases and 2560 controls | Danforth et al. [69] |
| rs1800629 | No overall association between pancreatic cancer risk and TNF‐α (rs1800629 G/A) | Pancreatitis significantly associated with rs1800629 GA + AA (OR, 3.1; 95% CI, 1.3–7.4) | San Francisco Bay Area | 532 cases and 1701 controls | Duell et al. [71] |
| rs1800630, rs361525 | rs1800630, rs361525 were significantly different between the patients with lung cancer and controls | 0.0001 | Taiwan | Patients 202, controls 205 | Shih et al. [72] |
| rs1800629 | rs1800629 G‐allele significantly higher in RCC patients compared to controls | Significant | Turkey | 29 patients, 50 controls | Baştürk et al. [73] |
| rs1800629 | rs1800629 was significantly associated with the risk of HCC | <0.001, odds ratio [OR] = 4.75 | Turkish | 110 patients and 110 control | Akkiz et al. [75] |
| rs1800629, rs361525 | Both SNPs of TNF‐α are not genetic risk factor for AP susceptibility | OR = 1.63; 1.13−4.01 for rs1800629 and OR = 0.86; 0.75−1.77 for rs361525 | Turkey | 103 patients with acute pancreatitis (AP) and 92 controls | Ozhan et al. [76] |
| rs1800629, rs1143627, rs1800896, rs361525 | rs1800629 confer a higher risk of HCC. Other polymorphisms were not related to risk of HCC in this study | OR = 1.74 | 20 studies Caucasian and Asian | 2763 patients with HCC and 4152 controls | Yang et al. [166] |
RCC, renal cell carcinoma; HCC, hepatic cell carcinoma; AP, acute pancreatitis; TNF‐α, tumor necrosis factor‐alpha.
Table 3.
Details of polymorphism, other disease association, P‐values and OR, population type and case studies with references.
| Polymorphism | Disease associations/No associations (others) | P‐values and OR | Population | Cases and controls (n) | References |
|---|---|---|---|---|---|
| TNF, IFNA10, IFNA17, IFNG genes | A statistically significant increase of CFTR mutation carriers in the population of patients with sarcoidosis versus the control population was found | 6.1 × 10−8 | Greek | 89 Greek patients with sarcoidosis and 212 control | Makrythanasis et al. [77] |
| rs1799724 | Significant increase in rs1799724 CC genotype in ms. The rs1800629 and rs361525 Not associated with MS | <0.001 | Turkish | 86 patients and 150 controls | Akcali et al. [78] |
| rs1799964, rs1799724, rs1800750 | Haplotype rs1799964 T/rs1800630 C/rs1799724 C/rs1800629 G increased the risk of susceptibility to AS compared to random controls | P (corr) < 0.001, OR = 2.756 | China | 119 patients,95 healthy controls, and 135 random healthy controls | Chung et al. [84] |
| rs1800629, rs361525, rs3093661 | G‐alleles of rs361525 and rs3093661 SNPs have been associated with higher risk of Graves’ disease as compared with A‐alleles. No significant difference of rs1800629 allelic frequency | OR = 2.385 | Chinese | 436 patients and 316 control subjects | Gu et al. [86] |
| rs1800629 | rs1800629 A‐allele is associated with overall susceptibility to asthma | OR = 1.37, P = 0.04 | China | 2409 patients and 3266 controls | Gao et al. [118] |
| rs1800629 | A‐allele increases the production of TNF‐α. TNF2A allele frequency was elevated in patients with PBC | OR = 1.21, P = 0.042 | US and Canada | 866 patients with PBC and 761 controls | Juran et al. [119] |
| rs1799964 | rs1799964 associated with bowel disease | 0.00004 | UK white Caucasoid | 133 patients with IBD and 354 healthy controls | Ahamad et al. [174] |
| rs1800629 | rs1800629 and an atopic history impact the severity of irritation | USA | 68 healthcare workers with irritant hand dermatitis | Davis et al. [121] | |
| rs1800629, rs361525 | Significantly increased risk was associated with the variant GA + AA genotypes of rs361525, compared with the GG genotype and significantly reduced psoriasis risk was associated with the variant GA + AA genotypes of the rs1800629, compared with the GG genotype | OR = 2.60 | Various ethnicities | 997 cases, 943 control for rs361525 and 1156 cases, 1083 control for rs1800629 | Li et al. [122] |
| rs1800630, rs1800629 | Association between TNF haplotype and plasma levels of plasminogen activator factor inhibitor 1 (PAI‐1) | Significant | Swedish | 1209 subjects with MI | Mellick [62] |
| rs1800629 | Cardiovascular risk factors did not differ between TNF‐alpha rs1800629 high‐/low‐producer genotype groups | Turkish | 102 non‐diabetic patients | Yilmaz et al. [124] | |
| rs1800630, rs1800629, rs1799964 | SDICH risks were positively associated with the minor alleles rs1799964 C and – rs1800629 A in men but inversely associated with rs1800630 A in females (P = 0.03) | P = 0.03 and P = 0.005, respectively | Taiwan | 260 SDICH patients and 368 controls | Chen et al. [125] |
| rs1800629, rs361525 | Association with homocysteine levels in patients with ischemic strokes and silent brain infarctions | <0.05 | Koreans | 257 patients with SBIs, and 216 control | Kim et al. [127] |
| rs1800629 | A significantly increased risk of SAD was observed in the carriers of A‐allele | OR = 2.635, P < 0.01 | Southern China | 112 patients and 121 controls | Yang et al. [129] |
| rs1800630, rs1800629, rs1799964 | No significant difference in genotype distribution of rs1800629 in AD was found and no association for rs1800630 and rs1799964. A significant association between −850 polymorphism and AD risk | TT vs. TC + CC: pooled odds ratio [OR], 1.61; 1.08–2.29; P = 0.02 | Caucasian Australians and Northern Europeans | Meta analysis | Di Bona et al. [130] |
| Promoter SNPs | No positive associations were found with AD | Italian cohort | 253 patients with AD and 356 controls | Tedde et al. [131] | |
| rs1800629 | Carriers of the A‐allele demonstrated better attentional processes as compared to G‐allele carriers. The distribution of rs1800629 genotypes did not significantly differ from the Hardy–Weinberg equilibrium | 0.202 | Caucasian | 67 genetically unrelated healthy participants | Beste et al. [136] |
| rs1800629 | rs1800629 A‐allele was associated with worsened labile anger | <0.05 | USA | 105 patients with hepatitis C | Lotrich et al. [137] |
| rs361525 | Associated with increased risk of new ICH | 0.003 | Northern California | 280 patients | Achrol et al. [139] |
| rs1799964 | Variability of the rs1799964 polymorphism may be associated with susceptibility to endometriosis. The frequency of the rs1799964 C allele was significantly lower in stage IV endometriosis cases than controls | P = 0.04; OR = 1.75, 1.019–3.01 | Japanese | 185 female neonates born at the Hayashi Clinic in Kobe, Japan | Asghar et al. [5] |
| rs1799964, rs180063, rs1799724, rs1800629, rs361525 | Alleles −1031 and −863, individually or in combination in the haplotype −1031C −863A −857C −308G 238G, were associated with lower muscle mass in men. Specifically, carriers of −1031C, compared with non‐carriers, exhibited lower arm muscle mass | 0.01 | European descent | 1050. Most participants are of European descent | Liu et al. [141] |
| rs361525, rs1800629 | No association with Paget’s disease of bone | ns | Spanish patients | 172 patients and 150 healthy controls | Corral‐Gudino et al. [142] |
| rs2229094 of LTA | Strong association between rs2229094 and development of Proliferative Vitreoretinopathy | 0.0283 | Spain | 450 patients and 312 controls | Rojas et al. [143] |
| rs1800629, rs361525, rs909253 | The rs361525 and rs909253 but not rs1800629, polymorphic variants are associated with early RM. Haplotype rs1800629 G/rs361525 A/rs909253 G show association with RM | Confirmed by regression analysis | Tunisia | 372 RM women and 274 age‐matched parous control women | Zammiti et al. [145] |
SAD, sporadic Alzheimer’s disease; ICH, intracranial haemorrhage; MS, multiple sclerosis; PBC, primary biliary cirrhosis; AD, Alzheimer’s disease; SDICH, spontaneous deep intracerebral hemorrhage; RM, recurrent miscarriage; CFTR, cystic fibrosis conductance regulator; ns, non‐significant; AS, ankylosing spondylitis; IBD, inflammatory bowel disease; MI, myocardial infarction; TNF, tumor necrosis factor.
In gene expression, the multiple TFs first assemble at the promoter site and the recruit RNA polymerase. These TFs bind to their cognate binding sites in the promoter region. The presence of polymorphism in regulatory region affects the interaction of TFs with transcription factor–binding site (TFBS), influencing the expression of gene and thus susceptibility/resistance to disease. We have also predicted several SNPs in the promoter of TNF‐alpha, computationally, which lies in TFBS of several TFs in upstream region of TNF‐alpha (Table 4). Therefore, we hypothesized that predicted SNPs interfere with gene regulation and will increase the susceptibility to disease.
Table 4.
Detected single nucleotide polymorphisms lies in transcription factor–binding site for transcription factors in tumor necrosis factor‐alpha upstream region.
| SNP ID | Position | TF | Allele 1 | Allele 2 | Conservation |
|---|---|---|---|---|---|
| rs5875327 | 31648480 | NRF‐2 | aGagaaagag | aCagaaagag | Yes |
| rs4647194 | 31648484 | NRF‐2 | aGagaaagag | aCagaaagag | Yes |
| rs2857713 | 31648535 | Thing1‐E47 | TGTGTGGCAC | TGCGTGGCAC | No |
| rs1041981 | 31648763 | AML‐1 | TTTGAGGTT | TTTGAGGGT | No |
| rs4647195 | 31648936 | Chop‐cEBP | CCCTGCCACCCC | CCCTGCCATCCC | No |
| rs3093544 | 31649758 | Spz1 | AGAGGAAGAGC | AGAGGAAGGGC | No |
| rs4645834 | 31649818 | Broad‐complex_4 | AAGGCaaaaaa | AAGTCaaaaaa | Yes |
| rs3093547 | 31649827 | Hunchback | GCaaaaaaat | TCaaaaaaat | Yes |
| rs4248157 | 31650122 | NF‐κB | GAGAACTTCC | GAGAACTTCT | No |
| rs9282875 | 31650245 | Thing1‐E47 | AGTCTGGCGG | AGTCTGGCAG | Yes |
| rs4647198 | 31650314 | COUP‐TF | TGACCCCCGCCCCT | TGACCCCCACCCCT | No |
| rs2507961 | 31650433 | TBP | CTATGGAAGTCGAGT | CTATGAAAGTCGAGT | No |
| rs1800630 | 31650455 | NF‐κB | GGGACCCCCA | GGGGGGTCCC | No |
| rs4645836 | 31650456 | p50 | GTGGGGGTCCC | GGGGGGGTCCC | No |
| rs4248158 | 31650512 | E74A | CTGGAGG | CTGGAAG | No |
| rs4987086 | 31650536 | bZIP910 | GGGACAT | GGGACGT | No |
| rs4248159 | 31650559 | SOX17 | CACAATGGG | CCCAGTGTG | No |
| rs2857712 | 31650632 | HFH‐2 | TAATGCTGGGTT | TAATGCTGGTTT | Yes |
| rs2736196 | 31650634 | HFH‐2 | TAATGCTGGGTT | TAATGCTGGTTT | Yes |
| rs2736195 | 31650670 | NF‐κB | GGGCCTGCCC | AGGCCTGCCC | Yes |
| rs4248160 | 31650672 | Snail | CAGGTC | CAGGCC | Yes |
| rs4248162 | 31650748 | Spz1 | GGGGTAGTAGG | GGGGTAGCAGG | No |
| rs2857711 | 31650750 | Spz1 | GGGGTAGTAGG | GGGGTAGCAGG | No |
| rs1800195 | 31650743 | Spz1 | GGGGTAGCAGG | GGGGGAGCAGG | No |
| rs3093548 | 31650799 | Myf | CCCCAGCTCCTT | AAAGAGCTGGGG | No |
| rs4248163 | 31650807 | E74A | CCCGCAG | CCGGCAG | No |
| rs4248164 | 31651327 | Hen‐1 | CAGCAGACGCTC | CAGCAGATGCTC | Yes |
| rs4645838 | 31651391 | E74A | CTGAAAA | CCTGAAA | No |
| rs2515924 | 31651470 | CREB | GAGGGTGAAGCC | GAGGGTGGAGCC | No |
| rs2228088 | 31651584 | Myf | AAGCACCGCCTG | AAGCAACGCCTG | Yes |
| rs4645839 | 31651804 | ARNT | aAcgtg | aTcgtg | No |
| rs1800610 | 31651806 | ARNT | aAcgtg | aTcgtg | No |
Tumour necrosis factor promoter polymorphism and susceptibility to falciparum malarial infection and pulmonary tuberculosis have been carried out in Indian population. In malaria, TNF‐α rs1799964 C and rs1800630 A‐alleles as well as homozygotes for the TNF enhancer haplotype CACGG correlated with enhanced plasma TNF levels in both patients and controls. Significantly, higher TNF levels were observed in patients with severe malaria. In tuberculosis, no significant differences of the allele frequencies between the patients with tuberculosis and controls have been reported but a significant difference in the serum TNF‐α level in the patients and the controls has been found. Two TNF polymorphisms rs1800629 and rs361525 show association in most of the diseases (if any association found). Probably, these polymorphisms affect the transcription of gene.
Polymorphisms of TNF are likely to contribute to disease, the complex pattern of associations that has been revealed could also be attributable to LD with another susceptibility locus in the vicinity of the gene. By examining LD patterns, we determined that the effect of TNF is independent of the known HLA–A and HLA–DRB1 associations (Fig. 4). The chromosomal region surrounding TNF, however, is abundant in genes of immunologic relevance. To identify true susceptibility genes, the genetic variation of the region must be studied, and extended haplotypes must be constructed and analysed.
Figure 4.
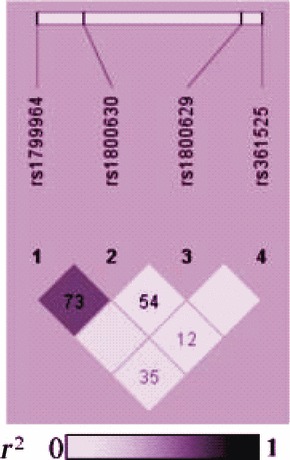
The Linkage disequilibrium (LD) between the four SNPs mentioned in the review [rs1799964 (−1031); rs1800630 (−863); rs1800629 (−308); rs361525 (−238)]. This LD plot is taken from falciparum malaria case control study in Indian population (Sinha et al. [59]).
Linkage disequilibrium patterns were estimated to determine whether the observed TNF SNP effects are attributable to the known HLA associations with disease or are independent contributors to disease. All of the associated TNF SNPs were tested against all HLA–A and HLA–DRB1 alleles. The results showed that none of the polymorphic positions in TNF are in LD with any of the associated HLA–A or HLA–DRB1 alleles (HLA–A*02, HLA–DRB1*08 or HLA–DRB1*1).
Acute anterior uveitis case–control study was carried out by Kuo et al. [159] in UK population. The association of the SNPs of TNF‐α, LT‐α, TNF‐R1 and TNF‐R2 genes in patients with idiopathic acute anterior uveitis (IAU) was investigated in this study. In addition, there was very little linkage disequilibrium between TNF‐α−857 and the other TNF SNPs, suggesting that the effect is largely attributable to TNFα−857. Results suggest that the uncommon TNF‐α−857T allele is a susceptibility marker for IAU (Fig. 5).
Figure 5.
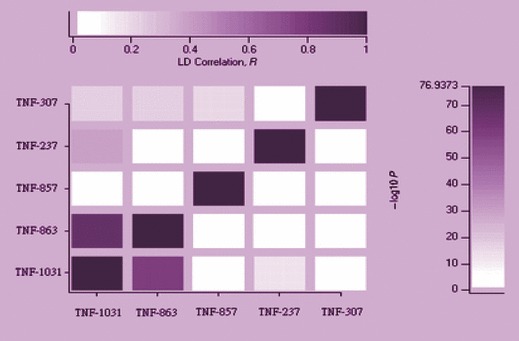
Linkage disequilibrium (LD) between TNF−1031, −863, −857, −307, and −237. Linkage disequilibrium was weak between TNF−857 and the other SNPs in the gene. The strongest evidence for LD was between TNF−857 and TNF−307 (r = 0.19). From acute anterior uveitis case control study in UK population (Kuo et al. [159]).
We have checked the conservation pattern in the promoter region and found that most of the region in the promoter is conserved (Fig. 6). It is also suggestive of the fact that if a polymorphism or any variation in the DNA sequence occurs in the conserved region, then it effects the interaction of TF with TF binding largely. Understanding the conservation and change of regulatory sequences is critical to our knowledge of the unity as well as diversity of animal development and phenotypes. It can be hypothesized from these data that as the number of organisms increases, the per cent conservation decreases although certain position in the sequence remains constant throughout. These conserved sequences are thought to be the essential sites that are controlling the regulatory activity for the normal expression of the gene.
Figure 6.
Consensus nucleotide pattern details of three conserved motifs, identified from 1500 bp upstream and 500 bp downstream promoter sequences of TNF gene belongs to Human, Mouse and Rat.
Contribution of promoter polymorphism in expression divergence, fitness and evolution
Wittkopp [160] reported that natural selection has played some role in expression divergence, but the relative frequency of adaptive and neutral changes remains unclear. Bradley et al. [161] observed differences in TFBS between species that were similar in regions of the genome. DNA sequence variation in TFBS affects gene expression, gene expression to phenotypic variation and phenotypic variation to fitness in the wild [161]. The variations in the DNA region alter the interaction between TF and TFBS, thereby modulating the host–parasite interaction. The genome tries to selects those variations, which provide resistance against the disease. Malaria is an example of evolutionary selection, in which sickle cell anaemia is selected against the pressure of malaria in endemic region. There is evidence of positive selection in early HIV‐1 infection, which appears to be driven in many cases by escape from early cytotoxic T‐lymphocyte (CTL) responses via mutations in the APOBEC sequence, suggesting a role for APOBEC in determining the pathway of immune escape [162].
The recruitment of different combinations of TFs to different genes allows expression of each gene to be regulated independently. Those changes that alter the activity or availability of TFs and the cis‐regulatory sequences, to which they bind, can change gene expression. Both types of changes result in evolution. The studies suggested that those changes that affect the cis‐regulatory activity are the predominant source of expression divergence between species [160, 163, 164, 165]. Bradley et al. [161] detected binding of the same TFs to regions of DNA in D. melanogaster and D. yakuba that have a common evolutionary origin; however, the relative affinity of these binding sites often differed between species. This suggests that evolutionary changes in the DNA sequence of cis‐regulatory regions have occurred that alter the strength of the interaction between TFs and their binding sites without eliminating binding.
In the light of these facts, we have hypothesized that TNF enhancer polymorphism plays important role in susceptibility/resistance to diseases and those polymorphism that lie in transcription factor–binding sites might play role in expression divergence, fitness and evolution.
Conclusion
As the TNF gene is tightly regulated at the level of transcription. The presence of polymorphism in the 5′ regulatory region might affect transcription of TNF gene. We concluded that low‐level TNF provides host defence, whereas high TNF level has been associated with severe manifestations. Alterations in the circulating levels of TNF might be a reason for differential association with diseases in different populations and also affect the expression divergence, fitness and evolution.
Acknowledgment
We acknowledge the Council of Scientific and Industrial Research, New Delhi, India, for providing research facility and supportive environment to carryout doctoral research work at Central Institute of Medicinal and Aromatic Plants, Lucknow, India.
References
- 1. Nedwin GE, Naylor SL, Sakaguchi AY et al. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res 1985;13:6361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF‐2/Jun and NFATp. Mol Cell Biol 1996;16:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsytsykova AV, Goldfeld AE. Inducer‐specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes. Mol Cell Biol 2002;22:2620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Heel DA, Udalova IA, De Silva AP et al. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF‐kB transcription factors. Hum Mol Genet 2002;11:1281–9. [DOI] [PubMed] [Google Scholar]
- 5. Asghar T, Yoshida S, Kennedy S et al. The tumor necrosis factor‐a promoter −1031C polymorphism is associated with decreased risk of endometriosis in a Japanese population. Hum Reprod 2004;19:2509–14. [DOI] [PubMed] [Google Scholar]
- 6. Bayley JP, Ottenhoff TH, Verweij CL. Is there a future for TNF promoter polymorphisms? Genes Immun 2004;5:315–29. [DOI] [PubMed] [Google Scholar]
- 7. Rasmussen SK, Urhammer SA, Jensen JN, Hansen T, Borch‐Johnsen K, Pedersen O. The −238 and −308G/A polymorphisms of tumor necrosis factor alpha gene promoter are not associated with features of the insulin resistance syndrome or altered birth weight in Danish Caucasians. J Clin Endocrinol Metab 2000;85:1731–4. [DOI] [PubMed] [Google Scholar]
- 8. Mizuki N, Ohno S, Sato T et al. Microsatellite polymorphism between the tumor necrosis factor and HLA‐B genes in Behcet’s disease. Hum Immunol 1995;43:129–35. [DOI] [PubMed] [Google Scholar]
- 9. Nadel S, Newport MJ, Booy R, Levin M. Variation in the tumor necrosis factor‐alpha gene promoter region may be associated with death from meningococcal disease. J Infect Dis 1996;174:878–80. [DOI] [PubMed] [Google Scholar]
- 10. Cabrera M, Shw MA, Sharples C et al. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med 1995;182:1259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fong KY, Howe HS, Tin SK, Boey ML, Feng PH. Polymorphism of the regulatory region of tumor necrosis factor alpha gene in patient with systemic lupus erythematosus. Ann Acad Med Singapore 1996;25:90–3. [PubMed] [Google Scholar]
- 12. Verjans GM, Brinkman BM, Van Doornik CE, Kijlstra A, Verweij CL. Polymorphism of tumour necrosis factor‐alpha (TNF‐alpha) at position −308 in relation to ankylosing spondylitis. Clin Exp Immunol 1994;97:45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson AG, Clay FE, Crane AM, Cork MJ, Duff GW. Comparative genetic association of human leukocyte antigen class II and tumor necrosis factor alpha with dermatitis herpetic formis. J Invest Dermatol 1995;104:856–8. [DOI] [PubMed] [Google Scholar]
- 14. Negoro K, Kinouchi Y, Hiwatashi N et al. Crohn’s disease is associated with Novel polymorphisms in the 50‐flanking region of the tumor necrosis factor gene. Gastroenterology 1999;117:1062–7. [DOI] [PubMed] [Google Scholar]
- 15. Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumor necrosis factor alpha (TNF‐alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet 1992;1:353. [DOI] [PubMed] [Google Scholar]
- 16. Koch M, Rett K, Volk E et al. The tumor necrosis factor alpha 2238G‐A and 2308G‐A promoter polymorphisms are not associated with insulin sensitivity and insulin secretion in young healthy relatives of type II diabetic patients. Diabetologia 2000;43:181–4. [DOI] [PubMed] [Google Scholar]
- 17. Orchard TR, Chua CN, Ahmad T, Cheng H, Welsh KI, Jewell DP. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology 2002;123:714–8. [DOI] [PubMed] [Google Scholar]
- 18. Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 1995;273:220–6. [PubMed] [Google Scholar]
- 19. World Health Organization . Global Tuberculosis Control–Surveillance, Planning, Financing. URL: http://www.who.int (Accessed on 15 November 2009), 2009. [Google Scholar]
- 20. Schurr E. Is susceptibility to tuberculosis acquired or inherited? J Intern Med 2007;261:106–11. [DOI] [PubMed] [Google Scholar]
- 21. Comstock GW. Tuberculosis in twins a re‐analysis of the Prophit survey. Am Rev Respir Dis 1978;117:621–4. [DOI] [PubMed] [Google Scholar]
- 22. Fernando SL, Britton WJ. Genetic susceptibility to mycobacterial disease in humans. Immunol Cell Biol 2006;84:125–37. [DOI] [PubMed] [Google Scholar]
- 23. Hoal EG. Human genetic susceptibility to tuberculosis and other mycobacterial diseases. IUBMB Life 2002;53:225–9. [DOI] [PubMed] [Google Scholar]
- 24. Malik S, Schurr E. Genetic susceptibility to tuberculosis. Clin Chem Lab Med 2002;40:863–8. [DOI] [PubMed] [Google Scholar]
- 25. Sharma S, Ghosh B, Sharma SK. Association of TNF polymorphisms with sarcoidosis, its prognosis and tumour necrosis factor (TNF)‐alpha levels in Asian Indians. Clin Exp Immunol 2008;151:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma S, Sharma A, Kumar S, Sharma SK, Ghosh B. Association of TNF haplotypes with asthma, serum IgE levels, and correlation with serum TNF alpha levels. Am J Respir Cell Mol Biol 2006;35:488–95. [DOI] [PubMed] [Google Scholar]
- 27. Ates O, Musellim B, Ongen G, Topal Sarikaya A. Interleukin‐10, and tumor necrosis factor‐alpha gene polymorphisms in tuberculosis. J Clin Immunol 2008;28:232–6. [DOI] [PubMed] [Google Scholar]
- 28. Oh JH, Yang CS, Noh YK et al. Polymorphisms of interleukin‐10 and tumour necrosis factor‐alpha genes are associated with newly diagnosed and recurrent pulmonary tuberculosis. Respirology 2007;12:594–8. [DOI] [PubMed] [Google Scholar]
- 29. Oral HB, Budak F, Uzaslan EK et al. Interleukin‐10 (IL‐10) gene polymorphism as a potential host susceptibility factor in tuberculosis. Cytokine 2006;35:143–7. [DOI] [PubMed] [Google Scholar]
- 30. Qu Y, Tang Y, Cao D et al. Genetic polymorphisms in alveolar macrophage response‐related genes, and risk of silicosis and pulmonary tuberculosis in Chinese iron miners. Int J Hyg Environ Health 2007;210:679–89. [DOI] [PubMed] [Google Scholar]
- 31. Scola L, Crivello A, Marino V et al. IL‐10 and TNF‐alpha polymorphisms in a sample of Sicilian patients affected by tuberculosis: implication for ageing and life span expectancy. Mech Ageing Dev 2003;124:569–72. [DOI] [PubMed] [Google Scholar]
- 32. Selvaraj P, Sriram U, Mathan Kurian S, Reetha AM, Narayanan PR. Tumour necrosis factor alpha (−238 and −308) and beta gene polymorphisms in pulmonary tuberculosis: haplotype analysis with HLA‐A, B and DR genes. Tuberculosis 2001;81:335–41. [DOI] [PubMed] [Google Scholar]
- 33. Vejbaesya S, Mahaisavariya P, Luangtrakool P, Sermduangprateep C. NRAMP1 and TNF‐alpha polymorphisms and susceptibility to tuberculosis in Thais. Respirology 2007;12:202–6. [DOI] [PubMed] [Google Scholar]
- 34. Sharma S, Rathored J, Ghosh B, Sharma SK. Genetic polymorphisms in TNF genes and tuberculosis in North Indians. BMC Infect Dis 2010;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goulart LR, Goulart IM. Leprosy pathogenetic background: a review and lessons from other mycobacterial diseases. Arch Dermatol Res 2009;301:123–37. [DOI] [PubMed] [Google Scholar]
- 36. Santos AR, Suffys PN, Vanderborght PR et al. Role of tumor necrosis factor‐alpha and interleukin‐10 promoter gene polymorphisms in leprosy. J Infect Dis 2002;186:1687–91. [DOI] [PubMed] [Google Scholar]
- 37. Shaw MA, Donaldson IJ, Collins A et al. Association and linkage of leprosy phenotypes with HLA class II and tumour necrosis factor genes. Genes Immun 2001;2:196–204. [DOI] [PubMed] [Google Scholar]
- 38. Franceschi DS, Mazini PS, Rudnick CC et al. Influence of TNF and IL10 gene polymorphisms in the immunopathogenesis of leprosy in the south of Brazil. Int J Infect Dis 2009;13:493–8. [DOI] [PubMed] [Google Scholar]
- 39. Vanderborght PR, Matos HJ, Salles AM et al. Single nucleotide polymorphisms (SNPs) at −238 and −308 positions in the TNFalpha promoter: clinical and bacteriological evaluation in leprosy. Int J Lepr Other Mycobact Dis 2004;72:143–8. [DOI] [PubMed] [Google Scholar]
- 40. Kaijzel EL, van Krugten MV, Brinkman BMN et al. Functional analysis of a human tumour necrosis factor alpha (TNF‐alpha) promoter polymorphism related to joint damage in rheumatoid arthritis. Mol Med 1998;4:724–33. [PMC free article] [PubMed] [Google Scholar]
- 41. Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin‐10 gene promoter. Eur J Immunogenet 1997;24:1–8. [DOI] [PubMed] [Google Scholar]
- 42. Fishman D, Faulds G, Jeffery R et al. The effect of novel polymorphisms in the interleukin‐6 (IL‐6) gene on IL‐6 transcription and plasma IL‐6 levels, and an association with systemic‐onset juvenile chronic arthritis. J Clin Invest 1998;102:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keuter M, Dharmana E, Gasem MH et al. Patterns of proinflammatory cytokines and inhibitors during typhoid fever. J Infect Dis 1994;169:1306–11. [DOI] [PubMed] [Google Scholar]
- 44. Dunstan SJ, Hue NT, Rockett KR et al. A TNF region haplotype offers protection from typhoid fever in Vietnamese patients. Hum Genet 2007;122:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meissner A, Mikkelsen TS, Gu H et al. Genome scale DNA methylation maps of pluripotent and differentiated cells. Nature 2008;454:766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pociot F, Briant L, Jongeneel CV et al. Association of tumour necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF‐α and TNF‐β by human mononuclear cells: a link to insulin dependent diabetes mellitus. Eur J Immunol 1993;23:224–31. [DOI] [PubMed] [Google Scholar]
- 47. Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med 1999;340:207–14. [DOI] [PubMed] [Google Scholar]
- 48. Mira JP, Cariou A, Grall F. Association of TNF2, a TNF‐a promoter polymorphism, with septic shock susceptibility and mortality. JAMA 1999;282:561–8. [DOI] [PubMed] [Google Scholar]
- 49. McGuire W, Knight JC, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Severe malarial anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles. J Infect Dis 1999;179:287–90. [DOI] [PubMed] [Google Scholar]
- 50. Forte GI, Scola L, Misiano G et al. Relevance of gamma interferon, tumor necrosis factor alpha, and interleukin‐10 gene polymorphisms to susceptibility to Mediterranean spotted fever. Clin Vaccine Immunol 2009;16:811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim YJ, Lee HS, Yoon JH et al. Association of TNF‐promoter polymorphisms with the clearance of hepatitis B virus infection. Hum Mol Genet 2003;12:2541–6. [DOI] [PubMed] [Google Scholar]
- 52. Somi MH, Najafi L, Noori NB et al. Tumor necrosis factor‐alpha gene promoter polymorphism in Iranian patients with chronic hepatitis B. Indian J Gastroenterol 2006;25:14–5. [PubMed] [Google Scholar]
- 53. Wang S, Wei M, Han Y et al. Roles of TNF‐α gene polymorphisms in the occurrence and progress of SARS‐Cov infection: a case–control study. BMC Infect Dis 2008;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mac Lean L, Chisi JE, Odiit M et al. Severity of Human African Trypanosomiasis in East Africa Is Associated with Geographic Location, Parasite Genotype, and Host Inflammatory Cytokine Response Profile. Infect Immun 2004;72:7040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guivier E, Galan M, Salvador AR et al. TNF‐alpha expression and promoter sequences reflect the balance of tolerance/resistance to Puumala hantavirus infection in European bank vole populations. Infect Genet Evol 2010;10:1208–17. [DOI] [PubMed] [Google Scholar]
- 56. Ubalee R, Suzuki F, Kikuchi M et al. Strong association of a tumor necrosis factor‐α promoter allele with cerebral malaria in Myanmar. Tissue Antigens 2001;58:407–10. [DOI] [PubMed] [Google Scholar]
- 57. Aidoo M, McElroy PD, Kolczak MS, Terlouw DJ, Kuile FO, Nahlen B. Tumor necrosis factor‐alpha promoter variant 2 (TNF2) is associated with pre‐term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet Epidemiol 2001;21:201–11. [DOI] [PubMed] [Google Scholar]
- 58. Hananantachai H, Patarapotikul J, Ohashi J et al. Significant association between TNF‐α (TNF) promoter allele (−1031C, −863C, and −857C) and cerebral malaria in Thailand. Tissue Antigens 2007;69:277–80. [DOI] [PubMed] [Google Scholar]
- 59. Sinha S, Mishra SK, Sharma S et al. Polymorphisms of TNF‐enhancer and gene for FcγRIIa correlate with the severity of falciparum malaria in the ethnically diverse Indian population. Malar J 2008;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mergani A, Khamis AH, Haboor AB et al. Lack of association between −308 tumor necrosis factor polymorphism and susceptibility to cerebral malaria among Central Sudanese children. Int J Genet Mol Biol 2010;2:67–71. [Google Scholar]
- 61. Aguiar EC, Prestes AL. TNF gene polymorphisms are associated with reduced survival in severe Chagas’ disease cardiomyopathy patients. Microbes Infect 2006;8:598–603. [DOI] [PubMed] [Google Scholar]
- 62. Mellick GD. TNF polymorphism and cardiovascular disease: TNF gene polymorphism and quantitative traits related to cardiovascular disease: getting to the heart of the matter. Eur J Hum Genet 2007;15:609–11. [DOI] [PubMed] [Google Scholar]
- 63. Sato H, Silveira L, Fingerlin T et al. TNF polymorphism and bronchoalveolar lavage cell TNF‐α levels in chronic beryllium disease and beryllium sensitization. J Allergy Clin Immunol 2007;119:687–96. [DOI] [PubMed] [Google Scholar]
- 64. Moffett SP, Zmuda JM, Oakley JI et al. Tumor necrosis factor‐α polymorphism, bone strength phenotypes, and the risk of fracture in older women. J Clin Endocrinol Metab 2005;90:3491–7. [DOI] [PubMed] [Google Scholar]
- 65. Azmy IA, Balasubramanian SP, Wilson AG et al. Role of tumour necrosis factor gene polymorphisms (−308 and −238) in breast cancer susceptibility and severity. Breast Cancer Res 2004;6:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaudet MM, Milne RL, Cox A et al. Five polymorphisms and breast cancer risk: results from the breast cancer association consortium. Cancer Epidemiol Biomarkers Prev 2009;18:1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jung JH, Chae YS, Moon JH et al. TNF superfamily gene polymorphism as prognostic factor in early breast cancer. J Cancer Res Clin Oncol 2010;136:685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deshpande A, Nolan JP, White PS et al. TNF‐alpha promoter polymorphisms and susceptibility to human papillomavirus 16‐associated cervical cancer. J Infect Dis, 2005;191:969–76. [DOI] [PubMed] [Google Scholar]
- 69. Danforth KN, Rodriguez C, Hayes RB et al. TNF polymorphisms and prostate cancer risk. Prostate 2008;68:400–7. [DOI] [PubMed] [Google Scholar]
- 70. Seidemann K, Zimmermann M, Book M et al. Tumor necrosis factor and lymphotoxin alfa genetic polymorphisms and outcome in pediatric patients with non‐Hodgkin’s lymphoma: results from Berlin‐Frankfurt‐Munster Trial NHL‐BFM 95. J Clin Oncol 2005;23:8414–21. [DOI] [PubMed] [Google Scholar]
- 71. Duell EJ, Casella DP, Burk RD, Kelsey KT, Holly EA. Inflammation, genetic polymorphisms in proinflammatory genes TNF‐A, RANTES, and CCR5, and risk of pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:726. [DOI] [PubMed] [Google Scholar]
- 72. Shih CM, Lee YL, Chiou HL et al. Association of TNF‐alpha polymorphism with susceptibility to and severity of non‐small cell lung cancer. Lung Cancer 2006;52:15–20. [DOI] [PubMed] [Google Scholar]
- 73. Başturk B, Yavaşçaoğlu I, Vuruşkan H, Goral G, Oktay B, Oral HB. Cytokine gene polymorphisms as potential risk and protective factors in renal cell carcinoma. Cytokine 2005;30:41–5. [DOI] [PubMed] [Google Scholar]
- 74. Onishi T, Ohishi Y, Goto H et al. Study on the relationship between tumour necrosis factor gene polymorphism and prognosis in the patients with renal cell carcinoma. Nihon Hinyokika Gakkai Zasshi 1998;89:413–20. [DOI] [PubMed] [Google Scholar]
- 75. Akkiz H, Bayram S, Bekar A et al. G−308A TNF‐alpha polymorphism is associated with an increased risk of hepatocellular carcinoma in the Turkish population: case–control study. Cancer Epidemiol 2009;33:261–4. [DOI] [PubMed] [Google Scholar]
- 76. Ozhan G, Yanar HT, Ertekin C, Alpertunga B. Polymorphisms in tumour necrosis factor alpha (TNFα) gene in patients with acute pancreatitis. Mediators Inflamm 2010;6:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Makrythanasis P, Tzetis M, Rapti A et al. Cystic fibrosis conductance regulator, tumor necrosis factor, interferon alpha‐10, interferon alpha‐17, and interferon gamma genotyping as potential risk markers in pulmonary sarcoidosis pathogenesis in Greek patients. Genet Test Mol Biomarkers 2010;14:577–84. [DOI] [PubMed] [Google Scholar]
- 78. Akcali A, Pehlivan S, Pehlivan M, Sever T, Akgul P, Neyal M. TNF‐alpha promoter polymorphisms in multiple sclerosis: no association with −308 and −238 alleles, but the −857 alleles in associated with the disease in Turkish patients. Int J Immunogenet 2010;37:91–5. [DOI] [PubMed] [Google Scholar]
- 79. Moffatt MF, Cookson WO. Tumour necrosis factor haplotypes and asthma. Hum Mol Genet 1997;6:551–4. [DOI] [PubMed] [Google Scholar]
- 80. Huizinga TW, Westendorp RG, Bollen EL et al. TNF‐alpha promoter polymorphisms, production, and susceptibility to multiple sclerosis in different groups of patients. J Neuroimmunol 1997;72:149–53. [DOI] [PubMed] [Google Scholar]
- 81. Lucotte G, Bathelier C, Mercier G. TNF‐alpha polymorphisms in multiple sclerosis: no association with −238 and −308 promoter alleles, but the microsatellite allele a11 is associated with the disease in French patients. Mult Scler 2000;6:78–80. [DOI] [PubMed] [Google Scholar]
- 82. Aguillón JC, Cruzat A, Aravena O, Salazar L, Llanos C, Cuchacovich M. Could single‐nucleotide polymorphisms (SNPs) affecting the tumour necrosis factor promoter be considered as part of rheumatoid arthritis evolution? Immunobiology 2006;211:75–84. [DOI] [PubMed] [Google Scholar]
- 83. Lee YH, Ji D, Song GG. Tumor necrosis factor‐alpha promoter −308A/G polymorphism and rheumatoid arthritis susceptibility: a metaanalysis. J Rheumatol 2007;34:43–9. [PubMed] [Google Scholar]
- 84. Chung WT, Choe JY, Jang WC et al. Polymorphisms of tumor necrosis factor‐alpha promoter region for susceptibility to HLA‐B27‐positive ankylosing spondylitis in Korean population. Rheumatol Int 2010;1–9. [DOI] [PubMed] [Google Scholar]
- 85. Lee YH, Song GG. Lack of association of TNF‐alpha promoter polymorphisms with ankylosing spondylitis: a meta‐analysis. Rheumatology 2009;48:1359–62. [DOI] [PubMed] [Google Scholar]
- 86. Gu LQ, Zhu W, Pan CM et al. Tumor necrosis factor alpha (TNF‐α) polymorphisms in Chinese patients with Graves’ disease. Clin Biochem 2010;43:223–7. [DOI] [PubMed] [Google Scholar]
- 87. Rich SS, Concannon P, Erlich H et al. The Type 1 Diabetes Genetics Consortium. Ann NY Acad Sci 2006;1079:1–8. [DOI] [PubMed] [Google Scholar]
- 88. Roach JC, Deutsch K, Li S et al. Swedish Childhood Diabetes Study Group; Diabetes Incidence in Sweden Study Group Genetic mapping at 3‐kilobase resolution reveáis inositol 1,4,5‐triphosphate receptor 3 as a risk factor for type 1 diabetes in Sweden. Am J Hum Genet 2006;79:614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smyth DJ, Cooper JD, Bailey R et al. A genome‐wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon‐induced helicase (IFIH1) región. Nat Genet 2006;238:617–9. [DOI] [PubMed] [Google Scholar]
- 90. Krikovszky D, Vásárhelyi B, Tóth‐heyn P, Körner A, Tulassay T, Madácsy L. Association between G−308A polymorphism of the tumor necrosis factor‐alpha gene and 24‐hour ambulatory blood pressure values in type 1 diabetic adolescents. Clin Genet 2002;62:474–7. [DOI] [PubMed] [Google Scholar]
- 91. Kumar R, Goswami R, Agarwal S, Israni N, Singh SK, Rani R. Association and interaction of the TNF‐alpha gene with other pro‐ and anti‐inflammatory cytokine genes and HLA genes in patients with type 1 diabetes from North India. Tissue Antigens 2007;69:557–67. [DOI] [PubMed] [Google Scholar]
- 92. Das SN, Baniasadi V, Kapuria V. Association of −308TNF‐alpha promoter polymorphism with type 1 diabetes in North Indians. Int J Immunogenet 2006;33:411–6. [DOI] [PubMed] [Google Scholar]
- 93. Noble JA, Valdés AM, Lane JA, Green AE, Erlich HA. Linkage disequilibrium with predisposing DR3 haplotypes accounts for apparent effects of tumor necrosis factor and lymphotoxin‐alpha polymorphisms on type 1 diabetes susceptibility. Hum Immunol 2006;67:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Deng GY, Maclaren NK, Huang HS, Zhang LP, She JX. No primary association between the −308 polymorphism in the tumor necrosis factor alpha promoter región and insulin‐dependent diabetes mellitus. Hum Immunol 1996;45:137–42. [DOI] [PubMed] [Google Scholar]
- 95. Boraska V, Skrabić V, Culić CV, Becić K, Kapitanović S, Zemunik T. Association of TNF promoter polymorphisms with type 1 diabetes in the South Croatian population. Biol Res, 2008;41:157–63. [PubMed] [Google Scholar]
- 96. Shin HD, Yang SW, Kim DH, Park Y. Independent association of tumor necrosis factor polymorphism with type 1 diabetes susceptibility. Ann N Y Acad Sci 2008;1150:76–85. [DOI] [PubMed] [Google Scholar]
- 97. Kubaszek A, Pihlajamaki J, Komarovaski V et al. Promoter polymorphisms of the TNF‐α (G−308A) and IL‐6 (C−174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes the Finnish Diabetes Prevention Study. Diabetes 2003;52:1872–6. [DOI] [PubMed] [Google Scholar]
- 98. Buraczynska K, Koziol‐Montewka M, Majdan M, Tokarz A, Ksiazek A. Genetic determination of TNF and myeloperoxidase production in dialyzed patients with diabetic nephropathy. Ren Fail 2004;26:633–9. [DOI] [PubMed] [Google Scholar]
- 99. Hampe J, Shaw SH, Saiz R et al. Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet 1999;65:1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dechairo B, Dimon C, van Heel D et al. Replication and extension studies of inflammatory bowel disease susceptibility regions confirm linkage to chromosome 6p (IBD3). Eur J Hum Genet 2001;9:627–33. [DOI] [PubMed] [Google Scholar]
- 101. Rioux JD, Silverberg MS, Daly MJ et al. Genome wide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet 2000;66:1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Westendorp RG, Langermans JA, Huizinga TW et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 1997;349:170–3. [DOI] [PubMed] [Google Scholar]
- 103. Kawasaki A, Tsuchiya N, Hagiwara K, Takazoe M, Tokunaga K. Independent contributions of HLA‐DRB1 and TNF‐α promoter polymorphisms to the susceptibility of Crohn’s disease. Genes Immun 2000;1:351–7. [DOI] [PubMed] [Google Scholar]
- 104. Tomita K, Chiba T, Sugai T, Habano W. Association between tumor necrosis factor‐alpha and Fc‐gamma receptor polymorphisms with infliximab in Crohn’s disease. Hepatogastroenterology 2010;57:535–9. [PubMed] [Google Scholar]
- 105. Chagani T, Paré PD, Zhu S et al. Prevalence of tumor necrosis factor‐α and angiotensin converting enzyme polymorphisms in mild/moderate and fatal/near‐fatal asthma. Am J Respir Crit Care Med 1999;160:278–82. [DOI] [PubMed] [Google Scholar]
- 106. Li Kam, Wa TC, Mansur AH, Britton J et al. Association between α−308 tumour necrosis factor promoter polymorphism and bronchial hyperreactivity in asthma. Clin Exp Allergy 1999;29:1204–8. [DOI] [PubMed] [Google Scholar]
- 107. Bilolikar H, Nam AR, Rosenthal M, Davies JC, Henderson DC, Balfour‐Lynn IM. Tumour necrosis factor gene polymorphism and childhood wheezing. Eur Respir J 2005;26:637–46. [DOI] [PubMed] [Google Scholar]
- 108. Gao JM, Lin YG, Qiu CC et al. The association between tumor necrosis factor α gene polymorphism and asthma. Chin Med Sci J 2003;18:248–53. [Google Scholar]
- 109. Sandford AJ, Chan HW, Wong GW, Lai CK, Chan‐Yeung M. Candidate genetic polymorphisms for asthma in Chinese schoolchildren from Hong Kong. Int J Tuberc Lung Dis 2004;8:519–27. [PubMed] [Google Scholar]
- 110. Witte JS, Palmer LJ, OConnor RD, Hopkins PJ, Hall JM. Relationship between tumour necrosis factor polymorphism TNFα‐308 and risk of asthma. Eur J Hum Genet 2002;10:82–5. [DOI] [PubMed] [Google Scholar]
- 111. Winchester EC, Millwood IY, Rand L, Penny MA, Kessling AM. Association of the TNF‐alpha‐308 (G→A) polymorphism with self‐reported history of childhood asthma. Hum Genet 2000;107:591–6. [DOI] [PubMed] [Google Scholar]
- 112. Wang TN, Chen WY, Wang TH, Chen CJ, Huang LY, Ko YC. Gene–gene synergistic effect on atopic asthma: tumour necrosis factor‐α‐308 and lymphotoxin‐α‐NcoI in Taiwan’s children. Clin Exp Allergy 2004;34:184–8. [DOI] [PubMed] [Google Scholar]
- 113. Louis R, Leyder E, Malaise M, Bartsch P, Louis E. Lack of association between adult asthma and the tumour necrosis factor alpha‐308 polymorphism gene. Eur Respir J 2000;16:604–8. [DOI] [PubMed] [Google Scholar]
- 114. Beghe B, Padoan M, Moss CT et al. Lack of association of HLA class I genes and TNF alpha‐308 polymorphism in toluene diisocyanate‐induced asthma. Allergy 2004;59:61–4. [DOI] [PubMed] [Google Scholar]
- 115. Bucková D, Hollá LI, Vasků A, Znojil V, Vácha J. Lack of association between atopic asthma and the tumor necrosis factor alpha‐308 gene polymorphism in a Czech population. J Investig Allergol Clin Immunol 2002;12:192–7. [PubMed] [Google Scholar]
- 116. Di Somma C, Charron D, Deichmann K, Buono C, Ruffilli A. Atopic asthma and TNF‐308 alleles: linkage disequilibrium and association analyses. Hum Immunol 2003;64:359–65. [DOI] [PubMed] [Google Scholar]
- 117. Shin HD, Park BL, Kim LH et al. Association of tumor necrosis factor polymorphisms with asthma and serum total IgE. Hum Mol Genet 2004;13:397–403. [DOI] [PubMed] [Google Scholar]
- 118. Gao J, Shan G, Sun B, Thompson PJ, Gao X. Association between polymorphism of tumour necrosis factor α−308 gene promoter and asthma: a meta‐analysis. Thorax 2006;61:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Juran BD, Atkinson EJ, Larson JJ et al. Carriage of a tumor necrosis factor polymorphism amplifies the cytotoxic T‐lymphocyte antigen 4 attributed risk of primary biliary cirrhosis: evidence for a gene‐gene interaction. Hepatology 2010;52:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ahmad T, Wallace GR, James T et al. Mapping the HLA association in Behcet’s disease. A role for tumor necrosis factor polymorphisms?. Arthritis Rheum 2003;48:807–13. [DOI] [PubMed] [Google Scholar]
- 121. Davis JA, Visscher MO, Wickett RR, Hoath SB. Influence of tumour necrosis factor‐alpha polymorphism −308 and atopy on irritant contact dermatitis in healthcare workers. Contact Dermatitis 2010;63:320–32. [DOI] [PubMed] [Google Scholar]
- 122. Li C, Wang G, Gao Y, Liu L, Gao T. TNF‐alpha gene promoter −238G>A and −308G>A polymorphisms alter risk of psoriasis vulgaris: a meta‐analysis. J Invest Dermatol 2007;127:1886–92. [DOI] [PubMed] [Google Scholar]
- 123. Cohen JD, Bournerias I, Buffard V et al. Psoriasis induced by tumor necrosis factor‐a antagonist therapy: a case series. J Rheumatol 2007;34:380–5. [PubMed] [Google Scholar]
- 124. Yilmaz R, Altun B, Ozer N, Hazirolan T, Turgan C. Impact of cytokine genotype on cardiovascular surrogate markers in hemodialysis patients. Ren Fail 2010;32:806–16. [DOI] [PubMed] [Google Scholar]
- 125. Chen YC, Hu JF, Chen P et al. Association of TNF‐α gene with spontaneous deep intracerebral hemorrhage in the Taiwan population: a case control study. BMC Neurol 2010;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rost NS, Greenberg SM, Rosand J. The Genetic Architecture of Intracerebral Hemorrhage. Stroke 2008;39:2166–73. [DOI] [PubMed] [Google Scholar]
- 127. Kim OJ, Lee JH, Choi JK et al. Association between Tumor Necrosis Factor‐Alpha (−308G→A and −238G→A) Polymorphisms and Homocysteine Levels in Patients with Ischemic Strokes and Silent Brain Infarctions. Cerebrovasc Dis 2010;30:483–90. [DOI] [PubMed] [Google Scholar]
- 128. Laws SM, Perneczky R, Wagenpfeil S et al. TNF polymorphisms in Alzheimer disease and functional implications on CSF beta‐amyloid levels. Hum Mutat 2005;26:29–35. [DOI] [PubMed] [Google Scholar]
- 129. Yang L, Lu R, Jiang L, Liu Z, Peng Y. Expression and genetic analysis of tumor necrosis factor‐alpha (TNF‐alpha) G−308A polymorphism in sporadic Alzheimer’s disease in a Southern China population. Brain Res 2009;9:178–81. [DOI] [PubMed] [Google Scholar]
- 130. DiDi Bona D, Candore G, Franceschi C et al. Systematic review by meta‐analyses on the possible role of TNF‐alpha polymorphisms in association with Alzheimer’s disease. Brain Res Rev 2009;61:60–8. [DOI] [PubMed] [Google Scholar]
- 131. Tedde A, Putignano AL, Nacmias B, Bagnoli S, Cellini E, Sorbi S. Lack of association between TNF‐alpha polymorphisms and Alzheimer’s disease in an Italian cohort. Neurosci Lett 2008;446:139–42. [DOI] [PubMed] [Google Scholar]
- 132. Pickering M, Cumiskey D, O’ Connor JJ. Actions of TNF‐alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol 2005;90:663–70. [DOI] [PubMed] [Google Scholar]
- 133. Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor‐alpha mediates one component of competitive, experience‐ dependent plasticity in developing cortex. Neuron 2008;58:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 2009;33:355–66. [DOI] [PubMed] [Google Scholar]
- 135. Baune BT, Ponath G, Rothermund M, Riess O, Funke H, Berger K. Association between genetic variants of IL‐beta, IL‐6 and TNF‐alpha cytokines and cognitive performance in the elderly general population of the MEMO‐study. Psychoneuroendocrinology 2008;33:68–76. [DOI] [PubMed] [Google Scholar]
- 136. Beste C, Heil M, Domschke K, Baune BT, Konrad C. Associations between the tumor necrosis factor alpha gene (−308G→A) and event‐related potential indices of attention and mental rotation. Neuroscience 2010;170:742–8. [DOI] [PubMed] [Google Scholar]
- 137. Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alpha treatment is associated with a polymorphism in Tumor Necrosis Factor alpha. Clin Neuropharmacol 2010;33:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Leung L, Cahill MC. TNF‐α and neuropathic pain. J Neuroinflammation 2010;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Achrol AS, Pawlikowska L, McCulloch CE et al. Tumor Necrosis Factor‐α 238G>A Promoter Polymorphism Is Associated with Increased Risk of New Hemorrhage in the Natural Course of Patients with Brain Arteriovenous Malformations. Stroke 2006;37:231–4. [DOI] [PubMed] [Google Scholar]
- 140. Kennedy SH. Is there a genetic basis to endometriosis? Semin Reprod Endocrinol 1997;15:309–17. [DOI] [PubMed] [Google Scholar]
- 141. Liu D, Metter EJ, Ferrucci L et al. TNF promoter polymorphisms associated with muscle phenotypes in humans. J Appl Physiol 2008;105:859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Corral‐Gudino L, del Pino‐Montes J, García‐Aparicio J, Alonso‐Garrido M, González‐Sarmiento R. Paget’s disease of bone is not associated with common polymorphisms in interleukin‐6, interleukin‐8, and tumor necrosis factor alpha genes. Cytokine 2010;52:146–50. [DOI] [PubMed] [Google Scholar]
- 143. Rojas J, Fernandez I, Pastor JC et al. A strong genetic association between the tumor necrosis factor locus and proliferative vitreoretinopathy: the retina 4 project. Ophthalmology 2010;117:2417–23. [DOI] [PubMed] [Google Scholar]
- 144. Kimkong I, Hirankarn N, Nakkuntod J, Kitkumthorn N. Tumour necrosis factor‐alpha gene polymorphisms and susceptibility to oral lichen planus. Oral Dis 2011;17:206–9. [DOI] [PubMed] [Google Scholar]
- 145. Zammiti W, Mtiraoui N, Khairi H, Gris JC, Almawi WY, Mahjoub T. Associations between tumor necrosis factor‐α and lymphotoxin‐α polymorphisms and idiopathic recurrent miscarriage. Reproduction 2008;135:397–403. [DOI] [PubMed] [Google Scholar]
- 146. Palmirotta R, La Farina F, Ferroni P et al. TNFA gene promoter polymorphisms and susceptibility to recurrent pregnancy loss in italian women. Reprod Sci 2010;17:659–66. [DOI] [PubMed] [Google Scholar]
- 147. Jones PA, Baylin SB. The Fundamental Role of Epigenetic Events in Cancer. Nat Genet 2002;3:415–28. [DOI] [PubMed] [Google Scholar]
- 148. Strunnikova M, Schagdarsurengin U, Kehlen A, Garbe JC, Stampfer MR, Dammann R. Chromatin inactivation precedes de novo DNA methylation during the progressive epigenetic silencing of the RASSF1A promoter. Mol Cell Biol 2005;25:3923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bird AP, Wolffe AP. Methylation‐induced repression‐belts, braces, and chromatin. Cell 1999;99:451–4. [DOI] [PubMed] [Google Scholar]
- 150. Zhang Y, Rohde C, Tierling S et al. DNA methylation analysis of chromosome 21 gene promoters at single base pair and single allele resolution. PLoS Genet 2009;5: e1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Weber M, Hellmann I, Stadler MB et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 2007;39:457–66. [DOI] [PubMed] [Google Scholar]
- 152. Eckhardt F, Lewin J, Cortese R et al. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet 2006;38:1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Milani L, Lundmark A, Kiialainen A et al. DNA Methylation for subtype classification and prediction of treatment outcome in patients with childhood acute lymphoblastic leukemia. Bood 2010;115:1214–25. [DOI] [PubMed] [Google Scholar]
- 154. Andersen MC, Engström PG, Lithwick S et al. In silico detection of sequence variations modifying transcriptional regulation. PLoS Comput Biol 2008;4:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kuriki C, Tanaka T, Fukui Y, Sato O, Motojima K. Structural and functional analysis of a new upstream promoter of the human FAT/CD36 gene. Biol Pharm Bull 2002;25:1476–8. [DOI] [PubMed] [Google Scholar]
- 156. Zabetian CP, Anderson GM, Buxbaum SG et al. A quantitative‐trait analysis of human plasma‐dopamine beta‐hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 2001;68:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rigat B, Hubert C, Alhenc‐Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I‐converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990;86:1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Skoog T, Vant Hoft FM, Kallin B et al. A Common functional polymorphism (C→A substitution at position −863) in the promoter region of tumour necrosis factor‐α (TNF‐α) gene associated with reduced circulating levels of TNF‐α. Hum Mol Genet 1999;8:1443–9. [DOI] [PubMed] [Google Scholar]
- 159. Kuo NW, Lympany PA, Menezo V et al. TNF‐α 857T, a genetic risk marker for acute anterior uveitis. Invest Ophthalmol Vis Sci 2005;46:1565–71. [DOI] [PubMed] [Google Scholar]
- 160. Wittkopp PJ. Variable Transcription Factor Binding: a Mechanism of Evolutionary Change. PLoS Biol 2010;8:e1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bradley RK, Li X‐Y, Trapnell C, Wayne ML, Nuzhdin SV. Binding site turnover produces pervasive quantitative changes in transcription factor binding between closely related Drosophila species. PLoS Biol 2010;8:e1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wood N, Bhattacharya T, Keele BF et al. HIV evolution in early infection: selection pressures, patterns of insertion and deletion, and the impact of APOBEC. PLoS Pathog 2009;5:1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wilson MD, Barbosa‐Morais NL, Schmidt D et al. Species‐specific transcription in mice carrying human chromosome 21. Science 2008;322:434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Graze RM, McIntyre LM, Main BJ, Wayne ML, Nuzhdin SV. Regulatory divergence in Drosophila melanogaster and D. simulans a genome‐wide analysis of allele‐specific expression. Genetics 2009;183:547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tirosh I, Reikhav S, Levy AA, Barkai N. A yeast hybrid provides insight into the evolution of gene expression regulation. Science 2009;324:659–62. [DOI] [PubMed] [Google Scholar]
- 166. Yang Y, Luo C, Feng R, Bi S. The TNF‐α, IL‐1B and IL‐10 polymorphisms and risk for hepatocellular carcinoma: a meta‐analysis. J Cancer Res Clin Oncol 2010;137:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. McCormick DP, Grady JJ, Diego A, et al. Acute otitis media severity: Association with cytokine gene polymorphisms and other risk factors. Int J Pediatr Otorhinolaryngol 2011;75:708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Emonts M, Veenhoven RH, Wiertsema SP, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics 2007;120:814–23. [DOI] [PubMed] [Google Scholar]
- 169. Majetschak M, Floh AS, Obertacke U, et al. Relation of a TNF gene polymorphism to severe sepsis in trauma patients. Ann Surg 1999;230:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Vejbaesya S, Chierakul N, Luangtrakool P, Sermduangprateep C. NRAMP1 and TNF‐alpha polymorphisms and susceptibility to tuberculosis in Thais. Respirology 2007;12:202–6. [DOI] [PubMed] [Google Scholar]
- 171. Drigo SA, Neto EC, Ianni B, et al. Goldberg AC Lack of association of tumour necrosis factor‐α polymorphisms with Chagas disease in Brazilian patients. Immuno Lett 2007;108:109–11. [DOI] [PubMed] [Google Scholar]
- 172. Cordeiro CA, Moreira PR, Andrade MS, et al. Interleukin‐10 gene polymorphism (‐1082G/A) is associated with toxoplasmic retinochoroiditis. Invest Ophthalmol Vis Sci 2008;49:1979–82. [DOI] [PubMed] [Google Scholar]
- 173. Kimkong I, Hirankarn N, Nakkuntod J, Kitkumthorn N. Tumour necrosis factor‐alpha gene polymorphisms and susceptibility to oral lichen planus. Oral Dis 2011;17:206–9. [DOI] [PubMed] [Google Scholar]
- 174. Ahamad A, Stevens CW, Smythe WR, et al. Promising early local control of malignant pleural mesothelioma following postoperative intensity modulated radiotherapy (IMRT) to the chest. Cancer J 2003;9:476–84. [DOI] [PubMed] [Google Scholar]


