Summary
Objective To determine the effect of handwashing on the risk of respiratory infection.
Methods We searched PubMed, CAB Abstracts, Embase, Web of Science, and the Cochrane library for articles published before June 2004 in all languages. We had searched reference lists of all primary and review articles. Studies were included in the review if they reported the impact of an intervention to promote hand cleansing on respiratory infections. Studies relating to hospital‐acquired infections, long‐term care facilities, immuno‐compromised and elderly people were excluded. We independently evaluated all studies, and inclusion decisions were reached by consensus. From a primary list of 410 articles, eight interventional studies met the eligibility criteria.
Results All eight eligible studies reported that handwashing lowered risks of respiratory infection, with risk reductions ranging from 6% to 44% [pooled value 24% (95% CI 6–40%)]. Pooling the results of only the seven homogenous studies gave a relative risk of 1.19 (95% CI 1.12%–1.26%), implying that hand cleansing can cut the risk of respiratory infection by 16% (95% CI 11–21%).
Conclusions Handwashing is associated with lowered respiratory infection. However, studies were of poor quality, none related to developing countries, and only one to severe disease. Rigorous trials of the impact of handwashing on acute respiratory tract infection morbidity and mortality are urgently needed, especially in developing countries.
Keywords: handwashing, respiratory infections, systematic review, meta‐analysis
Keywords: Lavage des mains, infections respiratoires, révision systématique, meta‐analyse
Keywords: lavado de manos, infecciones respiratorias, revisión sistemática, meta‐análisis
Abstract
Objectif Déterminer l'effet du lavage des mains sur le risque d'infections respiratoires.
Méthodes Nous avons effectué des recherches sur PubMed, CAB Abstracts, Embase, Web of Science et la bibliothèque Cochrane pour des articles publiés avant Juin 2004 en toutes langues. Nous avons aussi recherché manuellement les listes de références de toute publication primaire ou des articles de révision. Les études ont été incluses dans notre révision si elles rapportaient l'impact d'une intervention à promouvoir le lavage des mains sur les infections respiratoires. Les études portant sur les infections acquises à l'hôpital ou dans les services de santé avec prise en charge à long terme ou par des personnes à immunité compromise ou âgées ont été exclues. Nous avons évalué chaque étude indépendamment et les décisions pour l'inclusion ont été prises par consensus. D'une liste de départ de 410 articles, 8 études d'intervention ont satisfait aux critères d’éligibilitè.
Résultats Toutes les 8 études ont rapporté que le lavage des mains diminuait le risque d'infections respiratoire. La diminution du risque allait de 6 à 44% (valeurs cumulées 24%; IC95%: 6–40%). Le seul cumule des résultats de 7 études homogènes a donné un risque relatif de 1,19 (IC95%: 1,12–1,26%), suggérant que le lavage des mains peu réduire le risque d'infection respiratoire de 16% (IC95%: 11–21%).
Conclusion Le lavage des mains est associé avec une diminution des infections respiratoires. Cependant, les études étaient de pauvre qualité, aucune ne portait sur des pays en développement et une seule portait sur des maladies sévères. Des essais plus rigoureux de l'impact du lavage des mains sur la morbidité et la mortalité des infections respiratoires aigues sont urgemment nécessaires et plus particulièrement dans les pays en voie de développement.
Abstract
Objetivo Determinar el efecto del lavado de manos en el riesgo de infección respiratoria
Método Se hizo una búsqueda en PubMed, CAB Abstracts, Embase, Web of Science, y la Cochrane library de artículos publicados, en todos los idiomas, antes de Junio del 2004. Se buscó a mano las listas de referencias de todos los artículos primarios y de revisión. Se incluyeron en la revisión aquellos estudios que reportaran el impacto sobre infecciones respiratorias de una intervención para promover el lavado de manos. Se excluyeron los estudios relacionados con infecciones nosocomiales, instalaciones de cuidados a largo plazo, personas inmuno‐suprimidas o personas mayores. Nosotros, independientemente, evaluamos todos los estudios, y la decisión de incluirlos o excluirlos fue consensuada. De una lista inicial de 410 artículos, ocho estudios de intervención tenían los criterios para ser elegidos.
Resultados Los ocho estudios elegibles reportaban que el lavado de manos disminuía el riesgo de infecciones respiratorias, con reducción del riesgo de entre un 6 a un 44% (valor combinado 24% (95% IC 6%‐40%). Agrupando los resultados de los sete estudios homogéneos se obtenía un riesgo relativo del 1.19 (95% IC 1.12 – 1.26), lo cual implica que el lavado de manos puede disminuir el riesgo de infección respiratoria en un 16% (95% IC 11 a 21%).
Conclusiones El lavado de manos está asociado con una disminución de la infección respiratoria. Sin embargo, los estudios analizados eran de baja calidad, ninguno relacionado con países de baja renta, y solo uno de ellos con enfermedad severa. Se requieren con urgencia ensayos rigurosos sobre el impacto del lavado de manos en la morbilidad y mortalidad por infecciones respiratorias, especialmente en países de baja renta.
Introduction
Acute respiratory tract infections (ARIs) cause at least 2 million deaths a year (Guerrant & Blackwood 1999; WHO 2002). They are the leading cause of childhood morbidity and mortality in the world and the biggest cause of disability‐adjusted life years lost (DALYs) (Murray & Lopez 1997). It was estimated that 21% of deaths in the 42 countries with the highest mortality are because of pneumonia (Black et al. 2003). Mortality and morbidity is concentrated in the under‐fives and in the poorer countries (WHO 2002). Efforts are needed to identify interventions against ARIs if the international community is to achieve the millennium development goal of reducing child mortality by two‐thirds by 2015 (Jones et al. 2003). Hands are known to transport bacterial and viral respiratory pathogens. Microbiological studies have identified respiratory pathogens on hands (Hendley et al. 1973; Reed 1975; Gwaltney & Hendley 1978; 1978, 1980; Ansari et al. 1991) and fomites (environmental surfaces) are also implicated in the transmission chain (Mahl & Sadler 1975; Bean et al. 1982; Brady et al. 1990). Several viruses that were believed to use airborne or fomite routes exclusively are now thought also to be transmitted faeco‐orally. Enteric adenoviruses are responsible for 2–24% of respiratory viral disease in children (Cherry 1998). The coronavirus causing severe acute respiratory syndrome (SARS) is readily isolated from the faeces of infected patients (WHO 2003a, 2003b) and H5N1, the Asian ‘flu virus, has recently been found in faeces (de Jong et al. 2005). Carriage of pathogens on the hands is an important link in the faecal–oral route of disease transmission. Hands are thus disease vectors: carrying respiratory micro‐organisms shed from the nose, mouth or anus to the nasal mucosa, conjunctiva (Hendley et al. 1973), or mouth (WHO 2003b) of new hosts. Hands can be cleansed of viruses and bacteria by washing with soap (both plain and antibacterial) (Faix 1987; Ansari et al. 1989; Luby et al. 2001; Gibson et al. 2002; Montville et al. 2002; Larson et al. 2003) or other cleansers (Hoque & Briend 1991; Kaltenthaler et al. 1991; Hoque et al. 1995; Dyer et al. 2000; 2001, 2003). Furthermore, handwashing is known to reduce respiratory infection in healthcare settings (Isaacs et al. 1991; Falsey et al. 1999; Makris et al. 2000).
Handwashing is an effective (Curtis & Cairncross 2003), feasible (Khan 1982; Stanton & Clemens 1987; Pinfold & Horan 1996; Curtis et al. 2001) and cost‐effective (Borghi et al. 2002) means of preventing gastroenteric infection in developing country settings and may offer a promising new intervention against ARIs. We conducted this quantitative systematic review to answer the question: What is the effect of handwashing on the risk of respiratory infection in the general population?
Methods
This systematic review aimed to recover all work published before June 2004 that related hand cleansing or washing to the risk of respiratory infection in the healthy population living in the community. Figure 1 outlines the selection and review process.
Figure 1.

Selection and review process.
Identification of studies
We searched PubMed, CAB Abstracts, Embase, Web of Science and the Cochrane library for papers published before June 2004, in all languages. Papers in languages other than English, French and Spanish were translated into English. The medical subject heading (MeSH) and text words for the terms handwashing, or hand hygiene or hand cleansing or hand cleaning were used separately and in combination with MeSH terms for acute respiratory infections, respiratory disease, respiratory illness, sinusitis, common cold, otitis media, pharyngitis, influenza, coryza, laryngitis, epiglottitis, croup, pneumonia, bronchitis, bronchiolitis, pertussis and whooping cough. After hand searching of bibliographies of all relevant articles, including reviews, and removal of duplicates, the final citation total was 395. We also identified 15 additional articles from our own collections.
Eligibility criteria
We included interventional studies that calculated the risk of any respiratory outcome related to hand cleansing. Our focus was the impact of handwashing on the healthy general population and hence we excluded studies conducted in hospitals, healthcare or geriatric care settings, and studies with immuno‐compromised subjects or people suffering from genetic disorders.
Screening process
Initially, both authors reviewed the full citation list independently and then came to a consensus on 61 potentially relevant abstracts for retrieval. Three hundred and sixteen were rejected because they did not meet the eligibility criteria, concerned microbiology, vaccination, pharmacology, animal disease or organisms irrelevant to ARI, or because they were not peer‐reviewed reports.
Next, both authors then read all 61 abstracts and agreed to retrieve the 16 full papers that appeared potentially eligible to either, or both of them. Forty‐five abstracts were rejected as they concerned children with disabilities, hospital settings, microbiology, studies relating to handwashing but not ARIs, studies relating to ARIs but not handwashing, observational studies, or studies that included several components other than handwashing, so the effect of handwashing per se could not be disentangled.
Both authors then read all 16 remaining papers and reached a consensus on eight papers from which to extract data (Master et al. 1997; Niffenegger 1997; Ladegaard & Stage 1999; Dyer et al. 2000; Roberts et al. 2000; Ryan et al. 2001; 2001, 2003). Five studies were excluded because they used several interventions and it was not possible to attribute the reported effect to handwashing alone (Kotch et al. 1994; Krilov et al. 1996; Carabin et al. 1999; Uhari & Möttönen 1999; Ponka et al. 2004). Three studies concerned school absenteeism associated with not washing hands, but did not provide sufficient detail regarding respiratory infections and were also excluded (Kimel 1996; Hammond et al. 2000; Guinan et al. 2002).
Data abstraction
Data concerning the study design, sample size, measures of effect (abstracted or computed: see Box 1), nature of the intervention, location, study population and methodological shortcomings of the studies were abstracted and tabulated independently by both authors.
Figure Box 1 .

Computation of risk and rate ratios and 95% CI.
Entry to meta‐analysis
Studies were retained for the meta‐analysis if they provided risk or rate ratio estimates and 95% CI, or the means to calculate them, of hand cleansing in association with a respiratory tract infection. According to Deeks et al. (2003), pooling risk and rate ratios is acceptable provided that statistical heterogeneity is tested for.
Data synthesis
The estimates of the measures of effect from all eight studies were pooled in a meta‐analysis using a random effects model and tested for heterogeneity using Chi‐squared tests. Statistical analysis was performed using stata8 (Stata Statistical Software 2003). One study concerned three different outcomes and were combined (Ladegaard & Stage 1999). A forest plot and a random‐effect pooled estimate of the relative risk were generated (Egger et al. 1997). No further sub‐group pooled analyses were attempted because studies were too few.
Sensitivity analysis
We first tested for methodological heterogeneity by excluding the uncontrolled study (Ryan et al. 2001) and then the cross‐over trial (Dyer et al. 2000). This study had a 2‐week wash out period, and as we only included the first period to minimise the risk of bias originating from the carry‐over effect, this may have constituted a form of selection bias (Curtin et al. 2002). We further tested for methodological heterogeneity by excluding the two studies of poorest quality (Niffenegger 1997; White et al. 2001). We constructed a funnel plot and used Begg's‐adjusted rank correlation test (Begg & Mazumdar 1994; Egger et al. 1997) to assess the likelihood of publication bias.
Results
Table 1 shows the data abstracted from the eight qualifying studies. All were conducted in developed country settings, three concerned schools, three concerned childcare facilities, one a university hall of residence and one a navy base. All concerned hand cleansing interventions: three mentioned soap use (Master et al. 1997; Niffenegger 1997; Ryan et al. 2001) and 3 sanitisers (Dyer et al. 2000; 2001, 2003), while the remaining two did not specify either (Ladegaard & Stage 1999; Roberts et al. 2000). Only two studies were randomised‐controlled trials (RCTs) and all had methodological flaws (Table 1). Two studies were especially poor (Niffenegger 1997; White et al. 2001), having one intervention and one control group, but analysed as if children and episodes were independent of each other. The relative risk of ARIs associated with not washing hands ranged from 1.06 to 1.80. The combined random effects estimate of the relative risk was 1.32 (95% CI 1.06–1.66), implying that handwashing could cut the risk of respiratory infection by 24% (95% CI 6–40%).
Table 1.
Characteristics of the eight studies of handwashing included in the review
| Study | Location/setting | Study design | Exposure/intervention | Age group | Methodological shortcomings* | Outcome | Measure of effect (95% CI) | Sample size and follow‐up |
|---|---|---|---|---|---|---|---|---|
| (A) Dyer et al. (2000) | Private elementary school in California | Cross‐over intervention | Hand sanitiser given to all study children for supervised use after entering classroom, before eating, after sneezing or coughing, after using restroom | 5–12 | 1/2/5/6/8/9/ | Days of respiratory illness | Risk ratio 1.37 (0.78–2.40) | Four hundred and twenty children in 14 classes 4 weeks plus 4 weeks |
| (B) Ladegaard & Stage (1999) | Eight child day‐care centres, Denmark | Intervention trial using blocked randomisation | Training of centre personnel and distribution of t‐shirts with the imprint ‘Clean hands – yes please’ to all children. Older children were given handwashing exercises, heard a fairy tale about handwashing, coloured drawings from fairy tale, sang ‘wash your hands’ songs and received a copy of the fairy tale. Children also given material to pass on to parents about handwashing. | 0–6 | 4/5/6/8/9/ | Parents/personnel reporting of common cold/sore throat | Risk ratio 1.25 (0.81–1.92) | Four intervention centres with 212 children, four control centres with 263 children. 2 months baseline, 2 months intervention, and 2 months post‐intervention. |
| Parents/personnel reporting of bronchitis/pneumonia | Risk ratio 1.13 (0.36–3.51) | |||||||
| Parents/personnel reporting of otitis media Combined | Risk ratio 1.93 (0.69–5.41) Risk ratio 1.29 (0.90–1.84) | |||||||
| (C) Master et al. (1997) | Elementary school, MI, USA | Intervention in six classes, none in eight classes: experimental | Required to WHWS 4× a day plus after toilet | 5–12 | 1/2/5/6/8/9/ | URI days of illness | Risk ratio 1.06 (0.78–1.44) | Three hundred and five children in 14 clusters 37 days |
| (D) Niffenegger (1997) | Two childcare facilities, IN, USA | Intervention in one facility compared with none in control facility | Instructional programme on germs and WHWS for teachers and children | 3–5 | 1/4/5/8/9/ | Incidence of colds | Risk ratio 1.47 (1.01–2.13) | Twenty‐six intervention children, 12 control 70 days |
| (E) Roberts et al. (2000) | Childcare centres Australian Capital Territories | Cluster randomised‐ controlled trial | Training sessions for staff using ‘GloGerm’, visits and newsletters. Staff and child handwashing after toileting, before eating, after changing a diaper, after wiping a nose | 0–3 years | 2/5/ | Parental recall of illness symptoms over 2 weeks by telephone interview, plus illness calendars | Rate ratio compliant children 1.12 (1.03–1.22) | Eleven intervention, 12 control centres 458 children 113 677 child days |
| (F) Ryan et al. (2001) | US Navy Recruits, IL, USA | Intervention baseline and follow‐up | Directive to recruits to WHWS 5× daily, wet sinks allowed, liquid soap dispensers, monthly education and inspection | Young adults | 1/3/6/8/ | Self‐reporting of respiratory infection | Rate ratio 1.80 (1.78–1.82) | A total of 44797 recruits in pre‐intervention year – 45714 (average) in 2 years post‐intervention |
| (G) White et al. 2001 | One private and two elementary schools, CA, USA | Double‐blind placebo controlled | Hand sanitiser, placebo without active ingredients upon entering classroom, before and after eating, before leaving class | 5–12 | 1/2/4/6/7/8/9/ | Days of illness with respiratory symptoms | Risk ratio 1.34 (0.96–1.89) | Thirty‐two classes 769 children 5 weeks |
| (H) White et al. (2003) | Student residence halls, CO, USA | Intervention in two halls, not in two controls | Gel dispensers installed in rooms and public places of intervention halls plus handwashing campaign | Students | 1/2/5/6/7/8/9/ | Weekly self‐report of symptoms | Risk ratio 1.25 (1.14–1.37) | Four residence halls 430 students 8 weeks |
*Methodological shortcomings of studies: 1/intervention not randomised, 2/baseline incidences not given, 3/no concurrent control group, 4/unsatisfactory case definition, 5/no placebo intervention, 6/impact on behaviour not assessed, 7/high loss to follow‐up, 8/no correction for repeated episodes and 9/no control for clustering.
WHWS, wash hands with soap; URI, upper respiratory tract infection; RR, relative risk.
Evidence of heterogeneity
The pooled random effects model was tested for heterogeneity and showed a chi‐square of 194 on 7 degrees of freedom with a significant P‐value of >0.001, denoting that there was heterogeneity between the studies included in the meta‐analysis. After exclusion of the Ryan study, the chi‐square was 5.8 on 6 degrees of freedom with a P‐value = 0.45, showing that the heterogeneity was because of the inclusion of this study. The resultant pooled random effect estimate of the relative risk was 1.19 (95% CI 1.12–1.26).
Sensitivity analysis
Once this study had been removed from the pool, we conducted sensitivity analysis to determine whether the effect of removing studies for other reasons (cross‐over trial, poorest studies) affected the results. Table 2 shows that there was very little change in the pooled effect measure and no evidence of heterogeneity between the remaining studies.
Table 2.
Sensitivity analyses
| RR (95% CI) | Number of studies | Heterogeneity Q (P‐value) | Interpretation of results | |
|---|---|---|---|---|
| All studies pooled in meta‐analysis | 1.32 (1.07–1.66) | 8 | 194.07 (>0.001) | Results show a 24% (95% CI 6–40%) relative risk reduction associated with handwashing. However, there is an evidence of heterogeneity between the pooled estimates denoted by the high chi‐squared value with a statistically significant P‐value. |
| Excluding the uncontrolled study (Ryan et al. 2001) | 1.19 (1.12–1.26) | 7 | 5.76 (0.45) | After excluding the uncontrolled study, the pooled relative risk reduction associated with handwashing came down to 16% (95% CI 11–21%). There was no evidence of heterogeneity between the remaining seven data points. |
| Excluding cross‐over trial (Dyer et al. 2000) | 1.19 (1.11–1.27) | 6 | 5.51 (0.36) | Dyer et al. (2000) was the only cross‐over trial, all other studies followed a parallel group design. Effect of excluding it showed a relative risk reduction as above. No evidence of heterogeneity. |
| Excluding poorest studies (Niffenegger (1997) and White et al. 2001) | 1.18 (1.11–1.25) | 5 | 3.91 (0.41) | Niffenegger (1997) and White et al. (2001) were excluded to test the pooled effect without the two studies with poorest quality. The resultant relative risk reduction was as above. No evidence of heterogeneity. |
RR, relative risk.
We therefore retained the random effects estimate for the relative risk based on seven studies, yielding a relative risk of 1.19 (95% CI 1.12–1.26). This implies that the relative risk of respiratory infections associated with not cleansing hands is 16% (95% CI 11–21%). Figure 2 below gives the forest plot for the seven studies in the meta‐analysis. We plotted a funnel graph to test for publication bias and computed Begg's‐adjusted rank correlation. Our results showed no evidence of publication bias (Begg's‐adjusted rank correlation test, P = 0.55).
Figure 2.
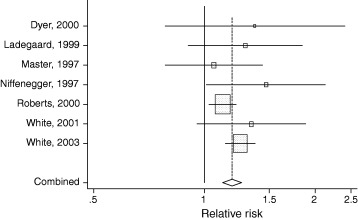
Forest plot of the seven studies pooled in meta‐analysis.
Discussion
Our results suggest that hand cleansing can cut the risk of respiratory tract infection by about 16% (95% CI 11–21%). The implications of this finding for preventing ARIs in developing countries are uncertain because of the poor quality of the studies, their geographical limitations and the use of non‐severe outcome measures.
Though we find a consistent pattern of impact of handwashing on ARI, our pooled estimate can only be seen as indicative, as studies were few, of poor quality and limited in geographical scope. Some major studies were also excluded. All of the studies included in the review had methodological flaws. Of the eight intervention studies, only two (Ladegaard & Stage 1999; Roberts et al. 2000) were RCT. Though all used a control group, only one used a placebo intervention to blind investigators and participants to the study hypotheses (White et al. 2001). Only one dealt with clustering and the non‐independent nature of subsequent illness episodes correctly in the analysis (Roberts et al. 2000). Only three studies gave an adequate description of the outcome measure (Niffenegger 1997; Roberts et al. 2000; Ryan et al. 2001). Very few reported a baseline risk of respiratory infections (Niffenegger 1997; Ryan et al. 2001) and most gave unsatisfactory case definitions (Niffenegger 1997; Ryan et al. 2001; White et al. 2001). There were too few studies to carry out subgroup analyses to determine which hand cleansing regimes were most effective. Few studies offered compliance data, and those that did showed low values (Roberts et al. 2000; White et al. 2003). It may be that handwashing, when complied with, can have a much bigger impact than reported in these studies.
Because of methodological issues, our review excluded a number of, otherwise eligible, studies showing strong effects of handwashing on respiratory infection. In what was a very large study, Ryan demonstrated a 45% reduction in the risk of clinical reports of ARI in navy recruits practising handwashing. St Sauver et al. (St Sauver et al. 1998) carried out a retrospective cross‐sectional study of reported handwashing by children and providers and showed two‐ to threefold average reductions in the odds of respiratory infection with handwashing.
Though the major burden of ARI disease falls in developing countries, clearly the major burden of research has fallen in developed countries, especially in the US. The poor geographical distribution of studies is surprising and may reflect the fact that in the US handwashing is commonly believed to protect against colds and flu, but not elsewhere (anecdotal evidence). Most studies concerned upper respiratory infections such as colds and influenza and few included patients with serious illness. This means that extrapolating the results of this review to developing countries, and to the severe pneumonias which are responsible for most ARI deaths in those settings, is uncertain. On the other hand, the fact that the pooled studies referred to communal settings may be relevant to the often more crowded and communal aspects of life in poorer settings within developing countries.
Despite these limitations, the results show a coherent and significant pattern of impact of hand cleansing on ARI infection. This is impressive, but possibly not surprising, given the substantial body of evidence that handwashing can cut the risk of microbial and viral hand contamination and prevent nosocomial infection. This study adds evidence to a pattern of findings suggesting that ARIs and other contagious illnesses can be prevented by handwashing. These include microbiological studies, hospital‐based studies and studies concerning other infections (Curtis & Cairncross 2003). Huge sums have been spent on the search for effective counter‐measures to ARIs, especially to develop vaccines against influenza, respiratory syncytial virus, Hib B, SARS (Chin et al. 1969; Fulginiti et al. 1969; Osterhaus & De Vries 1992) and now Asian ‘flu. As ARIs are caused by over 200 different organisms, any one vaccine will have a limited effect on the burden of disease.
If hand cleansing can reduce the risk of ARIs by 16% and diarrhoeal disease by almost half (Curtis & Cairncross 2003), then it may represent a feasible option for developing countries, where handwashing rates are currently low, but soap and water are, in most cases, readily available (Scott et al. 2003). Small‐scale studies have shown that improving handwashing is possible (Pinfold & Horan 1996; Curtis et al. 2001) and a recent global public–private partnership for handwashing (http://www.globalhandwash.org) is promoting handwashing in national scale programmes.
Although suggestive, the quality of the current evidence concerning handwashing and ARIs is very poor. Randomised‐controlled trials to rigorously investigate the impact of handwashing on morbidity and mortality in all settings, but especially in developing countries, are urgently needed.
Conflict of interest statement
The grant from Unilever PLC could be viewed as constituting a conflict of interest as we suggest that handwashing with soap could prevent respiratory tract infection. However, our main commercially related interest is in encouraging soap companies to do more to promote hygiene and public health.
Acknowledgements
We thank Wolf‐Peter Schmidt, Ian Roberts, Sara Thomas, Rachel Clarke, Eileen Chappel, Steve Luby, Simon Cousens, Helen Weiss, Kenny Hermansen and colleagues in the Hygiene Centre for their assistance. We are supported by grants from Unilever PLC, the World Bank and the Water and Sanitation Programme. The funders had no involvement in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
References
- Ansari SA, Sattar SA, Springthorpe VS, Wells GA & Tostowaryk W (1989) In vivo protocol for testing efficacy of hand‐washing agents against viruses and bacteria: experiments with rotavirus and Escherichia coli . Applied and Environmental Microbiology 55, 3113–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari SA, Springthorpe S, Sattar SA, Rivard S & Rahman M (1991) Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. Journal of Clinical Microbiology 29, 2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN & Balfour HH (1982) Survival of influenza viruses on environmental surfaces. The Journal of Infectious Diseases 146, 47–51. [DOI] [PubMed] [Google Scholar]
- Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- Black RE, Morris SS & Bryce J (2003) Where and why are 10 million children dying every year? Lancet 361, 2226–2234. [DOI] [PubMed] [Google Scholar]
- Borghi J, Guinness L, Ouedraogo J & Curtis V (2002) Is hygiene promotion cost‐effective? A case study in Burkina Faso. Tropical Medicine & International Health 7, 1–10. [DOI] [PubMed] [Google Scholar]
- Brady MT, Evans J & Cuartas J (1990) Survival and disinfection of parainfluenza viruses on environmental surfaces. American Journal of Infection Control 18, 18–23. [DOI] [PubMed] [Google Scholar]
- Carabin H, Gyorkos TW, Soto JC, Joseph L, Payment P & Collet J (1999) Effectiveness of a training program in reducing infections in toddlers attending day care centers. Epidemiology 10, 219–227. [PubMed] [Google Scholar]
- Cherry JD (1998) Adenoviruses In: Pediatric Infectious Diseases, Vol. 2 (ed Cherry JD.) WB Saunders, Philadelphia, pp. 1666–1684. [Google Scholar]
- Chin J, Magoffin RL, Shearer LA, Schifble JH & Lennette EH (1969) Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a paediatric population. American Journal of Epidemiology 89, 449. [DOI] [PubMed] [Google Scholar]
- Curtin F, Elbourne D & Altman DG (2002) Meta‐analysis combining parallel and cross‐over clinical trials. III: the issue of carry‐over. Statistics in Medicine 21, 2161–2173. [DOI] [PubMed] [Google Scholar]
- Curtis V & Cairncross S (2003) Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. The Lancet Infectious Diseases 3, 275–281. [DOI] [PubMed] [Google Scholar]
- Curtis V, Kanki B, Cousens S et al. (2001) Evidence for behaviour change following a hygiene promotion programme in West Africa. Bulletin of the World Health Organization 79, 518–526. [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (2003) Analysing and presenting results In: Cochrane Reviewers’ Handbook 4.2.2 [updated December 2003]; Section 8 (eds Alderson P, Green S. & Higgins J.) Available at: http://www.cochrane.org/resources/handbook/hbook.htm (accessed on January 31, 2004). [Google Scholar]
- Dyer DL, Shinder A & Shinder F (2000) Alcohol‐free instant hand sanitizer reduces elementary school illness absenteeism. Family Medicine 32, 633–638. [PubMed] [Google Scholar]
- Egger M, Smith GD & Phillips AN (1997) Meta‐analysis: principles and procedures. British Medical Journal 315, 1533–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix RG (1987) Comparative efficacy of handwashing agents against cytomegalovirus. Infection Control 8, 158–162. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Criddle MM, Kolassa JE, McCann RM, Brower CA & Hall WJ (1999) Evaluation of a handwashing intervention to reduce respiratory illness rates in senior day‐care centers. Infection Control and Hospital Epidemiology 20, 200–202. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M & Meiklejohn G (1969) Respiratroy virus immunization. 1. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum‐precipitated respiratory syncytial virus vaccine. American Journal of Epidemiology 89, 435. [DOI] [PubMed] [Google Scholar]
- Gibson LL, Rose JB, Haas CN, Gerba CP & Rusin PA (2002) Quantitative assessment of risk reduction from hand washing with antibacterial soaps. Journal of Applied Microbiology 92, 136S‐143S. [PubMed] [Google Scholar]
- Guerrant RL & Blackwood BL (1999) Threats to global health and survival: the growing crises of tropical infectious diseases – our ‘unfinished agenda’. Clinical Infectious Diseases 28, 966–986. [DOI] [PubMed] [Google Scholar]
- Guinan M, McGuckin M & Ali Y (2002) The effect of a comprehensive handwashing program on absenteeism in elementary schools. American Journal of Infection Control 30, 217–220. [DOI] [PubMed] [Google Scholar]
- Gwaltney Jr JM & Hendley JO (1978) Rhinovirus transmission: one if by air, two if by hand. American Journal of Epidemiology 107, 357–361. [DOI] [PubMed] [Google Scholar]
- Gwaltney Jr JM, Moskalski PB & Hendley JO (1978) Hand‐to‐hand transmission of rhinovirus colds. Annals of Internal Medicine 88, 463–467. [DOI] [PubMed] [Google Scholar]
- Gwaltney Jr JM, Moskalski PB & Hendley JO (1980) Interruption of experimental rhinovirus transmission. The Journal of Infectious Diseases 142, 811–815. [DOI] [PubMed] [Google Scholar]
- Hammond B, Ali Y, Fendler E, Dolan M & Donovan S (2000) Effect of hand sanitizer use on elementary school absenteeism. American Journal of Infection Control 28, 340–346. [DOI] [PubMed] [Google Scholar]
- Hendley JO, Wenzel RP & Gwaltney JM (1973) Transmission of rhinovirus colds by self‐inoculation. The New England Journal of Medicine 288, 1361–1364. [DOI] [PubMed] [Google Scholar]
- Hoque BA & Briend A (1991) A comparison of local handwashing agents in Bangladesh. The Journal of Tropical Medicine and Hygiene 94, 61–64. [PubMed] [Google Scholar]
- Hoque BA, Mahalanabis D, Alam MJ & Islam MS (1995) Post‐defecation handwashing in Bangladesh: practice and efficiency perspectives. Public Health 109, 15–24. [DOI] [PubMed] [Google Scholar]
- Isaacs D, Dickson H, O'Callaghan C, Sheaves R, Winter A & Moxon ER (1991) Handwashing and cohorting in prevention of hospital acquired infections with respiratory syncytial virus. Archives of Disease in Childhood 66, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS and the Bellagio Child Survival Study Group (2003) How many child deaths can we prevent this year? Lancet 362, 65–71. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ et al. (2005) Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. New England Journal of Medicine 352, 686–691. [DOI] [PubMed] [Google Scholar]
- Kaltenthaler E, Waterman R & Cross P (1991) Faecal indicator bacteria on the hands and the effectiveness of hand‐washing in Zimbabwe. The Journal of Tropical Medicine and Hygiene 94, 358–363. [PubMed] [Google Scholar]
- Khan MU (1982) Interruption of shigellosis by handwashing. Transactions of the Royal Society of Tropical Medicine and Hygiene 76, 164–168. [DOI] [PubMed] [Google Scholar]
- Kimel LS (1996) Handwashing education can decrease illness absenteeism. The Journal of School Nursing 12, 14–18. [DOI] [PubMed] [Google Scholar]
- Kotch JB, Weigle KA, Weber DJ et al. (1994) Evaluation of an hygienic intervention in child day‐care centers. Pediatrics 94, 991–994. [PubMed] [Google Scholar]
- Krilov LR, Barone SR, Mandel FS, Cusack TM, Gaber DJ & Rubino JR (1996) Impact of an infection control program in a specialized preschool. American Journal of Infection Control 24, 167–173. [DOI] [PubMed] [Google Scholar]
- Ladegaard MB & Stage V (1999) Hand‐hygiene and sickness among small children attending day care centers. An intervention study. Ugeskrift for Laeger 161, 4396–4400. [PubMed] [Google Scholar]
- Larson E, Aiello A, Lee LV, Della‐Latta P, Gomez‐Duarte C & Lin S (2003) Short‐ and long‐term effects of handwashing with antimicrobial or plain soap in the community. Journal of Community Health 28, 139–150. [DOI] [PubMed] [Google Scholar]
- Luby SP, Agboatwalla M, Raza A et al. (2001) Microbiologic effectiveness of hand washing with soap in an urban squatter settlement, Karachi, Pakistan. Epidemiology and Infection 127, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahl MC & Sadler C (1975) Virus survival on inanimate surfaces. Canadian Journal of Microbiology 21, 819–823. [DOI] [PubMed] [Google Scholar]
- Makris AT, Morgan L, Gaber DJ, Richter A & Rubino JR (2000) Effect of a comprehensive infection control program on the incidence of infections in long‐term care facilities. American Journal of Infection Control 28, 3–7. [DOI] [PubMed] [Google Scholar]
- Master D, Longe SH & Dickson H (1997) Scheduled hand washing in an elementary school population. Family Medicine 29, 336–339. [PubMed] [Google Scholar]
- Montville R, Chen Y & Schaffner DW (2002) Risk assessment of hand washing efficacy using literature and experimental data. International Journal of Food Microbiology 73, 305–313. [DOI] [PubMed] [Google Scholar]
- Murray CJL & Lopez AD (1997) Global mortality, disability and the contribution of risk factors: global burden of disease study. Lancet 349, 1436–1442. [DOI] [PubMed] [Google Scholar]
- Niffenegger JP (1997) Proper handwashing promotes wellness in child care. Journal of Pediatric Health Care 11, 26–31. [DOI] [PubMed] [Google Scholar]
- Osterhaus AD & De Vries P (1992) Vaccination against acute respiratory virus infections and measles in man. Immunobiology 184, 180–192. [DOI] [PubMed] [Google Scholar]
- Pinfold JV & Horan NJ (1996) Measuring the effect of a hygiene behaviour intervention by indicators of behaviour and diarrhoeal disease. Transactions of the Royal Society of Tropical Medicine and Hygiene 90, 366–371. [DOI] [PubMed] [Google Scholar]
- Ponka A, Poussa T & Laosmaa M (2004) The effect of enhanced hygiene practices on absences due to infectious diseases among children in day care centers in Helsinki. Infection 32, 2–7. [DOI] [PubMed] [Google Scholar]
- Reed SE (1975) An investigation of the possible transmission of rhinovirus colds through indirect contact. The Journal of Hygiene 75, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L, Smith W, Jorm L, Patel M, Douglas RM & McGilchrist C (2000) Effect of infection control measures on the frequency of upper respiratory infection in child care: a randomized controlled trial. Pediatrics 105, 738–742. [DOI] [PubMed] [Google Scholar]
- Ryan MAK, Christian RS & Wohlrabe J (2001) Handwashing and respiratory illness among young adults in military training. American Journal of Preventive Medicine 21, 79–83. [DOI] [PubMed] [Google Scholar]
- Scott B, Curtis V & Rabie T (2003) Protecting children from diarrhea and acute respiratory infections: the role of hand washing promotion in water and sanitation programmes. Regional Health Forum WHO South-East Asia Region 7, 42–47. [Google Scholar]
- St Sauver J, Khurana M, Kao A & Foxman B (1998) Hygienic practices and acute respiratory illness in family and group day care homes. Public Health Reports 113, 544–551. [PMC free article] [PubMed] [Google Scholar]
- Stanton BF & Clemens JD (1987) An educational intervention for altering water‐sanitation behaviors to reduce childhood diarrhea in urban Bangladesh: part II a randomised trial to assess the impact of the intervention on hygienic behaviors and rate of diarrhea. American Journal of Epidemiology 125, 292–333. [DOI] [PubMed] [Google Scholar]
- Stata Statistical Software (2003) Stata Corporation, Texas. [Google Scholar]
- Uhari M & Möttönen M (1999) An open randomized controlled trial of infection prevention in child day‐care centers. The Pediatric Infectious Disease Journal 18, 672–677. [DOI] [PubMed] [Google Scholar]
- White CG, Shinder FS, Shinder AL & Dyer DL (2001) Reduction of illness absenteeism in elementary schools using an alcohol‐free instant hand sanitizer. The Journal of School Nursing 17, 258–265. [PubMed] [Google Scholar]
- White C, Kolble R, Carlson R et al. (2003) The effect of hand hygiene on illness rate among students in university residence halls. American Journal of Infection Control 31, 364–370. [DOI] [PubMed] [Google Scholar]
- WHO (2002) The World Health Report 2002: Reducing Risks, Promoting Healthy Life. World Health Organization, Geneva. [Google Scholar]
- WHO (2003a) Update 33 – Update on Hong Kong and China, First SARS Case Reported in India. Available at: http://www.who.int/csr/sarsarchive/2003_04/_18/en/ (accessed on July 15, 2003). [Google Scholar]
- WHO (2003b) Update 47 – Studies of SARS Virus Survival, Situation in China. Available at: http://www.who.int/csr/sarsarchive/2003_05_05/en/ (accessed on July 16, 2003). [Google Scholar]


