Summary
In bronchoalveolar lavage fluid (BALF) of pigs originating from different herds bacteria, cells and the antibacterial peptide PR‐39 were examined to gain information about the lung health status. In a high health nucleus herd 56% and in low health herds 20–100% of the examined pigs were found positive for potentially pathogenic bacteria. Based on these findings, a novel definition for bacterial respiratory tract disease was established using an 8% cut‐off for the relative number of neutrophils in bronchoscopic and a 40% cut‐off in transtracheal BALF in combination with the occurrence of potentially pathogenic microorganisms. The antibacterial peptide PR‐39 was highly correlated to this definition of respiratory disease. An assessment of the bacteriological respiratory health status appears to be possibly based on the determination of PR‐39 concentrations in BALF using different cut‐off values according to the lavage method (2.5 nM for bronchoscopic and 5 nM for transtracheal BALF).
Introduction
Epidemiological investigations in the recent past have shown that next to diseases of the gastrointestinal system and locomotion disorders, most losses during the finishing period are due to multifactorial respiratory diseases (Straw et al., 1983; Schoder et al., 1993; Baumann and Bilkei, 2002). Swine are commonly the carriers of various microorganisms, which do not cause health problems under good husbandry conditions, so that microbiological findings are often difficult to interpret. Persistently infected carrier animals without clinical symptoms are the main cause for disseminating respiratory disease pathogens among individuals and between herds.
The porcine respiratory disease complex (PRDC) is caused by viral and bacterial pathogens and is modulated by several other factors including management, climate, air pollution, population and social factors, which, in part, are associated with the increased herd size in intensive pig production (Done, 1991; Tuovinen et al., 1997).
Despite the economic importance of respiratory disease in pigs no commonly accepted definition of ‘health’ and ‘disease’ exists. A challenging task for the veterinarian is the early detection of a disturbance in the balance between host immune system, environmental influences and microbial invaders, which is facilitated by the examination of respiratory disease parameters in bronchoalveolar lavage fluid (BALF). Classical microbiological methods or PCR analyses are used to investigate the presence of potential pathogens associated with PRDC in BALF (Palzer et al., 2005). The interpretation of results is often difficult, as pathogenic microorganisms also occur in healthy pigs (Hensel et al., 1994). In swine, the proportion of polymorphonuclear neutrophilic granulocytes (PMNs) in BALF is significantly increased in pneumonic pigs and correlated to the total cell count. A cut‐off of 8% PMNs in BALF has been suggested to differentiate between healthy pigs and pigs with respiratory disorders (Ganter and Hensel, 1997; Mombarg et al., 2002). In contrast to cellular components, soluble disease markers for lung alterations in swine are virtually unavailable. Up to now, most studies are limited to measurements of changes in cytokine levels and acute phase proteins (Van Reeth et al., 2002; Van Gucht et al., 2006). The porcine antibacterial peptide PR‐39 was found to be increased in BALF after experimental infection with Actinobacillus (A.) pleuropneumoniae, where a concentration of PR‐39 higher than 1 nM in BALF had been suggested as a cut‐off value for individual pigs (Hennig‐Pauka et al., 2006). PR‐39 in BALF as a diagnostic parameter for detecting bacterial respiratory tract disease in general is evaluated in this study where bacteriological findings and the relative number of PMNs were used to define the bacteriological respiratory health status of the pigs.
Materials and Methods
Animals and samples
The selected pigs from 11 different places of origin showed no or different gradations of respiratory disease. An overview of herds and their hierarchical position within the pork production process is given in Table 1. Lethality caused by respiratory disease was stated by the farmers on farm 2 (15%), farm 3 (5%) and farm 10 (2%) (Table 1). A recapitulatory evaluation of all pigs, which had been lavaged in the clinic and which originated from different farms, resulted in a lethality of 40%. Pigs chosen for bronchoalveolar lavage (BAL) had not been treated within at least the previous 9 days before sampling. With the exception of pigs originating from herd 1, some of them showed coughing, dyspnoea, elevated body temperature or a body condition below average. On most farms, additional diagnostic investigations had been performed as shown in Table 1.
Table 1.
Characterization of swine farms
| Farm | Character of farm | History of respiratory disease | History of diagnostic investigations on the farm | Dyspnoea/ coughing |
|---|---|---|---|---|
| 1 | High health nucleus herd, 500 sows, specified pathogen‐free (epizootics, endo‐ and ectoparasites, toxigenic Pasteurella multocida, A. pleuropneumonia, PRRS virus | No | Negative serological findings for toxin of Pasteurella multocida, A. pleuropneumoniae, PRRS virus | No |
| 2 | Finishing unit, 220 pigs | For 6 months | Positive PCR in BALF for M. hyopneumoniae, negative for influenza and PRRS virus | 15% |
| 3 | Farrow‐to‐finish, rearing unit | Acute | Necropsy and cultural isolation of H. parasuis and Sc. suis from lung tissue | 20% |
| 4 | Rearing, 2400 pigs | For 6 months | Negative serological findings for A. pleuropneumoniae, PRRS virus, influenza virus | 10% |
| 5 | Rearing, 2500 pigs | For 2 months | Negative serological findings for A. pleuropneumoniae, influenza and PRRS virus | 8% |
| 6 | Gilt rearing, 180 sows | For 4 months | Positive serological findings for A. pleuropneumoniae, influenza and PRRS virus | 80% |
| 7 | Gilt rearing, 200 sows | For several years | Negative serological findings for A. pleuropneumoniae, influenza and PRRS virus. Positive serological findings for M. hyopneumoniae | 70% |
| 8 | Gilt rearing, 100 sows | For several years | Negative serological findings for PRRS virus, toxin of Pasteurella multocida. Positive serological findings for A. pleuropneumoniae | 30% |
| 9 | Boar rearing, 1300 pigs | For several years | Necropsy and cultural isolation of H. parasuis from lung tissue. Negative serological findings for toxin of Pasteurella multocida, A. pleuropneumoniae, PRRS virus | 40% |
| 10 | Farrow‐to‐finish, rearing and growing unit, 2500 pigs | For 2 years | Positive serological findings for A. pleuropneumoniae, M. hyopneumoniae, influenza and PRRS virus | 3% |
| 11 | Clinic for swine and small ruminants, inhomogenous patient population, 100 pigs | Acute to chronic | Not known | 50% |
Pigs were lavaged either endotracheally or transtracheally without visual control using disposable material as described elsewhere (Delbeck et al., 1997; Nienhoff et al., 2006). For biosecurity reasons, bronchoscopic BAL was performed in the Clinic for Swine and Small Ruminants, University of Veterinary Medicine Hannover, Foundation, as described previously (Hennig‐Pauka et al., 2001). For collecting BALF, pigs were anaesthetized by intramuscular injection of azaperone 2 mg/kg (Stresnil®; Janssen GmbH, Neuss, Germany) and ketamine 15 mg/kg (Ursotamin®; Serumwerk Bernburg AG, Bernburg, Germany). The total fluid recovered was recorded as a percentage for each lavaged pig. Information about the pigs chosen for BAL and bacteriological findings in the respective BALF samples are given in Table 2.
Table 2.
Information about BALF samples taken on the farms
| Farm | Estimated body weight of animals examined | Number of BALF samples examined | Number of BALF samples positive for pathogenic microorganisms | BAL method | Pathogens detected |
|---|---|---|---|---|---|
| 1 | 15–30 kg | 10 | 3 | Endotracheal | Bordetella bronchiseptica, Sc. suis, Haemophilus parasuis, M. hyopneumoniae |
| 10 | 2 | Transtracheal | |||
| 16 | 15 | Bronchoscopic | |||
| 2 | 70 kg | 5 | 4 | Transtracheal | M. hyopneumoniae, Pasteurella multocida |
| 3 | 25 kg | 5 | 4 | Transtracheal | Sc. suis, Haemophilus parasuis |
| 4 | 10 kg | 5 | 4 | Transtracheal | Bordetella bronchiseptica, Sc. suis, Haemophilus parasuis |
| 5 | 25–30 kg | 4 | 4 | Transtracheal | Bordetella bronchiseptica, Pasteurella multocida, Sc. suis, Haemophilus parasuis |
| 6 | 40–45 kg | 10 | 6 | Endotracheal | M. hyopneumoniae, Haemophilus parasuis |
| 7 | 50 kg | 10 | 2 | Endotracheal | M. hyorhinis |
| 8 | 30 kg | 10 | 3 | Endotracheal | Sc. suis, Haemophilus parasuis |
| 9 | 25–30 kg | 10 | 7 | Endotracheal | Sc. suis |
| 10 | 10–45 kg | 10 | 9 | Transtracheal | M. hyopneumoniae, Sc. suis, Haemophilus parasuis |
| 11 | 10–50 kg | 29 | 25 | Bronchoscopic | M. hyopneumoniae, A. pleuropneumoniae, Pasteurella multocida, Arcanobacterium pyogenes, Haemophilus parasuis, Sc. suis, Bordetella bronchiseptica |
Bacteriological examination
Immediately after BALF collection, an aliquot of BALF was transferred to Mycoplasma selective nutrient broth (Mykoplasma Liquid Medium ML 10A; Mycoplasma experience Ltd., Reigate, UK). The bacteriological examination of BALF and the PCR for the detection of Mycoplasma (M.) hyopneumoniae were described previously (Hartwig, 1994; Hensel et al., 1994; Kurth et al., 2002).
Bacteriological findings were evaluated by summarizing detected microorganisms in two classes: microorganisms which are empirically associated with respiratory disease were classified as ‘pathogens’ (Haemophilus parasuis, Pasteurella multocida, Bordetella bronchiseptica, M. hyopneumoniae, Steptococcus suis, Arcanobacterium pyogenes, A. pleuropneumoniae and confluent growth of M. hyorhinis). Other microorganisms are classified as ‘commensals and environmental contaminants’ (Acinetobacter spp., Aeromonas spp., Alcaligenes spp., haemolytic and non‐haemolytic Streptococci, Bacillus spp., Citrobacter spp., Corynebacterium spp., Escherichia coli, Enterobacter spp., Enterococcus spp., Klebsiella spp., coagulase‐negative Staphylococci, Mannheimia haemolytica, Moraxella spp., Morganella spp., Myroides spp., Pantoea spp., Pasteurella spp., Proteus spp., Pseudomonas spp., Staphylococcus aureus, Staphylococcus hyicus and sporadic growth of M. hyorhinis). Confluent growth of an organism in bacterial culture was referred to as ‘high grade’.
Cytological examination
Cytological examinations were performed using routine methods (Kipper, 1990; Ganter and Hensel, 1997). Leucocytes were counted in three fields of a Tuerk counting chamber. After 200 g centrifugation of BALF the supernatant was stored at −80°C and the cell pellet was adjusted to a cell concentration of approximately 1 g/l with 154 mm sodium chloride. Cytocentrifuge slides were prepared by centrifugation at 200 g for 10 min. (Varifuge 20 RS and Zytosystem; Heraeus Sepatech GmbH, Osterode, Germany), air‐dried and stained with 10% May‐Gruenwald‐Giemsa solution. A total of 200 cells were differentiated and classified as PMNs, lymphocytes or macrophages.
Determination of the antibacterial peptide PR‐39 in BALF
A PR‐39 capture ELISA was performed as previously described (Zhang et al., 1997) and modified for BALF (Hennig‐Pauka et al., 2006). Briefly, 96‐well microtitre plates (MaxiSorb™ Surface; Nunc, Roskilde, Denmark) were coated overnight at 4°C with a mouse monoclonal antibody against PR‐39 as the capture reagent. All BALF supernatants were centrifuged at 5000 g for 10 min to remove cell debris. Samples, the polyclonal rat anti‐PR‐39 detecting antibody and the conjugate (peroxidase‐labelled goat‐anti‐rat antibody; Dianova, Hamburg, Germany) were suspended in phosphate‐buffered saline (10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, 1.76 mM KH2PO4) containing 1% BSA and 0.01% cetrimonium bromide (Panyutich et al., 1991; Zhang et al., 1997). The substrate consists of 1.2 mM 3,3′,5,5′‐tetramethylbenzidine (SeramunBlue® fast; Seramun Diagnostika GmbH, Wolzig, Germany) and hydrogen peroxide (3 mM). Colour development was determined at 450 nm using a microplate reader (SLT‐Spectra; SLT Lab instruments Deutschland GmbH, Crailsheim, Germany). PR‐39 concentrations were determined by the reference‐standard method (Butler et al., 1987) based on serial twofold dilutions of BALF (1:2 to 1:128) and synthetic PR‐39 (Affiniti Research Products Ltd, Exeter, UK) as standard.
Statistical analysis
Data were analysed using sas ® statistical software (SAS Institute Inc., Cary, NC, USA). P‐values <0.05 were considered significant. As parameters of interest seemed abnormally distributed, they were reported as median values, the 25 and 75 percentiles, and maximum and minimum values. Correlations between parameters were examined by calculating Spearman’s Correlation coefficients (r). The diagnostic impact of the relative number of PMNs and the concentrations of PR‐39 were investigated by constructing Receiver Operating Characteristics (ROC) plots resulting from the calculation of sensitivities and specificities for different potential cut‐off‐values (Zweig and Campbell, 1993). In ROC‐curves, diagnostic sensitivity (Y‐axis) and 100‐diagnostic specificity (X‐axis) are plotted at different cut‐off values, and the applicability of a test system to differentiate between healthy and diseased individuals at different cut‐off values can be estimated. In addition, for cut‐off values of interest odds ratios for dichotomic variables were calculated. The kappa‐index was calculated to determine the extent to which the defined respiratory health status tallies with the respiratory health status predicted (Landis and Koch, 1977).
Results
Pathogens were detected in 56% of the BALF samples from a high health nucleus herd without a history or clinical symptoms of respiratory disease. It must be assumed that the isolation of pathogenic microorganisms alone has no significance for the health status of the herd.
The detection rates of samples positive for pathogenic microorganisms were in the range of 20–100% in herds with respiratory disorders. As animals from different farms sampled by different lung lavage methods have been included in the study, the wide range of bacterial detection rates was not surprising.
The diagnostic significance of the potential disease parameters, relative number of PMNs and the concentration of PR‐39 in BALF for the prediction of the bacterial respiratory health status was examined. The relative number of PMNs could only be determined for bronchoscopic and transtracheal BALF. There was a high positive correlation between the relative number of PMNs and the PR‐39 concentration (r = −0.74, P < 0.0001). Furthermore, negative correlations were found between the relative numbers of PMNs and macrophages (r = −0.94, P < 0.001) as well as between PR‐39 and the relative number of macrophages (r = −0.67, P < 0.001).
Most specific cut‐offs for the relative number of PMNs in bronchoscopic and transtracheal BALF were determined using the isolation of bacterial pathogens as reference (Fig. 1). A 100% specificity was achieved using a cut‐off of 8% PMNs in bronchoscopic BALF (56% sensitivity). In transtracheal BALF a 100% specificity was achieved using a cut‐off of 40% (38% sensitivity). Based on these results, pigs with isolation of at least one pathogenic bacterial species, including PCR for M. hyopneumoniae, but only in combination with more than 8% PMNs in bronchoscopic BALF or 40% PMNs in transtracheal BALF, respectively, were defined as diseased, suggesting that in these animal pathogens had already provoked a cellular reaction and had damaged the respiratory tract.
Figure 1.
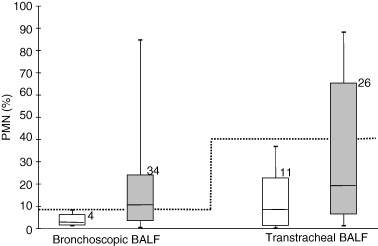
The relative numbers of PMNs in BALF samples either negative (□) or positive () for pathogenic isolates. The box represents the 25%, the 50% (median) and the 75% quartile, and the top and bottom forms mark the maximum and minimum values. Numbers on top of the bars are the number of animals examined. The dotted line indicates the cut‐offs with 100% specificity.
Using this definition in bronchoscopic BALF, 84% of the animals defined as diseased had a PR‐39 concentration of at least 2.5 nM; this corresponds to a significant 45‐fold increase in risk compared with animals with a PR‐39 concentration below the cut‐off. In transtracheal BALF 90% of the diseased animals had PR‐39 concentrations of at least 5 nM; this corresponds to a significant 72‐fold increase in risk (Fig. 2, Table 3). Odds ratios for different PR‐39 cut‐offs in the different lavage groups are shown in Table 3.
Figure 2.
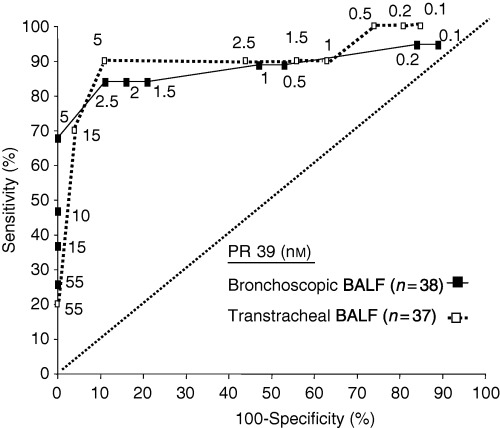
ROC‐curves for PR‐39 in bronchoscopic and transtracheal BALF with respect to bacteriological findings, which were weighted by cellular findings. Pigs which were positive for at least one pathogenic species in BALF, but only in combination with more than 8% PMNs (bronchoscopic BALF) or 40% PMNs, respectively (transtracheal BALF), were considered as diseased. For every cut‐off value the 100‐specifity on the x‐axis and the sensitivity on the y‐axis is shown. The y = x‐axis is depicted.
Table 3.
Odds ratios of PR‐39 cut‐offs, their significance, sensitivities and specificities and kappa coefficients with respect to the detection of pathogens in combination with more than 8% PMNs in bronchoscopic or 40% PMNs in transtracheal BALF
| Bronchoscopic BALF | Transtracheal BALF | |
|---|---|---|
| PR‐39 cut‐off | 1 nM | 1 nM |
| Number of animals | 38 | 37 |
| Odds ratio (95% CI) | 9.4 (1.7–52.7) | 5.3 (0.6–48.2) |
| Significance (Fisher’s Exact test) | 0.01 | 0.22 |
| % Sensitivity (95% CI) | 89.5 (66.9–98.7) | 90.0 (55.5–99.7) |
| % Specificity (95% CI) | 52.6 (28.8–75.6) | 37.0 (19.4–57.6) |
| Kappa coefficient (95% CI) | 0.42 (0.15–0.69) | 0.20 (−0.02–0.38) |
| PR‐39 cut‐off | 2.5 nM | 5 nM |
| Number of animals | 38 | 37 |
| Odds ratio (95% CI) | 45.3 (6.7–307.7) | 72.0 (6.6–785) |
| Significance (Fisher’s Exact test) | <0.0001 | <0.0001 |
| % Sensitivity (95% CI) | 84.2 (60.4–96.6) | 90.0 (55.5–99.8) |
| % Specificity (95% CI) | 89.5 (66.7–98.7) | 88.9 (70.8–97.7) |
| Kappa coefficient (95% CI) | 0.74 (0.52–0.95) | 0.74 (0.50–0.98) |
CI, confidence interval.
Kappa coefficient: <0.00, poor; 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1, almost perfect (Landis and Koch, 1977).
The evaluation of PR‐39 as a marker in BALF for the respiratory bacteriological status can be summarized as follows:
-
1
Using a definition for bacterial respiratory disease that combines bacteriological and cellular results, subclinically infected pigs without apparent respiratory symptoms can be identified.
-
2
Lavage method‐dependent cut‐offs for PR‐39 are appropriate for predicting the respiratory bacteriological status in swine with adequate sensitivity and specificity.
Discussion
In this study, BALF disease parameters were examined in healthy pigs or in pigs with ongoing or recently occurring respiratory diseases of various origins. The major question which should be answered by this field study was if PR‐39 was applicable as a disease marker for bacterial respiratory tract diseases.
In this study, it was not possible to ascertain the intrinsic causes for the respiratory problems on the farms, because neither abiotic factors nor virus infections were systematically examined. Some serological examinations had been initiated additionally to the diagnostic investigations on the farms as shown in Table 1. Virus infections had not been taken into consideration in this study, because changes in the PR‐39 concentration in BALF during viral infections of the respiratory tract have not been investigated so far. All pigs showing relative amounts of PMNs higher than the cut‐offs showed also positive findings for potentially pathogenic bacteria – independently of additional virus infection. With respect to the classification of individual pigs in this study, virological examinations would not have given additional information. The influence of viral pathogens as well as of abiotic factors on the PR‐39 concentration has to be tested on standardized animal models in future experiments. In general, lymphocytes and macrophages were intensely influenced by virus infections and both cell types were not chosen as allocation bases in this study.
The non‐recurring sampling procedure in this field study corresponded to a cross‐sectional study and resulted only in a snap‐shot of the animals’ state of health. In part, serological examinations at farm level had been performed but had not provided satisfactory results (Table 1). The interpretation of serological findings mostly requires additional anamnestic and clinical information as well as the bacteriological or virological examination of samples. Serum titres alone do not prove the causative involvement of a pathogen in a clinical disease problem, as they can be induced by maternally derived antibodies, after recovery from respiratory disease or by endemic latent disease. In general, costs of serological testings are often minimized by examining only a reduced number of animals with the consequence that the information gained in the findings is of limited value. Accurate calculation of an adequate sample size to detect an infected herd (identification of at least one positive reagent) resulted in approximately 30 serum samples per animal group (300–3000 pigs) assuming a prevalence of 10% seropositive reagents (Große Beilage, 2000). Additional serological tests during this study would have given information about the impact of virus infections and bacterial pathogens, which had not been detected by bacteriological examination. A clinical diagnosis for an individual can be confirmed by an increase in antibodies against the aetiological agent during a serological testing of paired serum samples in a 3‐ to 4‐weeks’ interval (Große Beilage, 2000). In this study, additional serological testing was not carried out for economic reasons. Serological examination of bacterial pathogens would have been possible for M. hyopneumoniae and A. pleuropneumoniae, which are both considered to be obligate pathogenic (Palzer, 2005). A differentiation between M. hyopneumoniae antibodies induced either by infection or by vaccination has not been possible so far (Nathues et al., 2006). Furthermore, serological findings are not appropriate for ascertaining M. hyopneumoniae as the causative agent for respiratory disease in single individuals (Runge et al., 1996). The detection of antibodies against A. pleuropneumoniae is of little diagnostic value in recent outbreaks (Nielsen, 1988). In subclinically infected herds, clinical signs can flare up due to other respiratory infections. Due to the fact that the cultural diagnostic of A. pleuropneumoniae is difficult in samples from subclinically infected pigs, serology provides an important diagnostic tool in these animals.
Antibody responses caused by virus infections of the respiratory tract are difficult to interpret: in general, high seroprevalences are found for influenza virus, the porcine respiratory coronavirus (PRCV), porcine circovirus 2 (PCV2) and the PRRS virus. Van Reeth and Pensaert (1994) found no association between infection and respiratory disease with respect to influenza virus and PRCV. The prevalences of positive serological findings, as well as of PCR positive results for PCV2, are also high in pigs with no clinical signs of respiratory disease. Antibody titres had been observed to be similar in pigs in herds with and without post‐weaning multi‐systemic wasting syndrome (Larochelle et al., 2003). Asymptomatic, persistent infections with PRRS virus can be detected by serology (Große Beilage, 2000).
A previous comparison of the three lavage methods in healthy animals taken from one nucleus herd with a good status of respiratory health had shown that the total cell count and the PR‐39 concentration in BALF obtained with all three methods and the differential cell count obtained in bronchoscopic and transtracheal BALF must be interpreted differently depending on the BAL method. Because of a low degree of contamination transtracheal BALF turned out to be well suited for a subsequent bacteriological examination (data not shown). In transtracheal BALF high PR‐39 values were observed (data not shown). These might be due to an additional synthesis by other bronchial cells as has been shown for lactoferrin and lysozyme as abundant antimicrobial proteins in the respiratory tract with a primary airway origin during bronchitis (Thompson et al., 1990).
In this study, a comparison of the three methods was not possible because of the inhomogenous pig groups originating from different farms. Nevertheless, a microbiological diagnosis was possible in only 45% of all diseased animals in the endotracheal BALF group, whereas in the bronchoscopic group a diagnosis was obtained in 89.2%, and in the transtracheal group in 86.3%. High PR‐39 concentrations were also observed in transtracheal BALF samples. On one hand, these differences might be due to the different bacterial burden and species the animals had been exposed to on their farms. On the other hand, these differences might also be caused by the different lavage methods. With respect to endotracheal and bronchoscopic BALF, results are similar to a previous comparison of endotracheal and bronchoscopic BALF (Delbeck et al., 1997). A low degree of commensal organisms and environmental contaminants in transtracheal BALF has been found by Nienhoff et al. (2006) and has also been observed in this study.
Only bacterial pathogens, but not virus infections and environmental noxae, were taken into consideration in this field study. A differentiation of facultative and obligate pathogenic microorganisms was avoided. All bacteria, which can empirically be associated with respiratory disease, were summarized as ‘pathogenic microorganisms’. All of them, with the exception of A. pleuropneumoniae, have also been found in clinically healthy animals (Kipper, 1990). In previous studies, neither the number nor the spectrum of bacterial species in BALF was found to be different between healthy pigs or pigs with acute or chronic respiratory disease (Kipper, 1990; Ganter et al., 1993; Hartwig, 1994; Hensel et al., 1994).
The definition of the respiratory status in pigs remains a problem, because porcine respiratory health cannot be defined solely based on microbiological findings as determined in this study. This is exemplified by our study where we isolated pathogens in 56% of the pigs from an excellently kept high health nucleus herd.
One sensitive marker for differentiating between healthy pigs and those with respiratory disorders is the relative number of PMNs in BALF (Ganter and Hensel, 1997). In healthy control herds, a mean percentage of 4% of endotracheal BALF samples exceeding the 8% reference limit for the relative number of PMNs was found (Mombarg et al., 2002). In this study, we observed that the number of PNMs could not be determined consistently using endotracheal BALF, and our data imply that the cut‐off value for PMNs from transtracheal BALF should be set at 40%.
Based on the results of this study, we suggest a combination of microbiological findings and the relative number of PMNs as definition for bacterial respiratory disease. This combination of bacteriological and cytological findings results in a weighting of the real negative impact of the pathogens in the respiratory tract. The highly positive correlation between PR‐39 and the relative number of PMNs supports the hypothesis of PMNs being one major source for PR‐39. Both parameters can be considered as positive disease markers. PMNs have been proven to be a sensitive marker for respiratory disease; this marker may be replaced by the determination of the PR‐39 concentration, especially in endotracheal BALF samples where cell differentiation is not possible.
In a previous study, both parameters have been tested in a homogenous pig group, which had been experimentally infected with A. pleuropneumoniae (Hennig‐Pauka et al., 2006). As a consequence of that study, the most important question to be answered was whether other bacteria also could induce increased PR‐39 concentrations. The answer, that increased PR‐39 concentrations were not specific for an infection with A. pleuropneumoniae, was given in this study. The chosen cut‐offs for the relative numbers of PMNs or for PR‐39 are appropriate for identifying pigs subclinically affected by bacterial respiratory pathogens and for pre‐selecting pigs for further diagnostics, e.g. multiplex PCR.
In conclusion, BAL sampling methods currently used in practice can be highly valuable tools for the assessment of the impact of bacteriological findings in the porcine respiratory tract. The determination of disease markers in pigs cannot replace diagnostic examinations for specific bacterial and viral pathogens, but can give additional helpful information for the interpretation of microbiological findings. Especially in experimental research the PR‐39‐ELISA is a valuable tool for diagnosing an infection of the respiratory tract. The concentration of PR‐39 in BALF – independent of the sampling method – appears to be a highly reliable surrogate marker for bacterial respiratory disease. If an appropriate test would be available, costs for BALF examination (and for the routine control of respiratory herd health) could be reduced considerably.
Acknowledgements
This work was funded by the Sonderforschungsbereich 587 (project A4) from the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.
References
- Baumann, B. , and Bilkei G., 2002: Emergency‐culling and mortality in growing/fattening pigs in a large Hungarian “farrow‐to‐finish” production unit. Dtsch. Tierärztl. Wochenschr. 109, 26–33. [PubMed] [Google Scholar]
- Butler, J. E. , Peterman J. H., Suter M., and Dierks S. E., 1987: The immunochemistry of solid‐phase sandwich enzyme‐linked immunosorbent assays. Fed. Proc. 46, 2548–2556. [PubMed] [Google Scholar]
- Delbeck, F. , Tegeler R., and Ganter M., 1997: Bronchoalveolar lavage on pig breeding farms. Dtsch. Tierärztl. Wochenschr. 104, 374–378. [PubMed] [Google Scholar]
- Done, S. H. , 1991: Environmental factors affecting the severity of pneumonia in pigs. Vet. Rec. 128, 582–586. [DOI] [PubMed] [Google Scholar]
- Ganter, M. , and Hensel A., 1997: Cellular variables in bronchoalveolar lavage fluids (BALF) in selected healthy pigs. Res. Vet. Sci. 63, 215–217. [DOI] [PubMed] [Google Scholar]
- Ganter, M. , Kipper S., Schottger‐Wegener H., Beckmann G., and Bunka S., 1993: Pneumonia diagnosis in living swine using lung lavage. Berl Münch Tierärztl Wochenschr. 106, 330–333. [PubMed] [Google Scholar]
- Große Beilage, E. , 2000: Die Planung serologischer Untersuchungen und Interpretation der Befunde am Beispiel porziner Atemwegserkrankungen. Tierärztl. Prax. 28, 40–46. [Google Scholar]
- Hartwig, W. , 1994: Die Eignung der Bronchoskopie und der bronchoalveolären Lavage (BAL) für die epidemiologische Untersuchung respiratorischer Erkrankungen im Schweinebestand. Doctoral Thesis, University of Veterinary Medicine, Hannover. [Google Scholar]
- Hennig‐Pauka, I. , Ganter M., Gerlach G. F., and Rothkotter H. J., 2001: Enzyme activities, protein content and cellular variables in the pulmonary epithelial lining fluid in selected healthy pigs. J. Vet. Med. A 48, 631–639. [DOI] [PubMed] [Google Scholar]
- Hennig‐Pauka, I. , Jacobsen I., Blecha F., Waldmann K. H., and Gerlach G. F., 2006: Differential proteomic analysis reveals increased cathelicidin expression in porcine bronchoalveolar lavage fluid after an Actinobacillus pleuropneumoniae infection. Vet. Res. 37, 75–87. [DOI] [PubMed] [Google Scholar]
- Hensel, A. , Ganter M., Kipper S., Krehon S., Wittenbrink M. M., and Petzoldt K., 1994: Prevalence of aerobic bacteria in bronchoalveolar lavage fluids from healthy pigs. Am. J. Vet. Res. 55, 1697–1702. [PubMed] [Google Scholar]
- Kipper, S. , 1990: Bronchoskopie bei Schweinen sowie mikrobiologische und zytologische Untersuchungen der bronchoalveolären Spülflüssigkeit. Doctoral Thesis, University of Veterinary Medicine, Hannover. [Google Scholar]
- Kurth, K. T. , Hsu T., Snook E. R., Thacker E. L., Thacker B. J., and Minion F. C., 2002: Use of a Mycoplasma hyopneumoniae nested polymerase chain reaction test to determine the optimal sampling sites in swine. J. Vet. Diagn. Invest. 14, 463–469. [DOI] [PubMed] [Google Scholar]
- Landis, J. R. , and Koch G. G., 1977: The measurement of observer agreement for categorical data. Biometrics 33, 159–174. [PubMed] [Google Scholar]
- Larochelle, R. , Magar R., and D’ Allaire S., 2003: Comparative serologic and virologic study of commercial swine herds with and without postweaning multisystemic wasting syndrome. Can. J. Vet. Res. 67, 114–120. [PMC free article] [PubMed] [Google Scholar]
- Mombarg, M. J. , Niewold T. A., Stockhofe‐Zurwieden N., Van Leengoed L. A., and Verheijden J. H., 2002: Assessment of respiratory herd health in weaner pigs by measuring cellular composition of bronchoalveolar lavage fluid. J. Vet. Med. B 49, 424–428. [DOI] [PubMed] [Google Scholar]
- Nathues, H. , Strutzberg‐Minder K., Kreienbrock L., and Große Beilage E., 2006: Möglichkeiten und Grenzen serologischer Diagnostik im Rahmen der Bestandsbetreuung von Schweinen am Beispiel der Mycoplasma‐hyopneumoniae‐Infektion. Dtsch. Tierärztl. Wschr. 113, 448–452. [PubMed] [Google Scholar]
- Nielsen, R. , 1988: Seroepidemiology of Actinobacillus pleuropneumoniae . Can. Vet. J. 29, 580–582. [PMC free article] [PubMed] [Google Scholar]
- Nienhoff, H. , Staszyk C., and Mumme J., 2006: Beschreibung eines praktikablen Verfahrens zur Gewinnung von Lungenspülproben bei Schweinen in schweinehaltenden Betrieben. Prakt. Tierarzt 87, 138–140. [Google Scholar]
- Palzer, A. , 2005: Keimspektrum und Erregerassoziationen bei gesunden und an Pneumonie erkrankten Schweinen. Doctoral Thesis, Ludwigs‐Maximilians‐Universität, München. [Google Scholar]
- Palzer, A. , Ritzmann M., Wolf G., and Heinritzi K., 2005: Erregernachweis aus bronchoalveolärer Lavage bei Schweinen mit Atemwegserkrankungen. Tierärztl. Umschau 60, 550–556. [Google Scholar]
- Panyutich, A. V. , Voitenok N. N., Lehrer R. I., and Ganz T., 1991: An enzyme immunoassay for human defensins. J. Immunol. Methods 141, 149–155. [DOI] [PubMed] [Google Scholar]
- Runge, M. , Ganter M., Delbeck F., Hartwick W., Rüffer A., Franz B., and Amtsberg G., 1996: Nachweis von Pneumonieerregern bei Schweinen aus Problembeständen: Kulturelle und immunfluoreszenz‐mikroskopische Untersuchungen der bronchoalveolären Lavage (BAL) und serologische Befunde. Berl. Münch. Tierärztl. Wschr. 109, 101–107. [PubMed] [Google Scholar]
- Schoder, G. , Maderbacher R., Wagner G., and Baumgartner W., 1993: Causes of losses in a pig fattening facility. Dtsch. Tierärztl. Wochenschr. 100, 428–432. [PubMed] [Google Scholar]
- Straw, B. E. , Neubauer G. D., and Leman A. D., 1983: Factors affecting mortality in finishing pigs. J. Am. Vet. Med. Assoc. 183, 452–455. [PubMed] [Google Scholar]
- Thompson, A. B. , Bohling T., Payvandi F., and Rennard S. I., 1990: Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J. Lab. Clin. Med. 115, 148–158. [PubMed] [Google Scholar]
- Tuovinen, V. K. , Grohn Y. T., and Straw B. E., 1997: Farrowing unit housing and management factors associated with diseases and disease signs of importance for feeder pig quality. Acta Agric. Scand. A 47, 117–125. [Google Scholar]
- Van Gucht, S. , Atanasova K., Barbe F., Cox E., Pensaert M., and Van Reeth K., 2006: Effect of porcine respiratory coronavirus infection on lipopolysaccharide recognition proteins and haptoglobin levels in the lungs. Microbes Infect. 8, 1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth, K. , and Pensaert M., 1994: Prevalence of infections with enzootic respiratory and enteric viruses in feeder pigs entering fattening herds. Vet. Rec. 135, 594–597. [PubMed] [Google Scholar]
- Van Reeth, K. , Van Gucht S., and Pensaert M., 2002: Correlations between lung proinflammatory cytokine levels, virus replication, and disease after swine influenza virus challenge of vaccination‐immune pigs. Viral Immunol. 15, 583–594. [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Ross C. R., Dritz S. S., Nietfeld J. C., and Blecha F., 1997: Salmonella infection increases porcine antibacterial peptide concentrations in serum. Clin. Diagn. Lab. Immunol. 4, 774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig, M. H. , and Campbell G., 1993: Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39, 561–577. [PubMed] [Google Scholar]


