Abstract
Parainfluenza virus (PIV) may cause life‐threatening pneumonia in lung transplant patients and there are no proven effective therapies. We report the use of inhaled DAS181, a novel sialidase fusion protein, to treat severe PIV type 3 pneumonia in a lung transplant patient. Treatment was well tolerated and associated with improvement in oxygenation and symptoms, along with rapid clearance of PIV. DAS181 should be systematically evaluated for treatment of PIV infection in transplant recipients.
Keywords: parainfluenza, DAS181, lung transplant, solid organ transplant
Parainfluenza virus (PIV) may infect 5–15% of lung transplant (LT) patients 1, 2, and severity can range from asymptomatic infection to severe lower respiratory tract disease 1, 2, 3. Viral clearance in transplant patients is also impaired. In addition to the direct morbidity from acute respiratory tract viral infections, increasing evidence suggests that many respiratory viruses, including PIV, may lead to both acute rejection and bronchiolitis obliterans syndrome, the latter being the single most common cause of allograft failure and death in LT recipients 2, 3, 4.
Currently there is no US Food and Drug Administration‐approved vaccine or treatment for PIV infection; treatment is supportive and includes supplemental oxygen, reduction in immunosuppression, and bronchodilators. While one study has reported a potential role for a multidrug regimen containing aerosolized ribavirin, intravenous immunoglobulin (IVIG), and methylprednisolone in LT recipients with respiratory syncytial virus (RSV) or PIV infection, it included only 1 case of lower respiratory tract PIV infection 5. Studies of PIV infection in the hematopoietic cell transplant (HCT) population have shown no decrease in mortality or duration of viral shedding with the use of aerosolized ribavirin 6. Thus, a great need exists for new effective therapies against PIV.
DAS181, a novel sialidase fusion protein, cleaves sialic acid‐containing receptors used by PIV and influenza to bind to respiratory epithelial cells 7. DAS181 has in vitro and in vivo activity against both PIV 8 and influenza 7, and has been shown to be safe in an animal model 9 and in phase‐1 human trials. Recently, Chen et al. 10 and Guzmán‐Suarez et al. 11 reported the use of DAS181 for treatment of PIV infection in 2 HCT recipients and 1 LT recipient. We describe a case of severe PIV type 3 pneumonia in a LT recipient treated with DAS181, associated with a significant clinical and virologic response.
Methods
DAS181 (NexBio Inc., San Diego, California, USA) was obtained under an emergency investigational new drug application that was approved by the US Food and Drug Administration and the University of Washington institutional review board. The patient provided written informed consent. DAS181 was administered as an inhaled dry powder at an emitted dose of 10 mg daily for 5 days using an oral inhaler (Cyclohaler; Teva Pharmaceuticals Ltd, North Wales, Pennsylvania, USA). Inhaled albuterol was administered before each dose of DAS181. Oropharyngeal wash samples were obtained in a standardized manner before starting therapy, daily while on therapy, and subsequently until the time of hospital discharge to monitor response to treatment. Samples were placed in 3 mL viral transport media and analyzed via quantitative reverse‐transcription polymerase chain reaction (PCR) at the University of Washington Molecular Virology Laboratory 12. Plasma samples were also analyzed via quantitative reverse‐transcription PCR 13.
Case report
A 64‐year‐old woman with a history of interstitial pulmonary fibrosis (IPF) diagnosed 18 years earlier underwent a right LT 11 months before this presentation. Her course was complicated by an episode of humoral rejection 7 days post transplant, treated with IVIG, plasmapheresis, and cyclophosphamide. Since the time of this initial complication she had been doing well and was not requiring any supplemental oxygen. She had been unable to tolerate mycophenolate mofetil or azathioprine, so her immunosuppressive regimen at the time of admission included tacrolimus and prednisone 10 mg daily. Ongoing antimicrobial medications included trimethoprim‐sulfamethoxazole, acyclovir, azithromycin, and clotrimazole.
Two weeks before admission, the patient's husband developed symptoms of bronchitis, and 1 week before admission the patient developed malaise, sore throat, and nasal congestion and was treated empirically with amoxicillin‐clavulanate. Two days before admission, she became increasingly dyspneic and developed a cough productive of yellow sputum. Two days later, her oxygen saturation on ambient air was 80%, and she was admitted.
On admission she was afebrile and required 5 L of supplemental oxygen to maintain oxygen saturations above 95%. Laboratory results were notable only for mild leukopenia (Table 1). Cytomegalovirus was not detectable in plasma by PCR. Two sets of blood cultures were sterile. A computed tomography scan of her chest showed diffuse ground‐glass opacities throughout her right lung with interlobular septal thickening and a small right pleural effusion. Her left (native) lung showed unchanged honeycombing and traction bronchiectasis consistent with her known history of IPF. She was initially treated with ceftriaxone and oseltamivir. The following day, bronchoscopy revealed erythematous, easily collapsible airways with diffuse thin secretions throughout her right lung consistent with tracheobronchitis. Cultures from bronchoalveolar lavage (BAL) were negative for bacteria, fungi, Legionella, and mycobacteria. Urine Legionella antigen was negative. BAL fluorescent antibody (FA) for Pneumocystis jirovecii pneumonia; PCR for Aspergillus fumigatus; cytomegalovirus and RSV shell vial cultures; and PCR for bocavirus, human metapneumovirus, PIV types 1, 2, and 4, RSV, influenza A and B, coronavirus, rhinovirus, and adenovirus were all negative. BAL and nasopharyngeal swab FA and PCR were positive for PIV type 3. Plasma samples taken on hospital days 4 and 5 showed no evidence of PIV viremia.
Table 1.
Laboratory values
| Variable | Reference range | Admission | DAS181 day 1 (hospital day 5) | DAS181 day 5 (hospital day 9) | Discharge (hospital day 15) |
|---|---|---|---|---|---|
| White blood cells (cells/μL) | 4300–10,000 | 3060 | 3120 | 4250 | 5400 |
| Absolute neutrophil count (cells/μL) | 1800–7000 | 2030 | |||
| Absolute lymphocyte count (cells/μL) | 1000–4800 | 500 | |||
| Platelet count (cells/μL) | 150,000–400,000 | 146,000 | 177,000 | 274,000 | 398,000 |
| Hemoglobin (g/dL) | 11.5–15.5 | 10.2 | 9.0 | 9.2 | 8.9 |
| Creatinine (mg/dL) | 0.38–1.02 | 0.85 | 0.98 | 0.92 | 0.94 |
| Total bilirubin (mg/dL) | 0.2–1.3 | 0.6 | 0.6 | 0.2 | 0.6 |
| Aspartate aminotransferase (U/L) | 15–40 | 50 | 36 | 42 | 50 |
| Alanine aminotransferase (U/L) | 6–40 | 14 | 17 | 19 | 22 |
| Alkaline phosphatase (U/L) | 31–132 | 107 | 148 | 166 | 83 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
On hospital day 2 she was transferred to the intensive care unit with increasing hypoxia (Fig. 1). Based on the BAL results, oseltamivir and ceftriaxone were discontinued. Her immunosuppression was not markedly changed. On hospital day 5, DAS181 treatment was initiated. Beginning on the fifth day of DAS181 therapy (hospital day 9), a significant reduction was seen in detectable PIV copies (Fig. 1), and by hospital day 11 PIV was no longer detectable in oropharyngeal washes (lower limit of detection is ≥1000 viral copies/mL). Over the 5 days of treatment (hospital days 5–9) with DAS181, she began to feel subjectively improved with decreased dyspnea and cough, and by hospital day 13 she was weaned off of high‐flow facemask to nasal cannula using 4 L of supplemental oxygen to maintain saturations in the high 90s. She noted some persistent dyspnea with exertion, but no longer while at rest. Throughout her hospital course, her laboratory values were only significant for a slight rise in alkaline phosphatase (Table 1).
Figure 1.
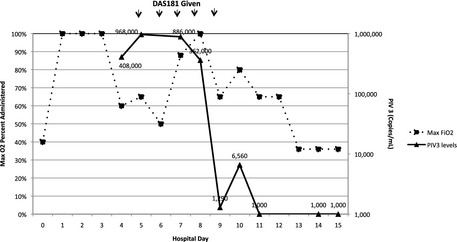
Quantitative oropharyngeal wash parainfluenza virus 3 (PIV3) viral loads and maximum administered oxygen percentage during hospitalization. FiO2, fraction of inspired oxygen.
One week after hospital discharge, she again noted increased dyspnea, desaturations with ambulation, and increased oxygen requirement to 6 L at rest. Spirometry (forced vital capacity [FVC] 1.15 L [30% of predicted], and forced expiratory volume in 1 s [FEV1] 0.77 L [30%]) was markedly decreased from prior assessment 2 months earlier (FVC 2.81 L [86%], FEV1 1.69 L [67%]). Repeat bronchoscopy showed A2B1 cellular rejection and she was found to have antidonor antibodies in serum. She was treated with methylprednisolone, plasmapheresis, IVIG, and rituximab, with improvement in her symptoms and oxygen requirements to 3 L at the time of discharge. Respiratory viral FA and PCR from BAL did not detect any evidence of PIV (or other viral) infection. Repeat spirometry 1 week post discharge showed improved results from prior, although still impaired from her baseline (FVC 1.53 L [47%], FEV1 0.98 L [39%]).
Discussion
We report a case of an LT recipient treated with DAS181 for severe PIV3 pneumonia. As was evident in our patient, PIV may cause severe lung disease in HCT and LT recipients 1, 2, 3, 6, with diffuse involvement of her transplanted lung, marked symptoms, and very high oxygen requirements nearly prompting intubation. Although copathogens may be commonly found in association with PIV in immunocompromised hosts 6, no additional pathogens were identified in our patient.
As no effective therapies are available to treat PIV, new active agents are clearly needed. DAS181 has in vitro and in vivo activity against PIV 8, and its use has been described in a small number of transplant recipients 10, 11. In our patient, a dramatic >2‐log decline was seen in quantitative viral PIV3 in oropharyngeal samples starting on day 5 of DAS181 therapy, with a diminution to undetectable levels 2 days after that. Overall, this indicates a ≥3‐log decline in viral load. The reduction in PIV viral load appeared to correlate with symptomatic improvement and reduction in oxygen requirements. Notably, no additional antiviral therapy was used, and immunosuppressive therapy was not significantly changed during this period.
An association may exist between respiratory viral infections and the subsequent development of acute and chronic rejection 2, 3, 14; interestingly, acute rejection was diagnosed shortly after PIV3 infection in our patient, with no evidence of recurrent PIV, and she subsequently responded to additional immunosuppressive treatment. It is not clear to what degree rejection was contributing to her initial severe lung process, but as she had marked clinical improvement without any change in immunosuppressive therapy, it is likely that rejection was not the predominant feature initially.
Our patient tolerated DAS181 well. During treatment with DAS181, it is common to see a mild increase in alkaline phosphatase levels, thought to be related to delayed clearance secondary to systemic protein desialylation 9; this was seen in our patient, and promptly resolved after discontinuation of the drug. No other toxicity was evident.
Three DAS181‐treated patients have been described previously, including 2 HCT recipients and 1 LT recipient 10, 11. Compared with the previously reported cases, our patient's lung involvement was dramatically more severe, with very high oxygen requirements. Initial oropharyngeal wash PIV viral load in our patient was similar to one of the HCT patients 10, but appreciably higher than that found in the other 2 patients 11, and we demonstrated a much greater decline in PIV viral load, as compared with the relatively small change seen in the previously described LT recipient 11. We used inhaled albuterol prior to DAS181 dosing, in an attempt to optimize local drug delivery, but cannot say if that made a significant difference in our patient's response. As presently formulated, DAS181 cannot be readily used in ventilated patients.
One obvious limitation of our report is that this is a description of a single patient. Thus, it is difficult to know the true benefit of DAS181, as opposed to simply the natural resolution of PIV3 infection. However, the rapid and large (≥3 log) virologic decline seen in our patient, which appeared to correlate with clinical improvement, suggests a potent, specific antiviral effect that resulted in clinical benefit. In addition, prolonged viral shedding with PIV infection is commonly seen in immunocompromised patients 15; our patient's clearance of virus fairly quickly after DAS181 treatment again suggests an in vivo antiviral effect. We were not able to start treatment with DAS181 until 3 days after her initial diagnosis. Whether earlier treatment would have led to more rapid improvement in symptoms and oxygenation is not clear, nor is the potential long‐term effects of earlier treatment on graft survival and the incidence of bronchiolitis obliterans syndrome.
The present case suggests that DAS181 is well tolerated and has potent in vivo antiviral effects against a major viral pathogen in LT recipients. Given the potential for both acute and long‐term benefits associated with treatment of PIV infection in solid organ transplant and HCT recipients, future controlled trials of DAS181 in these populations are warranted.
Acknowledgements
Thanks: We thank Sarah Johnson for excellent assistance with sample collection.
Financial support: HL093294 (to M.B.).
Disclosure: R.B.M., R.L.S., and C.H. are employees of NexBio, Inc.
Author contributions: D.R.D., A.P.L., and R.M.R.: Concept/design. D.R.D. and R.M.R.: Drafting article. D.R.D., A.P.L., and R.M.R.: Data collection. All authors: Critical revision of article, and approval of article.
Drozd D.R., Limaye A.P., Moss R.B., Sanders R.L., Hansen C., Edelman J.D., Raghu G., Boeckh M., Rakita R.M.. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transpl Infect Dis 2013: 15: E28–E32. All rights reserved
References
- 1. Weigt SS, Gregson AL, Deng JC, Lynch JP 3rd, Belperio JA. Respiratory viral infections in hematopoietic stem cell and solid organ transplant recipients. Semin Respir Crit Care Med 2011; 32: 471–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community‐acquired respiratory viruses in lung transplant recipients. Transplantation 2010; 89: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 3. Vilchez RA, Dauber J, McCurry K, Iacono A, Kusne S. Parainfluenza virus infection in adult lung transplant recipients: an emergent clinical syndrome with implications on allograft function. Am J Transplant 2003; 3: 116–120. [DOI] [PubMed] [Google Scholar]
- 4. Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest 2011; 140: 502–508. [DOI] [PubMed] [Google Scholar]
- 5. Liu V, Dhillon GS, Weill D. A multi‐drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart‐lung transplant recipients. Transpl Infect Dis 2010; 12: 38–44. [DOI] [PubMed] [Google Scholar]
- 6. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001; 98: 573–578. [DOI] [PubMed] [Google Scholar]
- 7. Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad‐spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother 2006; 50: 1470–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moscona A, Porotto M, Palmer S, et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo . J Infect Dis 2010; 202: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larson JL, Kang S‐K, Choi BI, et al. A safety evaluation of DAS181, a sialidase fusion protein, in rodents. Toxicol Sci 2011; 122: 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen YB, Driscoll JP, McAfee SL, et al. Treatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantation. Clin Infect Dis 2011; 53: e77–e80. [DOI] [PubMed] [Google Scholar]
- 11. Guzman‐Suarez BB, Buckley MW, Gilmore ET, et al. Clinical potential of DAS181 for treatment of parainfluenza‐3 infections in transplant recipients. Transpl Infect Dis 2012; 14 (4): 427–433. [DOI] [PubMed] [Google Scholar]
- 12. Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real‐time PCR assays with fluorescent‐antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44: 2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis 2010; 201: 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am J Transplant 2011; 11: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood 2007; 110: 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]


