Abbreviations
- B19V
human parvovirus B19
- CHIKV
chikungunya virus
- CoV(s)
coronavirus(‐es)
- DENV
dengue viruses
- ECDC
European Centres for Disease Control
- EID(s)
emerging infectious disease(s)
- MERS
Middle Eastern respiratory syndrome
- PARV4
parvovirus 4
- SARS
severe acute respiratory syndrome
- vCJD
variant Creutzfeldt‐Jakob disease
- WNV
West Nile virus
Past Efforts to Respond to Infectious Disease Threats to the Blood Supply
The introduction of blood donation screening for syphilis in the 1940s, followed by hepatitis B surface antigen (HBsAg) testing in the early 1970s and the subsequent conversion in the United States to an all‐volunteer blood supply led most to believe that, with the exception of residual posttransfusion non‐A, non‐B hepatitis, the blood supply was relatively safe and would remain so for the foreseeable future. However, with the recognition of AIDS/human immunodeficiency virus (HIV) as a worldwide threat to the safety of the blood supply in 1982 to 1983, times of complacency were over and there was a critical need to increase vigilance and decrease the response time to threats to recipient safety. Even before the current proactive focus on emerging infectious disease (EID) agents, the blood industry's progressive response to HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) was noteworthy. Our responses to established and EID agents transmissible by labile blood components continue to be based on two main interventions: donor selection and questioning, to remove those with recognized risk factors, and testing to deal with what remains. It is important to recognize that the earliest “first‐generation” tests for HBsAg, viral lysate‐based HIV antibody and single‐antigen HCV antibody tests, albeit less than optimal in sensitivity and specificity, successfully removed more than 90% of the pretesting risk within 1 to 2 years of discovery of the etiologic agents. Subsequent development of second‐ and third‐generation assays achieved our current expectations of test sensitivity of more than 99.9%. The public expects an absence of infectious disease transmissions, most notably for highly infectious and pathogenic agents like HIV. Thus, we must recognize and acknowledge the contributions of the test manufacturing industry, which have enabled the incredible gains in blood supply safety achieved over the past three decades. As an example, Figures 1A and 1B display the enormous effort and investment that industry has provided to the transfusion community with the release worldwide of more than 50 versions of HIV serologic assays since 1985. Together with nucleic acid testing (NAT) in the United States and most of the industrialized world, HIV residual risks (along with those of HBV and HCV) are currently estimated at less than 1 per million transfused units. Plasma‐derived products have had the additional benefit of pathogen reduction, which in combination with the other two interventions, has nearly eliminated infectious disease risks of plasma derivatives.
Figure 1.
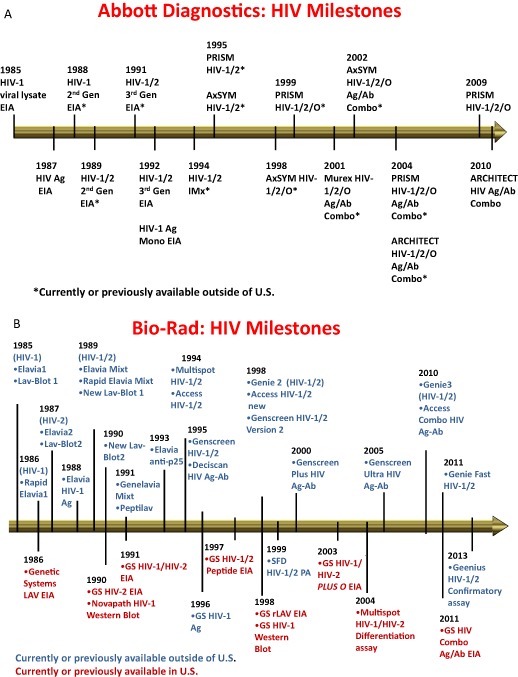
Successive versions of HIV immunoassays: (A) Abbott Diagnostics; (B) Bio‐Rad.
In August 2009, a group from the AABB Transfusion Transmitted Diseases (TTD) committee published a supplement to TRANSFUSION 1 (see also http://www.aabb.org/resources/bct/eid/Pages/default.aspx) that reviewed the definition and background of EID agents that pose a real or theoretical threat to transfusion safety, but for which an existing effective intervention is lacking. Emergence was recognized as due to multiple factors, including an increase in the incidence of a new agent, as occurred with HIV in the early 1980s and variant Creutzfeldt‐Jakob disease (vCJD) prions in the 1990s; recognition of a previously undetected agent or of an infection or clinical condition that is now linked to an infectious agent; or due to an agent that now has reemerged with pathogenic properties due to mutation, drug resistance, or global population or environmental changes. EIDs of concern represent numerous classes of agents with well over 60% from zoonotic sources, involve multiple transmission routes, result in chronic as well as acute infections, and derive from human activities in which transportation has a critical role.1, 2 Most notably, emergence is unpredictable. This problem is highlighted in Fig. 2 in which the transmission routes for “classical” transfusion‐transmissible agents are contrasted with those for some recent EID agents that have threatened blood safety. There are no consistent patterns to predict emergence or magnitude of threat to blood safety. As shown in Fig. 2 recent EID threats include vector‐borne agents such as those causing malaria, leishmaniasis, Chagas disease, babesiosis, dengue, or West Nile fever or neuroinvasive disease; respiratory agents such as the severe acute respiratory syndrome (SARS) coronavirus (CoV); those that may be transmitted sexually such as human herpesvirus type 8 and cytomegalovirus; those transmitted by, or originating from, food or water such as hepatitis A virus (HAV), hepatitis E virus (HEV), and vCJD; and those where infection results from direct contact with an infected source such as simian foamy viruses.
Figure 2.
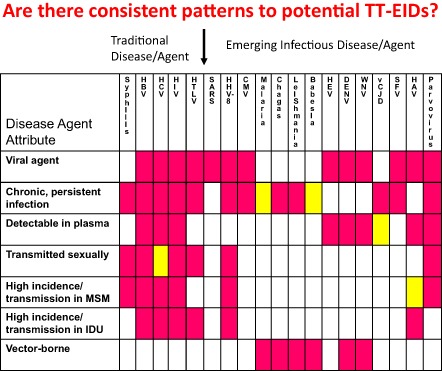
Patterns of key characteristics of transfusion‐transmissible (TT) EID agents. Those listed to the left of the arrow are the traditional TT agents of concern up to the year 2000, while those to the right are selected recent TT‐EID agents.
The necessary attributes for transfusion transmission were outlined in the TRANSFUSION supplement1 and elsewhere,2 including the presence of the agent in blood during an asymptomatic phase in the donor and the agent's survival or persistence in blood during processing and/or storage; moreover, the agent must be recognized as responsible for a clinically apparent disease outcome in at least a proportion of recipients who become infected. Without these attributes, agents were not considered as a transfusion threat and were excluded. Sixty‐eight agents were initially identified by the AABB EID subgroup, each with enough evidence or likelihood of transfusion transmission (e.g., blood phase) and potential for clinical disease to warrant further consideration. In the Supplement, Fact Sheets were published providing information on agent classification; background on the disease agent's importance; the clinical syndromes and/or diseases caused; transmission modes (including vectors and/or reservoirs); likelihood of transfusion transmission; and if proven to be transfusion transmitted, information on known transmission cases, the feasibility and predicted success of interventions that could be used for donor qualification (questioning), and tests available for diagnostics or that could be adapted for donor screening. Finally the efficacy, if known, of inactivation methods for plasma‐derived products was included. The Supplement also included a separate section on pathogen reduction technologies for all blood components using published data. Agents were prioritized relative to their scientific and/or epidemiologic threat as well as their perceived threat to the community including concerns expressed by the regulators of blood. Agents given the highest priority due to a known transfusion transmission threat and severe or fatal disease in recipients were the vCJD prion, dengue viruses (DENV), and the obligate red blood cell (RBC) parasite that causes babesiosis (Babesia microti and related species). Although the focus of the Supplement was toward the United States and Canada, many of the agents (and the processes) are applicable worldwide.
Even now, 4 years later, much of the original process and prioritization has not changed from both scientific and public perception perspectives, including the listing of the same three agents of greatest concern to recipient safety. A different list of agents posing a threat to transfusion safety would likely have been developed by other groups, depending on different conditions and geographic areas. For example, authors from the European Centres for Disease Control (ECDC) prioritized agents based on climate change driving an increased threat from certain agents to the blood supply in Europe; included in the listing, in order of priority, were West Nile virus (WNV), followed by DENV, Leishmania, chikungunya virus (CHIKV), malaria parasites, tick‐borne encephalitis virus, and the agent of Lyme disease.3 Of note, ECDC considered two agents (CHIKV and the agent of Lyme disease) of concern even though transfusion transmission of these agents has never been documented. Of necessity, any list of EID agents is not, and can never be, complete due to the nature of emergence. For example, it is believed that the number of recognized viral agents infecting humans in 1960 was 50, whereas in the year 2020 the number is projected to exceed 200.4 Another estimate is that there have been 5.3 new viruses discovered each year from 1940 to 2004, of which, as already mentioned, at least two‐thirds are zoonoses,5 with human activities forcing interactions with agents that are in equilibrium with their natural hosts but whose threat is new in humans.
Since the publication of the Supplement, five new Fact Sheets (yellow fever viruses [including vaccine breakthrough infections], miscellaneous arboviruses, XMRV [including a comprehensive table of published literature], and human parvoviruses and bocaviruses other than B19) were added and 11 existing Fact Sheets updated (Babesia, Bartonella, the chronic wasting disease prion, human prions other than vCJD, the vCJD prion, Coxiella burnetii [the agent of Q fever], DENV, HEV, Japanese encephalitis [JE virus complex], tick‐borne encephalitis viruses, and human parvovirus B19 [B19V]). Updates are being made to the Fact Sheets for the small, obligate‐intracellular bacteria previously known as rickettsia including Anaplasma and Erhlichia, the former now having reports of seven transfusion transmissions from infected RBCs or platelets (PLTs; with or without leukoreduction),6, 7, 8, 9, 10, 11 and Ehrlichia ewingii, which was recently reported as linked to transmission by contaminated, leukoreduced, and irradiated PLTs.12 Also, tables were released to update the pathogen reduction sections of the Supplement, including tabulations of pathogen reduction clinical trials and results (with only published data included) for PLTs, plasma, RBCs, and whole blood and the availability and commercial routine use of such technologies by country for PLTs and plasma.
Illustrative EID Research Articles in This Themed Issue
Within this first “themed issue” of TRANSFUSION some of these agents have been the focus of original research studies related to methods of detection, incidence, prevalence, clinical outcomes, removal, or need for an intervention. These include HEV, B19V, and related parvovirus 4 (PARV4), and CHIKV, all of which have associated Fact Sheets.1, 13, 14, 15, 16, 17 HEV is a small, nonenveloped, single‐stranded RNA virus in the Hepevirus genus consisting of four genotypes and a single serotype. Globally HEV represents the most common cause of acute viral hepatitis. Of the four genotypes, Genotypes 1 and 2 are transmitted by food or water (similar to HAV) whereas Genotypes 3 and 4 are more commonly associated with transmission from animals, especially swine, including by consumption of raw or undercooked food products primarily containing pork liver. It is these genotypes (3 and 4) that have been associated with transfusion and transplant transmissions.1 HEV infection in the immunocompromised host (such as solid organ transplant recipients) leads frequently to chronic infection (>60%) and eventually to cirrhosis in approximately 14% of those with chronic HEV infection;18 persistent viremia (RNA in plasma) can be recovered in approximately 1% of cases.19 The frequency of HEV as assessed by antibody (immunoglobulin [Ig]G) testing varies widely because of varying viral prevalence geographically (1% to >50%) and variable test performance characteristics. In the study by Xu and colleagues13 in this issue of TRANSFUSION, a Chinese‐manufactured antibody test was used that is recognized as having superior performance compared to other tests. In this study, of 1939 volunteer US blood donors, anti‐HEV IgG prevalence was 18.8% (95% confidence interval [CI], 17.0%‐20.5%) and IgM prevalence was 0.4%. The presence of antibody increased linearly with age representing a cohort effect or cumulative exposure. Testing for RNA was done in pools of seven to eight donor samples by real‐time reverse transcription–polymerase chain reaction (PCR) and nested PCR with 95% lower limits of detection of 400 and 200 IU/mL, respectively; no donor tested positive for HEV RNA. In addition, the study examined a separate repository of 362 recipients where samples from the linked donors of transfused products were available. Although there were two possible anti‐HEV IgG recipient seroconversions, neither was believed due to transfusion transmission. In one IgG‐positive recipient, no HEV RNA or IgM was detected in pretransfusion or posttransfusion samples. The source of IgG was determined to be one high‐titer, anti‐HEV–positive donor whose unit was transfused just before the recipient's positive IgG finding, and thus the IgG was likely acquired passively by transfusion and not as the result of HEV infection in the recipient. Although this recipient also received a different IgM‐ and RNA‐positive donor unit, the recipient died 4 days later and hence could not be evaluated further. Of note, the linked donor sample traced as part of this recipient's lookback was the only RNA‐positive donor identified among all donor samples tested in this study. The second recipient had a low‐level reactive IgG response after transfusion; however, the pretransfusion sample also had low‐level IgG reactivity (slightly below the cutoff). No donor sample received by this second recipient tested positive for IgM anti‐HEV or HEV RNA. Again, the anti‐HEV IgG could not be attributable to transfusion‐transmitted HEV. Thus, no transfusion transmissions were observed among the 362 recipients (95% CI, 0.0%‐0.8%).
A study on the use of a new detection method for the B19V, a DNA‐containing, nonenveloped Erythrovirus trophic for erythroid progenitor cells, is also reported in this issue of TRANSFUSION.14 B19V is resistant to inactivation; thus plasma for further manufacture is screened by NAT at a cutoff below which transmission has not been documented. Susceptible hosts for more severe disease include those who are immune compromised, patients with shortened RBC survival, and pregnant women (due to associated fetal damage). However, most adults are immune. Acute infection results in high viral loads, frequently at or above 1012 DNA IU/mL.1 Viremia precedes symptoms and has been associated with transfusion transmission; at least 12 cases are documented in the literature with eight from Japan (103‐108 IU/mL), some of which were from antibody‐positive units.1, 20 DNA prevalence in donors varies (<1% in most studies).21 High‐titer B19V, DNA‐positive RBCs from antibody‐negative donors are well known to be infectious.22, 23, 24 There is a consensus that pooled plasma products should be prepared from donations that have been screened to minimize the titer of B19V to fewer than 104 DNA copies/mL, an approach generally achieved by NAT.1 However, in this issue of TRANSFUSION, Sakata and colleagues14 suggest that this outcome may be achieved by the use of an immunoassay designed to detect viral antigens. The chemiluminescent antigen assay has sensitivity comparable to 6.4 log IU/mL B19V DNA, can detect all three B19V genotypes, and thus is suitable for testing plasma intended for further manufacture. The need for more sensitive testing to protect recipients of labile blood components, however, remains controversial.
Recently, another human parvovirus has been described, somewhat confusingly named PARV4. It has been tentatively placed in a genus named Partetravirus. PARV4 DNA and antibody have been identified at varying frequencies among blood donors and plasma pools undergoing further manufacture, but at higher levels in injection drug users and usually in association with HCV. PARV4, like B19V, is resistant to conventional viral inactivation procedures.1 In this issue of TRANSFUSION, Maple and coworkers15 describe the development and evaluation of an IgG test for PARV4 and document a 4.8% seroprevalence among a small sample of blood donors from the United Kingdom, but with little evidence of increased prevalence between 1999 and 2009. Prevalence in 184 injection drug users was 20.7%, with 68% of the PARV4 IgG‐positive samples also anti‐HCV positive. In contrast, B19V seroprevalence was 65.5% in injection drug users and 76.3% in blood donors.15 Also in this issue, Baylis and colleagues16 used a novel approach to show that PARV4 is somewhat less sensitive to heat inactivation than B19V. Currently, PARV4 has not been clearly associated with any disease state. Thus, the concern expressed in these articles for further research to protect the blood supply from risks posed by this virus may be overstated. As pointed out elsewhere in this commentary, disease causation is a critical characteristic of emerging infections that are of concern to transfusion safety.
CHIKV is a mosquito‐borne alphavirus that is endemic with sporadic outbreaks in Africa, India, Southeast Asia, and the Philippines. Several recent explosive outbreaks have spread from east Africa to the Indian Ocean islands of Comoros, Madagascar, Mayotte, Mauritius, Seychelles, and la Réunion Island, then spreading to several states in India.1 In la Réunion during the 2005 to 2007 outbreak it was estimated that 34% of the 766,000 residents were infected and another 1.3 million cases estimated to have occurred in India.25 Local transmission was identified in Italy in the summer of 2007.1, 26 It appears that a simple mutation event in the virus, derived from the southern and eastern African lineage of CHIKV, has emerged during these outbreaks; this mutation favors Aedes albopictus as the vector over A. aegypti, thus expanding the area at risk.1, 27 Experience with WNV and DENV suggests that CHIKV might offer a threat to blood safety and thus precautions were implemented during the outbreak in la Réunion. Local collections of whole blood were canceled and supplies were shipped from the French mainland. Apheresis PLTs were tested for viral nucleic acids and pathogen reduction was rapidly implemented.28 These precautions remained in force until the risk was judged to be no greater than that for HBV transfusion transmission. In this issue of TRANSFUSION, Appassakij and colleagues17 have provided relevant information about viral levels in symptomatic and asymptomatic cases from an epidemic of chikungunya in Songkhla, Thailand, in 2009. Symptomatic individuals were viremic for up to 8 days, at levels of 1.3 × 101 to 2.9 × 108 pfu/mL (median, 5.6 × 105 pfu/mL), whereas the asymptomatic cases had a range of 8.4 × 101 to 2.9 × 105 pfu/mL (median, 3.4 × 103 pfu/mL); due to small numbers of individuals studied, no significant difference was observed (1 pfu was equivalent to approx. 100 copies of RNA). The ratio of symptomatic to asymptomatic cases studied was 10:1. The authors concluded that, despite the absence of reported cases of transfusion‐transmitted chikungunya, likely due to the difficulty in differentiating mosquito‐borne illness from transfusion transmission in the setting of large outbreaks, there is significant risk of such transmission in outbreak areas.17
New AABB “Toolkit” Initiative
The AABB EID subgroup recognized that a system of assessing the risk or threat of EIDs and their potential impact on blood safety and availability should be formalized so that the process may be applied by the next generation of experts. This system must include a process for monitoring, identifying, evaluating, estimating severity, assessing risk quantitatively, and developing interventions. Thus, we are now developing a “toolkit” containing the necessary “tools” for EID monitoring (horizon scanning) and for validating and evaluating the effectiveness of proposed interventions. Our goal is to develop a systematic approach to risk assessment and intervention development for the impact of emerging infections on blood safety in North America. The system is primarily intended to educate and advise AABB members about risks and interventions in a timely and accurate fashion. Secondary audiences include North American blood systems, blood services, and transfusion services. Certainly this toolkit may be adapted to the needs of blood services and governments or regulatory agencies responsible for blood safety internationally. The process and final product (toolkit), including methods to monitor EID agent emergence, identify and recognize a transfusion transmission threat, processes to quantify risk, and the appropriate management of such threats, should be considered for implementation by all blood systems. Figure 3 provides a flow diagram outlining the scheme as envisioned currently. It starts with consideration of where threats arise and with monitoring those threats: horizon scanning. List servers such as ProMed (http://www.promedmail.org/) are likely the most useful source of current and accurate information and responsible commentary. Much of what is known globally regarding each new agent, whether transfusion transmitted or not, is posted routinely in ProMed.
Figure 3.
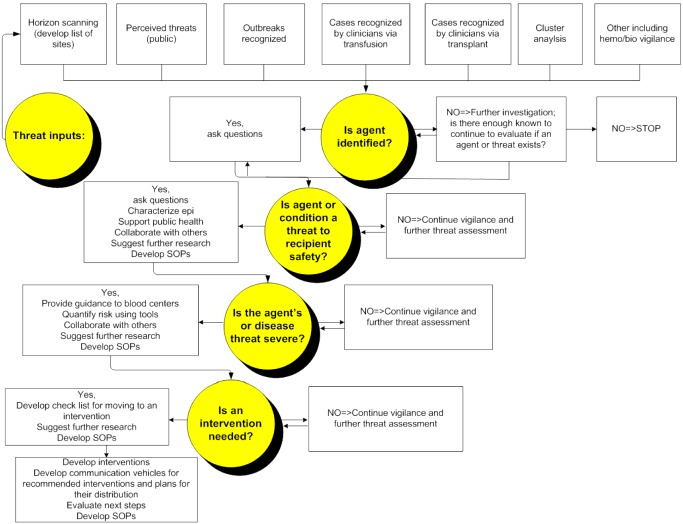
Outline of AABB EID subgroup's toolkit including the framework for recognition, assessment, and management of EID agents for risk of transfusion‐associated transmission and disease. SOP = standard operating procedure.
One recent example is the information provided by ProMed (and via the ECDC and World Health Organization [WHO]) on the newly described Middle Eastern respiratory syndrome (MERS), which was quickly linked to a novel CoV. CoV's are a family of viruses that cause illnesses ranging from the common cold to SARS, which sickened more than 8000 people and killed 774 in 2002 and 2003, according to the WHO. Thus far it is believed the MERS CoV, which was first recognized in Jordan in April 2012 although the first published case was from Saudi Arabia,29 is far less transmissible than the SARS CoV. While most laboratory‐confirmed cases have been identified in the Arabian peninsula including Saudi Arabia, Qatar, and the United Arab Emirates, cases have occurred due to travel of infected individuals in North Africa (Tunisia) and in Europe (the United Kingdom, France, Germany, and Italy). Globally, from September 2012 to July 27, 2013, WHO has been informed of a total of 91 laboratory‐confirmed cases of infection with MERS CoV, including 46 deaths (http://www.promedmail.org/direct.php?id=20130626.1793072; http://www.promedmail.org/direct.php?id=20130727.1848186); the vast majority of cases identified to date are in individuals with severe disease and hence asymptomatic cases are likely underrecognized. Cases outside of the Middle East are believed to be in individuals transferred to those countries for care of their disease or returned from the Middle East and who subsequently became ill. In France, Italy, Tunisia, and the United Kingdom, there has been limited local transmission among patients who had not been to the Middle East but had been in close contact with the laboratory‐confirmed or probable cases (http://www.ecdc.europa.eu/en/publications/Publications/MERS-CoV-novel-coronavirus-risk-assessment.pdf). Based on the current situation and available information, WHO encourages all Member States to continue their surveillance for severe acute respiratory infections and to carefully review any unusual patterns, including potential for transmission by transfusion or transplantation. These actions are consistent with those shown in Fig. 3 when little is known about an agent and surveillance remains the most appropriate action. The AABB EID subgroup is also developing a Fact Sheet for MERS CoV.
Once a disease has been characterized as a potential threat to human health, the toolkit in Fig. 3 continues with asking the question of whether (or not) an etiologic agent can be identified. Characterization of the agent is critical to understand the likely risk and actions moving forward; however, even in the absence of the identification of a definitive agent, we continue by asking the question of whether the disease threat is a risk to blood recipient safety. In the example of MERS CoV, the appropriate action at present is surveillance with no further action required to protect recipient safety, but those conditions may change. If a potential threat for recipient safety is identified, further actions would be recommended to assess the severity of the agent or disease threat. Tools are being developed to quantify risk, including research to develop models for quantitative risk assessments: for example, ECDC's EUFRAT tool (http://eufrattool.ecdc.europa.eu/). If the threat is perceived to be real and exceed a critical threshold, then the next phase of action would be triggered, which is development and evaluation of interventions. This scenario developed in 2002 when WNV caused the largest arboviral outbreak in the United States, with 4156 cases of disease according to the US Centers for Disease and Prevention (CDC) including 2946 cases of WNV neuroinvasive disease and 284 deaths (http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2012_CasesAndDeathsClinicalPresentationHumanCases.pdf). Also in 2002, WNV was shown to be transfusion transmitted with 23 cases of recipient infection linked to blood products from WNV‐infected donors.30 Although transfusion‐transmitted cases represent only approximately 5% of the total relative to mosquito‐transmitted cases, the large WNV outbreak, severity of disease, and documentation of transfusion transmission resulted in the rapid development and implementation of WNV RNA screening, which was in place within 8 months of recognition of transfusion transmission. The 2003 WNV season was comparable in size to that of 2002 but increased in geographic reach to the Rockies in the West.31, 32 Through the 2012 WNV transmission season in the United States (now covering nearly the entire United States), there have been 37,088 cases of WNV‐related disease of which 16,196 cases were neuroinvasive disease including 1549 deaths. In addition, 3725 WNV‐positive blood donors have been identified from 2003 to 2012 (according to the CDC through 2006 and since then via the AABB WNV Biovigilance website33). Blood centers first use minipool NAT for such screening, but soon recognized that increased testing sensitivity was needed during outbreak periods and thus convert to individual‐donation NAT,31 which is required to identify approximately 50% of infected donors.33 Before refinements in WNV testing policies from 2003 to 2008, a total of 11 breakthrough cases of WNV transfusion transmissions were recognized from screened blood.33 Subsequently, one transmission in 2010 from untested granulocytes later found to be WNV RNA positive was identified.34 At the time of this writing, one WNV transfusion transmission that occurred during the 2012 season, which was comparable to the large outbreaks in 2002 and 2003, is being investigated.
DENV are flaviviruses related to WNV, and hence one would predict a similar situation with respect to transfusion risk as that of WNV before the implementation of a testing intervention. Unexpectedly, however, relative to the expanse of dengue‐endemic areas and magnitude of annual outbreaks (in 2010, the estimated worldwide burden of dengue was approximately 400 million cases35), only three clusters of transfusion transmissions have been reported.36 Investigational testing has documented rates of DENV RNA positivity in donors of 0.03% to 0.31% in several endemic areas;37 however, few affected countries have implemented DENV RNA testing. In northern Queensland in Australia, another intervention is used that includes discard of RBCs from donors in geographic regions having localized outbreaks, but allows their cocomponent plasma to be used for fractionation;38 a similar policy has been in effect for many years in Australia for donors who have malaria risk.39 Thus, for many arthropod‐borne viruses including DENV and CHIKV, and other agents with varying transmission patterns, the cycle of ongoing surveillance and risk assessment described in Fig. 3 remains in effect.
Conclusions
As we have shown, the risk to public health by EIDs is applicable to considerations of blood safety. Indeed, the success in managing the traditional transfusion‐transmissible infections means that, in the absence of interventions, at least some EID agents (notably WNV31, 32 and Babesia 1) offer significantly more risk to blood recipients than currently exists for the classic transfusion‐transmitted viruses HIV, HCV, or HBV. Nevertheless, not all EID agents represent a threat, and it is important to have an effective approach to assess and manage potential risk. We believe that it is possible to formalize such an approach by examining the properties of EID agents with respect to their transmissibility and quantitative risk, along with the urgency (or otherwise) of need for action. Less tangible is the issue of public perception and fear, which may generate considerations beyond those that are quantifiable. Finally, we wish to point out that the continuing development of rapid viral discovery techniques, while critical to advancing our understanding of the etiologies of disease, also leads to the recognition of many commensal or incidental agents that pose no discernible threat to human health; these should not divert us from our mission to assure recipient safety.
Conflict of Interest
None.
References
- 1. Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KM, Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion 2009;49(Suppl 2):1S‐29S. [DOI] [PubMed] [Google Scholar]
- 2. Dodd RY. Emerging infections and transfusion safety. In: Murphy MF, Pamphilon DH, Heddle NM, editors. Practical transfusion medicine. 4th ed. Chichester: Wiley; 2013. p. 161‐167. [Google Scholar]
- 3. Semenza JC, Domanović D. Commentary; blood supply under threat. Nature Clim Change 2013;3:1‐6. [Google Scholar]
- 4. Woolhouse MEJ, Howey R, Gaunt E, Reilly L, Chase‐Topping M, Saville N. Temporal trends in the discovery of human viruses. Proc Roy Soc B 2008;275:2111‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature 2008;451:990‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eastlund T, Persing D, Mathiesen D, Kim D, Bieging J, McCann P, Heiler G, Raynovic S. Human granulocytic ehrlichiosis after red cell transfusion [abstract]. Transfusion 1999;39(Suppl):117S. [Google Scholar]
- 7. Centers for Disease Control and Prevention . Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. Morb Mortal Wkly Rep MMWR 2008;57:1145‐1148. [PubMed] [Google Scholar]
- 8. Annen K, Friedman K, Eshoa C, Horowitz M, Gottschall J, Straus T. Two cases of transfusion‐transmitted Anaplasma phagocytophilum . Am J Clin Pathol 2012;137:562‐565. [DOI] [PubMed] [Google Scholar]
- 9. Jareb M, Pecaver B, Tomazic J, Muzlovic I, Avsic‐Zupanc T, Premru‐Srsen T, Levicnik‐Stezinar S, Karner P, Strle F. Severe human granulocytic anaplasmosis transmitted by blood transfusion. Emerg Infect Dis 2012;18:1354‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alhumaidan H, Westley B, Esteva C, Berardi V, Young C, Sweeney J. Transfusion‐transmitted anaplasmosis from leukoreduced red blood cells. Transfusion 2013;53:181‐186. [DOI] [PubMed] [Google Scholar]
- 11. Townsend RL, Calhoun RD, Fialkow LB, Berardi V, Stramer SL. Probable transfusion‐transmission of Anaplasma phagocytophilum by leukoreduced platelets [abstract]. Transfusion 2013. 53:A13‐276. [DOI] [PubMed] [Google Scholar]
- 12. Regan J, Matthias J, Green‐Murphy A, Stanek D, Bertholf M, Pritt BS, Sloan LM, Kelly AJ, Singleton J, McQuiston JH, Hocevar SN, Whittle JP. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013;56:e105‐107. [DOI] [PubMed] [Google Scholar]
- 13. Xu C, Wang R, Schecherly C, Ge S‐X, Shih JW‐K, Xia N‐S, Luban N, Alter HJ. An assessment of hepatitis E virus in US blood donors and recipients: no detectable HEV RNA in 1939 donors tested and no evidence of HEV transmission to 362 prospectively followed recipients. Transfusion 2013. 53:2505‐2511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakata H, Matsubayashi K, Ihara H, Sato S, Kato T, Wakisaka A, Tadokoro K, Yu M‐YW, Baylis SA, Ikeda H, Takamoto S. Impact of chemiluminescent enzyme immunoassay screening for human parvovirus B19 antigen in Japanese blood donors. Transfusion 2013. 53:2556‐2566. [DOI] [PubMed] [Google Scholar]
- 15. Maple P, Beard S, Parry R, Brown K. Testing UK blood donors for exposure to human parvovirus 4 using a time‐resolved fluorescence immunoassay to screen sera and Western blot to confirm reactive samples. Transfusion 2013. 53:2575‐2584. [DOI] [PubMed] [Google Scholar]
- 16. Baylis SA, Tuke PW, Miyagawa E, Blümel J. Studies on the inactivation of human parvovirus 4. Transfusion 2013. 53:2585‐2592. [DOI] [PubMed] [Google Scholar]
- 17. Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion 2013. 53:2567‐2574. [DOI] [PubMed] [Google Scholar]
- 18. Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto‐Viguier E, Thervet E, Contiflebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque‐Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140:1481‐1489. [DOI] [PubMed] [Google Scholar]
- 19. Pas SD, de Man RA, Mulders C, Balk AHMM, van Hal PTW, Weimar W, Koopmans MPG, Osterhaus ADME, van der Eijk AA. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis 2012;18:869‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satake M, Hoshi Y, Taira R, Momose S‐Y, Hino S, Tadokoro K. Symptomatic parvovirus B19 infection caused by blood component transfusion. Transfusion 2011;51:1887‐1895. [DOI] [PubMed] [Google Scholar]
- 21. Kleinman SH, Glynn SA, Lee T‐H, Tobler L, Montalvo L, Todd D, Kiss JR, Shyamala V, Busch MP. Prevalence and quantitation of parvovirus B19 levels in blood donors with a sensitive polymerase chain reaction screening assay. Transfusion 2007;47:1756‐1764. [DOI] [PubMed] [Google Scholar]
- 22. Hourfar MK, Mayr‐Wohlfart U, Themann A, Sires W, Seifried E, Schrezenmeier H, Schmidt M. Recipients potentially infected with parvovirus B19 by red blood cell products. Transfusion 2011;51:129‐136. [DOI] [PubMed] [Google Scholar]
- 23. Kleinman SH, Glynn SA, Lee T‐H, Tobler LH, Schlumpf KS, Todd DS, Qiao H, Yu M‐yW, Busch MP. A linked donor‐recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood 2009;114:3677‐3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu M‐yW, Alter HJ, Virata‐Theimer MLA, Geng Y, Ma L, Schechterly CA, Colvin CA, Luban NLC. Parvovirus B19 infection transmitted by transfusion of red blood cells confirmed by molecular analysis of linked donor and recipient samples. Transfusion 2010;50:1712‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charrel RM, de Lamballerie X, Raoult D. Chikungunya outbreaks—the globalization of vectorborne diseases. New Engl J Med 2007;356:769‐771. [DOI] [PubMed] [Google Scholar]
- 26. Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelinio P, Dottori M, Clufolini MG, Majori GC, Cassone A, CHIKV Study Group . Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 2007;370:1840‐1846. [DOI] [PubMed] [Google Scholar]
- 27. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. Plos Pathog 2007;3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasonglès P, Angelini‐Tibert MF, Simon P, Currie C, Isola H, Kientz D, Slaedts M, Jacquet M, Sundin D, Lin L, Corash L, Cazenave JP. Transfusion of platelet components prepared with photochemical pathogen inactivation treatment during a chikungunya virus epidemic in Ile de la Réunion. Transfusion 2009;49:1083‐1091. [DOI] [PubMed] [Google Scholar]
- 29. Zaki AM, van Boheemen S, Bestebroer T, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367:1814‐1820. [DOI] [PubMed] [Google Scholar]
- 30. Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, Goodman JL, Chamberland M; West Nile Virus Transmission Investigation Team . Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med 2003;349:1236‐1245. [DOI] [PubMed] [Google Scholar]
- 31. Dodd RY. Emerging infections, transfusion safety, and epidemiology. N Engl J Med 2003;349:1205‐1206. [DOI] [PubMed] [Google Scholar]
- 32. Petersen LR, Epstein JE. Problem solved? West Nile virus and transfusion safety. N Engl J Med 2005;353:516‐517. [DOI] [PubMed] [Google Scholar]
- 33. Stramer SL, Markowitz MA. AABB Association Bulletin #13‐02—West Nile Virus Nucleic Acid Testing—Revised Recommendations. 2013. [cited 2013 Jun 29]. Available from: URL: http://www.aabb.org/resources/publications/bulletins/Pages/ab13-02.aspx
- 34. Meny GM, Santos‐Zabala L, Szallasi A, Stramer SL. West Nile virus infection transmitted by granulocyte transfusion. Blood 2011;117:5778. [DOI] [PubMed] [Google Scholar]
- 35. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jeanisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature 2013;496:504‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stramer SL, Linnen JM, Carrick JM, Foster GA, Krysztof DE, Zou S, Dodd RY, Tirado‐Marrero LM, Hunsperger E, Santiago GA, Muñoz‐Jordan JL, Tomashek KM. Dengue viremia in blood donors identified by RNA and detection of transfusion transmission during the 2008 dengue outbreak in Puerto Rico. Transfusion 2012;52:1657‐1666. [DOI] [PubMed] [Google Scholar]
- 37. Stramer SL, Foster G, Brodsky JP, Muñoz‐Jordan JL, Hunsperger E, Lanteri M, Añez‐Germán J, Rios M, Cory R, Linnen J. Investigational dengue testing yields high rates of RNA‐positive donors in Puerto Rico [abstract]. Transfusion 2013. 53:A13‐276 . [Google Scholar]
- 38. Faddy HM, Seed CR, Fryk JJ, Hyland CA, Ritchie SA, Taylor CT, van der Merwe KL, Flower RLP, McBride WJH. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis 2013;19:787‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seed CR, Kee G, Wong T, Law M, Ismay S. Assessing the safety of a test‐based, targeted donor screening strategy to minimize transfusion‐transmitted malaria. Vox Sang 2010;98:182‐192. [DOI] [PubMed] [Google Scholar]


