Abstract
Cytomegalovirus (CMV) pneumonitis occurs frequently among solid organ transplant recipients and is classically associated with significant viral replication in both blood and bronchoalveolar lavage (BAL) samples. We present a case of a 64‐year‐old lung transplant recipient who presented with CMV pneumonitis that was diagnosed based on the association of viral inclusion in the BAL sample, rapid response to ganciclovir, and absence of other infectious etiology. Surprisingly, we observed very low or undetectable viral load both in blood and BAL samples. Diagnosis of CMV pneumonitis should rely on the association of clinical, pathological, radiological, and microbiological signs, while quantitative nucleic acid amplification testing should be interpreted with caution.
Keywords: cytomegalovirus, nucleic acid amplification testing, lung transplantation, viral load, polymerase chain reaction, pneumonitis
Cytomegalovirus (CMV) infection is the most common opportunistic infection after lung transplantation, where it provides both direct effects of tissue injury and infection and indirect effects (e.g., acute and chronic allograft injuries, CMV‐induced immunosuppression). Practically, CMV disease is defined as the association of CMV infection (i.e., replication) and attributable symptoms 1.
Diagnosis of tissue‐invasive disease (e.g., CMV pneumonitis) relies on the detection of CMV in the concerned organ. Identification of inclusion bodies and/or viral antigens by immunohistochemistry is a commonly accepted diagnostic method.
Quantitative nucleic acid amplification testing (NAAT) is a highly sensitive tool that is now widely used. As a consequence, CMV tissue‐invasive disease is rarely associated with very low or undetectable viral load (VL) in the blood 2. The so‐called compartmentalization process has mainly been described in gastrointestinal CMV disease and retinitis 2, 3, 4.
We report a case of CMV pneumonitis associated with very low or undetectable VL in both blood and bronchoalveolar lavage (BAL) samples and discuss the limitations of NAAT in such cases.
Case report
We present a case of a 64‐year‐old female lung transplant recipient (LTR) who was admitted in our hospital in July 2015 with fever >38.5°C, cough, dyspnea, and hypoxemia 15 months after transplantation.
The patient underwent bilateral lung transplantation in April 2014 for chronic obstructive pulmonary disease. Her past medical history included a pre‐transplant episode of diverticulitis. The CMV serostatus at the time of transplantation indicated a moderate risk of infection, with both donor and recipient being seropositive. Early CMV prophylaxis included intravenous ganciclovir (GCV) (followed by oral valganciclovir [valGCV]) and CMV‐specific hyperimmune globulins. Initial immunosuppression included anti‐thymocyte globulin, methylprednisolone, cyclosporine, and azathioprine.
In April 2015, the patient was first admitted with fever, diarrhea, and abdominal pain. Immunosuppression consisted of methylprednisolone and tacrolimus. Computed tomography (CT) scan showed pericolic infiltration compatible with a recurrence of diverticulitis. However, repeated antibiotic therapies with amoxicillin/clavulanic acid and piperacillin/tazobactam did not lead to any improvement. Owing to persistence of infectious digestive signs of unclear origin, a laparoscopy was performed and resulted in partial colectomy.
Analysis of the resected colon revealed histological evidence of CMV colitis (i.e., viral inclusions and positive immunohistochemistry) despite low‐level viremia (repeated polymerase chain reaction [PCR] showing < 1500 copies/mL on whole blood samples) using real‐time CMV DNA PCR R‐Gene™ kit (bioMérieux, Brussels, Belgium).
Interestingly, a scheduled post‐transplant check‐up performed just before the colectomy revealed the presence of CMV on a BAL sample (VL 9269 copies/mL), without any sign of pneumonitis on the CT scan, normal transbronchial biopsies, and no cytological evidence of CMV infection on the BAL (direct examination and immunochemistry).
After demonstration of CMV colitis, the patient received a 2‐week course of intravenous GCV 5 mg/kg twice daily, followed by a 1‐month course of oral valGCV 900 mg/day (dose adjusted according to renal function) with a good clinical and biological response.
In July 2015, the patient was readmitted with fever >38.5°C, cough, dyspnea, and hypoxemia. ValGCV had been stopped a week before. Blood analysis showed acute onset of inflammatory syndrome (C‐reactive protein 261 mg/L) and elevated alanine and aspartate aminotransferases (respectively 86 and 69 IU/L). A thoracic CT scan demonstrated bilateral infiltrates (Fig. 1A). Blood cultures remained sterile. A very low CMV VL was observed in blood (<1250 copies/mL). A BAL performed in a lung segment identified by abnormalities on CT scan was inflammatory and lymphocytic (527 cells/mm3, lymphocytes 39%). No bacterial/mycobacterial pathogen or fungal agent was identified, despite the use of direct examination, appropriate cultures, and galactomannan antigen. A CMV PCR was performed on the BAL sample, showing a low VL (665 copies/mL; control 1767 copies/mL), significantly decreased as compared to the analysis performed 3 months earlier, in the absence of clinical and radiological signs of pneumonia. An empirical 5‐day antibiotic therapy with piperacillin/tazobactam was unsuccessful, with persistence of fever, inflammatory syndrome (C‐reactive protein 272 mg/L), and worsening of infiltrates on the CT scan (Fig. 1B).
Figure 1.
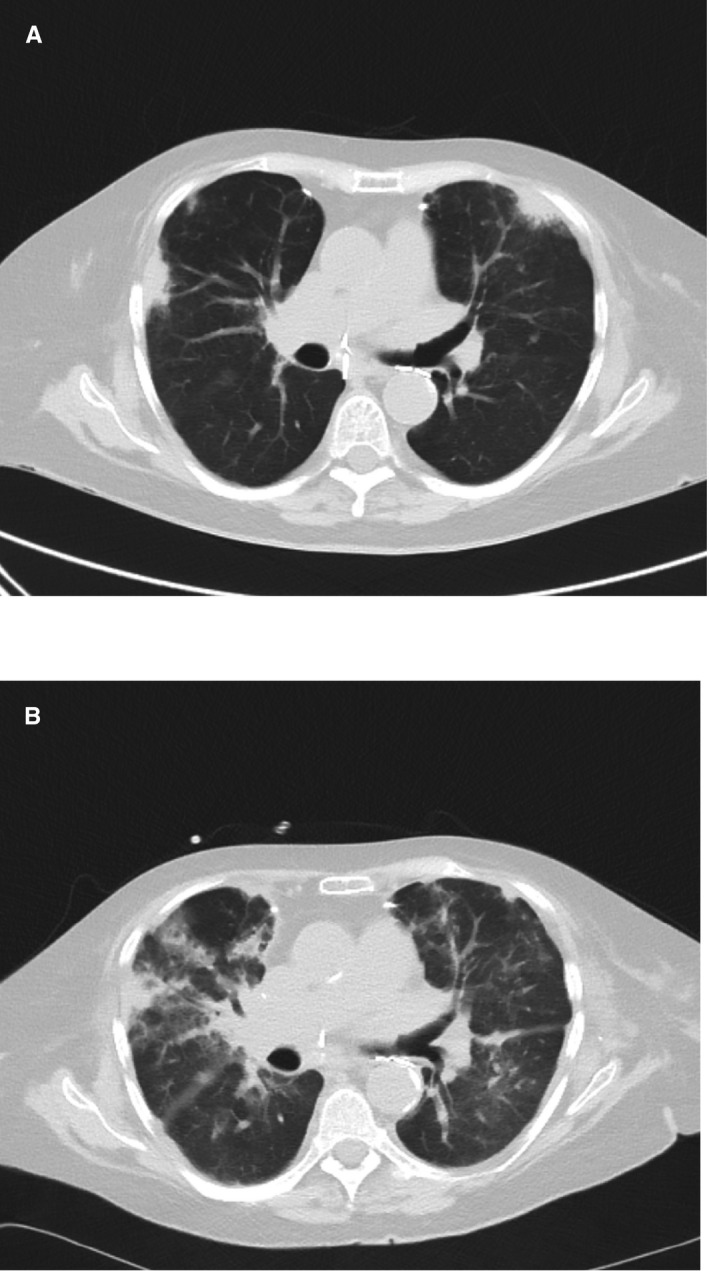
Evolution on computed tomography scans of pulmonary infiltrates (A) at admission and (B) after 5 days of empirical antibiotic therapy with piperacillin/tazobactam.
Pathologist identified, on the BAL sample, a macrophage whose morphologic changes were characteristic of viral infection with CMV (Fig. 2). Despite the very low‐level CMV VL observed both in the blood and in the BAL sample, a CMV pneumonitis was therefore suspected. No therapeutic intervention was added, apart from initiation of anti‐CMV therapy with GCV (5 mg/kg twice daily), leading to resolution of fever, dyspnea, cough, and hypoxemia within 10 days. Biological abnormalities and radiological infiltrates also resolved.
Figure 2.
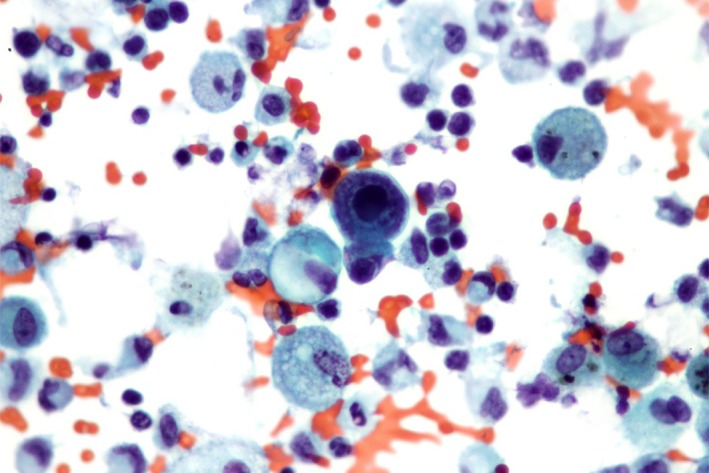
Bronchoalveolar lavage specimen examined after cytocentrifugation (magnification ×40). Besides numerous pulmonary alveolar macrophages and various leukocytes, the specimen revealed a macrophage whose morphologic changes were characteristic of a viral infection with cytomegalovirus. The cell was larger and showed a huge amphophilic intranuclear inclusion with surrounding halo associated to a marked margination of chromatin on the inner surface of the nuclear membrane. The texture of the cytoplasm was enhanced.
A posteriori, the BAL sample was tested with a customized Taqman® Array Card (TAC) real‐time PCR method, targeting 24 viruses, 8 bacteria, and 2 fungi simultaneously 5. The TAC assay included testing for the following pathogens: CMV; herpes simplex viruses (HSV); influenza A virus (H1, H3, H7); influenza B virus; respiratory syncytial virus (RSV) A, RSV B; parainfluenza virus 1–4; adenoviruses; rhinoviruses; enteroviruses (including EV D68); human metapneumovirus; coronaviruses (229E, HKU1, OC43, NL63); bocavirus; parechovirus; mumps virus; measles virus; Mycoplasma pneumoniae; Chlamydophila pneumoniae, Chlamydophila psittaci; Bordetella pertussis, Bordetella parapertussis, Bordetella holmesii/bronchiseptica; Coxiella burnetii; Legionella pneumophila; Pneumocystis jirovecii; and Aspergillus species. In addition to the TAC assay, a single‐plex PCR for 2 other herpesviridae (varicella zoster virus [VZV] and human herpesvirus‐6 [HHV‐6]) was performed. All these analyses were negative (including CMV, HSV, VZV, and HHV‐6), with the exception of the presence of Pneumocystis jirovecii, which was considered to be a colonizer because of the resolution of pneumonitis without anti‐Pneumocystis therapy.
Discussion
CMV pneumonitis is an important event in LTRs. In our patient, the diagnosis of CMV pneumonitis was considered highly probable, given the following features: (a) the presence of compatible signs and symptoms in an LTR with recent history of proven CMV colitis; (b) the absence of any other infectious etiology, despite an extensive workup, including a customized Taqman® Array Card assay; (c) the identification of a viral inclusion body suggestive of CMV infection on the BAL sample; (d) the lack of improvement after broad‐spectrum antibacterial therapy; and (e) a complete clinical and biological response to GCV monotherapy.
While identification of inclusion bodies or viral antigens in biopsies is considered the diagnostic method of choice for CMV tissue‐invasive disease 1, qualitative and quantitative NAAT are widely used after transplantation in blood, tissue, and/or BAL samples as a diagnostic tool 6. Such molecular methods are known to be highly sensitive, but have limited positive predictive value for several reasons. First, detection of CMV is very common in blood as well as in BAL samples in both symptomatic and asymptomatic LTRs 7, 8. Second, a positive NAAT made on a tissue specimen or a BAL sample may either reflect active disease or shedding 1. Third, high VLs are sometimes observed in asymptomatic transplant patients, which underline the imperfect degree of correlation between VLs and occurrence of invasive disease 9, 10, 11.
On the other hand, cases of invasive CMV diseases have sometimes been described with very low to undetectable VL in the blood, mostly in retinal and gastrointestinal diseases 2, 3, 4. This situation is illustrated by the episode of colitis described in our patient. Finally, some authors have described a much better correlation between high VL in BAL samples and CMV pneumonitis 8, 10, 12. All in all, the place of quantitative NAAT in the positive diagnosis of CMV pneumonitis remains unclear.
Our case illustrates that invasive CMV pneumonitis may not only be associated with very low or undetectable blood VL but also with low or undetectable levels in BAL samples. In conclusion, diagnosis of CMV pneumonitis should rely on the association of clinical, pathological, radiological, and microbiological findings, while quantitative NAAT should be interpreted with caution in LTRs.
Acknowledgements
Funding: No funding was received for the study or its publication.
Conflicts of interest: None of the authors has any conflict of interest with regard to the study or its publication.
Coussement J., Steensels D., Nollevaux M.‐C., Bogaerts P., Dumonceaux M., Delaere B., Froidure A.. When polymerase chain reaction does not help: cytomegalovirus pneumonitis associated with very low or undetectable viral load in both blood and bronchoalveolar lavage samples after lung transplantation. Transpl Infect Dis 2016: 18: 284–287. All rights reserved
References
- 1. Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation 2013; 96 (4): 333–360. [DOI] [PubMed] [Google Scholar]
- 2. Razonable RR, Humar A; AST Infectious Diseases Community of Practice . Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13 (Suppl 4): 93–106. [DOI] [PubMed] [Google Scholar]
- 3. Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 2013; 57 (11): 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fica A, Cervera C, Perez N, et al. Immunohistochemically proven cytomegalovirus end‐organ disease in solid organ transplant patients: clinical features and usefulness of conventional diagnostic tests. Transpl Infect Dis 2007; 9 (3): 203–210. [DOI] [PubMed] [Google Scholar]
- 5. Steensels D, Reynders M, Descheemaeker P, et al. Clinical evaluation of a multi‐parameter customized respiratory Taqman® array card compared to conventional methods in immunocompromised patients. J Clin Virol 2015; 72: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev 2013; 26 (4): 703–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manuel O, Kumar D, Moussa G, et al. Lack of association between beta‐herpesvirus infection and bronchiolitis obliterans syndrome in lung transplant recipients in the era of antiviral prophylaxis. Transplantation 2009; 87 (5): 719–725. [DOI] [PubMed] [Google Scholar]
- 8. Schlischewsky E, Fuehner T, Warnecke G, et al. Clinical significance of quantitative cytomegalovirus detection in bronchoalveolar lavage fluid in lung transplant recipients. Transpl Infect Dis 2013; 15 (1): 60–69. [DOI] [PubMed] [Google Scholar]
- 9. Cope AV, Sabin C, Burroughs A, Rolles K, Griffiths PD, Emery VC. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor‐recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J Infect Dis 1997; 176 (6): 1484–1490. [DOI] [PubMed] [Google Scholar]
- 10. Chemaly RF, Yen‐Lieberman B, Castilla EA, et al. Correlation between viral loads of cytomegalovirus in blood and bronchoalveolar lavage specimens from lung transplant recipients determined by histology and immunohistochemistry. J Clin Microbiol 2004; 42 (5): 2168–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Humar A, Gregson D, Caliendo AM, et al. Clinical utility of quantitative cytomegalovirus viral load determination for predicting cytomegalovirus disease in liver transplant recipients. Transplantation 1999; 68 (9): 1305–1311. [DOI] [PubMed] [Google Scholar]
- 12. Chemaly RF, Yen‐Lieberman B, Chapman J, et al. Clinical utility of cytomegalovirus viral load in bronchoalveolar lavage in lung transplant recipients. Am J Transplant 2005; 5 (3): 544–548. [DOI] [PubMed] [Google Scholar]


