Abstract
Objectives
To describe the prevalence of human rhinovirus (RV) species in children hospitalised with pneumonia in Manhiça, Mozambique, and the associations between RV species and demographic, clinical and laboratory features.
Methods
Nasopharyngeal aspirates were collected from children 0 to 10 years of age (n = 277) presenting to Manhiça District Hospital with clinical pneumonia. Blood samples were collected for HIV and malaria testing, blood culture and full blood counts, and a chest X‐ray was performed. A panel of common respiratory viruses was investigated using two independent multiplex RT‐PCR assays with primers specific for each virus and viral type. RV species and genotypes were identified by seminested PCR assays, sequencing and phylogenetic tree analyses.
Results
At least one respiratory virus was identified in 206 (74.4%) children hospitalised with clinical pneumonia. RV was the most common virus identified in both HIV‐infected (17 of 38, 44.7%) and HIV‐uninfected (74 of 237, 31.2%; P = 0.100) children. RV‐A was the most common RV species identified (47 of 275, 17.0%), followed by RV‐C (35/275, 12.6%) and RV‐B (8/275, 2.9%). Clinical presentation of the different RV species was similar and overlapping, with no particular species being associated with specific clinical features.
Conclusions
RV‐A and RV‐C were the most common respiratory viruses identified in children hospitalised with clinical pneumonia in Manhiça. Clinical presentation of RV‐A and RV‐C was similar and overlapping.
Keywords: rhinovirus, pneumonia, Mozambique, children
Abstract
Objectifs
Décrire la prévalence des espèces du rhinovirus (RV) humain chez les enfants hospitalisés pour une pneumonie à Manhiça, au Mozambique, et les associations entre les espèces de RV et les caractéristiques démographiques, cliniques et de laboratoire.
Méthodes
Des prélèvements nasopharyngés ont été recueillis chez des enfants de 0 à 10 ans (n = 277) présentés à l'hôpital de District de Manhiça avec une pneumonie clinique. Des échantillons de sang ont été prélevés pour le dépistage du VIH et du paludisme, la culture du sang et la numération de la formule sanguine, et une radiographie pulmonaire a été réalisée. Un panel des virus respiratoires courants a été investigué en utilisant deux tests indépendants de RT‐PCR multiplex avec des amorces spécifiques pour chaque virus et chaque type viral. Les espèces et génotypes du RV ont été identifiés par des tests de PCR semi‐imbriquées, par le séquençage et par l'analyse des arbres phylogénétiques.
Résultats
Au moins un virus respiratoire a été identifié chez 206 (74,4%) enfants hospitalisés pour une pneumonie clinique. Le RV était le virus le plus couramment identifié à la fois chez les enfants infectés (17/38; 44,7%) et chez ceux non‐infectés (74/237; 31,2%; P = 0,100) par le VIH. Le RV‐A était l'espèce de RV la plus couramment identifiée (47/275; 17,0%), suivie du RV‐C (35/275; 12,6%) et du RV‐B (8/275; 2,9%). La présentation clinique des différentes espèces du RV était semblable et superposée et aucune espèce particulière n’était associée à des caractéristiques cliniques spécifiques.
Conclusions
RV‐A et RV‐C étaient les virus respiratoires les plus couramment identifiés chez les enfants hospitalisés pour une pneumonie clinique à Manhiça. La présentation clinique de RV‐A et RV‐C était similaire et superposée.
Keywords: rhinovirus, pneumonie, Mozambique, enfants
Abstract
Objetivos
Describir la prevalencia de las especies de rinovirus (RV) humano en niños hospitalizados con neumonía en Manhiça, Mozambique; y las asociaciones entre las especies RV y características demográficas, clínicas, y de laboratorio.
Métodos
Se recolectaron aspirados nasofaríngeos de niños con 0‐10 años de edad (n = 277) que se presentaron en el Hospital Distrital de Manhiça con neumonía clínica. Se tomaron muestras de sangre para realizar pruebas para VIH y malaria, hemocultivos y hemogramas completos, y se tomó una placa de tórax. Se investigó la presencia de un panel de virus respiratorios comunes mediante dos ensayos independientes de multiplex RT‐PCR con cebadores específicos para cada virus y tipo de virus. Se identificaron las especies de RV y sus genotipos mediante una PCR semi‐anidada, secuenciación y análisis de árbol filogenético.
Resultados
Se identificó al menos un virus respiratorio en 206 (74.4%) niños hospitalizados con neumonía clínica. El RV fue el virus más comúnmente identificado, tanto en niños infectados con VIH (17/38, 44.7%) como en aquellos sin infectar (74/237, 31.2%; P = 0.100). La especie de RV más común era el RV‐A (47/275, 17.0%), seguida por RV‐C (35/275, 12.6%) y RV‐B (8/275, 2.9%). Las presentaciones clínicas de las diferentes especies de RV eran similares y se solapaban, sin que ninguna especie en particular estuviese asociada con características clínicas específicas.
Conclusiones
RV‐A y RV‐C fueron los virus respiratorios más comúnmente identificados en niños hospitalizados con neumonía clínica en Manhiça. Las presentaciones clínicas de RV‐A y RV‐C eran similares y se solapaba.
Introduction
Acute lower respiratory infections (ALRI) such as pneumonia account for an estimated 1.3 million deaths each year in children under 5 years of age, 43% of which occur in sub‐Saharan Africa 1. Management and prevention efforts against pneumonia in developing countries have traditionally focused on bacterial pathogens. The introduction of effective conjugate vaccines globally has led to decreasing trends in bacterial pneumonia and a subsequent increased interest in the role of virus‐associated ALRI. Recent advances in molecular diagnostics such as PCR have also led to the discovery of new viruses and viral species, highlighting the prominence of viruses in respiratory disease. Respiratory viruses are widely acknowledged to be the most common cause of both upper and lower respiratory tract infections in the developed world 2. However, advanced diagnostics are largely limited in the developing world, and studies including a comprehensive range of viral pathogens are scarce in many African countries. The majority of respiratory viral aetiological studies in Africa have relied on traditional cell culture and serological methods with PCR data only becoming available in recent years. Data beyond respiratory syncytial virus (RSV) are still scarce, and aetiological studies of ALRI in African children published as recently as 2013 did not screen for common respiratory viruses such as RV, coronavirus and bocavirus 3, or were unable to distinguish RV from enterovirus 4.
RV is the most common cause of childhood respiratory infections worldwide and responsible for almost two‐thirds of cases of the common cold 5 and lower respiratory tract infections such as pneumonia and bronchiolitis. The identification of RV‐C as the third RV species, first reported in 2006, led to several investigations on the prevalence of RV species, conducted predominantly in developed countries. The majority of these studies on children hospitalised with ALRI or asthma found that RV‐C was the most prevalent RV species and was often associated with more severe illness 6, 7, 8, 9, 10, 11, 12, 13. While there have been previous reports on the overall prevalence of RV in children with acute respiratory infections or pneumonia in Mozambique, none have specifically investigated RV species 14, 15. In the two Mozambican studies of respiratory viral prevalence, RV was the most commonly identified virus, identified in 26% of children with acute respiratory infections and in 24% with pneumonia. Only three other studies have investigated the prevalence of RV species in African children 16, 17, 18. While these studies confirmed the importance of RV in African children, they were inconclusive with respect to the role of RV species in ALRI in African children.
Much of our understanding on the viral aetiology of childhood pneumonia in Africa is based on studies conducted prior to the HIV epidemic that has engulfed many African countries. Evidence suggests that HIV infection is now driving both the frequency and outcome of pneumonia and that pneumonia is the leading cause of morbidity and mortality in HIV‐infected children 19. A recent study from South Africa reported that a respiratory virus was identified in the majority of both HIV‐infected and HIV‐uninfected children, with RV being the most frequently identified virus 20. No studies have investigated RV species among HIV‐infected children.
Given the limitations of routine microbiology facilities in the majority of African countries, most clinicians rely on examination of clinical features to determine the probable aetiology of ALRI in children. Clinical features, which are often indistinguishable for different respiratory viruses 21, 22, have not been comprehensively investigated in childhood ALRI in Africa. A few studies from Africa and the Middle East found that RV‐C was associated with wheezing 12, 18. The clinical relevance of viral co‐infections is also not well established, and there is conflicting evidence regarding the association between multiple viral identifications and disease severity. Some studies have reported an association between viral co‐infections and disease severity 23, 24, 25, 26, 27, 28, 29, while others have reported no differences in disease severity between single and multiple viral infections 30, 31.
The aim of this study was to describe the prevalence of respiratory viruses, in particular RV and RV species, and the association of RV species with HIV status, clinical features and seasonality in children with clinical pneumonia from Manhiça, Mozambique.
Materials and methods
Study setting and design
This study was conducted by the Manhiça Health Research Centre (Centro de Investigação em Saúde da Manhiça, CISM) at Manhiça District Hospital (MDH), a public hospital in Southern Mozambique. Manhiça district in Southern Mozambique has an estimated population of 143 000. The area has a subtropical climate with two distinct seasons: a warm and rainy season between November and April and a cool and dry season during the rest of the year. The HIV prevalence among newborns has been estimated between 2.9 and 8% 15. A demographic surveillance system (DSS) including 500 km2 surrounding the area, developed by CISM, has been running since 1996 and covers a population of around 92 000 inhabitants. Each individual living within the DSS area is issued a unique permanent identification number, and information on vital events is collected during routine household visits 32. Further characteristics of the DSS and study area are described elsewhere 32.
Children were recruited as part of a larger project aiming to investigate the underlying aetiology of children with respiratory symptoms. Between September 2010 and April 2013, 277 children 0–10 years of age presenting to the Manhiça District Hospital with fever (or a history of fever (>37.5 °C axillary temperature) in the preceding 24 h) and clinical pneumonia according to the WHO definition (increased respiratory rate and cough and/or difficulty in breathing), and sick enough to warrant hospital admission, were recruited into the study. Increased respiratory rate was defined according to Integrated Management of Childhood (IMCI) guidelines: ≥60 breaths per minute in children ≤2 months, ≥50 in 2–12 months, ≥40 in 1–5 years and ≥30 in 5–10 years. Exclusion criteria included prior enrolment in this study, use of antibiotics or antimalarial drugs during the preceding 2 weeks, history of cough for more than 2 weeks duration, active tuberculosis or history of direct contact with a documented tuberculosis case, and children with an oxyhaemoglobin saturation of less than 85% on examination on admission, as a proxy for possible Pneumocystis jirovecii infection.
Clinical and questionnaire data and samples were obtained from the enrolled cases on the day of recruitment. An NPA was collected from each child by a trained study health assistant. HIV testing was routinely conducted, and all patients underwent chest X‐ray and extensive clinical and laboratorial screening, including malaria testing, blood culture and full blood counts.
Written informed consent was obtained from parents or guardians prior to participation, and the study was approved by the University of Western Australia Human Research Ethics Committee, the Ethics Committee of the Hospital Clínic (Barcelona, Spain) and the Comité Nacional de Bioética para a Saúde (Maputo, Mozambique), prior to commencement.
Laboratory methods
Virus detection
NPAs were stored at −80 °C in Manhiça, Mozambique, until shipped to the study laboratories, on dry ice, for processing. Identification of common respiratory viruses (adenovirus, RSV, bocavirus, coronavirus, parainfluenza viruses, influenza viruses and metapneumovirus) was carried out using two independent multiplex RT‐PCR assays with primers specific for each virus and viral type. RV identification and genotyping were based on a published molecular method to determine RV genotypes and to differentiate closely related enteroviruses from RV 33. Viral RNA was first extracted from a 240 μl volume of NPAs using the QIAGEN QIAamp Viral RNA Mini Kit (Spin protocol) and reverse‐transcribed to cDNA. This was used for the PCR amplification of a 260‐bp variable sequence in the 5′ non‐coding region of the RV genome using in‐house designed primers. PCR products were then sequenced commercially by the Australian Genome Research Facility. Genotypes were assigned based on comparisons of the 5′ non‐coding region sequences with those of 101 classical serotypes as well as 52 newly identified genotypes using ClustalX software (Conway Institute, University College Dublin, Dublin, Ireland). Representative samples of each genotype have previously been sequenced at the VP4‐VP2 coding region to confirm the species assignment 34, 35.
HIV‐specific procedures
Recruited study children were referred for HIV counselling and testing, which required, for study purposes, an additional parental consent. HIV‐1 serodiagnosis was performed using a sequential testing algorithm with two rapid HIV‐1 antibody tests (Determine® and Unigold®). HIV infection was confirmed when necessary by an HIV‐1 DNA Amplicor test (version 1.5; Roche Molecular Systems, Inc., Branchburg, NJ, USA). Children identified as HIV positive were followed up according to national guidelines.
Statistical analyses
Demographic and clinical features (categorical variables) associated with viral identification were examined using chi‐squared (χ2) or Fisher's exact tests. Continuous variables were analysed using variance (anova) models (adjusting for age) and presented as means with standard deviation. Variables that were not normally distributed were logarithm‐transformed and presented as means. Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA), and a P‐value <0.05 was considered statistically significant.
Results
Population demographics
Two hundred and seventy‐seven cases (51.6% male) enrolled between September 2010 and April 2013 were included in this analysis. The mean age of the study population was 20.7 months. Thirty‐eight (13.7%) children were HIV infected. HIV‐infected children were older (mean age 27.8 months, 95% CI: 19.4–36.2) than HIV‐uninfected children (mean age 19.5 months, 95% CI: 17.0–22.0; P = 0.021). There was no difference in gender between HIV‐infected and HIV‐uninfected children. Of the 277 cases with WHO‐defined clinical pneumonia, 31 (11.2%) had a co‐infection with malaria. A total of 22 of 277 (7.9%) cases had a positive blood culture, with pneumococcus, Haemophilus influenzae type b and non‐typhoidal Salmonella being the three major causes of invasive bacterial disease. Chest X‐rays showed alveolar condensations compatible with bacterial pneumonia (WHO ‘primary end‐point pneumonia’) 36.
Respiratory viral identification
NPAs from 277 children were available for identification of respiratory viruses. At least one respiratory virus was identified in 206 (74.4%) children. Of the 206 children with at least one respiratory virus identified, 139 (67.5%) had a single virus infection, while co‐infection of 2, 3 or 4 viruses was identified in 51 (24.6%), 13 (6.3%) and 3 (1.5%) children, respectively. RV was the most common respiratory virus, identified in 92 (33.2%) children, followed by adenovirus (19.1%) and RSV (15.5%; Figure 1). RSV‐positive children (mean age 8.9 months, SD 3.0) were younger than RSV‐negative children (mean age 13.4 months, SD 2.9 P = 0.022). Adenovirus‐positive children (mean age 18.6 months, SD 2.6) were older than adenovirus‐negative children (mean age 11.5 months, SD 3.0). There were no other age or gender differences between children with and without any particular virus. Adjusting for age effects, there were no significant differences in the frequency of any viral pathogen between HIV‐infected and HIV‐uninfected children (Figure 1).
Figure 1.
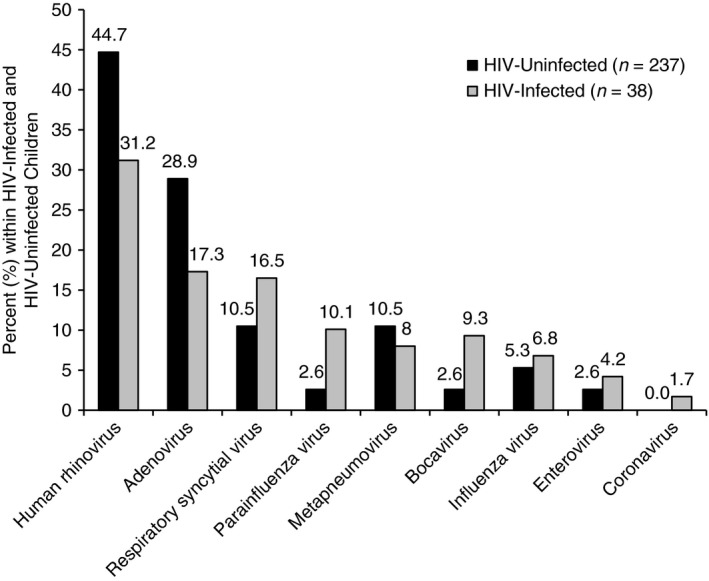
Respiratory viruses identified in nasopharyngeal aspirates of HIV‐infected and HIV‐uninfected children (n = 275). Two cases with unknown HIV status excluded.
Clinical features in children with each respiratory virus identified are shown in Table S1. There were no differences between demographic characteristics, past morbidities and comorbidities or current hospitalisation (Table 1), clinical features or laboratory and microbiology findings (Table 2) between RV‐positive and RV‐negative children. Of all respiratory viruses, RSV was positively associated with the most number of clinical features including wheeze, oxygen saturation, lower chest wall indrawing, nasal flare and deep breathing.
Table 1.
Demographic characteristics, past morbidity and comorbidity and current hospitalisation of human rhinovirus (RV)‐positive and RV‐negative children
| RV positive (n = 92) | RV negative (n = 185) | P‐value | |
|---|---|---|---|
| Demographic | |||
| Age in months: mean (SD) | 21.2 (22.1) | 20.6 (20.1) | 0.837 |
| Age <12 months: n (%) | 40 (43.5) | 82 (44.3) | 0.894 |
| Gender, male: n (%) | 47 (51.1) | 87 (47.0) | 0.524 |
| Past morbidity and comorbidity | |||
| HIV infectiona: n (%) | 17 (18.5) | 21 (11.4) | 0.100 |
| Previous admission for pneumonia: n (%) | 5 (5.4) | 4 (2.2) | 0.305 |
| Current hospitalisation and co‐infections | |||
| Length of admission (days): mean (SD) | 4.06 (2.65) | 4.49 (3.13) | 0.260 |
| Co‐infection – malaria: n (%) | 10 (10.9) | 21 (11.4) | 0.905 |
HIV status unknown from two cases.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Clinical features and laboratory and microbiology findings of human rhinovirus (RV)‐positive and RV‐negative children hospitalised with clinical pneumonia
| Clinical features | RV positive (n = 92) | RV negative (n = 185) | P‐value |
|---|---|---|---|
| Respiratory signs and symptoms | |||
| Fever on admission: n (%) | 91 (98.9) | 184 (99.5) | 0.613 |
| Hyperpyrexia (temperature > 39 °C): n (%) | 20 (22.0) | 59 (32.1) | 0.082 |
| Axillary temperature (°C): mean (SD) | 37.9 (1.2) | 38.4 (1.1) | 0.002 |
| Respiratory rate: median (IQR) | 58.5 (56.1–60.9) | 57.5 (55.7–59.3) | 0.490 |
| Oxygen saturation: mean (SD) | 96.1 (2.5) | 96.1 (2.4) | 0.988 |
| Hypoxemia (Sat02 < 90%): n (%) | 1 (1.1) | 7 (3.9) | 0.202 |
| Cyanosis: n (%) | 4 (4.3) | 5 (2.7) | 0.671 |
| Lower chest wall indrawing: n (%) | 60 (65.2) | 117 (62.3) | 0.818 |
| Nasal flaring: n (%) | 51 (55.4) | 83 (44.9) | 0.205 |
| Rhinorrhoea: n (%) | 23 (25.0) | 51 (27.6) | 0.802 |
| Grunting: n (%) | 14 (15.2) | 23 (12.4) | 0.708 |
| Rhonchi: n (%) | 31 (33.7) | 58 (31.4) | 0.804 |
| Wheezing: n (%) | 22 (23.9) | 29 (15.7) | 0.212 |
| Prolonged expiration: n (%) | 9 (9.8) | 9 (4.9) | 0.254 |
| Crackles: n (%) | 55 (59.8) | 105 (56.8) | 0.763 |
| Digital Clubbing: n (%) | 1 (1.1) | 1 (0.5) | 0.773 |
| Hepatomegaly: n (%) | 2 (2.2) | 6 (3.2) | 0.779 |
| Splenomegaly: n (%) | 6 (6.5) | 11 (5.9) | 0.863 |
| Pallor: n (%) | 7 (7.6) | 11 (5.9) | 0.569 |
| Nutritional Status | |||
| Height (cm): mean (SD) | 75.6 (16.0) | 75.4 (16.4) | 0.933 |
| Weight (kg): mean (SD) | 9.17 (3.99) | 9.23 (3.95) | 0.904 |
| Malnutrition: n (%) | 10 (10.9) | 20 (10.8) | 0.879 |
| Severe malnutrition (WAZ<−3DS): n (%) | 19 (20.7) | 34 (18.4) | 0.901 |
| Weight for age Z score (WAZ): mean (SD) | −1.64 (1.70) | −1.60 (2.03) | 0.835 |
| Laboratory and microbiology findings | |||
| X‐ray primary end‐point pneumonia: n (%) | 25 (27.2) | 48 (25.9) | 0.827 |
| White blood cell count (103/μl): mean (SD) | 17.4 (11.0) | 16.3 (9.5) | 0.382 |
| Abnormal WBC (<5 or >20): n (%) | 27 (30.7) | 40 (22.3) | 0.140 |
| Red blood cell count (106/μl): mean 106/μl (SD) | 3.72 (0.93) | 3.81 (1.07) | 0.535 |
| Haemoglobin (g/l): mean (SD) | 8.81 (2.36) | 8.84 (2.12) | 0.913 |
| Haematocrit (g/l): mean (SD) | 26.3 (6.35) | 27.0 (7.55) | 0.445 |
| Moderate or severe anaemia (Haematocrit <25 g/l): n (%) | 34 (38.6) | 64 (36.2) | 0.694 |
| Plasmodium density (parasites/μl): mean (SD) |
3.11 × 104 (9.87 × 104) |
2.32 × 104 (8.00 × 104) |
0.481 |
| Lymphocyte count (103/μl): mean (SD) | 5.47 (2.77) | 5.60 (3.20) | 0.765 |
| Monocyte count (103/μl): mean (SD) | 1.82 (1.34) | 1.80 (1.43) | 0.904 |
| Eosinophil count (103/μl): mean (SD) | 0.273 (0.562) | 0.175 (0.288) | 0.083 |
| Basophil count (103/μl): mean (SD) | 0.07 (0.06) | 0.06 (0.07) | 0.402 |
| Bacteraemia | |||
| All cause: n (%) | 5 (0.05) | 17 (0.09) | 0.742 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
RV species and genotypes
Ninety RV‐positive NPAs were successfully genotyped, of which 47 (52.2%) were RV‐A, 8 (8.9%) were RV‐B and 35 (38.9%) were RV‐C, respectively. Of the 47 RV‐A specimens, 45 were assigned to one of 24 known genotypes, while two specimens could not be assigned as the sequences were equally related to two genotypes. Of the eight RV‐B specimens, seven were assigned to one of four genotypes, while one was not assigned. All 35 RV‐C specimens were assigned to one of 16 genotypes. No single genotype was identified more than five times in this population, with only one RV‐A genotype and one RV‐C genotype being identified five times. There was no difference in the distribution of RV species between HIV‐infected and HIV‐uninfected children (P = 0.765).
There were no differences between demographic characteristics, past morbidities and comorbidities or current hospitalisation (Table 3), clinical features or laboratory and microbiology findings (Table 4) between RV‐A and RV‐C. RV‐B was excluded from analyses due to low numbers.
Table 3.
Demographic characteristics, past morbidity and comorbidity and current hospitalisation of human rhinovirus (RV)‐A and RV‐C positive children
| RV‐A (n = 47) | RV‐C (n = 35) | P‐value | |
|---|---|---|---|
| Demographic | |||
| Age in months: mean (SD) | 18.5 (20.4) | 22.8 (24.5) | 0.391 |
| Age <12 months: n (%) | 23 (48.9) | 15 (42.9) | 0.585 |
| Gender, male: n (%) | 19 (40.4) | 19 (54.3) | 0.213 |
| Past morbidity and comorbidity | |||
| HIV infectiona: n (%) | 8 (17.0)a | 7 (20.0) | 0.765 |
| Previous admission for pneumonia: n (%) | 5 (10.6) | 0 (0.0) | 0.090 |
| Current hospitalisation and co‐infections | |||
| Length of admission (days): mean (SD) | 4.47 (3.09) | 3.88 (1.95) | 0.324 |
| Co‐infection – malaria: n (%) | 6 (12.8) | 3 (8.6) | 0.548 |
HIV status unknown from one case.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 4.
Clinical features and laboratory and microbiology findings of human rhinovirus (RV)‐A and RV‐C‐positive children hospitalised with clinical pneumonia
| Clinical features | RV‐A (n = 47) (%) | RV‐C (n = 35) (%) | P‐value |
|---|---|---|---|
| Respiratory signs and symptoms | |||
| Fever on admission: n (%) | 46 (97.9) | 35 (100.0) | 0.385 |
| Hyperpyrexia (temperature > 39 °C): n (%) | 9 (19.6) | 7 (20.0) | 0.961 |
| Axillary temperature (°C): mean (SD) | 37.7 (1.0) | 37.9 (1.0) | 0.467 |
| Respiratory rate: median (IQR) | 58.9 (52.0–65.0) | 56.0 (52.0–74.0) | 0.405 |
| Oxygen saturation: mean (SD) | 95.7 (1.0) | 96.4 (1.0) | 0.214 |
| Hypoxemia (Sat02 < 90%): n (%) | 1 (2.2) | 0 (0.0) | 0.380 |
| Cyanosis: n (%) | 2 (4.3) | 1 (2.9) | 0.645 |
| Lower chest wall indrawing: n (%) | 32 (68.1) | 24 (68.6) | 0.683 |
| Nasal flaring: n (%) | 28 (59.6) | 21 (60.0) | 0.684 |
| Rhinorrhoea: n (%) | 14 (29.8) | 8 (22.9) | 0.514 |
| Grunting: n (%) | 9 (19.1) | 4 (11.4) | 0.420 |
| Rhonchi: n (%) | 16 (34.0) | 13 (37.1) | 0.670 |
| Wheezing: n (%) | 13 (27.7) | 7 (20.0) | 0.476 |
| Prolonged expiration: n (%) | 2 (4.3) | 4 (11.4) | 0.331 |
| Crackles: n (%) | 28 (59.6) | 24 (68.6) | 0.530 |
| Digital Clubbing: n (%) | 0 (0.0) | 1 (2.9) | 0.352 |
| Hepatomegaly: n (%) | 0 (0.0) | 2 (5.7) | 0.178 |
| Splenomegaly: n (%) | 2 (4.3) | 3 (8.6) | 0.505 |
| Pallor: n (%) | 6 (12.8) | 1 (2.9) | 0.279 |
| Nutritional status | |||
| Height (cm): mean (SD) | 73.5 (15.6) | 76.1 (15.7) | 0.417 |
| Weight (kg): mean (SD) | 8.00 (1.5) | 8.58 (1.5) | 0.426 |
| Malnutrition: n (%) | 4 (8.5) | 4 (11.4) | 0.631 |
| Severe malnutrition (WAZ<‐3DS): n (%) | 9 (19.1) | 8 (22.9) | 0.682 |
| Weight for age Z score (WAZ): mean (SD) | −1.62 (1.87) | −1.78 (1.44) | 0.604 |
| Laboratory and microbiology findings | |||
| X‐ray primary end‐point pneumonia: n (%) | 15 (31.9) | 8 (22.9) | 0.366 |
| White blood cell count (103/μl): mean (SD) | 14.16 (1.67) | 17.00 (1.75) | 0.137 |
| Abnormal WBC (<5 or >20): n (%) | 15 (33.3) | 10 (29.4) | 0.920 |
| Red blood cell count (106/μl): mean 106/μl (SD) | 3.81 (0.93) | 3.72 (0.89) | 0.717 |
| Haemoglobin (g/l): mean (SD) | 8.86 (2.44) | 8.97 (2.19) | 0.738 |
| Haematocrit (g/l): mean (SD) | 26.0 (1.3) | 25.6 (1.3) | 0.794 |
| Moderate or severe anaemia (Haematocrit <25 g/l): n (%) | 16 (36.4) | 13 (38.2) | 0.865 |
| Plasmodium density (parasites/μl): mean (SD) | 3.71 × 104 (5.4) | 15.7 × 104 (3.4) | 0.103 |
| Lymphocyte count (103/μl): mean (SD) | 5.12 (1.7) | 4.74 (1.6) | 0.543 |
| Monocyte count (103/μl): mean (SD) | 1.27 (2.9) | 1.40 (2.8) | 0.705 |
| Eosinophil count (103/μl): mean (SD) | 0.10 (2.9) | 0.17 (3.5) | 0.077 |
| Basophil count (103/μl): mean (SD) | 0.05 (2.3) | 0.06 (2.2) | 0.716 |
| Bacteraemia | |||
| All cause: n (%) | 2 (4.3) | 3 (8.6) | 0.492 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
RV co‐infections with other respiratory viruses
Of the 92 RV‐positive children, 35 (38%) had co‐infections with one other respiratory virus, 11 had co‐infections with two other respiratory viruses and three had co‐infections with three other respiratory viruses. The most common RV co‐infections were with adenovirus (21.7%) and RSV (15.2%). There were no differences in clinical features between children with a single virus identification compared with children who had multiple viruses identified. RV‐C‐infected children were more likely to have co‐infection with metapneumovirus than RV‐A‐ or RV‐B‐infected children (20.0% in RV‐C vs. 4.3% in RV‐A and 0% in RV‐B; (P = 0.039)). There were no other differences in RV species and viral co‐infections (data not shown).
Seasonality
One hundred and eighty‐four (66.4%) children were recruited during the warm and wet season (November–April) and 93 (33.6%) during the cool and dry season (May–October). The monthly distribution of RV species in comparison with RSV is shown in Figure 2. Overall, RV showed seasonal variation (P = 0.023) and was less frequent from June to August (with the exception of May). RSV showed strong seasonality (P < 0.001), being most prevalent between January and May with 70% of all RSV identifications occurring during February and March. When we classified the months into two seasons, children were more likely to be RSV positive during the warm and wet than cool and dry season (20.7% vs. 5.4%; P < 0.01) and more likely to be RV‐C positive (18.5% vs. 9.8%, P = 0.042) and enterovirus positive (9.7% vs. 1.1%, P < 0.01) during the cool than the warm season. There were no other significant seasonal or monthly patterns for any other respiratory virus.
Figure 2.
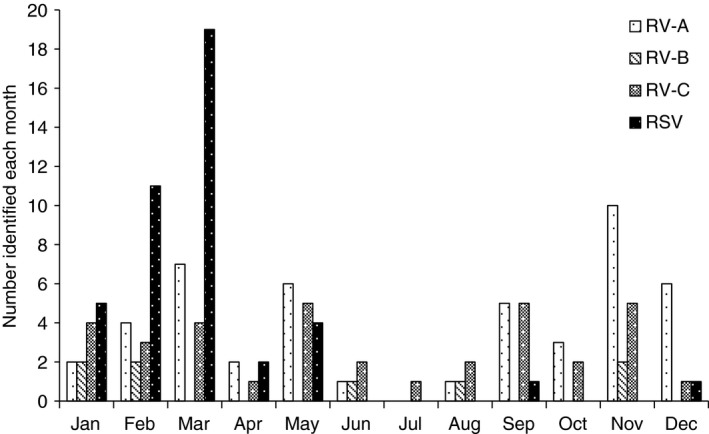
Human rhinovirus (RV) species and respiratory syncytial virus (RSV) identified in ALRI cases each month.
Discussion
Consistent with previous reports from Mozambique, RV was the most common respiratory virus identified in Mozambican children from Manhiça with clinical pneumonia 14, 15. RV‐A was the most commonly identified RV species followed by RV‐C and RV‐B. Only three studies have investigated RV species in children with respiratory illness in Africa. Consistent with our findings, two studies of children with ALRI from Kenya and Burundi reported that RV‐A was the most common species identified, followed by RV‐C and RV‐B 17, 18. In contrast, a study from South Africa investigated acute wheezing illness in young children 16 and reported RV‐C as the most common RV species. Other studies of children hospitalised with asthma or wheezing, predominantly from developed countries, have also found RV‐C to be the most frequently identified species 10, 11, 12, 37. Several RV genotypes from each species were identified suggesting that a large number of genotypes are circulating in the community and that no single RV genotype predominates at any given time. Hence, our findings support the majority of African studies that report RV‐A to be more common than RV‐C in children with ALRI.
A respiratory virus was identified in almost three‐quarters of children hospitalised with clinical pneumonia, which is higher than previous results from Mozambique 14, 15 but comparable with results from other similar settings 38, 39. We also identified higher prevalence of viral co‐infections (24.2%) than the previous reports from Mozambique which may be due to screening with a larger panel of respiratory viruses as well as more sensitive molecular techniques 14, 15. However, comparable rates have been reported in paediatric populations outside Mozambique 40. Given the high rate of viral identifications in children hospitalised with ALRI, our findings support current literature on the importance of respiratory viruses in the pathogenesis of ALRI. However, like the majority of viral aetiology investigations, our study was limited by the use of NPAs to identify respiratory viruses, which may not be entirely representative of respiratory viruses in the lower airway. Further investigations using lower airway samples as well as the inclusion of a contemporaneous control group may facilitate better understanding of the role of viruses in clinical pneumonia.
This is the first study to describe the prevalence of RV species among HIV‐infected and HIV‐uninfected children in Mozambique with WHO‐defined clinical pneumonia. Thirty‐eight (13.7%) cases were HIV infected, of which 26 (68.4%) had at least one respiratory virus. Contrary to our hypothesis, respiratory virus (including RV species) identification was not more common among HIV‐infected than HIV‐uninfected children. However, previous studies from Africa have reported increased viral identification rates among HIV‐infected children 15, 41. Madhi et al. reported that viral identification among HIV‐infected children varied according to respiratory virus and was lowest for RSV 41. There are limited viral data on HIV‐infected children with ALRI, and, like ours, most of these studies were limited to relatively small sample sizes and were unable to draw conclusions about the role of HIV. Further investigations including a larger HIV‐positive cohort are needed.
Overall, we did not find RV‐A or RV‐C to be associated with any particular clinical feature. Other studies have reported that overall, RV identification in children with ALRI was associated with unique clinical characteristics such as wheeze 42 and atopic dermatitis 43. We also did not observe any differences in associated clinical features between the three RV species. However, this could be due to the small numbers within each RV species, particularly in the RV‐B group, or to our study population representing a moderate‐to‐severe subset of all respiratory infections. In concordance with our findings, Luchsinger et al. 44 did not observe any differences between RV species according to clinical features or severity of illness in infants in Chile with ALRI. We also investigated associations between respiratory viruses and clinical features. Of all other respiratory viruses, RSV was positively associated with the most number of clinical features including wheeze, oxygen saturation, lower chest wall indrawing, nasal flare and deep breathing. Although not significant, we observed that children identified only with RSV were slightly younger than those with RV (15.4 months vs. 22.6 months; P = 0.120), which may partly explain differences in clinical severity observed. Similarly, other studies have also reported that RSV was associated with more severe disease than RV 44, 45. However, these studies investigated children with bronchiolitis rather than pneumonia and may also be confounded by age.
Two‐thirds of children were recruited during the warm and wet months. This is consistent with previous studies from Mozambique that reported an increase in the number of outpatient visits associated with malaria 46 and lower respiratory infection 47 during the rainy months of the year. Furthermore, O'Callaghan et al. 14 showed that viral respiratory infections contributed to the high burden of hospital visits during these months RV was prevalent throughout the year, with no significant differences between the seasons. Although we observed slightly lower RV prevalence during the cool and dry season, particularly from June to August, this was not significant and possibly due to population size. Our findings are supported by previous investigations of RV species in Africa, which found no seasonality patterns for RV or RV species identification 3, 17. In contrast to RV, RSV did show clear seasonal patterns and was most prevalent during the warm and wet season. This finding has been supported by previous studies from Mozambique 48 and Ghana 3 that reported RSV epidemics during the rainy season. In contrast, other studies from Burkina Faso, 49 Senegal 50 and South Africa 48 found RSV infections peaked in the dry season.
The main strengths of this study are the long recruitment period (over two and a half years) and the inclusion of both HIV‐infected and HIV‐uninfected children. This study also has a few limitations. Firstly, we identified respiratory viruses in NPAs. Although nasopharyngeal identification of viruses has been associated with lower respiratory tract infections, it also occurs among healthy, asymptomatic individuals. Because the mechanisms that lead to lower respiratory infection remain poorly understood, viral identification in the upper airway may not be entirely representative of that of the lower airway. Furthermore, as we are unable to differentiate asymptomatic infection from clinical (symptomatic) infection using molecular methods of detection, a virus‐positive NPA suggests but does not prove causation.
Secondly, our study population is comprised of a group of moderate–severe ALRI cases admitted to a hospital and fulfilling a strict pre‐defined set of clinical criteria for pneumonia as defined by WHO guidelines. Hence, our findings are not necessarily representative of the overall ALRI population and do not include children with isolated symptoms such as wheeze. Thirdly, our study did not include a contemporaneous control group to compare the prevalence of respiratory viruses between sick and healthy children. A useful control group may include children from the community without respiratory illness as well as children with an upper respiratory illness not severe enough to present to hospital. Nonetheless, this study provides important data on the prevalence of RV species in children with WHO‐defined clinical pneumonia in Mozambique.
Supporting information
Table S1. Clinical features of children hospitalized with clinical pneumonia according to virus identified.
Acknowledgements
The authors would like to thank all the children and families who agreed to take part in the study. This study resulted from the collaborative work of groups from the School of Paediatrics and Child Health, University of Western Australia, the Manhiça Health Research Centre and the Barcelona Institute for Global Health (ISGlobal). This study was funded by grants from the Bill and Melinda Gates Foundation, National Health and Medical Research Council grant and Asthma Foundation Western Australia.
References
- 1. Walker CLF, Rudan I, Liu L et al Global burden of childhood pneumonia and diarrhoea. Lancet 2013: 381: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 2008: 2008: 716–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwofie T, Anane Y, Nkrumah B, Annan A, Nguah S, Owusu M. Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol J 2012: 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feikin DR, Njenga MK, Bigogo G et al Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007–2010. Pediatr Infect Dis J 2013: 32: 14–19. [DOI] [PubMed] [Google Scholar]
- 5. Douglas RG. Pathogenesis of rhinovirus common colds in human volunteers. Ann Otolaryngol 1970: 79: 563–571. [DOI] [PubMed] [Google Scholar]
- 6. Lau SKP, Yip CCY, Lin AWC et al Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis 2009: 200: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamson D, Renwick N, Kapoor V et al MassTag polymerase‐chain‐reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza‐ like illness in New York State during 2004‐2005. J Infect Dis 2006: 194: 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 2006: 78: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renwick N, Schweiger B, Kapoor V et al A recently identified rhinovirus genotype is associated with severe respiratory‐tract infection in children in Germany. J Infect Dis 2007: 196: 1745–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bizzintino JA, Lee WM, Laing IA et al Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 2011: 37: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller EK, Edwards KM, Weinberg GA et al A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immun 2009: 123: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller EK, Khuri‐Bulos N, Williams JV et al Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Microbiol 2009: 46: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linsuwanon P, Payungporn S, Samransamruajkit R et al High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infection 2009: 59: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Callaghan‐Gordo C, Díez‐Padrisa N, Abacassamo F et al Viral acute respiratory infections among infants visited in a rural hospital of southern Mozambique. Trop Med Int Health 2011: 16: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 15. O'Callaghan‐Gordo C, Bassat Q, Morais L et al Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J 2011: 30: 39–44. [DOI] [PubMed] [Google Scholar]
- 16. Smuts H, Workman L, Zar H. Human rhinovirus infection in young African children with acute wheezing. BMC Infect Dis 2011: 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onyango CO, Welch SR, Munywoki PK et al Molecular epidemiology of human rhinovirus infections in Kilifi, coastal Kenya. J Med Virol 2012: 84: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esposito S, Daleno C, Baggi E et al Circulation of different rhinovirus groups among children with lower respiratory tract infection in Kiremba, Burundi. Euro J Clin Microbiol 2012: 31: 3251–3256. [DOI] [PubMed] [Google Scholar]
- 19. Graham SM. HIV and respiratory infections in children. Curr Opin Pulm Med 2003: 9: 215–220. [DOI] [PubMed] [Google Scholar]
- 20. Nunes MC, Kuschner Z, Rabede Z et al Clinical epidemiology of bocavirus, rhinovirus, two polyomaviruses and four coronaviruses in HIV‐infected and HIV‐uninfected South African children. PLoS One 2014: 9: e86448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011: 377: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams O, Weis J, Jasinska K, Vogel M, Tenenbaum T. Comparison of human metapneumovirus, respiratory syncytial virus and Rhinovirus respiratory tract infections in young children admitted to hospital. J Med Virol 2014: 87: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richard N, Komurian‐Pradel F, Javouhey E et al The impact of dual viral infection in infants admitted to pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J 2007: 27: 213–217. [DOI] [PubMed] [Google Scholar]
- 24. Semple MG, Cowell A, Dove W et al Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 2005: 2005: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calvo C, García‐García ML, Blanco C et al Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol 2008: 42: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rhedin S, Hamrin J, Naucler P et al Respiratory Viruses in Hospitalized Children with Influenza‐Like Illness during the H1n1 2009 Pandemic in Sweden. PLoS One 2012: 7: e51491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Echenique IA, Chan PA, Chapin KC, Andrea SB, Fava JL, Mermel LA. Clinical characteristics and outcomes in hospitalized patients with respiratory viral co‐infection during the 2009 H1N1 influenza pandemic. PLoS One 2013: 8: e60845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marcone DN, Ellis A, Videla C et al Viral etiology of acute respiratory infections in hospitalized and outpatient children in Buenos, Aires, Argentina. Pediatr Infect Dis J 2013: 32: e105–e110. [DOI] [PubMed] [Google Scholar]
- 29. Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol Infect 2014: 1: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schnepf N, Resche‐Rigon M, Chaillon A et al High burden of non‐influenza viruses in influenza‐like illness in the early weeks of H1N1v epidemic in France. PLoS One 2011: 6: e23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bicer S, Giray T, Col D et al Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr 2013: 39: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alonso PL, Saúte F, Aponte JJ et al Manhiça DSS, Mozambique. Population and Health in Developing Countries. International Development Research Centre: Ottawa; 2002. [Google Scholar]
- 33. Lee W, Kiesner C, Pappas T et al A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illness in infants. PLoS One 2007: 2: e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiang D, Kalra I, Yagi S et al Assay for 5′ noncoding region analysis of all human rhinovirus prototype strains. J Clin Microbiol 2008: 46: 3736–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee W‐M, Lemanske RF, Evans MD et al Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 2012: 186: 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cherian T, Mulholland EK, Carlin JB et al Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005: 83: 353–359. [PMC free article] [PubMed] [Google Scholar]
- 37. Cox DW, Bizzintino J, Ferrari G et al Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med 2013: 188: 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jroundi I, Mahraoui C, Benmessaoud R et al The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J Trop Pediatr 2014: 60: 270–278. [DOI] [PubMed] [Google Scholar]
- 39. Mermond S, Zurawski V, D'Ortenzio E et al Lower respiratory infections among hospitalized children in New Caledonia: a pilot study for the Pneumonia Etiology Research for Child Health Project. Clin Infect Dis 2012: 54 (Suppl. 2): S180–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chidlow GR, Laing IA, Harnett GB et al Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J Clin Virol 2012: 54: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type‐1. J Pediatr 2000: 137: 78–84. [DOI] [PubMed] [Google Scholar]
- 42. Mansbach JM, McAdam AJ, Clark S et al Prospective Multicenter Study of the Viral Etiology of Bronchiolitis in the Emergency Department. Acad Emerg Med 2008: 15: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korppi M, Kotaniemi‐Syranen A, Waris M, Vainionpaa R, Reijonen T. Rhinovirus‐associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 2004: 23: 995–999. [DOI] [PubMed] [Google Scholar]
- 44. Luchsinger V, Ampuero S, Palomino MA et al Comparison of virological profiles of respiratory syncytial virus and rhinovirus in acute lower tract respiratory infections in very young Chilean infants, according to their clinical outcome. J Clin Virol 2014: 61: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marguet C, Lubrano M, Gueudin M et al In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One 2009: 4: e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guinovart C, Bassat Q, Sigauque B et al Malaria in rural Mozambique. Part I: children attending the outpatient clinic. Malar J 2008: 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loscertales MP, Roca A, Ventura PJ et al Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. Pediatr Infect Dis J 2002: 21: 148–155. [DOI] [PubMed] [Google Scholar]
- 48. Robertson SE, Roca A, Alonso PL et al Respiratory syncytial virus infection: denominator‐based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ 2004: 82: 914–922. [PMC free article] [PubMed] [Google Scholar]
- 49. Ouédraogo S, Traoré B, Nene Bi ZAB et al Viral Etiology of Respiratory Tract Infections in Children at the Pediatric Hospital in Ouagadougou (Burkina Faso). PLoS One 2014: 9: e110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Niang MN, Diop OM, Sarr FD et al Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal: the EVIRA project. J Med Virol 2010: 82: 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical features of children hospitalized with clinical pneumonia according to virus identified.


