Abstract
Background
The use of porcine islets to replace insulin‐producing islet β‐cells, destroyed during the diabetogenic disease process, presents distinct challenges if this option is to become a therapeutic reality for the treatment of type 1 diabetes. These challenges include a thorough evaluation of the microbiological safety of the islets. In this study, we describe a robust porcine islet‐screening program that provides a high level of confidence in the microbiological safety of porcine islets suitable for clinical trials.
Methods
A four‐checkpoint program systematically screens the donor herd (Large White – Yorkshire × Landrace F1 hybrid animals), individual sentinel and pancreas donor animals and, critically, the islet macrobeads themselves. Molecular assays screen for more than 30 known viruses, while electron microscopy and in vitro studies are employed to screen for potential new or divergent (emergent) viruses.
Results
Of 1207 monthly samples taken from random animals over a 2‐year period, only a single positive result for Transmissible gastroenteritis virus was observed, demonstrating the high level of biosecurity maintained in the source herd. Given the lack of clinical signs, positive antibody titers for Porcine reproductive and respiratory syndrome virus, Porcine parvovirus, and Influenza A confirm the efficacy of the herd vaccination program. Porcine respiratory coronavirus was found to be present in the herd, as expected for domestic swine. Tissue homogenate samples from six sentinel and 11 donor animals, over the same 2‐year period, were negative for the presence of viruses when co‐cultured with six different cell lines from four species. The absence of adventitious viruses in separate islet macrobead preparations produced from 12 individual pancreas donor animals was confirmed using validated molecular (n = 32 viruses), in vitro culture (cells from four species), and transmission electron microscopy assays (200 cell profiles per donor animal) over the same 2‐year period. There has been no evidence of viral transmission following the implantation of these same encapsulated and functional porcine islets into non‐immunosuppressed diabetic cynomolgus macaques for up to 4 years. Isolated peripheral blood mononuclear cells from all time points were negative for PCV (Type 2), PLHV, PRRSV, PCMV, and PERV‐A, PERV‐B, and PERV‐C by PCR analysis in all six recipient animals.
Conclusion
The four‐checkpoint program is a robust and reliable method for characterization of the microbiological safety of encapsulated porcine islets intended for clinical trials.
Keywords: pancreatic islets, xenotransplantation, zoonoses
Abbreviations: Viruses
- BDV
Borna disease virus
- BVDV‐1
‐2, Bovine viral diarrhea virus type 1 & 2
- PAV
Porcine adenovirus
- PCV
Porcine circovirus
- PCMV
Porcine cytomegalovirus
- PEV
Porcine enterovirus
- PERV‐A/B/C
Porcine endogenous retrovirus A B C
- PHEV
Porcine hemagglutinating encephalomyelitis virus
- PHoV
Porcine hokovirus
- PLHV‐1
‐2 ‐3, Porcine lymphotropic herpesvirus type 1 2 & 3
- PPV
Porcine parvovirus
- PRRSV
Porcine reproductive & respiratory syndrome virus
- PRCV
Porcine respiratory coronavirus
- PRotA
Porcine rotavirus A
- PRotC
Porcine rotavirus C
- PRV
Pseudorabies virus
- PTV
Porcine teschovirus
- RV
Rabies virus
- REO‐1
‐2 ‐3, Reovirus type 1 2 & 3
- SEOV
Seoul virus
- SIV
Swine influenza virus
- SNV
Sin Nombre virus
- EMCV
Swine encephalomyocarditis virus
- HEV
Swine hepatitis E virus
- SVDV
Swine vesicular disease virus
- SWPOX
Swine pox virus
- TTV
Torque tenovirus
- TGEV
Transmissible gastroenteritis virus
- EEEV
Eastern equine encephalomyelitis virus
- VEEV
Venezuelan equine encephalomyelitis virus
- WEEV
Western encephalomyelitis virus
- VSV‐IN
Vesicular stomatitis Indiana virus
- VSV‐NJ
Vesicular stomatitis New Jersey virus
- WNV
West Nile virus
Cell Lines
- BHK‐21
Baby hamster kidney cell line
- BT
Bovine turbinate cell line
- MA104
African green monkey kidney cell line
- MARC‐145
Monkey kidney cell line
- MDCK
Madin Darby canine kidney cell line
- MRC‐5
Human fetal lung fibroblast cell line
- PAM
Pulmonary alveolar macrophages
- PK‐15
Porcine kidney cell line
- PPK
Primary porcine kidney cell line
- ST
Swine testicular cell line
Other
- ATMP
Advanced Therapy Medicinal Products
- CBER
Center for Biologicals Evaluation and Research
- CFR
Code of Federal Regulations
- cGMP
Current Good Manufacturing Practices
- CHMP
Committee for Medicinal Products for Human Use
- cpe
Cytopathic effect
- DPF
Designated pathogen‐free
- df
Dilution factor
- ELISA
Enzyme linked immunosorbent assay
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- HA
Hemadsorption test
- HEPA
High‐efficiency particulate arrestance
- HI
Hemagglutination inhibition
- IEQ
Islet equivalent; an islet of diameter 150 μm.
- ICH
International Council of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IHC
Immunohistochemistry
- IND
Investigational new drug application
- IXA
International Xenotransplantation Association
- ISO
International Organization for Standardization
- n.d.
Not done
- MVDL
University of Minnesota Veterinary Diagnostic Laboratory
- NAHLN
National Animal Health Laboratory Network
- NHP
Non‐human primate
- PAR‐1
PERV‐A receptor 1
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- PCR
Polymerase chain reaction
- qPCR
Quantitative PCR
- Ph. Eur.
European Pharmacopeia
- PQA Plus®
Pork Quality Assurance Plus
- PRNT
Plaque reduction neutralization test
- RBC
Red blood cells
- qRT‐PCR
Quantitative reverse transcription PCR
- SN
Serum neutralization
- SOP
Standard operating procedures
- SPF
Specific pathogen free
- TEM
Transmission electron microscopy
- USDA
United States Department of Agriculture
- USP
United States Pharmacopeia
- VI
Virus isolation
- VN
Virus neutralization
- WHO
World Health Organization
1. Introduction
The ultimate goal for the treatment of type 1 diabetes is the restoration of physiological blood glucose regulation without the requirement for lifelong therapy such as immunosuppression or immunoregulation. There are numerous challenges for the attainment of this goal, and diverse approaches ranging from insulin pumps to gene therapy to stem cell strategies are all being pursued.1, 2, 3, 4, 5 In another approach, the replacement of lost islet cells through human islet allotransplantation has established the clinical utility of islet grafting.6, 7 Although only 13% of human islet recipients remain insulin‐independent at 5 years, the majority of patients (67%) retain some graft function as evidenced by persistent C‐peptide secretion.8 Importantly, islet transplantation largely eliminates hypoglycemic unawareness post‐grafting. Further improvement in long‐term insulin independence is likely; data from a few select institutions indicate that an increasing number of islet recipients (approaching 50%) are able to maintain external insulin independence for 5 years.9 Nonetheless, the availability of suitable human pancreases for either whole organ or isolated islet transplantation is entirely inadequate as evidenced by the recovery of only 1293 donor pancreases in the United States in 2015.10
The use of xenogeneic islets could provide a solution to the limited supply of human islets for transplantation. Porcine islets are particularly suitable as a source of insulin‐producing cells because porcine insulin differs from human insulin by only a single amino acid and porcine insulin has been shown to provide reliable and safe glycemic control in human diabetic patients. Furthermore, only the transplantation of whole pancreas or intact islets of Langerhans can be expected to replicate normal and precise physiological glucose control, which is dependent on a variety of cell types and proteins from pancreatic islets. This enormously complex regulatory machinery is absent in all other approaches to the re‐establishment of normoglycemia.
The use of animal‐derived islet tissue, however, will necessitate either suppression of the patient's immune system or the physical isolation of the islets to prevent their rejection. We have previously described the ability of agarose‐agarose encapsulated porcine islets to function in diabetic animal models.11, 12, 13, 14, 15 These encapsulated islet macrobeads are at least partially immune isolated as an outer layer of dense agarose prohibits direct cell access to the xenogeneic islets. Although small immune mediators, such as cytokines, can still enter the macrobead, this encapsulation approach has been sufficiently robust to allow spontaneously diabetic BB rats to survive for more than 6 months without immunosuppression and without exogenous insulin administration.13 Ongoing studies in non‐immunosuppressed diabetic cynomolgus macaques also demonstrate porcine islet macrobead function for more than 6 months in 5 of 6 animals (unpublished data, see Checkpoint 4 below).
The clinical use of islets from animals also requires a well‐thought‐out strategy to assure the microbiological safety of the animal tissue. A strategy to provide an aseptic product and to reduce the probability of inadvertent transmission of adventitious viruses is critical to the successful implementation of this exciting therapy. Our approach takes advantage of the unique aspects of the islet macrobead, namely its long‐term viability in culture and lack of patient immunosuppression, to limit the microbiological safety risk of porcine islets for transplantation. Essential to our approach is the establishment of four checkpoints that span the macrobead production and transplantation processes. The checkpoint approach is built upon an increasing level of microbiological screening from the donor herd up to the islet product, using multiple assay formats with the ability to screen for known and unknown pathogens. Using the checkpoint approach, we provide evidence to support the use of donor animals housed in high hygienic but not HEPA‐filtered environments, not only for islet xenotransplantation but also other xeno‐cell therapies. In this study, we present and document the effectiveness of our microbiological safety strategy to minimize the microbiological risks of agarose encapsulated porcine islets for the treatment of type 1 diabetes.
2. Materials and Methods
2.1. Source animal facility
Donor pigs were Large White – Yorkshire × Landrace F1 hybrid animals obtained from well‐characterized, non‐genetically modified Choice Genetics (formerly Newsham) source animals. The Source Animal Facility is an independently owned herd located in rural Southwest Ohio. The breeding herd numbers approximately 2000 sows and utilizes a two‐tiered quarantine testing system through a modern multisite production strategy. Three segregated facilities (nursery, gilt isolation, and farrowing and gestation) maintain data records and follow standard operating procedures (SOPs) for animal husbandry, vaccination, housing, cleaning and waste management, and biosecurity measures. Controlled transportation of animals, supplies and semen, feeding and watering, pest and rodent control, gravel barrier around facility perimeter, and caretaker qualifications including routine health assessments are all components of the comprehensive biosecurity program. Routine Quality Assurance site audits are used to verify compliance. Corn‐based pig feed was produced exclusively with corn grown and ground onsite at the source animal facility. Gilts, gestation sows, and lactating sows were given the same base feed, consisting of corn with various supplements (eg, amino acids, vitamins, minerals, protein), with slight variations based on the breeding status of the animal. All ingredients used in the production of complete feed were certified to be free of any restricted use protein products as defined in the FDA Code of Federal Regulation Title 21 Part 589.2001 (21 CFR 589.2001) pertaining to cattle materials prohibited in animal feed. Complete medical histories are archived for all source animals. The facility holds Pork Quality Assurance Plus (PQA Plus®) certification, which is a producer‐driven program to ensure U.S. pork products are of the highest quality and safe.16 The PQA Plus program mandates Good Production Practices with an emphasis on biosecurity and practices to reduce the risk of introducing and spreading of infectious agents. All retired breeding sows not used as pancreas donors are sent to a local abattoir for pork products.
Source animals are routinely vaccinated for various agents (Table 1). Killed vaccines are used when available. Modified live virus vaccines are used with no evidence for reversion to virulence in back‐passage studies (PARASAIL and ProSystem RCE) or when no USDA‐licensed killed vaccines are available (Enterisol Ileitis). The modified live virus vaccine Ingelvac PRRS is the exception to the above [vaccine virus has been found in one study with non‐vaccinated pigs in contact with vaccinated animals17] and is alternated quarterly with a killed PRRS vaccine.
Table 1.
Vaccine information and vaccination schedule
| Vaccine | Target agent | Form | Manufacturer | Weaning | Post‐weaning | Gilts | Pre‐farrowing | Post‐breeding | Whole herd | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 wk | 6 wk | |||||||||
| PARASAIL® | Haemophilus parasuis | Avirulent live | Newport Laboratories | X | X | |||||
| CircoFLEX® | Circovirus | Killed | Boehringer Ingelheim | X | ||||||
| MycoFLEX® | Mycoplasma hyopneumonia | Killed | Boehringer Ingelheim | X | ||||||
| Myco Shield® | M. hyopneumonia | Killed | Novartis | X | ||||||
| Pneumostar® SIV | SIV H1N1 and H3N2 | Killed | Novartis | X | X d | |||||
| Enterisol Ileitis | Lawsonia intracellularis | Attenuated | Boehringer Ingelheim | X | ||||||
| Parvo Shield® L5E | Parvovirus, 5 strains of Leptospira and Erysipelas | Killed | Novartis | X | X | |||||
| Prefarrow Shield® 9a | E. coli (K99, K88, 987P, F41), C. perfringens type C, Bordetella bronchiseptica, Pastruella multocidatypes A/D | Killed | Novartis | X | ||||||
| ProSystem® RCEb | Rotavirus G serotypes 5 and 4 of Serogroup A, C. perfringens Type C, E. coli (K99, K88, 987P, F41) | Modified live | Merck | X | ||||||
| Ingelvac® PRRSc | Porcine Reproductive and Respiratory Syndrome Virus | Modified live | Boehringer Ingelheim | X | ||||||
| PRRSd | Porcine Reproductive and Respiratory Syndrome Virus | Killed | Newport | X | ||||||
5 wk pre‐farrowing.
2 wk pre‐farrowing.
January and July.
April and October.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The facility operates a Source Animal Health Screening Program to continuously assess the health status of the herd. Between January 2013 and December 2014, the screening program was composed of random antemortem monthly herd testing and a sentinel animal program that includes both antemortem and postmortem testing. Target agents, test methods, and sample types for the monthly and sentinel antemortem screening are shown in Table 2. The sentinel animal program consists exclusively of randomly selected breeding sows as they are the only animals housed long term (>2 years) at the facility. Blood, nasal swabs, and fecal samples are collected from sentinel animals for microbiological screening. Gilts selected from the nursery for breeding are first quarantined and qualified for introduction to the gestation facility. Once inside the breeding facility, breeding sows of all ages are candidates for the sentinel program. Trained boars used for heat‐checking are not part of the sentinel program as they are not eligible for pancreas donation, although these animals are part of the routine herd health screening performed monthly.
Table 2.
Monthly herd and quarterly sentinel antemortem screening
| Target agent | Test method | Sample type | JAN | FEB | MAR | APR | MAY | JUN | JUL | AUG | SEPT | OCT | NOV | DEC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | |||||||||||||||
| Monthly Herd | TGEV | ELISA | Serum | 0/49 | 0/50 | 0/50 | 0/55 | 0/50 | 0/50 | 0/56 | 1/50 | 0/51 | 0/50 | 0/50 | 0/50 |
| PRCV | ELISA | Serum | 44/49 | 49/50 | 47/50 | 50/55 | 46/50 | 50/50 | 55/56 | 34/50 | 25/51 | 29/50 | 10/50 | 16/50 | |
| PRRSV* | ELISA | Serum | 32/49 | 26/50 | 48/50 | 47/55 | 30/50 | 27/50 | 29/56 | 36/50 | 32/51 | 26/50 | 48/50 | 45/50 | |
| Quarterly sentinel | Brachyspira sp. | Culture | Feces | n.d. | n.d. | 0/11 | 0/10 | ||||||||
| Salmonella spp. | Culture | Feces | 2/10 | 0/10 | 0/11 | 0/10 | |||||||||
| Enteric viruses | TEM | Feces | 0/10 | 10/10a | 2/8b | 10/10c | |||||||||
| Influenza A* | qPCR | Nasal swab | 0/10 | 4/10 | 0/11 | 0/10 | |||||||||
| PCMV | qPCR | Buffy coat | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| PPV* | HI | Serum | 10/10d | 10/10e | 11/11f | 9/10g | |||||||||
| PRCV | ELISA | Serum | 10/10 | 10/10 | 10/11 | 2/10 | |||||||||
| PRRSV* | ELISA | Serum | 9/10 | 9/10 | 9/11 | 10/10 | |||||||||
| PRV | ELISA | Serum | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| TGEV | ELISA | Serum | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| BVDV‐1. ‐2 | SN | Serum | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| EMCV | SN | Serum | 2/10h | 0/10 | 2/11i | 0/10 | |||||||||
| VSV‐IN | SN | Serum | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| VSV‐NJ | SN | Serum | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| EEEV | PRNT | Serum | 0/10 | 1/10j | 2/11j | 0/10 | |||||||||
| VEEV | PRNT | Serum | 2/10j | 1/10j | 3/11j | 1/10j | |||||||||
| WEEV | PRNT | Serum | 2/10j | 1/10j | 3/11 | 1/10j | |||||||||
| WNV | PRNT | Serum | 0/10 | 0/10 | 0/11 | 0/10 | |||||||||
| 2014 | |||||||||||||||
| Monthly herd | TGEV | ELISA | Serum | 0/51 | 0/50 | 0/49 | 0/50 | 0/50 | 0/50 | 0/50 | 0/47 | 0/50 | 0/51 | 0/50 | 0/48 |
| PRCV | ELISA | Serum | 22/51 | 0/50 | 14/49 | 33/50 | 23/50 | 20/50 | 14/50 | 9/47 | 6/50 | 15/51 | 11/50 | 7/48 | |
| PRRSV* | ELISA | Serum | 37/51 | 45/50 | 35/49 | 31/50 | 25/50 | 46/50 | 48/50 | 38/47 | 21/50 | 30/51 | 25/50 | 19/48 | |
| Quarterly sentinel | Brachyspira sp. | Culture | Feces | 0/10 | 0/10 | 0/10 | 0/10 | ||||||||
| Salmonella spp. | Culture | Feces | 0/10 | 2/10 | 0/10 | 0/10 | |||||||||
| Enteric viruses | TEM | Feces | 10/10c | 0/10 | 10/10c | 0/10 | |||||||||
| Influenza A* | qPCR | Nasal swab | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| PCMV | qPCR | Buffy coat | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| PPV* | HI | Serum | 9/10k | 10/10l | 10/10m | 10/10n | |||||||||
| PRCV | ELISA | Serum | 0/10 | 8/10 | 4/10 | 4/10 | |||||||||
| PRRSV* | ELISA | Serum | 9/10 | 8/10 | 9/10 | 8/10 | |||||||||
| PRV | ELISA | Serum | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| TGEV | ELISA | Serum | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| BVDV‐1, ‐2 | SN | Serum | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| EMCV | SN | Serum | 3/10p | 0/10 | 0/10 | 0/10 | |||||||||
| VSV‐IN | SN | Serum | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| VSV‐NJ | SN | Serum | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
| EEEV | PRNT | Serum | 1/10j | 0/10 | 0/10 | 0/10 | |||||||||
| VEEV | PRNT | Serum | 3/10j | 1/10j | 0/10 | 5/10j | |||||||||
| WEEV | PRNT | Serum | 0/10 | 0/10 | 2/10j | 0/10 | |||||||||
| WNV | PRNT | Serum | 0/10 | 0/10 | 0/10 | 0/10 | |||||||||
*Vaccination, n.d. (not done), abacteriophage, bfew picornaviridae (n = 2), ccaudovirales, ddilution factor (df): 128 (n = 1), 512 (n = 2), 1024 (n = 4), 2048 (n = 3), edf: 512 (n = 3), 1024 (n = 1), 2048 (n = 3), 4096 (n = 3), fdf: 64 (n = 1), 1024 (n = 2), 2048 (n = 6), 4096 (n = 1), 8192 (n = 1), gdf: 256 (n = 1), 1024 (n = 1), 2048 (n = 3), 4096 (n = 2), 8192 (n = 2), hdf: 64, idf: 64 (n = 1), 128 (n = 1), jdf: 10, kdf: 256 (n = 2), 512 (n = 3), 1024 (n = 1), 2048 (n = 2), 8192 (n = 1), ldf: 256 (n = 2), 1024 (n = 3), 2048 (n = 3), 4096 (n = 2), mdf: 64 (n = 1), 512 (n = 1), 1024 (n = 1), 2048 (n = 2), 4096 (n = 1), 8192 (n = 1), ndf: 64 (n = 1), 256 (n = 1), 512 (n = 2), 1024 (n = 2), 2048 (n = 2), 4096 (n = 2), pdf: 32.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.2. Postmortem microbiological screening of sentinel and donor animals
Following electrical stun and exsanguination at a USDA‐regulated abattoir in compliance with all federal regulations concerning animal handling and welfare, numerous tissues including brain, heart, lung, liver, tonsil, lymph node, spleen, ileum, kidney, pancreas, bone marrow, PBMCs, feces, and serum were collected and archived as fixed and frozen aliquots at the time of pancreas procurement. Aliquots of isolated islets were also frozen and archived. Donor animal tissue samples were sent to the University of Minnesota, Veterinary Diagnostic Laboratory (MVDL) to screen for the bacterial and viral pathogens listed in Table 3. The MVDL is one of the three major swine diagnostic laboratories in the United States, and swine veterinarians use the MVDL's assays to monitor heard health and identify new outbreaks of infectious disease. The laboratory is a member of the National Animal Health Laboratory Network (NAHLN) of the USDA. A complete qualification report for all assays within the Herd Health Program, “Performance Characteristics of Diagnostic Assays Used to Screen Source Animals for Xenotransplantation,” has been submitted to the FDA as part of an Investigational New Drug (IND) application for the use of agarose‐agarose encapsulated porcine islets to treat patients with type 1 diabetes. The qualification report contains detailed information on all diagnostic assays used to screen source animals and includes assay protocols and data regarding sensitivity, specificity, and repeatability.
Table 3.
Sentinel and pancreas donor postmortem screening
| Target agent | Test method(s) | Sample type | 2013 | 2014 | ||
|---|---|---|---|---|---|---|
| Sentinels | Pancreas donors | Sentinels | Pancreas donors | |||
| Aerobic | Culture | Lung | 0/3 | 0/6 | n.d. | 0/2 |
| Brachyspira sp. | Culture | Feces | 0/3 | 0/7 | n.d. | 0/1 |
| Salmonella spp. | Culture | Feces, mesenteric lymph node, spleen, tissue poola | 0/3 | 0/7 | n.d. | 0/2 |
| Leptospira * | IHC | Kidney (formalin‐fixed) | 0/3 | 0/7 | 0/3 | 0/4 |
| L. intracellularis * | IHC | Ileum (formalin‐fixed) | 0/3 | 0/7 | 0/2 | 0/4 |
| TGEV | IHC | Ileum (formalin‐fixed) | 0/2 | 0/7 | 0/3 | 0/3 |
| Enteric viruses | TEM | Feces | 2/3b | 1/7c | 0/3 | 1/3d |
| Influenza A* | qRT‐PCR | Lung | 0/3 | 0/7 | 0/3 | 0/4 |
| PCMV | qPCR | Buffy coat | 0/3 | 0/7 | 0/3 | 0/4 |
| BVDV‐1, ‐2 | qRT‐PCR | Tissue homogenatee | 0/3 | 0/6 | 0/3 | 0/4 |
| BVDV‐1, ‐2 | VI (culture: BT) | Tissue homogenatee | 0/3 | 0/7 | 0/3 | 0/4 |
| EMCV | VI (culture: BHK) | Serum, heart | 0/3 | 0/7 | 0/3 | 0/4 |
| PHoV | qPCR | Pancreas | n.d. | 2/7 | 0/1 | 1/1 |
| PHoV | qPCR | Mesenteric lymphnode | 1/3 | 2/7 | 1/3 | 3/3 |
| PERV‐A | qRT‐PCR | Tissue homogenatee | 3/3 | 7/7 | 3/3 | 4/4 |
| PERV‐B | qRT‐PCR | Tissue homogenatee | 3/3 | 7/7 | 3/3 | 4/4 |
| PERV‐C | qRT‐PCR | Tissue homogenatee | 2/3 | 5/7 | 3/3 | 4/4 |
| PRRSV | qRT‐PCR | Serum, tissue homogenatee | 0/3 | 0/7 | 0/3 | 0/4 |
| PRRSV* | ELISA | Serum | 2/3 | 7/7 | 3/3 | 3/4 |
| PRCV | ELISA | Serum | 2/3 | 6/7 | 2/3 | 2/4 |
| PRV | ELISA | Serum | 0/3 | 0/7 | 0/3 | 0/4 |
| TGEV | ELISA | Serum | 0/3 | 0/7 | 1/3 | 0/3 |
| VSV‐IN | SN | Serum | 0/3 | 0/7 | 0/3 | 0/4 |
| VSV‐NJ | SN | Serum | 0/3 | 0/7 | 0/3 | 0/4 |
| EEEVf | PRNT | Serum | 1/3g | 0/7 | 1/3g | 0/4 |
| VEEVf | PRNT | Serum | 0/3 | 1/7g | 1/3g | 0/4 |
| WEEVf | PRNT | Serum | 0/3 | 2/7g | 0/3 | 0/4 |
| WNVf | PRNT | Serum | 0/3 | 0/7 | 0/3 | 0/4 |
| PPV* | HI | Serum | 3/3h | 6/6i | 3/3j | 4/4k |
| PHEV | HI | Serum | 2/2l | 3/3m | n.d. | n.d. |
| Porcine viruses | Culture: MDCK | Tissue homogenaten | 0/3 | 0/7 | 0/3 | 0/4 |
| Porcine viruses | Culture: PAM | Tissue homogenaten | 0/3 | 0/7 | 0/3 | 0/4 |
| Porcine viruses | Culture: PPK | Tissue homogenaten | 0/3 | 0/7 | 0/3 | 0/4 |
| Porcine viruses | Culture: PK‐15 | Tissue homogenaten | 0/3 | 0/7 | 0/3 | 0/4 |
| Porcine viruses | Culture: MARC‐145 | Tissue homogenaten | 0/3 | 0/7 | 0/3 | 0/4 |
| Porcine viruses | Culture: BT | Tissue homogenaten | 0/3 | 0/7 | 0/3 | 0/4 |
*Vaccination, [n.d. (not done)], acolon, ileum, liver, spleen, lung, and mesenteric lymph node, bcoronavirus (n = 1), caudovirales (n = 1), csmall round viral particle, ddilution factor (df): 10, ekidney, lung, mesenteric lymph node, tonsil, and spleen, fTesting performed at National Veterinary Service Laboratory, gbacteriophage, hdf: 512 (n = 1), 1024 (n = 2), idf:1024 (n = 2), 2048 (n = 2), 8192 (n = 2), ≥8192 (n = 1), jdf: 1024 (n = 1), 2048 (n = 1), 4056 (n = 1), kdf: 512 (n = 2), 2048 (n = 2), ldf: 20 (n = 1), 40 (n = 1), mdf: 40 (n = 1), 320 (n = 1), 640 (n = 1), nheart, intestine, liver, pancreas, kidney, lung, mesenteric lymph node, tonsil, and spleen.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The MVDL assays are validated according to guidelines established by the American Association of Veterinary Laboratory Diagnosticians, and Standard Operating Procedures are available from the MVDL upon request (vdl@umn.edu). Detection of the different target agents included the following methods: Bacterial Culture, Transmission Electron Microscopy (TEM), Immunohistochemistry (IHC), conventional or quantitative Polymerase Chain Reaction (PCR or qPCR, respectively) and quantitative Reverse Transcription (qRT‐PCR), Hemagglutination Inhibition (HI), Serum Neutralization (SN), Virus Isolation (VI), and Plaque Reduction Neutralization Test (PRNT). In addition, virus isolation assays were performed on the infection susceptible cells Madin‐Darby canine kidney (MDCK) cells, porcine alveolar macrophages (PAM), primary porcine kidney cells (PPK), porcine kidney cells (PK‐15), African green monkey kidney cells (MARC‐145), and bovine turbinate cells (BT) to look for unknown porcine viruses. Each cell type was cultured in the presence of tissue homogenate (brain, ileum, kidney, lung, liver, mesenteric lymph node, spleen, and tonsil) from sentinel animals or donor animals and monitored for morphological changes indicative of viral infection. The PRNT testing for EEEV, VEEV, WEEV, and WNV occurred at the National Veterinary Service Laboratory (Ames, IA, USA).
2.3. Porcine islet isolation and encapsulation
Donor pancreases were procured from sows that were over 2 years of age with a history of multiple parities. Following electrical stunning and exsanguination of the donor, abdominal viscera were retrieved by a sterile‐gowned technician and transferred to a sterile container for transport. The viscera were then placed in a custom built, HEPA‐filtered, laminar flow work station for tissue retrieval. Surface monitoring was performed on the work surface prior to retrieval, and settle plates were placed inside the workstation during dissection to monitor for microbiological contamination. Intact pancreas was dissected from the viscera by a sterile‐gowned technician and immediately transferred to cold HBSS (CellGro, Manassas, VA, USA). The tissue was then surface‐rinsed by submersion in cold povidone‐iodine 10% solution (VetOne, Boise, ID, USA), followed by two submersion rinses in cold HBSS. The pancreas was transported to the islet isolation laboratory in a sterile container submerged in cold HBSS. The container was then transferred to a biological safety cabinet where the pancreas was removed and a transport media sample collected for total bioburden testing (Avista Pharma Solutions, Inc., Agawam, MA, USA). Warm ischemia times (beginning of exsanguination to pancreas immersion in cold transport solution) ranged from 10 to 15 minutes. Cold ischemia times (pancreas immersion in cold transport solution until placement in digestion chamber) ranged from 42 to 57 minutes.
Pancreases were perfused with 7.5 Wunsch U/g collagenase (Collagenase MA; Vitacyte, Indianapolis, IN, USA), 12 000 Neutral Protease U/g pancreas (BP Protease; Vitacyte), and 2.5 mg/pancreas of Pulmozyme (Genentech, South San Francisco, CA, USA) solution prepared in Cold Storage Purification Stock Solution (CellGro). Islet counts were expressed as islet equivalents (IEQ), based on a standard islet size of 150 μm, and 500 IEQ were encapsulated in agarose‐agarose macrobeads as previously described.18 Following quantification, islets were separated into 2000 IEQ aliquots before being resuspended in 0.5 mL of 0.8% (w/v) SeaKem Gold agarose (derived from Gelidium species of seaweed; Lonza Rockland, Inc., Rockland, MD, USA) prepared in Minimal Essential Medium plus 25 m/mol L HEPES buffer (Sigma‐Aldrich, St. Louis, MO, USA). The islet‐agarose suspension was expelled beneath the surface of sterile mineral oil (Sigma‐Aldrich) to produce four 0.125‐mL spherical beads of approximately 5‐6 mm in diameter, each containing 500 IEQ. The 1st coat macrobeads were cultured at 37°C in a humidified atmosphere of 5% CO2. After 3‐5 days, a second coat of 5% SeaKem Gold agarose was applied, giving each macrobead a final diameter of 8‐9 mm. The determination of the average pore size of the agarose macrobeads is difficult at best, given the gelation mechanism of random polymer coils forming aggregated double helices, which results in pores of various diameters with ill‐defined walls. For this reason, the macrobead has not been considered to provide absolute viral sequestration although the encapsulated islets are protected from direct immune cell contact. Agarase, the enzyme necessary to break down agarose, is abundant in ocean‐dwelling bacteria but has not been found in mammals.19 The macrobeads, in the absence of trauma, remain intact indefinitely following implantation in the abdominal cavity.
Macrobeads were then cultured at 37°C in a humidified atmosphere of 5% CO2 until collection for microbiological screening or implantation. Culture medium (RPMI 1640; Life Technologies, Grand Island, NY, USA; pre‐screened for endotoxin <0.03 EU/mL and for sterility) containing 11 mmol/L glucose, 2.5% heat‐inactivated porcine serum (Biologos, Montgomery, IL, USA; pre‐screened for sterility, and the absence of mycoplasma and adventitious viruses per 9CFRs and endotoxin <50 EU/mL) and 1% antibiotic/anti‐mycotic (Life Technologies) was changed weekly and 24‐hour post‐change media samples were taken for porcine insulin ELISA assays (Mercodia, Uppsala, Sweden). Islet isolation and encapsulation procedures were performed in Class II biosafety cabinets within an ISO Class 5 (at rest) and ISO Class 7 (active processing) laboratory by sterile‐gowned technicians. Routine environmental monitoring included particle counts, viable particle counts, settling plates, and surface monitoring and was performed for every islet isolation procedure. Macrobeads from a given donor were assigned a unique islet isolation number and cultured separately from islet macrobeads produced from other donor animals.
2.4. Viral screening of porcine islet macrobeads
All viral screening of islet macrobeads was performed at SGS Vitrology (Glasgow, UK). This group has extensive experience (approximately 15 years) with the testing of both the islet macrobeads and cancer macrobeads (see Discussion) and has been essential in the development of the viral screening programs for each product. Representative macrobeads were sealed in sterile screw top tubes, frozen, and shipped on dry ice to SGS Vitrology for screening using both molecular and in vitro co‐culture assays. All shipments were received and released by the Office of the Scottish Executive Rural Directorate following appropriate content and importation license verification. Aliquots of islet macrobeads, rather than isolated free islets, were selected for screening to insure any potential viral activation due to the stress of the encapsulation procedure was accounted for. Similar aliquots were also frozen and archived for possible future investigative use. For PCR assays, DNA was extracted from 6 macrobeads pooled from 3 individual donors (two macrobeads per donor) after homogenization in Buffer ATL (Qiagen, Valencia, CA, USA), lysis, and adsorption to a silica membrane. A Qiagen RNeasy kit was used for RNA extraction according to the manufacturer's instructions. The viral RNA and DNA extraction methods and the TaqMan® real‐time PCR assays were validated to ICH Q2 guidelines. All testing was carried out with validated assays, and all PCR tests were performed in compliance with, and as described by Ph. Eur. 2.6.2120 and USP <1127>21 (Figure 1). Each reaction contained 15 000‐30 000 islet cell equivalents (qPCR) or 13 000‐26 000 islet cell equivalents (qRT‐PCR).
Figure 1.
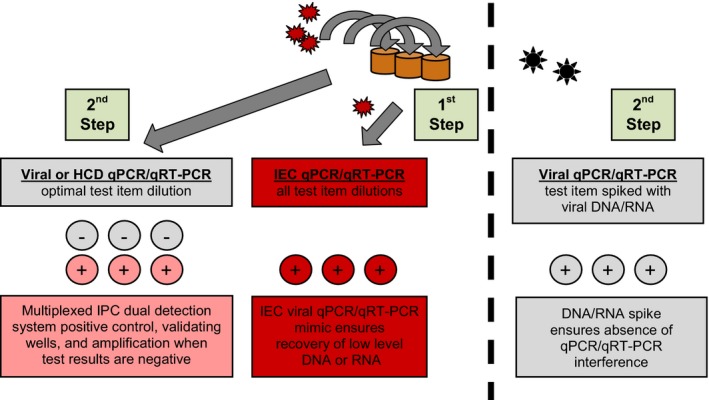
Viral Screening of Porcine Islets by qPCR / qRT‐PCR. Overview of the qPCR/qRT‐PCR (as appropriate) testing strategy used in the viral screening of porcine islet macrobeads (Checkpoint 3). 1st Step: each test sample is spiked with an internal extraction control (IEC; plant RNA or DNA) at the detection limit, mimicking the target nucleic acid. This process allows the level of nucleic acid recovery in the presence of the test sample to be assessed, which ensures that low levels of RNA or DNA can be recovered. 2nd Step: Test samples contain viral target primers/probes, multiplexed with internal positive control (IPC) reagents during amplification. In a separate assay, test samples are spiked with target nucleic acid (viral RNA or DNA) at the detection limit. This process ensures any negative results are not caused by sample matrix interference of the qPCR assay. This method of viral screening complies with the specific testing approach using PCR detection as described in Ph. Eur. 2.6.21, USP <1237>, and FDA Vaccine Guidance to Industry (2010)
In vitro assays for the detection of adventitious virus contaminants were performed as follows. Six islet macrobeads per donor sample, in culture medium, were used to generate each test sample. The test material was thawed, supernatant retained, and the macrobeads then ground in a sterile tissue grinder and taken through two cycles of freezing and thawing. The ground beads were then mixed with the culture supernatant and the pooled material filtered through a 100 μm filter to remove large debris and clarified by centrifugation. The clarified supernatant material was then used as the lysate sample for inoculation. Four indicator cell lines consisting of human lung fetal fibroblast cells (MRC‐5), baby hamster kidney cells (BHK‐21), swine testis cells (ST), and African green monkey kidney cells (MA104) were inoculated with each test sample, incubated at 36°C (±1°C), and monitored for a period of at least 28 days for development of virus‐induced cytopathic effect (cpe). Negative controls included monitoring the health of the indicator cell lines in tissue culture medium only throughout the in vitro assay. Cytopathic (cpe) virus positive controls were inoculated on the same day as the test samples and were included for each indicator cell line: MRC‐5 cells inoculated with Herpes simplex virus type 1; BHK‐21 cells inoculated with EMCV; ST cells inoculated with PPV; MA104 cells inoculated with Reo‐3. The positive control viruses were inoculated at 100 TCID50 units/vessel. At the end of the assay, negative controls (tissue culture medium only) and a positive control (Influenza A virus) for the hemadsorption (HA) assay were included to qualify each of the red blood cell preparations, using a pool of guinea pig, chicken, and human type O red blood cells, at 2‐8°C. These controls were inoculated onto separate batches of indicator cells nearer the end of the assay.
Transmission electron microscopy (TEM) was performed using RPMI/2.5% Glutaraldehyde fixed islet cells in macrobeads. The cells were post‐fixed in osmium tetroxide, stained en bloc in uranyl acetate, dehydrated in an ethanol series and propylene oxide before being infiltrated to Araldite resin and polymerized. Semi‐thin sections (~1 μm) were cut and mounted on glass slides, stained with toluidine blue, and examined by light microscopy for general quality of fixation, gross cell morphology, and for the presence of mitotic cells and dead or dying cells in the culture. Ultrathin sections (0.1 μm) were cut, mounted on electron microscope grids, and stained with uranyl acetate and lead citrate solutions. Minimums of 200 median cell profiles were examined for the presence of viruses, virus‐like particles, and other extraneous agents. The ultra structural morphology of the cells was also recorded.
2.5. Sterility, mycoplasma, and endotoxin screening of islet macrobeads
Prior to islet macrobead implantation, representative samples of macrobeads were sent for sterility testing per USP <71>22 and Ph. Eur. 2.6.123 via membrane filtration and additionally assessed for the presence of Mycoplasma using direct and indicator cell culture methods (USP <63>24 and Ph. Eur. 2.6.725 Mycoplasma Tests; Avista Pharma Solutions, Inc. Agawam, MA, USA). Macrobeads were also screened for the presence of bacterial endotoxins per USP <85>26 and Ph. Eur. 2.6.14.27 Microbiological testing (sterility, endotoxin, and mycoplasma) was repeated 2‐4 weeks prior to implant, as well as 24‐hour prior to implant.
2.6. Macrobead implant and viral screening of diabetic non‐human primates
Six non‐immunosuppressed streptozotocin‐induced diabetic male cynomolgus macaques of Mauritian or Asian origin with a median age of 6.7 years (range 4.0 to 8.9 years) and median body weight of 7.6 kg (range 5.5 to 8.4 kg) each received 198‐315 (mean 246) porcine islet macrobeads implanted intraperitoneally (day 0), corresponding to a mean of 123 000 islet equivalents per animal. All six animals received a second dose of 152‐362 (mean 200) islet macrobeads between 138 and 372 days from the first transplant corresponding to a mean of 100 000 islet equivalents per animal. Blood samples for PBMC isolation were collected prior to and post‐transplant at scheduled intervals for subsequent PCR analysis to determine potential porcine viral transmission including PCV, PLHV, PRRSV, PCMV, and PERV‐A, PERV‐B, and PERV‐C infection. Time points evaluated were 1 month (±7 days) and 6 months (±15 days) post‐transplant. Additionally, samples from 1 year (±60 days), 2 years (±60 days), and 3 years (±60 days) post‐transplant were evaluated in a subset of recipients who reached these time points during extended follow‐up. All procedures in animals were approved by the University of Minnesota Institutional Animal Care and Use Committee, conducted in compliance with the Animal Welfare Act, and adhered to principles stated in the Guide for Care and Use of Laboratory Animals.
3. Results
3.1. Multiple checkpoints for microbiological safety
Multiple checkpoints during the process of porcine islet isolation, encapsulation, culture, and transplantation were implemented to assess the microbiological safety of islet macrobeads (Figure 2).
Figure 2.
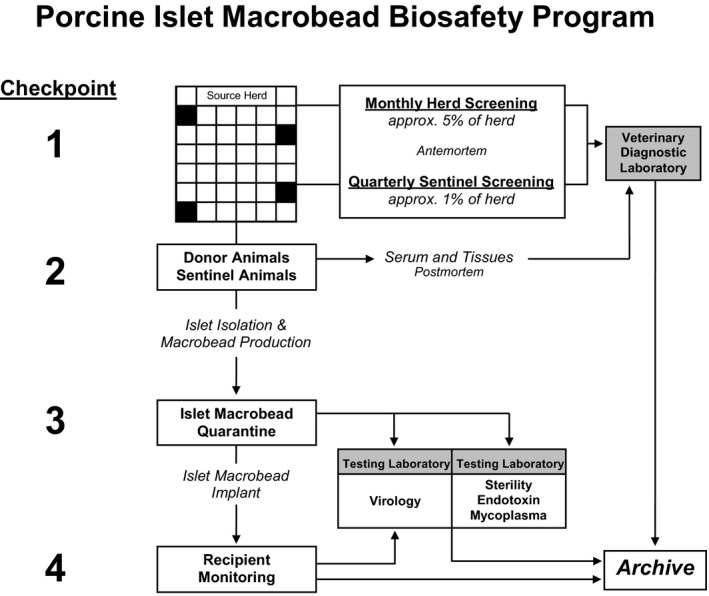
Porcine Islet Macrobead Biosafety Program. Various checkpoints are established during the lifecycle of porcine islet macrobeads to support microbiological safety. Checkpoint 1 encompasses a comprehensive set of biosecurity protocols for the management of the Source Animal Facility and includes monthly viral screening of randomly selected animals as well as quarterly screening of Sentinel Animals. In Checkpoint 2, all pancreas donor animals and culled Sentinel Animals undergo thorough necropsies with numerous tissues screened and archived. In Checkpoint 3, islet macrobeads are quarantined in tissue culture, while representative samples of each production lot are screened for the presence of 32 known viruses using validated molecular assays. Co‐culture assays and electron microscopy screening of islet macrobeads are also used to screen for unknown viruses in Checkpoint 3. Samples from recipients of porcine islet macrobeads can be similarly screened under Checkpoint 4
3.2. Checkpoint 1: High health status animals
The health status of all animals at the Source Animal Facility is maintained at a high level and continuously evaluated through the use of monthly health checks from randomly selected animals and the utilization of a Sentinel Animal Program in which selected animals located throughout the facility act as environmental monitors through extensive and repeated screenings. Monthly screening of randomly selected animals during the years of 2013 and 2014 for the presence of antibodies to PRRSV confirmed the efficacy of the vaccination program [826/1207 PRRSV antibody‐positive (68%)] given the absence of PRRSV RNA in sentinel and donor animal screening (Table 2). Antibodies were also routinely detected for PRCV (52%), demonstrating the widespread prevalence of this virus. Because the donor animal facility is negative for the presence of TGEV, screening for the introduction of this virus was used to continuously document the biosecurity of the Source Animal Facility: the one positive finding from 1207 samples was considered a false positive given the absence of any further positive findings.
Quarterly antemortem screening results of sentinel animals also demonstrate the efficacy of the vaccination program and overall herd health as evidenced by positive findings of antibodies to PPV and PRRSV in 150 of 162 samples (92%) that are part of the vaccination program, and no evidence of Influenza A virus as monitored by the presence of the RNA, which is also part of the vaccination program (0 of 81 nasal swab samples; Table 2). Additional results from sentinel animals included 42 positive identifications from 78 fecal samples (54%) for bacteriophage/Picornaviridae/Caudovirales in feces using electron microscopy. Antibodies to PRCV were found in 48 of 81 serum samples (59%). A total of 29 results were considered suspect for the Equine encephalomyelitis viruses (Venezuelan, Western, and Eastern) owing to virus growth variation and interpretation of low dilution factor results with these serology‐based assays. Seven positive results for the serology‐based EMCV assay and four findings of Salmonella spp. in feces were also reported.
3.3. Checkpoint 2: Postmortem screening of sentinel animals and pancreas donor animals
Numerous tissues were collected from all sentinel and donor animals at sacrifice for viral screening at the MVDL. Postmortem viral screening of six sentinel animals and 11 pancreas donor animals during 2013 and 2014 yielded 34 positive results from 301 samples (11%), excluding the viruses that are part of the vaccination program, the three PERV subtypes, and the samples screened with co‐culture assays (Table 3). In 2013, the presence of enteric viruses was detected in 2 of 3 sentinel animals (Coronaviridae, n = 1; Caudovirales, n = 1), and a small, round viral particle was observed but not identified in the feces of 1 of 7 pancreatic donor animals. In 2014, a bacteriophage was identified in 1 of 2 pancreas donor animals tested, while no positive findings were observed in the feces of the three sentinel animals tested. Screening for PHEV antibodies yielded positive findings in 2 of 2 sentinel animals and 3 of 3 pancreas donor animals in 2013. Further screening was discontinued in source animals in 2014 as this virus has been shown to be ubiquitous in pig herds and has no public health significance given that the pig is the only known host.28 Six positive results observed for the three Equine encephalomyelitis viruses are considered suspect‐positive results given the low dilution factor reported (df = 10). A single positive result was reported for antibodies to TGEV in the postmortem serum sample of a sentinel animal in 2014. The antemortem serum from this same animal was consistently negative, and immunohistochemistry staining of the ileum was also negative. The presence of PHoV sequences by PCR analysis was detected in both sentinel and donor animals (3 of 9 pancreases and 7 of 16 lymph node samples). In subsequent assessment of isolated porcine islets, there was no evidence of this virus. No evidence of viral transmission was observed in six different cell lines from four species using tissue homogenate samples from each of the sentinel and donor animals.
3.4. Checkpoint 3: Quarantine of islet macrobeads for microbial and viral screening
Given evidence of satisfactory insulin production by cultured islet macrobeads (≥75 mU/macrobead/24‐hour), the macrobeads were sent for viral screening to SGS Vitrology prior to use in pre‐clinical animal studies. All islet macrobeads were negative for viruses screened by PCR (n = 32 viruses) and by co‐culture assays using four different susceptible cell lines (n = 12 pancreas donor animals; Table 4). Transmission scanning electron microscopy was incorporated as an additional viral screening tool in 2013 and no viruses, virus‐like particles or extraneous agents including mycoplasma, yeasts, fungi, or bacteria were found in the 6 pancreas donors comprising the two macrobead Lots produced in 2013 and 2014 (Table 4 and Figure 3). Conditioned media samples taken at 4 weeks post‐islet encapsulation were negative for bacterial growth and for the presence of endotoxin and mycoplasma (Table 4).
Table 4.
Porcine islet macrobead screening
| Porcine viral screening by qPCR/qRT‐PCR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Lot (isolation dates): | 062812‐092012‐101012 | 101612‐112912‐121312 | 010913‐012413‐041113 | 121213‐011614‐021314 | ||||
| Target agent | Sensitivitya | Result | Sensitivitya | Result | Sensitivitya | Result | Sensitivitya | Result |
| BDV | 1000 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| BVDV‐1 | 1000 | (‐) | 100 | (‐) | 100 | (‐) | 1000 | (‐) |
| BVDV‐2 | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) |
| PEV | 1000 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| PAdV | 10 000 | (‐) | 10 000 | (‐) | 10 000 | (‐) | 10 000 | (‐) |
| PCV | 50 | (‐) | 50 | (‐) | 50 | (‐) | 50 | (‐) |
| PCMV | 10 | (‐) | 10 | (‐) | 10 | (‐) | 10 | (‐) |
| PHEV | 25 | (‐) | 25 | (‐) | 25 | (‐) | 25 | (‐) |
| PLHV‐1, ‐2, ‐3 | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| PPV | 10 | (‐) | 10 | (‐) | 10 | (‐) | 10 | (‐) |
| PRotA | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| PRotC | 10 000 | (‐) | 10 000 | (‐) | 10 000 | (‐) | 10 000 | (‐) |
| PRV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| PTV | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) |
| Rabies | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| REO‐1, ‐2, ‐3 | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) |
| SEOV | 10 | (‐) | 10 | (‐) | 10 | (‐) | 10 | (‐) |
| SNV | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) |
| EMCV | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) | 1000 | (‐) |
| HEV | 1000 | (‐) | 1000 | (‐) | 100 | (‐) | 100 | (‐) |
| SVDV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| SWPOX | 10 | (‐) | 10 | (‐) | 10 | (‐) | 10 | (‐) |
| TTV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| TGEV | 10 | (‐) | 10 | (‐) | 10 | (‐) | 10 | (‐) |
| EEEV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| VEEV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| WEEV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| WNV | 100 | (‐) | 100 | (‐) | 100 | (‐) | 100 | (‐) |
| 28‐day in vitro co‐culture assay for detection of viral contaminants | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolation Date: | 062812 | 092012 | 101012 | 101612 | 112912 | 121312 | 010913 | 012413 | 041113 | 121213 | 011614 | 021314 | ||||||||||||
| Cell Line | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA | cpe | HA |
| MRC‐5 | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| BHK‐21 | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| ST | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| MA104 | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| Microbiological screening of porcine islet macrobeads | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolation Date: | 062812 | 092012 | 101012 | 101612 | 112912 | 121312 | 010913 | 012413 | 041113 | 121213 | 011614 | 021314 |
| Test | ||||||||||||
| Sterility | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| Endotoxinb | 0.006 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Mycoplasm | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| TEM | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
Cpe, cytopathic effect; HA, hemadsorption.
sensitivity = limit of detection (gene copies per PCR).
EU/macrobead, transmission electron microscopy (TEM), not done (n.d.).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
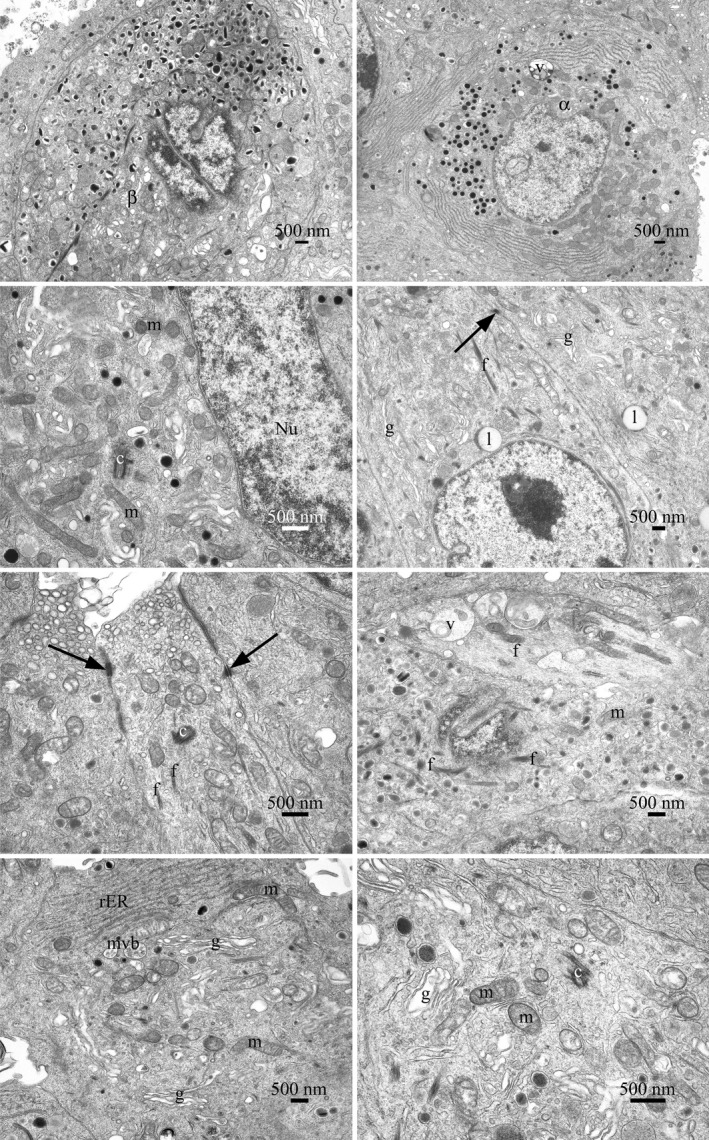
Transmission electron microscopy of islet cell profiles, typical of those present in the sample of cells examined. The islet cells were generally adherent with intercellular connections, cell junctions, and desmosomes observed. The cell surface appearances varied, smooth surfaces, blebs, and cell processes were observed. Various cell types were present, including α and β cells. No mitotic cells were observed by light or electron microscopy. No significant numbers of dead or dying cells were present in the population of cells examined. Nuclear profiles varied in size. In some cells, more than one nuclear profile was visible. They were often indented and invaginated or contained lacunae of cytoplasmic material. Nucleoli were prominent and sometimes more than one was observed. Heterochromatin was abundant, clumped, and found on the inner nuclear membrane and sporadically throughout the nucleus. The cells had a normal range of organelles. The mitochondria were numerous and varied in size and shape. The presence of cristae was noted although no matrix granules were seen. The Golgi body, when observed, was prominent and exhibited stacked cisternae. The rough‐surfaced endoplasmic reticulum was extensive and occurred in varied lengths. Lipid bodies, peroxisomes, and multivesicular bodies were present. Free ribosomes, polysomes, and fibrils were found throughout the cytoplasm. Vacuoles, with electron dense and flocculent material, were observed. Coated, un‐coated, and secretory vesicles, of varying types, were seen. Centrioles and microtubules were present. The cell structure is consistent with the morphology expected of secretory cells. No viruses, virus‐like particles or extraneous agents, including mycoplasmas, yeasts, fungi, or bacteria, were found. Abbreviations: f, fibrils; mitochondria (m); desmosome (arrow); centriole (c); nucleus (Nu); Golgi bodies (g), lipid bodies (l), vacuoles (v), multivesicular body (mvb), and rough‐surfaced endoplasmic reticulum (rER)
3.5. Checkpoint 4: Recipient screening for transmission of infectious agents
Although not a source material or product release screen, recipient monitoring post‐macrobead implantation provides valuable data that could be used to not only treat individual patients but also as a check for the efficacy and possible modification of the pre‐implantation checkpoints and is an important component of a thorough safety program. As an example of islet macrobead recipient monitoring, 6 diabetic cynomolgus macaques received porcine islet macrobead implantations and were routinely monitored for graft function and evidence of viral transmission and/or infection. Five of 6 animals implanted have shown a measurable clinical benefit in terms of reduced exogenous insulin requirements (an approximately 50% reduction as designed to model a proposed Phase 1 IND trial) with improved and/or stable blood glucose readings and HbA1c levels for up to 4 years. No significant adverse events have been observed in these animals to date. Body weight has remained stable or increased in all animals in line with the overall health of these animals. Screening for selected viruses was performed at the MVDL and was based on the presence of the virus in the source herd (PCV [Type 2], PERV‐A, PERV‐B, PERV‐C), viruses with uncertain herd status (PRRSV) or viruses of concern given similarity to infectious human viruses or activation in pig to immunosuppressed primate xenotransplantation (PLHV‐1, 2, and 3 and PCMV, respectively). Isolated peripheral blood mononuclear cells from all time points were negative for PCV (Type 2), PLHV‐1, 2, and 3, PRRSV, PCMV, PERV‐A, PERV‐B, and PERV‐C by PCR analysis (Table 5). Moreover, there has been no evidence of viral infection as observed and documented in the health status of the animals.
Table 5.
PCR screening of implanted non‐human primates
| Target agent | PCV, PLHV, PRRSV, PCMV, PERV | |||||
|---|---|---|---|---|---|---|
| Time (Post‐transplant) | ||||||
| Animal ID | 1 month | 6 months | 1 year | 2 years | 3 years | 4 years |
| 08FP6 | (‐) | (‐) | (‐) | (‐) | (‐) | (‐) |
| 08KP5 | (‐) | (‐) | (‐) | (‐) | (‐) | |
| 12JP3 | (‐) | (‐) | (‐) | (‐) | ||
| 12JP2 | (‐) | (‐) | (‐) | (‐) | ||
| 08FP10 | (‐) | (‐) | (‐) | |||
| 08FP17 | (‐) | (‐) | (‐) | |||
Time points evaluated were: 1 month (±7 days) and 6 months (±15 days) post‐transplant. Additionally, samples from 1 year (±60 days), 2 years (±60 days), 3 years (±60 days) and 4 years (±60 days) post‐transplant were evaluated in a subset of recipients who reached these time points during extended follow‐up.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. Discussion
The four‐checkpoint microbiological safety program outlined utilizes four separate assessments for the microbiological safety of agarose encapsulated porcine islets. These occur sequentially from the source animal herd and individual donor animals to the intended transplant product and finally the recipient following implantation. The donor herd, designated sentinel animals, pancreas donor animals, and islet macrobeads are extensively screened to exclude latent and adventitious contaminants. The encapsulation method supports islet viability over extended culture periods, which permits ample time for the conduct of numerous screening assays for the detection of specific viruses, electron microscopic detection of viral particles and/or cell damage, and the lengthy in vitro culture screens for both known and unknown viruses. This is a significant advance over the indirect testing of animals raised under gnotobiotic conditions and allows for screening at multiple points throughout the islet macrobead manufacturing process. Finally, samples from patients implanted with porcine islet macrobeads can be screened for the evidence of xenozoonosis using validated assays similar to those we have employed for sentinel and donor animals and for macrobead testing as part of a post‐implant surveillance program.
This integrated safety program has been developed with the help of numerous scientists, clinicians, and experts in veterinary care, toxicology, quality assurance, veterinary diagnostics, islet isolation, clinical medicine, microbiology, and virology. The program has evolved from the adventitious agent screening assessments used for vaccines and biotechnology products described in the USP, Ph. Eur., and in the ICH guidelines. Certain adaptations have been made due to the nature of the samples and type of product. The ability of this program to ensure that the health status of donor animals is high and that islet macrobeads are consistently negative for bacterial or viral contamination is now firmly established. The safety program is open to continual review and refinement in consultation with relevant experts throughout the world. For example, the use of transmission electron microscopy (TEM) to provide an additional screen of islet macrobeads for known and unknown viruses and for other extraneous agents has been recently implemented as it is routinely applied in vaccine production, even though TEM has a considerably lower sensitivity compared to PCR. Any positive TEM findings will be investigated to identify known agents using immunohistochemistry or molecular techniques. The involvement of various specialists extends beyond the development of the microbiological safety program and encourages continual program improvement. Regular meetings, for example, could prompt the addition of new viruses of concern to be added to the screening panels or new/optimized procedures from animal husbandry to clinical care could be implemented under specialist guidance.
In the United States, the Food and Drug Administration (FDA) has regulatory responsibility for somatic cell therapy that includes xenogeneic cells and therefore porcine islets.29 The FDA Center for Biologics, Evaluation and Research (CBER) regulates biological products including cell therapies and released a final Guidance for Industry regarding xenotransplantation products in 2003.30 The European Medicines Agency (EMA) oversees Advanced Therapy Medicinal Products (ATMPs) including cell transplantation in Europe.31, 32, 33 The World Health Organization34, 35 as well as the International Conference on Harmonisation36, 37, 38, 39 have also issued xenotransplantation guidance. The International Xenotransplantation Association (IXA) recently published a first update to the original consensus statements on the use of porcine islets for clinical trials.40, 41, 42, 43, 44, 45, 46, 47 The focus of all these guidelines, including the IXA statements, is on the safety of xenotransplantation.30, 33 An invited review of US xenogeneic regulations was published by FDA staff in 2010 and provides additional details on FDA guidelines.48 Schuurman has more recently published a thorough overview of worldwide xenogeneic regulations with an emphasis on the European requirements.49
Our microbiological safety program is in‐line with the issues for consideration in the FDA xenotransplantation guideline, as well as the IXA statements. In regard to donor animals, it is important to note that the guidelines do not state that source animals must be raised in HEPA‐filtered environments. This is significant because, in addition to the difficulty of obtaining and maintaining pathogen‐free swine,50, 51, 52 there is no guarantee that the pancreas from individual animals will be suitable for islet isolation. In fact, it is becoming increasing clear that only about 25% of porcine pancreases yield a sufficient number of islets for clinical use.18, 53, 54, 55 Given these limitations, our approach is to raise source animals in high hygienic environments, biopsy individual pancreases for islet isolation suitability and perform extensive microbiological screening of all selected donor animal tissues and the islet macrobeads themselves.
Our methodology, however, employs an alternative approach to the FDA‐recommended 21‐day quarantine period for individual donor animals that deserves some discussion. A minimum 7‐day quarantine period for animals used in the processing of biological products is also stated in 21 CFR part 600.56 The rationale for the quarantine period is to provide time for an acute infection to become clinically apparent.57 During a quarantine period, cohort animals would be housed in a HEPA‐filtered environment with strict husbandry practices designed to eliminate the introduction of adventitious agents, similar to a laboratory specific pathogen‐free (SPF) animal facility. Such animals would be monitored for the development of disease symptoms and undergo blood, fecal, and nasal swab sampling for microbiological status assessment. The development of disease symptoms or unsatisfactory diagnostic results in any animal would disqualify all animals in a given cohort from pancreas donation. This donor qualification process is most appropriate when there is not sufficient time to perform thorough screening of the islets to be transplanted, for example, when free islets are to be transplanted within a few days of isolation. The islets encapsulated in the agarose macrobeads, in contrast, survive for more than 1 year during in vitro culture13 allowing ample time to conduct the numerous and lengthy assays designed to detect both known and unknown microbiological agents. Assays that screen the islet product directly are more likely to detect a pathological agent than the assessment of the clinical condition of the donor which may not present disease symptoms (eg, PPV) or the detection of a latent virus (eg, PLHV) that might be readily observed in particular organs but not as reliably in peripheral blood cells.58 Thus, it is the islet macrobeads themselves, that is, the tissue to be implanted, that undergoes quarantine and it is the ability to thoroughly screen the islets using a wide variety of specific and non‐specific assays that obviates the need to raise or quarantine donor animals in HEPA‐filtered environments. As discussed by Schuurman in the first IXA consensus statement on source animals, “…it can be suggested that with longer time periods of islet cell culture the relevance of health status of the source pig becomes less, and that the focus of DPF status is more on the cultured cells.”.59 This ideology is again reflected in the first update of the IXA consensus statements in relation to encapsulated islets allowing the time necessary for thorough microbial screening and the potential to move the safety criteria further toward the islets.44
This design supports the use of animals that have spent their adult life in the same high biosecurity facility, employing protocol‐driven husbandry practices to provide donor animals of high health. The use of aseptic techniques and standard operating procedures from the procurement of the pancreas through to the isolation and encapsulation of the islets ensures freedom from adventitious virus contamination. The microbiological safety program presented here screens numerous tissues from all pancreas donor and sentinel animals for more than 25 pathogens. When combined with the use of pre‐screened media and porcine serum, this approach controls major routes of introduction for adventitious agents that could enter the macrobead production process. This is the emphasis of Checkpoints 1 and 2. Monitoring the source herd provides information on potential breaks in biosecurity that may warrant additional testing or the temporary halting of macrobead production (Checkpoint 1). This is also true of individual donor animal screening and in this case may necessitate the discard of any macrobeads produced from suspect tissue (Checkpoint 2). Any positive viral findings in the macrobeads, with the exception of PERV, would automatically result in the destruction of those macrobeads (Checkpoint 3). Finally, patient monitoring under Checkpoint 4 provides not only valuable information for potential treatment options in the case of suspected pathogen transmission but also manufacturing decisions as well as feedback to Checkpoints 1‐3 that may result in the modification of one or more checkpoints.
As with any medical procedure, there will always be risk with the transplantation of porcine islets. The challenge we face is to understand those risks such that they can be minimized to an acceptable level in light of the expected clinical benefits. The potential risk of viral transfer from the transplanted cells to the recipient has been the major safety concern. To address this issue, a testing rationale was developed to screen sentinel and donor animals for viruses that are present in the herd, are of special interest such as known zoonotic agents, or are known to infect swine in the USA. An initial characterization of the PERV infectivity status of the source herd was not performed given the unlikely ability, at least at that time, to eliminate these endogenous retroviruses and the growing conclusion that the risk of PERV transmission to human xenotransplantation trial participants is low.60, 61, 62 Viruses excluded from screening, to date, are those that are not known to be present in the United States (eg, Japanese encephalitis virus), unlikely to be present in the source facility (eg, vesicular exanthema of swine virus), or unlikely to be found in the pancreas or to survive during extended macrobead culture (Porcine torovirus). Many of these same screening exclusion criteria can apply to other infectious agents (eg, Toxoplasma gondii is unlikely to be present in the biosecure source herd). This is similar to the rationale used for pathogen screening as reported by Wynyard et al for the Auckland Island strain of swine63 in which agents present or with an uncertain status in New Zealand, or the relevance in a biosecure facility or infectious potential post‐transplantation, or the ability of the agent to survive in the environment of the pancreas were used to develop a list of screened pathogens. This herd, which originated from animals isolated on a remote island south of New Zealand, may be considered DPF because they are routinely screened and documented to be free of specific infectious bacteria, fungi, protozoa, and viruses. Although the rationale behind the development of this pathogen list was similar to our approach, the applications of the screening programs differ. Ultimately, the New Zealand group tested the source animal colony for 15 viruses, 10 bacterial species, and one protozoa. A reduced number of pathogens were screened for in the donor piglets and islet preparations. The Göttingen minipig herd in Ellegaard, Denmark, also qualifies as a DPF source animal facility as these animals have been shown to be free of at least 88 infectious agents including 20 viruses through PCR methodology. The one exception was the finding of swine Hepatitis E virus in 3 sow‐piglet pairs and in 3 animals under one year of age.52 These data suggest that in this case it may be possible to select donor animals based on the viral profile of individual animals.
Our approach favors routine screening of the donor herd with a greater emphasis on the screening of sentinel and donor animals. An even greater emphasis is placed on the qualification of the islet macrobeads (Checkpoint 3) in which an increased number of viruses are screened for using a variety of assays including specific PCR methods. Additionally, co‐culture and electron microscopy assays are employed to look for unknown viruses, which would then prompt efforts to identify the virus as either a known or unknown agent (eg, PRRSV inducing cpe in MA104 cells as one component of the islet macrobead screen). Also, as part of their release criteria, the islet macrobeads are confirmed to be sterile and free from mycoplasma and any significant level of endotoxin. Although the current paper reports porcine islet macrobead screening results for the previous 2 years, we have found no evidence for the presence of known or unknown transmissible viruses in the islet macrobeads during screening of porcine pancreatic islets isolated from 30 donor animals over the previous 5 years. Moreover, no evidence of xenogeneic infection has been found in numerous tissues from animals implanted with the islet macrobeads.
New or emergent viruses are routinely investigated to optimize screening at the various checkpoints. For example, the presence of the PHoV (also known as porcine partetravirus) nucleotide sequence was investigated in source animals and in islet macrobeads. To date, this virus has not been cultured or isolated and the only evidence of its existence are the findings of the genomic sequence.64, 65 We have found evidence of the PHoV sequence in various porcine tissues including pancreas, mesenteric lymph nodes, donor serum, and frozen vendor supplied porcine serum used during islet isolation and macrobead culture. This sequence was not found in purified islets isolated in the absence of porcine serum. Because we also detected the sequence in domestic and international porcine serum sources, PHoV screening of islet macrobeads cultured in these sera was postponed until a suitable PHoV‐free source could be obtained.
With regard to PERV, the identification of this virus and its ability to cross the species barrier has been the principal concern with porcine xenotransplantation. It is worth mentioning that the NHP is not an ideal model to assess the risk of PERV transmission. Cynomolgus monkeys lack the PERV‐A receptor 1 (PAR‐1) that is the major receptor for PERV‐A and PERV‐A/C entry.66 The actual risk of PERV infection in the clinic remains unclear, but so far there has been no transmission of PERV or other porcine microorganisms in humans that have been exposed to pig islet cells.63, 67, 68, 69 We consider these findings, as well as the negative viral findings from diabetic dogs exposed to porcine islet macrobeads for 2.4 years, to be evidence that viral transmission following macrobead implantation is very low risk.15 Studies with D17 canine cells have shown these cells to have the PERV‐A receptor,70 but apparently not to the same degree as human 293 cells.71 Thus, there may be some question as to the relevance of the dog model for PERV transmission studies, although canine cells and subjects are susceptible to other porcine viruses including TGEV,72, 73 PRV,74 and SIV75, 76, 77 among others.
Other, non‐microbiological risks to the patient also exist and must be considered as part of the comprehensive safety evaluation. These include procedural risks that accompany pre‐ and post‐implantation screening, as well as the general anesthesia and laparoscopic surgery required to implant the macrobeads. The procedural risks are well known and, although real, are modest. We have been implanting cancer macrobeads (encapsulated mouse renal adenocarcinoma cells) to treat patients with various malignancies for 10 years without adverse events attributable to the macrobeads either as an intraperitoneal irritant or as a microbiological hazard.78 See also ClinicalTrials.gov Identifiers NCT00283075, NCT01053013, and NCT02046174.
A final important consideration is the immune status of the recipients. Recent progress in clinical islet allotransplantation has confirmed the potential of islet grafting to reduce exogenous insulin administration and the occurrence of hypoglycemic unawareness.6, 7 While these results are encouraging, numerous adverse events including intrahepatic bleeding, neutropenia, mouth ulcers, anemia, diarrhea, edema, hypercholesterolemia, and pharyngitis have been reported.79 Most of the reported adverse events are primarily associated with immunosuppressive therapy. Encapsulating porcine islets using the agarose‐agarose method eliminates the need for immunosuppressive therapy, and a fully competent immune system is expected to significantly reduce the risk of xenozoonotic infection.80 Likewise, it is generally agreed that non‐genetically modified Source Animals reduce the risk of zoonotic infection because unmodified viral particles would be more likely to activate human complement‐mediated destruction.81, 82, 83 In our non‐immunosuppressed streptozotocin‐induced diabetic NHP surveillance, there has been no evidence of viral infection with PCV (Type 2), PLHV‐1, 2 and 3, PRRSV, PCMV, or PERV in samples collected longitudinally post‐transplant. Together, these data demonstrate a reduced risk in the absence of immunosuppression and suggests that the viral safety profile is acceptable for agarose encapsulated porcine islets.
During the last 20 years, an understanding of the risks associated with porcine xenotransplantation, and methodologies to manage those risks, including theoretical risks and the potential presence of unknown pathogens, have significantly progressed. The recent IXA consensus update notes that “theoretical risk” still exists in relation to the transmission of infectious agents when employing appropriate safeguards, but that such events will likely be rare should they occur.44 Although the actual risks of islet xenotransplantation cannot be not known in the absence of clinical trials, as stated by Fishman, “In clinical xenotransplantation, a level of safety has been developed beyond that available for human organs…”.84 Islet xenotransplantation is now at a point where clinical trials in the United States and Europe can once again be considered in light of the expected patient benefits. One particularly important benefit, based on our preclinical animal studies and islet transplantation in general, is the likely reduction in glycemic excursions that can be life threatening and that are experienced too often by patients with hypoglycemic unawareness. The microbiological safety program presented here screens a comprehensive set of tissues from all pancreas donor and sentinel animals for numerous infectious agents and, when combined with the macrobead screening assays, provides an unparalleled level of safety. These results support the use of a multicheckpoint biological safety program in the screening of encapsulated porcine islets intended for clinical trials for the treatment of hypoglycemic unawareness or unstable disease in patients with type 1 diabetes.
Author Contributions
LSG, JC, AL, MJM, DG, HVV, MMM, DH, SR, RDH, and BHS performed the concept/design. LSG, JC, AL, RWH, DG, MG, MAL, DGM, and BHS performed the critical revision of article. RWH, MAL, CM, JB, EWM, DGM, and MMM performed the data collection/organization. All authors performed data analysis/interpretation, drafting the article, and approval of the article.
Acknowledgments
We thank our colleagues at the Xenia Division of The Rogosin Institute, especially Brian Doll and Ashley Ewing and at Bob Evans Farms, Inc. We are also indebted to the porcine islet isolation team of Hollie Adkins, Lisa Circle, Steven G. Harbeck and Eric D. Meyer.
Gazda, L. S. , Collins, J. , Lovatt, A. , Holdcraft, R. W. , Morin, M. J. , Galbraith, D. , Graham, M. , Laramore, M. A. , Maclean, C. , Black, J. , Milne, E. W. , Marthaler, D. G. , Vinerean, H. V. , Michalak, M. M. , Hoffer, D. , Richter, S. , Hall, R. D. and Smith, B. H. (2016), A comprehensive microbiological safety approach for agarose encapsulated porcine islets intended for clinical trials. Xenotransplantation, 23: 444–463. doi: 10.1111/xen.12277
Funding information
This work was supported by Metromedia Bio‐Science LLC.
References
- 1. Heinemann L, Fleming GA, Petrie JR, et al. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs. A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia. 2015;58:862–870. [DOI] [PubMed] [Google Scholar]
- 2. Norgaard K, Shin J, Welsh JB, Gjessing H. Performance and acceptability of a combined device for insulin infusion and glucose sensing in the home setting. J Diabetes Sci Technol. 2015;9:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Handorf AM, Sollinger HW, Alam T. Insulin gene therapy for type 1 diabetes mellitus. Exp Clin Transplant. 2015;13(Suppl. 1):37–45. [PubMed] [Google Scholar]
- 4. Li R, Buras E, Lee J, et al. Gene therapy with neurogenin3, betacellulin and SOCS1 reverses diabetes in NOD mice. Gene Ther. 2015;22:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johannesson B, Sui L, Freytes DO, Creusot RJ, Egli D. Toward beta cell replacement for diabetes. EMBO J. 2015;34:841–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goss JA, Schock AP, Brunicardi FC, et al. Achievement of insulin independence in three consecutive type‐1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation. 2002;74:1761–1766. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. [DOI] [PubMed] [Google Scholar]
- 8. Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990–1997. [DOI] [PubMed] [Google Scholar]
- 9. McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Organ Procurement and Transplantation Network . U.S. Dept. of Health and Human Services. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Cited: 31AUG2016.
- 11. Jain K, Asina S, Yang H, et al. Glucose control and long‐term survival in biobreeding/Worcester rats after intraperitoneal implantation of hydrophilic macrobeads containing porcine islets without immunosuppression. Transplantation. 1999;68:1693–1700. [DOI] [PubMed] [Google Scholar]
- 12. Jain K, Yang H, Cai BR, et al. Retrievable, replaceable, macroencapsulated pancreatic islet xenografts. Long‐term engraftment without immunosuppression. Transplantation. 1995;59:319–324. [PubMed] [Google Scholar]
- 13. Gazda LS, Vinerean HV, Laramore MA, et al. Encapsulation of porcine islets permits extended culture time and insulin independence in spontaneously diabetic BB rats. Cell Transplant. 2007;16:609–620. [DOI] [PubMed] [Google Scholar]
- 14. Gazda LS, Vinerean HV, Laramore MA, et al. Pravastatin improves glucose regulation and biocompatibility of agarose encapsulated porcine islets following transplantation into pancreatectomized dogs. J Diabetes Res. 2014;2014:Article ID 405362. doi: 10.1155/2014/405362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gazda LS, Vinerean HV, Laramore MA, et al. No evidence of viral transmission following long‐term implantation of agarose encapsulated porcine islets in diabetic dogs. J Diabetes Res. 2014;2014:Article ID 727483. doi: 10.1155/2014/727483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pork Quality Assurance Plus Certification. Pork Checkoff; National Pork Board. Available at: http://www.pork.org/pqa-plus-certification/. Cited: 31AUG2016.
- 17. Torrison J, Knoll M, Wiseman B. Evidence of pig‐to‐pig transmission of a modified‐live PRRS virus vaccine. In: Proc Am Assoc Swine Vet, Annual Meeting. 1996:89–91. [Google Scholar]
- 18. Gazda LS, Adkins H, Bailie JA, et al. The use of pancreas biopsy scoring provides reliable porcine islet yields while encapsulation permits the determination of microbiological safety. Cell Transplant. 2005;14:427–439. [DOI] [PubMed] [Google Scholar]
- 19. Fu XT, Kim SM. Agarase: review of major sources, categories, purification method, enzyme characteristics and applications. Mar Drugs. 2010;8:200–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Pharmacopoeia . Nucleic Acid Amplification Techniques. Section 2.6.21, Current Edition.
- 21. U.S. Pharmacopoeia . Nucleic Acid‐Based Techniques‐Amplification. Chapter 1127, Current Edition.
- 22. U.S. Pharmacopoeia . Sterility Tests. Chapter 71, Current Edition.
- 23. European Pharmacopoeia . Sterility. Section 2.6.1, Current Edition.
- 24. U.S. Pharmacopoeia . Mycoplasmas Test. Chapter 63, Current Edition.
- 25. European Pharmacopoeia . Mycoplasmas. Section 2.6.7, Current Edition.
- 26. U.S. Pharmacopoeia . Bacterial Endotoxins Test. Chapter 85, Current Edition.
- 27. European Pharmacopoeia . Bacterial Endotoxin. Section 2.6.14, Current Edition.
- 28. Saif LJ, Maurice B, Sestak K, Yeo S‐G, Jung K. Coronaviruses In: Zimmerman JJK, Locke A, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine, 10th edn Chichester, UK: Wiley‐Blackwell; 2012. [Google Scholar]
- 29. Application of current statutory authorities to human somatic cell therapy products and gene therapy products. FDA: Federal Register. 1993. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/UCM148113.pdf.
- 30. Source animal, product, preclinical, and clinical issues concerning the use of xenotransplantation products in humans. 2003. ed: FDA: Guidance for Industry, 2003.
- 31. Regulation (EC) No 1394/2007 of the European Parliament and of the Council on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Official J of the Eur Union. 2007. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:0121:0137:EN:PDF.
- 32. Guideline on Human Cell‐Based Medicinal Products. European Medicines Agency: Committee for Medicinal Products for Human Use, 2008.
- 33. Guideline on Xenogeneic Cell‐Based Medicinal Products. European Medicines Agency: Committee for Medicinal Products for Human Use. 2009. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/12/WC500016936.pdf.
- 34. First WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials. World Health Organization. 2008. Available at: http://www.who.int/transplantation/xeno/ChangshaCommunique.pdf.
- 35. Second WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials. World Health Organization. 2011. Available at: http://www.who.int/transplantation/xeno/report2nd_global_consultation_xtx.pdf.
- 36. Test procedures and acceptance criteria for biotechnological/biological products (Q6B). ICH Harmonised Tripartite Guideline. 1999. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q6B/Step4/Q6B_Guideline.pdf.
- 37. Quality of biotechnological biological products: derivation and characterization of cell substrates used for production of biotechnological/biological products (Q5D). ICH Harmonised Tripartite Guideline. 1998. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5D/Step4/Q5D_Guideline.pdf. [PubMed]
- 38. Safety preclinical evaluation of biotechnology: derived pharmaceuticals for biotechnological product (S6). ICH Harmonised Tripartite Guideline. 1997. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S6_R1/Step4/S6_R1_Guideline.pdf.
- 39. Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin (Q5A). ICH Harmonised Tripartite Guideline. 1998. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5A_R1/Step4/Q5A_R1__Guideline.pdf. [PubMed]
- 40. Hering BJ, Cozzi E, Spizzo T, et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes‐executive summary. Xenotransplantation. 2016;23:3–13. [DOI] [PubMed] [Google Scholar]
- 41. Cozzi E, Tonjes RR, Gianello P, et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes ‐ chapter 1: update on national regulatory frameworks pertinent to clinical islet xenotransplantation. Xenotransplantation. 2016;23:14–24. [DOI] [PubMed] [Google Scholar]
- 42. Spizzo T, Denner J, Gazda L, et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes ‐ chapter 2a: source pigs‐preventing xenozoonoses. Xenotransplantation. 2016;23:25–31. [DOI] [PubMed] [Google Scholar]
- 43. Cowan PJ, Ayares D, Wolf E, Cooper DK. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes. Chapter 2b: genetically modified source pigs. Xenotransplantation. 2016;23:32–37. [DOI] [PubMed] [Google Scholar]
- 44. Rayat GR, Gazda LS, Hawthorne WJ, et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes. Chapter 3: porcine islet product manufacturing and release testing criteria. Xenotransplantation. 2016;23:38–45. [DOI] [PubMed] [Google Scholar]
- 45. Cooper DK, Bottino R, Gianello P, et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes. Chapter 4: pre‐clinical efficacy and complication data required to justify a clinical trial. Xenotransplantation. 2016;23:46–52. [DOI] [PubMed] [Google Scholar]
- 46. Denner J, Tonjes RR, Takeuchi Y, Fishman J, Scobie L. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes. Chapter 5: recipient monitoring and response plan for preventing disease transmission. Xenotransplantation. 2016;23:53–59. [DOI] [PubMed] [Google Scholar]
- 47. Hering BJ, O'Connell PJ. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes. Chapter 6: patient selection for pilot clinical trials of islet xenotransplantation. Xenotransplantation. 2016;23:60–76. [DOI] [PubMed] [Google Scholar]
- 48. Arcidiacono JA, Evdokimov E, Lee MH, et al. Regulation of xenogeneic porcine pancreatic islets. Xenotransplantation. 2010;17:329–337. [DOI] [PubMed] [Google Scholar]
- 49. Schuurman HJ. Regulatory aspects of clinical xenotransplantation. Int J Surg. 2015;23:312–321. [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto H, Li TC, Koshimoto C, et al. Serological evidence for Hepatitis E virus infection in laboratory monkeys and pigs in animal facilities in Japan. Exp Anim. 2008;57:367–376. [DOI] [PubMed] [Google Scholar]
- 51. Abrahante JE, Martins K, Papas KK, et al. Microbiological safety of porcine islets: comparison with source pig. Xenotransplantation. 2011;18:88–93. [DOI] [PubMed] [Google Scholar]
- 52. Morozov VA, Morozov AV, Rotem A, et al. Extended microbiological characterization of Gottingen minipigs in the context of xenotransplantation: detection and vertical transmission of Hepatitis E virus. PLoS ONE. 2015;10:e0139893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holdcraft RW, Green ML, Breite AG, et al. Optimizing porcine islet isolation to markedly reduce enzyme consumption without sacrificing islet yield or function. Transplant Direct. 2016;2:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krickhahn M, Buhler C, Meyer T, Thiede A, Ulrichs K. The morphology of islets within the porcine donor pancreas determines the isolation result: successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant. 2002;11:827–838. [PubMed] [Google Scholar]
- 55. Sabat M, Godlewska E, Kinasiewicz J, Urbanowicz A, Orlowski T. Assessment of some porcine strains as donors of islets of Langerhans. Transplant Proc. 2003;35:2343–2344. [DOI] [PubMed] [Google Scholar]
- 56. Code of Federal Regulations Title 21 Volume 7 (21CFR600). U.S. Food and Drug Administration. 2015. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=600&showFR=1&subpartNode=21:7.0.1.1.1.2.
- 57. PHS Guideline on infectious disease issues in xenotransplantation. U.S. Food and Drug Administration. 2001. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/UCM092858.pdf.
- 58. Chmielewicz B, Goltz M, Franz T, et al. A novel porcine gammaherpesvirus. Virology. 2003;308:317–329. [DOI] [PubMed] [Google Scholar]
- 59. Schuurman HJ. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes–chapter 2: source pigs. Xenotransplantation. 2009;16:215–222. [DOI] [PubMed] [Google Scholar]
- 60. Fishman JA, Patience C. Xenotransplantation: infectious risk revisited. Am J Transplant. 2004;4:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Denner J, Tonjes RR. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev. 2012;25:318–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation‐associated infectious risk: a WHO consultation. Xenotransplantation. 2012;19:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. [DOI] [PubMed] [Google Scholar]
- 64. Lau SK, Woo PC, Tse H, et al. Identification of novel porcine and bovine parvoviruses closely related to human parvovirus 4. J Gen Virol. 2008;89:1840–1848. [DOI] [PubMed] [Google Scholar]
- 65. Souza CK, Streck AF, Goncalves KR, et al. Phylogenetic characterization of the first Ungulate tetraparvovirus 2 detected in pigs in Brazil. Braz J Microbiol. 2016;47:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mattiuzzo G, Takeuchi Y. Suboptimal porcine endogenous retrovirus infection in non‐human primate cells: implication for preclinical xenotransplantation. PLoS ONE. 2010;5:e13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Paradis K, Langford G, Long Z, et al. Search for cross‐species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999;285:1236–1241. [DOI] [PubMed] [Google Scholar]
- 68. Elliott RB, Escobar L, Garkavenko O, et al. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell Transplant. 2000;9:895–901. [DOI] [PubMed] [Google Scholar]
- 69. Valdes‐Gonzales R, Dorantes LM, Bracho‐Blanchet E, Rodiquez‐Ventura A, White DJG. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long‐term porcine islet xenotransplantation. J Med Virol. 2010;82:331–334. [DOI] [PubMed] [Google Scholar]
- 70. Takeuchi Y, Patience C, Magre S, et al. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998;72:9986–9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilson CA, Wong S, VanBrocklin M, Federspiel MJ. Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J Virol. 2000;74:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Horzinek MC, Lutz H, Pedersen NC. Antigenic relationships among homologous structural polypeptides of porcine, feline, and canine coronaviruses. Infect Immun. 1982;37:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Decaro N, Mari V, Campolo M, et al. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of Swine are circulating in dogs. J Virol. 2009;83:1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pensaert MB, Kluge JP. Pseudorabies virus (Aujeszky's disease). Virus Infections of Porcines. New York: Elsevier; 1989. [Google Scholar]
- 75. Gaush CR, Smith TF. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl Microbiol. 1968;16:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lombardo T, Dotti S, Renzi S, Ferrari M. Susceptibility of different cell lines to Avian and Swine Influenza viruses. J Virol Methods. 2012;185:82–88. [DOI] [PubMed] [Google Scholar]
- 77. Chen Y, Mo YN, Zhou HB, et al. Emergence of human‐like H3N2 influenza viruses in pet dogs in Guangxi. China Virol J. 2015;12:10. doi: 10.1186/s12985‐015‐0243‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith BH, Parikh T, Andrada ZP, et al. First‐in‐human phase 1 trial of agarose beads containing murine RENCA cells in advanced solid tumors. Cancer Growth Metastasis. 2016;9:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hirshberg B, Rother KI, Digon BJ III, et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid‐sparing immunosuppression: the National Institutes of Health experience. Diabetes Care. 2003;26:3288–3295. [DOI] [PubMed] [Google Scholar]
- 80. Fishman JA. Infection and xenotransplantation. Developing strategies to minimize risk. Ann N Y Acad Sci. 1998;862:52–66. [DOI] [PubMed] [Google Scholar]
- 81. Chapman LE, Wilson CA. Implications of the advent of homozygous alpha l, 3‐galactosyltransferase gene‐deficient pigs on transmission of infectious agents. Xenotransplantation. 2003;10:287–288. [DOI] [PubMed] [Google Scholar]
- 82. Magre S, Takeuchi Y, Langford G, et al. Reduced sensitivity to human serum inactivation of enveloped viruses produced by pig cells transgenic for human CD55 or deficient for the galactosyl‐alpha(1‐3) galactosyl epitope. J Virol. 2004;78:5812–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Quinn G, Wood JC, Ryan DJ, et al. Porcine endogenous retrovirus transmission characteristics of galactose alpha1‐3 galactose‐deficient pig cells. J Virol. 2004;78:5805–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fishman JA. Assessment of infectious risk in clinical xenotransplantation: the lessons for clinical allotransplantation. Xenotransplantation. 2014;21:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]


