Abstract
The incidence of skin and soft tissue infections (SSTI) due to multi‐drug resistant pathogens is increasing. The concomitant increase in antibiotic use along with the ease with which organisms develop mechanisms of resistance have together become a medical crisis, underscoring the importance of developing innovative and effective antimicrobial strategies. Nitric oxide (NO) is an endogenously produced molecule with many physiologic functions, including broad spectrum antimicrobial activity and immunomodulatory properties. The risk of resistance to NO is minimized because NO has multiple mechanisms of antimicrobial action. NO's clinical utility has been limited largely because it is highly reactive and lacks appropriate vehicles for storage and delivery. To harness NO's antimicrobial potential, a variety exogenous NO delivery platforms have been developed and evaluated, yet limitations preclude their use in the clinical setting. Nanotechnology represents a paradigm through which these limitations can be overcome, allowing for the encapsulation, controlled release, and focused delivery of NO for the treatment of SSTI. WIREs Nanomed Nanobiotechnol 2013. doi: 10.1002/wnan.1230
This article is categorized under:
-
1
Therapeutic Approaches and Drug Discovery > Nanomedicine for Infectious Disease
-
2
Nanotechnology Approaches to Biology > Nanoscale Systems in Biology
INTRODUCTION
The incidence of skin and soft tissue infections (SSTI) due to multi‐drug resistant (MDR) pathogens is continuing to rise.1 As a result, antibiotic use has increased in parallel to this trend. For example, a population‐based study in Canada demonstrated a 15% increase in physician visits for SSTI and an associated 49% increase in antibiotic prescriptions between 1996 and 2008.2 Unfortunately, increasing antibiotic use has become a major driving force in the development of resistant organisms, undermining their very purpose.3
Staphylococcus aureus is the etiologic agent and endemic cause of the majority of SSTI in the United States.4, 5 The growing rate of methicillin‐resistant S. aureus (MRSA) presents an emergent treatment challenge. Moreover, whereas the majority of MRSA infections between the 1960s and 1990s were hospital‐acquired, there has been an exponential increase in community‐associated MRSA since the late 1990s,2, 5 which has lead to a greater social and financial burden resulting from hospitalization.6 Other pathogens have also demonstrated emerging resistance to many antibiotics. For example, a group of MDR bacteria referred to as the ‘ESKAPE’ pathogens (Enterococcus faecalis, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), appropriately named as they ‘escape’ the effect of a variety of antibacterial drugs, have further complicated the SSTI landscape.1 This ongoing crisis warrants the development of innovative therapeutic strategies to combat MRSA and resistant microbes implicated in SSTI.
There are several mechanisms through which pathogens overcome antibiotic activity. Resistance to antibiotics can occur via inherent resistance in certain species, such as species that produce penicillinase and are therefore resistant to β‐lactam antibiotics. Resistance can also occur via de novo mutations or by the acquisition of resistance genes via horizontal transfer between microbes.1, 7 Some resistance mechanisms include direct removal of the drug from the intracellular space, decreased diffusion of the drug via modification or loss of porins, alterations or upregulation of drug target sites, bacterial enzyme drug degradation.8 Because excessive antibiotic use is associated with the emergence of and selection for resistance,3 antibiotic overuse and misuse also contributes to the growing problem of bacterial resistance.1, 4, 9
Nitric oxide (NO) is a diatomic gaseous molecule endogenously produced which, among other properties, exhibits broad spectrum antimicrobial activity. Antibiotic agents that exert multiple mechanisms of antimicrobial action limit pathogens' ability to develop resistance; such drugs are advantageous for this reason. The risk of bacterial resistance to both innate production and exogenous delivery of NO is minimized because NO exhibits multiple mechanisms of antimicrobial action.8 However, NO's utility in the clinical setting has been restricted because it is highly reactive and lacks proper vehicles for its delivery and storage,10, 11, 12 A variety of exogenous NO sources have been developed and studied for antimicrobial efficacy, but limitations preclude their use in the clinical setting. Nanotechnology offers a platform for targeted drug delivery, and extensive research has been conducted to evaluate the efficacy of antibacterial nanoparticles (nps). NO's broad antimicrobial properties and successful incorporation into nps offers a promising solution to the treatment of SSTI.
NITRIC OXIDE
Structure and Chemical Properties
NO is one of the smallest biologically active molecules13 and acts on virtually every cell in the body.14 Because of its lipophilic character and low molecular weight, NO traverses most physiologic barriers with relative ease to reach target cells.14, 15 Additionally, NO diffuses along its concentration gradient and can therefore cross cell membranes without the need for transport proteins.16, 17, 18 NO is a natural yet free radical‐forming gas and is highly unstable in an oxygen environment: it spontaneously reacts with oxygen or superoxide, forming reactive nitrogen oxide species (RNOS).
NO is endogenously synthesized when one of three distinct nitric oxide synthase (NOS) enzymes induces the oxidation of arginine to citrulline (Figure 1).15, 17, 19, 20, 21, 22 Two NOS isoforms, NOS1 and NOS3, are constitutively expressed4, 15, 18, 22, 23 and are also known by the cell types in which they were enzymatically identified: NOS1 or neuronal NOS (nNOS) from neuronal cells, and NOS3 or endothelial NOS (eNOS), from endothelial cells. Both eNOS and nNOS are calcium‐dependent, calmodulin‐regulated enzymes,23, 24 meaning their activity is tightly regulated. When activated, both of these enzymes produce low quantities of NO for short time periods.15 In these small quantities, NO functions as a signaling molecule.22 NOS2 or inducible NOS (iNOS) was originally discovered in macrophages4, 24 but is now known to be expressed in many cell types.23, 24 iNOS generates large quantities of NO in a noncalcium‐dependent fashion, and is induced by a wide array of stimulants including proinflammatory cytokines,13, 20, 21, 23, 24, 25 bacterial polysaccharides and endotoxins,13, 21, 23, 25 and neuropeptides23 (Figure 1), which often act synergistically in iNOS activation.15
Figure 1.
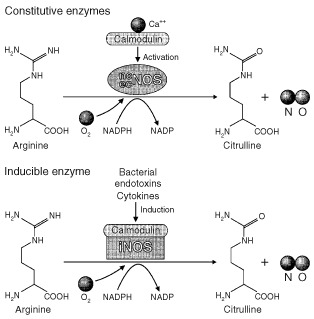
Synthesis of nitric oxide (NO). Endothelial nitric oxide synthase (eNOS) and neuronal NOS (nNOS) calcium‐dependent, calmodulin‐regulated enzymes. They are constitutively expressed and catalyze the conversion of arginine to citrulline. Inducible NOS (iNOS) converts arginine to citrulline in a calcium‐independent fashion, and is activated by bacterial endotoxins and proinflammatory cytokines. (Reprinted with permission from Ref 24. Copyright 1998 Nature Publishing Group)
Physiologic Function
NO plays a variety of important physiologic roles including blood pressure regulation, neurotransmission, inhibition of platelet aggregation, immune response, and wound healing.12, 26, 27 Because of its short half‐life, measured on the order of seconds, NO's biological impact is determined primarily by its rate of formation.14, 16 Its site of action is often close to its site of generation, as NO is rapidly scavenged by hemoglobin and myoglobin.13, 16
NO initiates multiple cellular signaling cascades, most notably via the soluble guanylyl cyclase (sGC) pathway. In this paradigm, NO binds to sGC, causing increased cyclic GMP levels and activation of protein kinase G. This signaling cascade leads to many downstream effects that facilitate both NO's local biologic activity as a vasodilator and neurotransmitter, as well as its distant impacts as an anti‐pyretic.23 NO can interact with many molecular targets, including protein thiols, heme, nonheme iron, tyrosyl radical proteins, deoxynucleotides, and deoxynucleosides.14 It can react with glutathione (GSH) and other thiol‐containing molecules to form S‐nitrosothiols (RSNOs), which function as NO carriers and donors.28 As a free radical, NO can generate potent nitrosylating agents capable of both signaling and cellular damage, such as peroxynitrite (OONO−) in the presence of superoxide.23 This effect only occurs at higher concentrations, since as mentioned above, NO is rapidly scavenged in most physiological conditions. RSNOs, S‐nitrosylated proteins, nitrosyl‐metal complexes, and nitrite may assist in long distance transport of NO. However, the actual NO species, once liberated from these carriers, are short lived.23
The majority of cutaneous cell types, including adipocytes, endothelial cells, melanocytes, keratinocytes, fibroblasts, Langerhans cells, neutrophils, and macrophages express some isoform of NOS and are therefore able to generate and release NO for a broad array of physiologic processes.16, 24, 26, 29 Keratinocytes are the major constituent of the epidermis and express all three NOS isoforms. They produce NO and hydrogen peroxide (H2O2) in response to inflammatory stimuli. This likely acts as one of the chief protective mechanisms of the skin, as the epidermis is constantly exposed to foreign matter and organisms. Additionally, NO synthesis on the skin surface may also regulate the growth of cutaneous commensal organisms.24 In the acidic skin environment, reactive nitrogen intermediates are formed, such as nitrous acid (HNO2), dinitrogen trioxide (H2NO3), and peroxynitrite (ONOO−), which may serve as a nonspecific defense mechanism against cutaneous pathogens.30 In addition, finely regulated responses are also exhibited by NOS species; wound healing is one example. Fibroblasts, found in the dermis, are key regulators of dermal remodeling by synthesizing extracellular matrix, collagen, and fibrin, while orchestrating many of the complex steps of wound healing. Fibroblasts express eNOS, nNOS, and iNOS,24 but this expression is inconsistent across different cells and possibly depends on cell maturation. Due to its widespread distribution, NO can help regulate basic physiological roles such as establishing and maintaining blood flow, protective responses against invading microorganisms, ultraviolet light‐induced melanogenesis, and development of erythema and edema in the setting of a sunburn.24
Antimicrobial Properties and Immune Function
NO has several intrinsic antimicrobial properties and is therefore vital to the body's innate immune response in the defense against invading microbes.17, 18, 24, 26, 31 One of the main mechanisms is its ability to generate RNOS via spontaneous reactions with oxygen or superoxide. These RNOS include peroxynitrite (OONO−), RSNOs, nitrogen dioxide (NO2), dinitrogen trioxide (N2O3 −), and dinitrogen tetroxide (N2O4).13, 19, 25 These RNOS are thought to exert NO's antimicrobial effects because they induce nitrosative and oxidative stress that is toxic to microbes.8 Peroxynitrite is formed during the oxidative burst in macrophages and is the most highly reactive and potentially cytotoxic of these RNOS.32 RNOS nitrosate protein thiols and modify amino acid residues and thus can inactivate essential enzymes.15 They can also nitrosylate metal centers (Fe‐S), further modifying protein functioning and depleting intracellular iron stores. These events ultimately block essential microbial processes.1, 21, 29, 33, 34 RNOS also damage microbial DNA, and they do so via a variety of mechanisms, including direct RNOS interaction with DNA, inhibition of DNA repair and replication, and increased synthesis of genotoxic mediators such as alkylating agents and H2O2.15, 28 OONO− can also induce DNA strand breaks and abasic sites, among other alterations.13, 21, 28 OONO− and NO2 have also been implicated in lipid damage and peroxidation with subsequent disruption of the microbial membrane.15, 33
Importantly, NO's ability to execute its antimicrobial properties is dictated by its concentration. At low concentrations, NO exerts its antimicrobial properties by acting as a potent immunostimulatory molecule.33 In this role, NO mediates immune cell differentiation, proliferation and apoptosis, cytokine production, expression of adhesion and co‐stimulatory molecules, and synthesis and deposition of extracellular matrix constituents.35 As NO concentration builds secondary to iNOS activation, its inherent antimicrobial properties come into play.15 The importance of iNOS activation to combat infection was demonstrated by iNOS knockout mice having greater susceptibility to herpes simplex virus infection, higher frequency of viral reactivation, and delayed viral clearance from dorsal root ganglia as compared to infected heterozygous mice.36 iNOS knockout mice are also more susceptible to Dengue virus infection, and were found to have significantly higher viral loads and greater mortality compared to wildtype mice.37 Similar results were seen in mice treated with the iNOS inhibitor aminoguanidine: treated mice were more susceptible to Salmonella typhimurium infection and death.38, 39 NO provides less feedback inhibition to iNOS compared to eNOS and nNOS, allowing for a bolus production of high NO levels to thwart a microbial threat.15
Antimicrobial Spectrum
NO has demonstrated activity against a variety of pathogens,14 including bacteria, viruses, parasites, and fungi.29
Bacteria
A variety of methods of NO delivery have demonstrated its antibacterial effect. Gaseous NO (gNO) was bactericidal against S. aureus, MRSA, Escherichia coli, Group B Streptococcus, and P. aeruginosa in vitro.40 In vitro studies of acidified nitrite, an NO donor, have demonstrated efficacy against P. aeruginosa,41 Burkholderia cepacia,41 S. aureus 30, 41 and Propionibacterium acnes.30 The NO‐donor β‐galactosyl‐pyrrolidinyl diazeniumdiolate (β‐Gal‐NONOate) was bactericidal against E. coli.42 S‐nitrosothiol NO donors demonstrated activity against P. aeruginosa,28 coagulase‐negative Staphylococci,28 S. aureus,28 Serratia marcescens,28 Enterobacter aerogenes,28 S. typhimurium 13 and E. coli.13 Finally, iNOS‐deficient mice failed to inhibit replication of Listeria monocytogenes, and succumbed to Listeria inocula that were at least 10‐fold lower than those lethal to wildtype mice.43
Viruses
NO has demonstrated antiviral activity via a variety of different NO donor molecules. S‐nitroso‐acetylpenicillamine (SNAP) and 3‐morpholinosydnonimine (SIN‐1) inhibited Epstein‐Barr virus (EBV) protein synthesis and DNA amplification.44 SNAP also inhibited the severe acute respiratory syndrome coronavirus replication cycle in a concentration‐dependent manner45 and reduced porcine parvovirus DNA, protein synthesis, and replication in vitro.46 Acidified nitrite cream demonstrated a 75% cure rate in patients treated for molluscum contagiosum.47
Parasites
There is evidence that microglia inhibit Toxoplasma gondii replication by an effector mechanism that utilizes NO; this is important in cerebral toxoplasmosis.29 In murine macrophages and mice, Leishmania proliferation increased when NO synthesis was inhibited.29 Indeed, survival of Leishmania within host macrophages depends on the parasite's ability to inhibit host iNOS expression or activity.48 Zeina et al.49 successfully treated a male patient with cutaneous leishmaniasis with topical glyceryl trinitrate, an exogenous NO donor. The S‐nitrosoglutathione (GSNO), S‐nitroso‐N‐acetyl‐l‐cysteine (SNAC),48 and peroxynitrite50 have demonstrated leishmanicidal activity in vitro. NO‐donors can kill other parasites including Trypanosoma cruzi 51 and Plasmodium falciparum.52
Fungi
NO impedes the growth of Cryptococcus neoformans,14, 53, 54, 55 and when NG‐mono‐methyl‐l‐arginine (l‐NMMA, a competitive inhibitor of NO synthesis) was added to activated murine macrophages, the in vitro production of NO and cryptostatic activity of the macrophages was suppressed.53 NO donor molecules also demonstrate antifungal activity: DETA‐NO inhibited the growth of six Candida species11 and the NO liberated from acidified sodium nitrite was effective against Candida albicans, Trichophyton mentagrophytes, and Trichophyton rubrum.30 Finally, a gNO‐producing probiotic patch was fungicidal to T. mentagrophytes and T. rubrum.25
NO‐RELEASING PLATFORMS
The use of exogenous NO for antimicrobial purposes has predominantly been designed to mimic the action of iNOS, i.e., both are designed to synthesize high quantities of NO for an extended period of time.15 Ideally, NO‐generators or donors would be stable at room temperature for easy storage, released predictably at therapeutic doses, delivered effectively to target sites, and cause minimal toxicity.15, 18 Several classes of natural and synthetic NO donors exist; they include gNO, organic nitrites and nitrates, acidified nitrites, RSNOs, diazeniumdiolates (NONOates), NO‐metal complexes, an NO‐releasing probiotic patch, and zeolites. Those that have been evaluated for their antimicrobial efficacy are highlighted below.
Gaseous NO
Ghaffari et al.26 designed a gNO exposure chamber to test the antimicrobial efficacy of gNO on common clinical pathogens. Constant exposure to 80 ppm of gNO inhibited P. aeruginosa and S. aureus growth, and gNO was bactericidal at 160 ppm.26 gNO delivered at 200 ppm for 24 h was bactericidal against a variety of clinically relevant pathogens, including S. aureus, MRSA, E. coli, Group B Streptococcus, P. aeruginosa, and C. albicans.40 When gNO was administered intermittently to S. aureus, P. aeruginosa, and E. coli in short durations and at high doses (160 ppm), the same bactericidal effect was demonstrated compared to continuous gNO delivery. However, it took 10 h longer to achieve this effect.7 Furthermore, the utility of intermittent gNO treatment may be limited in vivo because the time between treatments may permit bacterial replication; this possibility warrants further investigation. The efficacy of continuous gNO treatment was also evaluated in vivo: S. aureus was inoculated into full‐thickness wounds in the New Zealand white rabbit; wounds were then treated with 200 ppm of gNO for 8 h a day for three consecutive days. Treatment caused significant reduction in wound bacterial burden.56 Although effective as an antimicrobial, gNO is limited because of its expense, required delivery from a gas tank, length of time required for treatment, requirement for nonambulation during therapy, and potential toxicity to host cells from the production of NO2 and development of methemoglobinemia.7, 23, 25, 27, 40 Furthermore, gNO is not the best candidate for topical antimicrobial therapy because its short half‐life prevents delivery to deep wounds.8
Organic NO Donors: Nitrates and Nitrites
Organic NO donors include nitroglycerin, isosorbide dinitrate, isosorbide 5‐mononitrate, and sodium nitroprusside and have long been used to treat cardiovascular disease. There is a paucity of research examining the potential of these NO donors as antimicrobials, although two reports indicate that they have limited antibacterial and biofilm disrupting capabilities.15 Organic nitrates are limited because of the well‐known side effect of tachyphylaxis after continuous and prolonged use, and sodium nitroprusside has the feared side effect of cyanidosis.23 The availability of alternative NO donors that are more easily administered and cause fewer side effects decreases the likelihood that organic NO donors will be further investigated for antimicrobial efficacy.
Acidified Nitrite
Acidified nitrite creams generate NO via the reaction between an acid and nitrite.15 In an in vitro investigation, the addition of nitrite increased the microbicidal activity of acid solutions containing common cutaneous pathogens, including S. aureus, P. acnes, C. albicans, T. rubrum, and T. mentagrophytes.30 This NO donor is also effective in killing P. aeruginosa and B. cepacia.41 Acidified nitrite cream has demonstrated efficacy in human studies of tinea pedis,57 tinea versicolor,58 molluscum contagiosum47 and MRSA.59 These creams are advantageous because they are easily applied and because they are effective against several pathogens. However, they are limited because the ingredients must be mixed together immediately prior to use15 and because they have been associated with skin irritation after application.15, 18, 21
S‐nitrosothiols
RSNO include a variety of NO donors that all possess an NO moiety bound to a thiol (sulfhydryl group)23; NO is released when this bond is cleaved. Although NO release does not occur spontaneously, it can transpire in physiologic conditions. NO release can be induced by light with a wavelength of 550–600 nm, direct reaction with ascorbate, or copper ion‐mediated decomposition.15, 23 In addition to releasing NO, RSNO can participate in transnitrosylation, the process of transferring NO to another thiol group.15 This has important implications in the skin, as thiol groups are abundant in the cysteine‐rich stratum corneum.18 Two RSNOs (see Figure 2), S‐nitrosoglutathione (GSNO), and S‐nitroso‐N‐acetylcysteine (SNAC) were evaluated for antimicrobial efficacy, and demonstrated effective inhibitory and bactericidal effects against P. aeruginosa, coagulase‐negative Staphylococci, S. aureus, Serratia marcescens, and E. aerogenes. SNAC had greater antimicrobial activity compared to GSNO in all clinical isolates tested.28 GSNO and SNAC are also active against Leishmania major and Leishmania amazonensis.48 Despite demonstrated antimicrobial efficacy, RSNO are limited in their utility to treat SSTI because thiols spontaneously form disulfide bonds in the presence of heat and water, requiring their refrigeration as powder until they are ready for use.15 Additionally, light, heat and enzymes such as superoxide dismutase and a variety of dehydrogenases can induce premature NO release from the NO‐thiol bond.4
Figure 2.
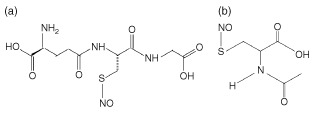
(a) S‐nitrosoglutathione (GSNO) structure and (b) S‐nitroso‐N‐acetylcysteine (SNAC) structure. (Reprinted with permission from Ref 60. Copyright 2004 Wiley‐Blackwell)
Diazeniumdiolates
These synthetic NO donors are easily produced via a reaction between NO and a variety of different amines. NONOates are stable under ambient conditions but release two molar equivalents of NO spontaneously when exposed to aqueous solution.19, 23, 61, 62 Rates of NO release can be controlled by modulating various parameters including pH, temperature, and the structure of the nucleophile to which the NO is complexed.19, 27 β‐Gal‐NONOate demonstrated higher bactericidal activity against E. coli compared to conventional NONOate.42 (Z)‐1‐[N‐(2‐aminoethyl)‐N‐(2‐ammonioethyl)amino]diazen‐1‐ium‐1,2‐diolate (DETA‐NO) is a NONOate that inhibited growth of six Candida species, and is synergistic when used in concert with azole antifungal drugs.11 NONOates are advantageous because they spontaneously release NO in biological milieus at predictable and dependable rates,11, 19, 63 they are easy to prepare, have an excellent shelf life62 and structural diversity.63 Yet the formation of methemoglobin potentially limits their use, as well as the risk of pulmonary and systemic toxicity secondary to the production of NONOate metabolites.27 For example, the N‐nitroso byproduct of O(2)‐vinyl 1(pyrrolidin‐1‐yl)diazen‐1‐ium‐1,2‐diolate (V‐PYRRO/NO) is a hepatocarcinogen.15, 63 The availability of other, less toxic NO donors with antimicrobial efficacy minimizes NONOate use for this purpose.15
NO Probiotic Patch
The probiotic patch is a simple and cost effective method for generating gNO at effective doses. It exploits the metabolic activity of Lactobacillus fermentum, a lactic acid‐producing bacterium. The lactic acid reacts with nitrite salts present in the gas‐permeable patch to produce gNO. The patch was bactericidal against E. coli, S. aureus, P. aeruginosa, and MRSA, and resulted in almost complete death of A. baumannii. It was also fungicidal toward T. mentagrophytes and T. rubrum.25 Patch application to S. aureus‐infected full‐thickness wounds in the New Zealand white rabbit caused significant decrease in wound area but a nonsignificant decrease in wound bacterial burden compared to controls.64 A major limitation to this system is the fact that the rate of gNO production depends on the activity of L. fermentum in each patch; this introduces variability in peak NO synthesis between patches.15
Zeolites
These are a new class of NO donors and consist of a framework of metal ions that can bind gNO and store it until exposure to water.23 The rate and extent of NO release can be altered by modifying pore size and the metal ions within the lattice.18 They are advantageous because of their stability, large storage capacity for NO and modifiable rate of NO release.4, 15, 23 Zeolites are effective against MSSA, MRSA, P. aeruginosa, and C. difficile,65 among others.15 More investigations are necessary to further elucidate zeolites' antimicrobial properties and potential for utility in the treatment of SSTI.4
Despite the efficacious antimicrobial activity of these NO donors, many have limitations, including instability on the skin surface,60 release of NO in low or inconsistent concentrations,15 short duration of action,8, 60 expense,23 and toxicity.40 Nanoparticulate platforms represent a unique way of circumventing some of these limitations.
NANOTECHNOLOGY AND NITRIC OXIDE
Nanotechnology represents a platform from which to deliver drugs to promote wound healing and treat infections, including SSTI. Because of their small size and high surface‐to‐volume ratio, npsallow for targeted delivery of antimicrobial products.66 As previously mentioned, nps can be exploited for antibacterial use in two main ways: some nps have inherent antimicrobial properties, whereas others can serve as vehicles to deliver traditional antibiotics. The efficacy of antimicrobial nps is promising and suggests that the encapsulation of nontraditional antimicrobial agents may be similarly efficacious. The incorporation of NO into nps presents an innovative avenue for the treatment of SSTI.
The nps that either generate or donate NO are advantageous over previously developed NO donor molecules for several important reasons. Firstly, the rate and duration of NO release can be modified by alterations in np size, composition and surface hydrophobicity.10 Secondly, toxicity can be minimized by varying the ingredients used for np synthesis. Thirdly, np synthesis can incorporate specific functional groups to maximize targeted delivery as well as to enable medical imaging.10 Finally, nps are advantageous because their small size enables them to surpass biological barriers that impede targeted delivery of drugs in other forms.
Nitric Oxide‐releasing Nanoparticles
Hybrid NO‐releasing Nanoparticles
Friedman et al.16 developed hybrid hydrogel/glass composite NO‐releasing nanoparticles (NO‐nps) through which encapsulated sodium nitrite is thermally reduced to NO within the polymeric nps.16 This platform is based on established silane‐based sol–gels made from either tetramethoxysilane (TMOS) or tetraethoxysilane (TEOS). Sol–gel refers to the transition of a system from a liquid ‘sol’ into a solid gel phase.23, 67 Sol––gels are capable of trapping proteins and other large molecules, yet they remain porous to smaller molecules like NO, which can limit their drug delivery capabilities. To overcome this limitation and minimize porosity, glass‐forming sugars and polysaccharides like chitosan can be added during sol–gel synthesis to essentially plug up these pores.23, 67 The resulting glassy properties are also of benefit because the matrix promotes the thermal reduction of nitrite to NO, as well as NO retention and sustained release.16, 23 The final NO‐np formulation is stored in a powder form. The NO remains trapped in the matrix when dry, permitting easy storage. Upon exposure to an aqueous environment, NO release is initiated as the nps swell from taking on water.4, 16, 31 The rate and total quantity of NO release can be modified by altering the synthesis steps, such as changing the concentration of nitrite or polyethylene glycol's (PEG) molecular weight and/or concentration.4, 15 For example, the utilization of larger PEGs increase pore size, allowing for a rapid bolus‐type NO release pattern, whereas NO‐nps made with smaller PEGs demonstrated a slower, sustained NO release over time. NO‐nps were minimally toxic to treated human lung fibroblasts and reconstituted human epidermis in vitro.16, 68 Human lung fibroblasts treated with NO‐nps in vitro demonstrated minimal toxicity compared to those cultured with media and control particles. This suggests that these NO‐nps are therapeutic agents safe for topical application.16 Furthermore, no clinical adverse events were reported in murine models of infection treated with NO‐nps. The ease of synthesis, storage, administration and control over NO release makes NO‐nps attractive for a broad range of clinical scenarios, including the treatment of SSTIs.
NO‐releasing Silica Nanoparticles
Shin et al.19 prepared synthetic NO‐releasing silica nps via a sol–gel process. The drug delivery potential of silica is attractive because of its chemical and structural versatility, as well as the fact that it is nontoxic.19 TEOS or TMOS was combined with aminoalkoxysilane, ethanol or methanol, water and ammonia; the amine functional groups were then converted to NONOates. This technology is advantageous for two reasons: first, because it is capable of storing large quantities of NO. Second, because np size (20–500 nm), half‐life (0.1–12 h) and release kinetics (15–30 h) can be altered by modifications in the synthetic process such as temperature, pH and the type and concentration of ingredients.10, 19
NITRIC OXIDE‐RELEASING NANOPARTICLES IN THE TREATMENT OF SOFT TISSUE INFECTIONS
In Vitro Data
The hybrid NO‐nps developed by Friedman et al. exhibited in vitro efficacy against a variety of gram‐positive and gram‐negative bacteria, including MSSA,69 MRSA,69 Streptococcus pyogenes,1 E. faecalis,1 A. baumannii,70 K. pneumoniae,1 E. coli 1 and P. aeruginosa.1 This nanoparticle platform was also effective against C. albicans in vitro.71
NO‐releasing silica nps demonstrated greater bactericidal efficacy against P. aeruginosa when compared to a nonencapsulated small molecule NO donor 1‐[2‐carboxylato)pyrrolidin‐1‐yl]diazen‐1‐ium‐1,2‐diolate (PROLI/NO).10 Cytotoxicity studies with mouse fibroblasts confirmed that NO‐releasing silica nps are nontoxic to these mammalian cells at concentrations capable of killing P. aeruginosa, while PROLI/NO was toxic to host cells at bactericidal concentrations.10 Smaller NO‐releasing silica nps (50 nm) were more effective in killing P. aeruginosa compared to larger nps with identical NO release profiles.12 Importantly, several bacterial species tested (MSSA, MRSA, S. epidermidis, E. coli, and P. aeruginosa) were unable to develop resistance to NO from silica nps after multiple exposures and colony passages.8
The silica‐based nps effectively killed biofilm‐forming pathogens, demonstrating greater than a 99% kill rate of biofilm cells of P. aeruginosa, E. coli, S. aureus, S. epidermidis, and C. albicans, with the greatest efficacy against P. aeruginosa and E. coli. Biofilms represent a serious therapeutic impediment given their ability to block drug penetration as well as permit transfer of resistance genes between communal cells.72 It is hypothesized that the ease with which NO diffuses across biological membranes may allow for its enhanced penetration into biofilms compared to traditional antibiotics.32 Therefore, an NO‐delivering platform may be one avenue to address this challenge.72
In Vivo Models
Excisional Wound Infections
Friedman et al.'s hybrid NO‐nps have been investigated in multiple murine infection models. When applied to a murine model of MRSA‐infected full‐thickness wounds, NO‐np‐treated wounds clinically demonstrated accelerated wound closure (Figure 3) and significantly lower bacterial burden as compared to controls. Histological examination of wounded tissue showed that those infected wounds treated with NO‐np had less inflammation, more organized granulation tissue, and less destructive changes to dermal architecture than in controls.69
Figure 3.
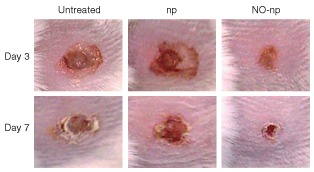
Nitric oxide‐releasing nanoparticles (NO‐nps) accelerated healing in methicillin‐resistant Staphylococcus aureus (MRSA)‐infected excisional wounds. Wounds were untreated, treated with nanoparticles without NO (np), or treated with NO‐np. (Reprinted with permission from Ref 69. Copyright 2009 Nature Publishing Group)
In an analogous study, these NO‐nps were applied to a murine model of MDR A baumannii‐infected full‐thickness excisional wounds. Similar to their effect on MRSA‐infected wounds, NO‐nps significantly increased the rate of wound healing (Figure 4), even more so than in the MRSA‐infected wounds, decreased wound bacterial loads, and inhibited collagen degradation.70 A. baumannii is an increasingly common etiologic agent of nosocomial infections, and is also implicated wound infections in soldiers deployed in Iraq and Afghanistan. Its resistance to many antibiotics complicates treatment of such infections;70 therefore, the success of topical NO‐nps in the treatment of A. baumannii wound infections is promising.
Figure 4.
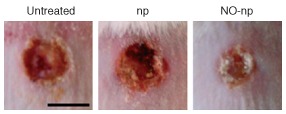
Nitric oxide‐releasing nanoparticles (NO‐nps) accelerated healing in Acinetobacter baumannii‐infected excisional wounds. Wounds were untreated, treated with nanoparticles without NO (np), or treated with NO‐np, 3 days post‐infection. (Reprinted with permission from Ref 70. Copyright 2010 Landes Bioscience)
Burn Wound Infections
The hybrid NO‐nps were found to be effective in treating burn wounds infected with C. albicans. Treated wounds healed significantly faster than control wounds (Figure 5) and had significantly lower fungal burden. Histological analysis demonstrated less suppurative inflammation and more fibrin deposition in NO‐np‐treated groups, with an associated increase in collagen content. Interestingly, mice in the control groups clinically demonstrated fungal transmission from the burn site (on their backs) to their paws as indicated by erythema and white maceration. This finding highlights the importance of quickly and effectively treating these infections to eliminate potential dissemination.71
Figure 5.
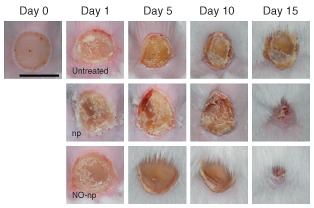
Nitric oxide‐releasing nanoparticles (NO‐nps) accelerated healing in Candida albicans‐infected burn wounds. Wounds were untreated, treated with nanoparticles without NO (np), or treated with NO‐np. Bar = 5 mm. (Reprinted with permission from Ref 71. Copyright 2012 Frontiers)
Abscesses
MRSA is a common pathogen also associated with deeper bacterial infections, such as intradermal, and intramuscular abscesses. Because of their biofilm‐like character and poor perfusion, abscesses are often difficult to treat with conventional antibiotics. In light of this, Friedman et al.'s hybrid NO‐nps were evaluated for the treatment of both of these clinical entities in murine model. Both topical and intradermal NO‐np application significantly reduced intradermal abscess area (Figure 6) and bacterial burden. Treatment resulted in improved preservation of dermal and subcutaneous architecture, with less inflammation, and bacterial presence on histologic exam.68
Figure 6.
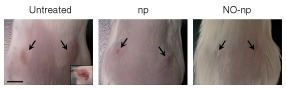
Nitric oxide‐releasing nanoparticles (NO‐nps) decrease methicillin‐resistant Staphylococcus aureus (MRSA)‐infected intradermal abscess area. Abscesses were untreated, treated with nanoparticles without NO (np), or treated with NO‐np, day 4. Arrows denote abscesses; inset demonstrates a representative purulent abscess 4 days after MRSA infection. Bar = 5 mm. (Reprinted with permission from Ref 68. Copyright 2009 Public Library of Science)
In a mouse model of MRSA‐infected intramuscular abscesses, both topical and intralesional administration of the hybrid NO‐nps also significantly decreased MRSA burden within the muscle compared to control mice and, in animals treated with systemic vancomycin, a commonly used systemic antibiotic for MRSA SSTIs. NO‐np‐treated mice demonstrated clinically accelerated abscess clearance based on visual decrease in abscess size and purulence compared to other treatment groups (Figure 7). Histologically, intralesional NO‐np administration resulted in less muscle necrosis, granulomatous inflammation, and decreased bacterial load compared to control mice. While vancomycin did have a significant impact on the intramuscular abscesses as compared to untreated, the outcome was not to the extent as those animals treated with the NO‐nps.33
Figure 7.
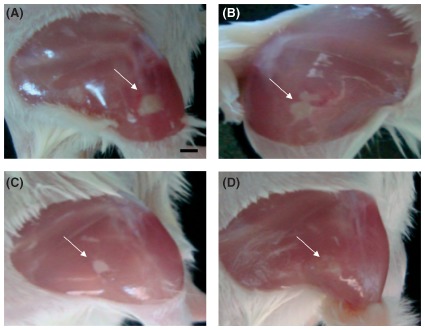
Nitric oxide‐releasing nanoparticles (NO)‐nps decrease methicillin‐resistant Staphylococcus aureus (MRSA)‐infected intramuscular abscess area. Induced MRSA‐infected intramuscular abscesses were clinically evaluated on day 4 after infection. These images, untreated (a), treated with vancomycin (b), treated topically with NO‐nps (c), or treated intralesionally with NO‐nps (d) are representative of the clinical appearance of these lesions Arrows denote abscesses. (Reprinted with permission from Ref 33. Copyright 2012 Landes Bioscience)
RSNO NANOPARTICLES
RSNOs are NO‐donating compounds that are generated from the reaction of NO with a thiol. S‐nitrosoglutathione (GSNO) is an S‐nitrosothiol, and functions as an NO donor that can transfer the nitrosonium ion to thiol moieties on proteins in a process called trans S‐nitrosylation.15 GSNO's main activity is nitrosation of sulfhydryl‐containing cellular proteins. In doing so, GSNO can reversibly block enzyme and protein functioning and disable key pathogen machinery. To counteract the threat to cell viability that results from nitrosation of critical cellular elements, bacteria employ GSNO reductases40 and nitroreductases, and also regenerate GSH.34
GSNO serves as a stable reservoir for NO donation and is advantageous compared to NO because S‐nitrosothiol half lives are measured in minutes to hours, compared to the seconds‐long half‐life of free NO.27 Additionally, as described above, GSNO is a potent nitrosating agent, conferring it with antimicrobial activity that threatens microorganism viability. In fact, the antimicrobial efficacy of GSNO in solution against E. coli has been previously reported.34 To elucidate GSNO's impact on bacterial growth and survival, Friedman et al. evaluated the ability of the hybrid NO‐nps to generate GSNO in the presence of GSH.34 When combined with GSH, NO‐np not only formed GSNO, but also produced significant concentrations of GSNO over an extended time period (greater than 24 h). This is likely secondary to the controlled and sustained release of NO from the NO‐np, which corresponds to steady GSNO formation. The mixture of NO‐np with GSH significantly inhibited the growth and/or survival of E. coli, K. pneumoniae, and P. aeruginosa compared to controls and NO‐np alone. K. pneumoniae was the most resistant to this formulation, whereas P. aeruginosa was the most susceptible and exhibited no growth over 24 h.34
Given the static and cidal activity of NO‐np‐generated GSNO in vitro, the efficacy of this platform was evaluated in the previously described excisional wound model infected with an MDR clinical isolate of P. aeruginosa. Wounds treated with NO‐np + GSH exhibited significantly accelerated wound closure clinically and histologically, as well as lower bacterial burden based on tissue cultures when compared to NO‐np‐treated and control wounds. The finding that NO‐np + GSH was more effective than NO‐np correlates to the in vitro data in that P. aeruginosa may be more sensitive to nitrosothiols as opposed to NO. In both the in vitro and in vivo setting, NO‐nps + GSH had greater antimicrobial activity compared to NO‐nps alone. This may be because GSNO is a more stable reservoir for NO and because it is a potent nitrosating agent, capable of rendering microbial proteins inactive. Additionally, GSNO can be actively taken up by microbial systems that usually function to import GSH. This enables GSNO to reach intracellular bacterial targets that NO cannot access. These results are promising and further highlight the versatility and applicability of NO‐nps to a wide array of clinical scenarios.73
CONCLUSION
The rise of pathogen resistance to our antimicrobial armamentarium and the economic burden of infections due to MDR organisms underscore the need for the development of innovative therapeutics to circumvent this problem.10, 68 Nitric oxide is an attractive approach to combating this medical epidemic due to its multiple mechanisms of both static and cidal activity against a broad range of organisms. NO's small size and hydrophobic nature enable it to rapidly traverse bacterial membranes, where it can significantly impact and interfere with cell function. Importantly, it has been shown that multiple bacterial species do not develop resistance to exogenous NO even after multiple exposures and cell passages—it is therefore unlikely that resistance would develop, as it would require multiple mutations to occur simultaneously.8 Despite the proven antimicrobial efficacy of a variety of NO donors, many have limitations that preclude their use in clinical settings. Recent advances in NO delivery, particularly the use of nanotechnology, are promising. The ease of nanoparticle production, storage, administration, and modulation render it an attractive therapeutic modality for SSTI. Its design for local application minimizes the risk for systemic toxicity associated with traditional, systemically administered antibiotics. The proven in vitro and in vivo antimicrobial efficacy provide further evidence that NO‐based nanotechnologies have the potential to treat SSTI caused by a variety of pathogens, including those with resistance to traditional antibiotics. Their therapeutic use in combat and/or disaster situations in which specialized medical care or technology is not readily available would be ideal given the breadth of physiologic, and importantly, antimicrobial, activities.17 NO‐nps are an innovative approach and promising solution to the treatment of SSTI in the setting of escalating bacterial resistance.
Conflict of interest: Co‐inventor of hybrid NO‐np.
References
- 1. Friedman A, Blecher K, Sanchez D, Tuckman‐Vernon C, Gialanella P, Friedman J, Martinez L, Nosanchuk J. Susceptibility of Gram‐positive and ‐negative bacteria to novel nitric oxide‐releasing nanoparticle technology. Virulence 2011, 2:217–221. [DOI] [PubMed] [Google Scholar]
- 2. Marra F, Patrick D, Chong M, McKay R, Hoang L, Bowie W. Population‐based study of the increased incidence of skin and soft tissue infections and associated antimicrobial use. Antimicrob Agents Chemother 2012, 56:6243–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huttner B, Goossens H, Verheij T, Harbarth S, on behalf of the CHAMP consortium . Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high‐income countries. Lancet Infect Dis 2010, 10:17–31. [DOI] [PubMed] [Google Scholar]
- 4. Englander L, Friedman A. Nitric oxide nanoparticle technology: a novel antimicrobial agent in the context of current treatment of skin and soft tissue infection. J Clin Aesthet Dermatol 2010, 3:45–50. [PMC free article] [PubMed] [Google Scholar]
- 5. Lautz T, Raval M, Barsness K. Increasing national burden of hospitalizations for skin and soft tissue infections in children. J Pediatr Surg 2011, 46:1935–1941. [DOI] [PubMed] [Google Scholar]
- 6. Frei C, Makos B, Daniels K, Oramasionwu C. Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in United States children. J Pediatr Surg 2010, 45:1967–1974. [DOI] [PubMed] [Google Scholar]
- 7. Miller C, McMullin B, Ghaffari A, Miller J, Stenzler A, Pick N, Roscoe D, Ghahary A, Road J, Av Gay Y. Gaseous nitric oxide bactericidal activity retained during intermittent high‐dose short duration exposure. Nitric Oxide 2009, 20:16–23. [DOI] [PubMed] [Google Scholar]
- 8. Privett B, Broadnax A, Bauman S, Riccio D, Schoenfisch MH. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Rosso J. Wound care in the dermatology office: where are we in 2011? J Am Acad Dermatol 2011, 64:S1–S7. [DOI] [PubMed] [Google Scholar]
- 10. Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH. Bactericidal efficacy of nitric oxide‐releasing silica nanoparticles. ACS Nano 2008, 2:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McElhaney‐Feser G, Raulli R, Cihlar R. Synergy of nitric oxide and azoles against Candida species in vitro. Antimicrob Agents Chemother 1998, 42:2342–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carpenter A, Slomberg D, Rao K, Schoenfisch M. Influence of scaffold size on bactericidal activity of nitric oxide‐releasing silica nanoparticles. ACS Nano 2011, 5:7235–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide‐related antimicrobial activity. J Clin Invest 1997, 99:2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green S, Nacy C. Antimicrobial and immunopathologic effects of cytokine‐induced nitric oxide synthesis. Curr Opin Infect Dis 1993, 6:384–396. [Google Scholar]
- 15. Schairer D, Chouake J, Nosanchuk J, Friedman A. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedman A, Han G, Navati M, Chacko M, Gunther L, Alfieri A, Friedman J. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008, 19:12–20. [DOI] [PubMed] [Google Scholar]
- 17. Cabrales P. Nanoengineering bactericidal nitric oxide therapies. Virulence 2011, 2:185–187. [DOI] [PubMed] [Google Scholar]
- 18. Weller R. Nitric oxide‐containing nanoparticles as an antimicrobial agent and enhancer of wound healing. J Invest Dermatol 2009, 129:2335–2337. [DOI] [PubMed] [Google Scholar]
- 19. Shin J, Metzger S, Schoenfisch M. Synthesis of nitric oxide‐releasing silica nanoparticles. J Am Chem Soc 2007, 129:4612–4619. [DOI] [PubMed] [Google Scholar]
- 20. Pacelli R, Wink D, Cook JK, Krishna MC, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell J. Nitric oxide potentiates hydrogen peroxide‐induced killing of Escherichia coli . J Exp Med 1995, 182:1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ormerod A, Copeland P, Hay I, Husain A, Ewen S. The inflammatory and cytotoxic effects of a nitric oxide releasing cream on normal skin. J Invest Dermatol 1999, 113:392–397. [DOI] [PubMed] [Google Scholar]
- 22. Medeiros R, Figueiredo C, Passos G, Calixto J. Reduced skin inflammatory response in mice lacking inducible nitric oxide synthase. Biochem Pharmacol 2009, 78:390–395. [DOI] [PubMed] [Google Scholar]
- 23. Friedman A, Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv 2009, 6:1113–1122. [DOI] [PubMed] [Google Scholar]
- 24. Bruch‐Gerharz D, Ruzicka T, Kolb‐Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol 1998, 110:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Jones ML, Ganopolsky JG, Labbe A, Prakash S. A novel nitric oxide producing probiotic patch and its antimicrobial efficacy: preparation and in vitro analysis. Appl Microbiol Biotechnol 2010, 87:509–516. [DOI] [PubMed] [Google Scholar]
- 26. Ghaffari A, Neil D, Ardakani A, Road J, Ghahary A, Miller C. A direct nitric oxide gas delivery system for bacterial and mammalian cell cultures. Nitric Oxide 2005, 12:129–140. [DOI] [PubMed] [Google Scholar]
- 27. Nacharaju P, Tuckman‐Vernon C, Maier KE, Chouake J, Friedman A, Cabrales P, Friedman JM. A nanoparticle delivery vehicle for S‐nitroso‐N‐acetyl cysteine: sustained vascular response. Nitric Oxide 2012, 27:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cariello A, Bispo P, de Souza G, Pignatari A, de Oliveira M, Hofling‐Lima A. Bactericidal effect of S‐nitrosothiols against clinical isolates from keratitis. Clin Ophthalmol 2012, 6:1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Groote MA, Fang FC. NO inhibitions: antimicrobial properties of nitric oxide. Clin Infect Dis 1995, 21(suppl 2):S162–S165. [DOI] [PubMed] [Google Scholar]
- 30. Weller R, Price R, Ormerod A, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J Appl Microbiol 2001, 90:648–652. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez D, Nosanchuk J, Friedman A. The purview of nitric oxide nanoparticle therapy in infection and wound healing. Nanomedicine 2012, 7:933–936. [DOI] [PubMed] [Google Scholar]
- 32. Flatley J, Barrett J, Pullman S, Hughes M, Green J, Poole R. Transcriptional responses of Escherichia coli to S‐nitrosoglutathione under defined chemostat conditions reveal major changes in methionine biosynthesis. J Biol Chem 2005, 280:10065–10072. [DOI] [PubMed] [Google Scholar]
- 33. Schairer D, Martinez L, Blecher K, Chouake J, Nacharaju P, Gialanella P, Friedman J, Nosanchuk J, Friedman A. Nitric oxide nanoparticles: pre‐clinical utility as a therapeutic for intramuscular abscesses. Virulence 2012, 3:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedman AJ, Blecher K, Schairer D, Tuckman‐Vernon C, Nacharaju P, Sanchez D, Gialanella P, Martinez LR, Friedman JM, Nosanchuk JD. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S‐nitrosoglutathione. Nitric Oxide 2011, 25:381–386. [DOI] [PubMed] [Google Scholar]
- 35. Bogdan C. Nitric oxide and the immune response. Nat Immunol 2001, 2:907–916. [DOI] [PubMed] [Google Scholar]
- 36. MacLean A, Wei X, Huang F, Al‐Alem U, Chan W, Liew F. Mice lacking inducible nitric‐oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J Gen Virol 1998, 79:825–830. [DOI] [PubMed] [Google Scholar]
- 37. Fagundes C, Costa V, Cisalpino D, Amaral FS, Souza PR, Souza RS, Ryffel B, Vieira L, Silva T, Atrasheuskaya A, et al. IFN‐γ production depends on IL‐12 and IL‐18 combined action and mediates host resistance to dengue virus infection in a nitric oxide‐dependent manner. PLoS One Trop Dis 2011, 5:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou X, Potoka D, Boyle P, Nadler E, McGinnis K, Ford H. Aminoguanidine renders inducible nitric oxide synthase knockout mice more susceptible to Salmonella typhimurium infection. FEMS Microbiol Lett 2002, 206:93–97. [DOI] [PubMed] [Google Scholar]
- 39. MacFarlane A, Schwacha M, Eisenstein T. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect Immun 1999, 67:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghaffari A, Miller CC, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006, 14:21–29. [DOI] [PubMed] [Google Scholar]
- 41. Major T, Panmanee W, Mortensen J, Gray L, Hoglen N, Hassett D. Sodium nitrite‐mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob Agents Chemother 2010, 54:4671–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen C, Shi Y, Song J, Qi Q, Gu L, Wang P. Delivery of nitric oxide released from β‐Gal‐NONOate activation by β‐galactosidase and its activity against Escherichia coli . Biol Pharm Bull 2006, 29:1239–1241. [DOI] [PubMed] [Google Scholar]
- 43. MacMicking J, Nathan C, Hom G, Chartrain N, Fletcher D, Trumbauer M, Stevens K, Xie Q, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 1995, 81:641–650. [DOI] [PubMed] [Google Scholar]
- 44. Kawanishi M. Nitric oxide inhibits Epstein‐Barr virus DNA replication and activation of latent EBV. Intervirology 1995, 38:206–213. [DOI] [PubMed] [Google Scholar]
- 45. Akerstrom S, Mousavi‐Jazi M, Klingstrom J, Leijon M, Lundkvist A, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol 2005, 79:1966–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei Z, Wang X, Ning X, Wang Y, Zhang H, Wang D, Chen H, Cui B. Nitric oxide inhibits the replication cycle of porcine parvovirus in vitro. Arch Virol 2009, 154:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ormerod A, White MI, Shah SAA, Benjamin N. Molluscum contagiosum effectively treated with a topical acidified nitrite, nitric oxide liberating cream. Br J Dermatol 1999, 141:1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Souza G, Yokoyama‐Yasunaka J, Seabra A, Miguel D, De Oliveira M, Uliana S. Leishmanicidal activity of primary S‐nitrosothiols against Leishmania major and Leishmania amazonensis: implications for the treatment of cutaneous leishmaniasis. Nitric Oxide 2006, 15:209–216. [DOI] [PubMed] [Google Scholar]
- 49. Zeina B, Banfield C, al‐Assad S. Topical glyceryl trinitrate: a possible treatment for cutaneous leishmaniasis. Clin Exp Dermatol 1997, 22:244–245. [PubMed] [Google Scholar]
- 50. Augusto O, Linares E, Giorgio S. Possible roles of nitric oxide and peroxynitrite in murine leishmaniasis. Braz J Med Biol Res 1996, 29:853–862. [PubMed] [Google Scholar]
- 51. Vespa G, Cunha F, Silva J. Nitric oxide is involved in control of Trypanosoma cruzi‐induced parasitemia and directly kills the parasite in vitro. Infect Immun 1994, 62:5177–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rockett K, Awburn M, Cowden W, Clark I. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun 1991, 59:3280–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tohyama M, Kawakami K, Futenma M, Saito A. Enhancing effect of oxygen radical scavengers on murine macrophage anticryptococcal activity through production of nitric oxide. Clin Exp Immunol 1996, 103:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alspaugh J, Granger D. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage‐mediated cytostasis. Infect Immun 1991, 59:2291–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Granger D, Hibbs JJ, Perfect J, Durack D. Metabolic fate of l‐arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest 1990, 85:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ghaffari A, Jalili R, Ghaffari M, Miller C, Ghahary A. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 2007, 15:368–377. [DOI] [PubMed] [Google Scholar]
- 57. Weller R, Ormerod A, Hobson R, Benjamin N. A randomized trial of acidified nitrite cream in the treatment of tinea pedis. J Am Acad Dermatol 1998, 38:559–563. [DOI] [PubMed] [Google Scholar]
- 58. Jowkar F, Jamshidzadeh A, Pakniyat S, Namazi M. Efficacy of nitric‐oxide liberating cream on pityriasis versicolor. J Dermatolog Treat 2010, 21:93–96. [DOI] [PubMed] [Google Scholar]
- 59. Ormerod A, Shah A, Li H, Benjamin N, Ferguson G, Leifert C. An observational prospective study of topical acidified nitrite for killing methicillin‐resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Res Notes 2011, 4:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Seabra A, Fitzpatrick A, Paul J, De Oliveira M, Weller R. Topically applied S‐nitrosothiol‐containing hydrogels as experimental and pharmacological nitric oxide donors in human skin. Br J Dermatol 2004, 151:977–983. [DOI] [PubMed] [Google Scholar]
- 61. Davies K, Wink D, Saavedra J, Keefer L. Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. J Am Chem Soc 2001, 123:5473–5481. [DOI] [PubMed] [Google Scholar]
- 62. Keefer L. Nitric oxide (NO)‐ and nitroxyl (HNO)‐generating diazeniumdiolates (NONOates): emerging commercial opportunities. Curr Top Med Chem 2005, 5:625–636. [DOI] [PubMed] [Google Scholar]
- 63. Keefer L. Progress toward clinical application of the nitric oxide‐releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol 2003, 43:585–607. [DOI] [PubMed] [Google Scholar]
- 64. Jones M, Ganopolsky J, Labbe A, Gilardino M, Wahl C, Martoni C, Prakash S. Novel nitric oxide producing probiotic wound healing patch: preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int Wound J 2012, 9:330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fox S, Wilkinson T, Wheatley P, Xiao B, Morris R, Sutherland A, Simpson A, Barlow PG, Butler A, Megson I, et al. NO‐loaded Zn(2+)‐exchanged zeolite materials: a potential bifunctional anti‐bacterial strategy. Acta Biomater 2010, 6:1515–1521. [DOI] [PubMed] [Google Scholar]
- 66. Blecher K, Nasir A, Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2:395–401. [DOI] [PubMed] [Google Scholar]
- 67. Han G, Tar M, Kuppam D, Friedman A, Melman A, Friedman J, Davies K. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med 2010, 7:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS One 2009, 4:e7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martinez L, Han G, Chacko M, Mihu M, Jacobson M, Gialanella P, Friedman A, Nosanchuk J, Friedman J. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol 2009, 129:2463–2469. [DOI] [PubMed] [Google Scholar]
- 70. Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence 2010, 1:62–67. [DOI] [PubMed] [Google Scholar]
- 71. Macherla C, Sanchez DA, Ahmadi MS, Vellozzi EM, Friedman AJ, Nosanchuk JD, Martinez LR. Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Front Microbiol 2012, 3:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti‐biofilm efficacy of nitric oxide‐releasing silica nanoparticles. Biomaterials 2009, 30:2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chouake J, Schairer D, Kutner A, Sanchez D, Makdisi J, Blecher‐Paz K, Nacharaju P, Tuckman‐Vernon C, Gialanella P, Friedman J, et al. Nitrosoglutathione generating nitric oxide nanoparticles as an improved strategy for combating Pseudomonas aeruginosa‐infected wounds. J Drugs Dermatol 2012, 2:1471–1477. [PubMed] [Google Scholar]


