Abstract
An emphasis on biosecurity in the cattle industry was made over the years to improve animal and public health. Nevertheless, the level of implementation of biosecurity measures (BSM) remains largely insufficient due to certain constraints. It is therefore necessary to prioritize the different BSM to be applied in accordance with the individual context and the main infectious diseases affecting cattle. Previous prioritization exercises of infectious diseases were neither specific to Belgium nor based on an exhaustive list of diseases. This study aimed at classifying the most important infectious diseases affecting cattle in Belgium. A list of 74 cattle infectious diseases reported in Europe was compiled based on a literature review. Through an online survey, Belgian rural veterinary practitioners (RVP) were asked to assign a score to each disease according to their frequency (question 1), their trends estimated between 2013‐15 (question 2), and finally to list the five most important diseases for adult cattle (question 3). Respectively, 107 and 93 RVP answered the first two questions and the last one. Results of the survey were used to classify the diseases based on their frequency, trends, and importance through an additional weighting system and a subsequent regression tree analysis. Belgian laboratory databases and previous disease prioritization exercises were also analysed and taken into account as additional data sources. For the most important diseases identified (those ranked as important by the three data sources), a literature review was performed in PubMed to identify their related risk factors and BSM. A total of 48 infectious diseases were classified as important in Belgium with six of them considered as important from the three data sources: bovine respiratory diseases (BRD), bovine respiratory syncytial virus (BRSV), bovine viral diarrhoea (BVD), infectious bovine rhinotracheitis (IBR), Q fever, and salmonellosis. Their related BSM should be prioritized in terms of BSM implementation.
Keywords: Belgium, biosecurity, cattle, classification, diseases, laboratory, prioritization, ranking, survey, trend, veterinarians
1. INTRODUCTION
Cattle farming is one of the main food‐production species in Belgium. Over the last few years, a shift from curative towards preventive medicine has been observed in the livestock sector and represents a key element of the European Union Animal Health Strategy since 2007 (European Comission, 2007). Nevertheless, several surveys highlight a low implementation level of biosecurity measures (BSM) by the farmers with different constraints expressed such as cost, usefulness, workload, and lack of clarity on the measures (Brennan & Christley, 2013; Gunn, Heffernan, Hall, McLeod, & Hovi, 2008; Hoe & Ruegg, 2006; Kristensen & Jakobsen, 2011; Nöremark, Frössling, & Lewerin, 2010; Sarrazin, Cay, Laureyns, & Dewulf, 2014; Sayers et al., 2013). The rate of implementation of BSM seems even lower in cattle farms versus pig or poultry production facilities (Sarrazin et al., 2014). To better advise cattle farmers and increase their level of implementation, it is essential to prioritize the biosecurity measures, according to the most important infectious diseases affecting or threatening Belgian cattle.
Based on the need to prioritize the infectious diseases (further referred to as diseases only) to address in terms of disease surveillance, control and eradication programs, many prioritization, or categorization exercises were conducted over the last few years. Given the lack of prevalence data for most cattle diseases, most of them followed the Delphi methodology (WHO, 2006) based on: (a) the establishment of an initial list of diseases, (b) the development of a prioritization methodology translated into a questionnaire, and (c) ranking or scoring of the different diseases by a panel of experts. The Delphi method based on a consensus approach has many advantages (e.g., no need of scientific evidence as it relies on experts’ opinion which can be modified through debates and avoids personal and political influence as a consensus is needed) and is recognized by the scientific community worldwide since its development by the RAND Corporation in the late 1960's. The recent prioritization exercises identified in the literature (ANSES, 2012; Ciliberti, Gavier‐Widén, Yon, Hutchings, & Artois, 2015; DISCONTOOLS, 2016; Havelaar et al., 2010; Humblet et al., 2012; McIntyre et al., 2014) were quantitative, semiquantitative, or qualitative and based on the Delphi method with the exception of two. One of them was based on the H‐index (McIntyre et al., 2014) and the second one on a literature review with a scoring and weighting system applied and validated by a panel of experts (Humblet et al., 2012) (Supporting Information Table S1).
Nevertheless, these scoring systems rely solely on expert's opinion and results will vary depending on: initial list of diseases to be assessed, criteria used, ranking methodology proposed, objective of the prioritization exercise, and available resources (e.g., time and quality of the expert panel involved). In addition, most of them did not consider multipathogen diseases such as mastitis, respiratory diseases, and diarrhoea, which are usually a major concern for both animal and public health and should not be automatically omitted.
The objectives of this study are to (a) identify major diseases of concern for Belgian cattle holders and their related BSM using a prioritization methodology based on the outcomes of a veterinary survey, the analysis of 3‐year laboratory databases and the review of previous prioritization articles and (b) summarize BSM related to the six most important diseases of concern, i.e., the only diseases defined as important by the three data sources following the classification process described in Figure 1.
Figure 1.
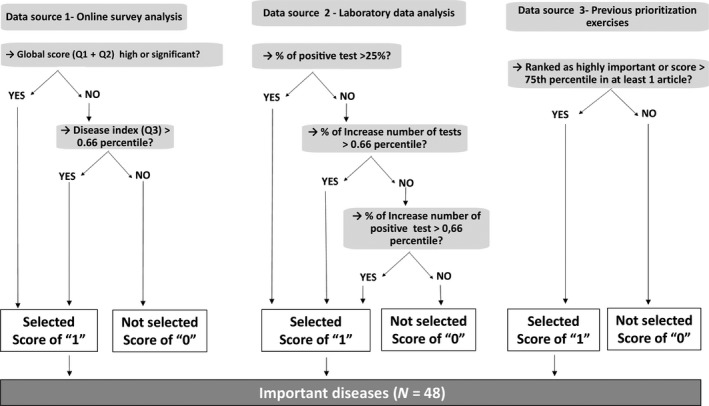
Selection criteria for the most important diseases to consider
2. MATERIAL AND METHODS
2.1. Initial list of cattle infectious diseases
An initial list of infectious cattle diseases was established based on several sources. The list provided by the Center for Food Security and Public Health, Iowa State University (http://www.cfsph.iastate.edu/DiseaseInfo/index.php) was used and completed by the review of five reference books on cattle diseases (Andrews, Blowey, Boyd, & Roger, 2008; Francoz & Yvon, 2014; Institut de l'Elevage, 2000; Kahrs, 2001; Scott, Penny, & Macrae, 2011), prioritization articles (Ciliberti et al., 2015; McIntyre et al., 2014; Phylum, 2010), diseases listed by the World Organisation for Animal Health (OIE), as well as diseases notifiable to the Belgian Federal Agency for the Safety of the Food Chain (FASFC).
Information regarding disease occurrence and importance in Europe and Belgium was collected from the FASFC (AFSCA, 2017a,b), European Centre for Disease Prevention and Control (ECDC, 2017; EFSA‐ECDC, 2015) and OIE websites and last reports (FAAV/WIV/CODA‐CERVA, 2015). The diseases for which the occurrence or existence in Europe or Belgium was not specified in those sources, a literature review was performed based on a web search in PubMed with the following combinations of terms: “name of the disease” or “name of the pathogen” and “Belgium” and/or “Europe” to complete the information. A list of 90 diseases was established with their occurrence in Europe and in Belgium, their OIE status in Belgium and basic epidemiological data (last occurrence in Belgium and zoonotic character) (Supporting Information Table S2).
2.2. Veterinary survey (Datasource 1, DS1)
In order to maintain the length of the questionnaire addressed to the rural veterinary practitioners (RVP) to the minimum, 31 diseases were excluded from the initial list of 90 diseases (16 diseases with no occurrences in Europe and 15 diseases with no occurrences in Belgium). The RVP were contacted on line through the two regional animal health organizations of the country, i.e., Association Régionale de Santé et d'Identification Animale (ARSIA) in Wallonia (southern part of the country) and Dierengezondheidszorg Vlaanderen (DGZ) in Flanders (northern part of the country) with monthly reminders over 4 months. The questionnaire was anonymous, available in French and Dutch version and could only be filled once by the same IP address. The number of persons included in the mailing list of the two organizations are respectively of 1876 and 1356 including both rural and small animal's practitioners as it was not possible to identify the part of RVP within these mailing lists.
In Wallonia, the RVP workforce (534 veterinarians having a rural practice out of 1876 veterinarians), was provided for each of the five provinces, by the Board of Veterinary Practitioners. A chi square test has been performed to assess that the sample of responding RVP is not unbalanced from one province to another. The counterpart workforce for Flanders was not available; indeed, in that region, veterinary practitioners have no obligation to provide details on their practices to the Regional Board.
The survey was pretested by four veterinarians before its final validation and included three questions in order to assess the frequency, 3‐year trend and the importance of each disease for the Belgian cattle sector. In the first question (Q1), RVP had to assign a score to each disease related to their average frequency based on the following scoring system: (a) never suspected, (b) suspected but never confirmed, (c) several times a year/occasionally, (d) at least once per quarter, and (e) several times a month.
In the second question (Q2), RVP were asked if the disease trend over the last 3 years was decreasing (score of 0), constant (score of 1), or increasing (score of 2).
The third question (Q3) was an open question where RVP were asked to list, in decreasing order of importance, the five main diseases affecting adult cattle; that information would help triangulating the information and identifying eventual diseases of importance omitted in the initial list. Each disease was assigned a score of 1 to 5, depending on its position in the list: (1) fifth disease listed, (2) fourth disease listed, (3) third disease listed, (4) second disease listed, and (5) first disease listed.
The answers to Q1 and Q2 were respectively used to calculate an average frequency score (af) and average trend score (at), for each disease. A global score per disease (GS) was then calculated by adding both averages.
| (1) |
A regression tree analysis based on the GS of the different diseases identified and classified the most important diseases to consider, from the RVP's perspective. The regression tree methodology is a nonlinear and nonparametric test increasingly used by the scientific community in public and animal health. It divides the population (in our case, the diseases) into different subgroups in relation to the GS with minimal within‐variance by using cross‐validation (Lemon, Roy, Clark, Friedmann, & Rakowski, 2003; Saegerman, Porter, & Humblet, 2011; Salford Systems, 2001).
Q3 was analysed separately. The analysis excluded noninfectious diseases such as foreign bodies and metabolic disorders, as mentioned by the RVP. The list of diseases was standardized in terms of disease denomination and consolidated. A disease index was then calculated for each disease by adding all its scores based on RVPs’ ranking. In order to identify the most important diseases, the 66th centile of the disease indexes was used as a threshold (index above 66th centile).
From the veterinary survey (DS 1), a score of “1” was attributed to (a) all diseases with a GS classified as high or important in the regression tree analysis (Table 1), and (b) all diseases having a disease index above the calculated 66th centile).
Table 1.
Classification of diseases, per category, based on the Regression Tree analysis of global score (GS), according to participants’ responses for questions 1 and 2 (N = 107)
| High GS (score = 4) | Significant GS (score = 3) | Moderate GS (score = 2) | Low GS (score = 1) |
|---|---|---|---|
|
BRD BRSV Coccidiosis (Inter)digital dermatitis Diarrhoea Intestinal Parasitism Lice and ectoparasitism Mastitis Mycoplasmosis Neosporosis Paratuberculosis Pasteurellosis Scabies |
BVD Dermatophytosis Distomatosis Enterotoxaemia Giardiasis Haemorragic enteritis Infectious bovine keratoconjuntivitis Necrobacillosis Q fever Salmonellosis Colibacillosis (verotoxic E. Coli) |
Actinobacillosis Actinomycosis Anaplasmosis Babesiosis Bluetongue Botulism Chlamydiosis Cryptococcosis Cysticercosis Dermatophilosis Enterotoxaemia (Clostridium spp) IBR Leptospirosis Listeriosis Lyme disease Papillomatosis Streptococcosis Toxi‐infections Tetanos |
Ankylostomosis Anthrax Aspergillosis Aujeszky's disease BSE BoTB Besnoitiosis Brucellosis Campylobacteriosis Cowpox Echinococcosis Encephalitis FMD Hypodermosis Malignant catarrhal fever Parafilariasis Pseudocowpox Rabies Sarcocystosis Schmallenberg disease Yersiniosis |
BoTB: bovine tuberculosis; BRD: bovine respiratory diseases; BRSV: disease caused by the bovine respiratory syncytial virus; BSE: bovine spongiform encephalopathy; BVD: bovine viral diarrhoea; FMD: foot and mouth disease; IBR: infectious bovine rhinotracheitis.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.3. Laboratory databases (DS2)
Due to the subjective character of the veterinary survey (DS1), the risk of underestimating some important diseases was not to be neglected, e.g., (re)emerging diseases with no occurrence in Belgium, and major zoonoses with a slight impact on cattle. These diseases were initially identified through the analysis of laboratory databases (DS2) provided by two regional animal health organizations, i.e., ARSIA in Wallonia (southern part of the country) and DGZ in Flanders (northern part of the country). These regional databases compiled the number of tests performed on cattle, per year and age category (adult, calves, and newborns) and their result (positive/negative), over a 3‐year period (2013 to 2015). The Veterinary and Agrochemical Research Centre (CODA‐CERVA), the national reference laboratory, provided additional data covering the period between 2012 and 2014. For each disease, the annual number of tests and the proportion of positive results were analysed. Diseases were considered equally important and attributed a score of “1” if, in at least one laboratory, one of the following arbitrary conditions was recorded: (i) >100 tests performed, (ii) >25% positive results, (iii) increasing number of tests requested over the period of concern (>66th centile), or (iv) increasing number of positive results (>66th centile). The tests linked to specific research projects were excluded from the analysis but the tests related to the official sampling scheme have been included.
2.4. Review of recent diseases prioritization exercises (DS 3)
As a third data source (DS3), six recent prioritization exercises (Supporting Information Table S1) were assessed to identify important diseases in regards to different criteria: zoonotic character (Havelaar et al., 2010; McIntyre et al., 2014), ruminants‐wildlife interactions (Ciliberti et al., 2015), European Union policies and priorities (5) and focus on food‐producing animals (ANSES, 2012; Humblet et al., 2012). As scoring and/or classification system differed in all articles, diseases were re‐classified (Table 2), as follows: 0 (not listed) to 4 (highest score/importance). Class 4 diseases of the different exercises as well as Class 3 diseases of the two articles focusing on food‐producing animals, including zoonosis (due to the importance in terms of potential economic impact on farms) (ANSES, 2012; Humblet et al., 2012) were defined as important and assigned a final score of “1”.
Table 2.
Scoring system for the reviewing of recent prioritization exercises (literature) and selection criteria
| References | Class 0 | Class 1 | Class 2 | Class 3 | Class 4 | Selection criteria DS 3 |
|---|---|---|---|---|---|---|
| Ciliberti et al. (2015) | Not listed | <25th percentile | < median | <75th percentile | >75th percentile | Class 4 |
| ANSES (2012) | Not listed | <25th percentile | < median | <75th percentile | >75th percentile | Class 3 and 4 |
| Humblet et al. (2012) | Not listed | Low imp. | Moderate imp. | Sign. Imp. | High imp. | Class 3 and 4 |
| DISCONTOOLS project | Not listed | <25th percentile | < median | <75th percentile | >75th percentile | Class 4 |
| Global ranking zoonoses (1) + (2) | Not listed | Low imp. | Moderate imp. | Medium imp. | High imp. | Class 4 |
| (1) McIntyre et al. (2014) | Not listed | <25th percentile | < median | <75th percentile | >75th percentile | |
| (2) Havelaar et al. (2010) | Not listed | Low score | Medium score | High score |
DS: data source; Imp.: importance.
In bold: classes defined as important and being assigned a score of 1 for DS 3.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.5. Databases consolidation and analysis
An overall score (OS) was calculated by adding the scores of the three DS (veterinary survey, laboratory databases, and prioritization exercises) (Table 3). Following the process of disease selection (Figure 1), all diseases with an OS > 0 (defined as important by at least one DS) were added to the list of important diseases.
Table 3.
Classification of the 48 most important diseases
| Disease | DS 1 (online survey) | DS 2 (Laboratories) | DS 3 (Previous prioritization exercises) | Score DS 3 | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global score (RT category) | Disease index > 0.66 percentile | Score DS 1 | Score DS 2 | Ciliberti et al. (2015) | ANSES, (2012) | Humblet et al. (2012) | DISCONTOOLS (2016) | McIntyre et al. (2014) and Havelaar et al. (2010) | |||
| Diseases selected from the three data sources | |||||||||||
| Bovine respiratory diseases | 4 | 1 | 1 | 1 | 4 | 2 | 2 | 1 | 3 | 1 | 3 |
| Disease caused by the bovine respiratory syncytial virus | 4 | 1 | 1 | 1 | 4 | 2 | 2 | 1 | 3 | 1 | 3 |
| Bovine viral diarrhoea | 3 | 1 | 1 | 1 | 3 | 3 | 1 | 2 | 2 | 1 | 3 |
| Infectious bovine rhinotracheitis | 2 | 1 | 1 | 1 | 3 | 3 | 0 | 1 | 2 | 1 | 3 |
| Q Fever | 3 | 0 | 1 | 1 | 4 | 4 | 4 | 2 | 6 | 1 | 3 |
| Salmonellosis | 3 | 0 | 1 | 1 | 4 | 4 | 3 | 4 | 6 | 1 | 3 |
| Diseases selected from two data sources | |||||||||||
| Coccidiosis | 4 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 2 |
| Colibacillosis (verotoxic E. Coli) | 3 | 0 | 1 | 0 | 0 | 4 | 3 | 4 | 7 | 1 | 2 |
| Cryptosporidiosis | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 4 | 4 | 1 | 2 |
| Diarrhoea/enteritis | 4 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Distomatosis | 3 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 3 | 0 | 2 |
| Giardiasis | 3 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Intestinal parasitism | 4 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Leptospirosis | 2 | 0 | 0 | 1 | 3 | 3 | 3 | 3 | 4 | 1 | 2 |
| Lice and ectoparasitism | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Listeriosis | 2 | 0 | 0 | 1 | 3 | 4 | 0 | 0 | 4 | 1 | 2 |
| Mycoplasmosis | 4 | 1 | 1 | 0 | 4 | 2 | 2 | 1 | 3 | 1 | 2 |
| Paratuberculosis | 4 | 1 | 1 | 0 | 4 | 4 | 2 | 3 | 2 | 1 | 2 |
| Pasteurellosis | 4 | 1 | 1 | 0 | 4 | 2 | 2 | 1 | 3 | 1 | 2 |
| Schmallenberg disease | 1 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 1 | 2 |
| Coccidiosis | 4 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 2 |
| Diseases selected from one data source only | |||||||||||
| Anaplasmosis/ Ehrlichiosis | 2 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 5 | 1 | 1 |
| Anthrax | 1 | 0 | 0 | 0 | 4 | 4 | 4 | 3 | 4 | 1 | 1 |
| Aujeszky's Disease | 1 | 0 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 1 | 1 |
| Babesiosis (bovine) | 2 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 1 | 1 | 1 |
| Botulism | 2 | 0 | 0 | 0 | 3 | 2 | 4 | 0 | 6 | 1 | 1 |
| Bovine enzootic leucosis (BEL) | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| Bovine Herpes virus 4 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ||||
| Bovine Spongiform Encephalopathy | 1 | 0 | 0 | 0 | 0 | 4 | 3 | 2 | 7 | 1 | 1 |
| Brucellosis | 1 | 0 | 0 | 0 | 4 | 0 | 3 | 4 | 5 | 1 | 1 |
| Campylobacteriosis | 1 | 0 | 0 | 0 | 2 | 1 | 2 | 2 | 8 | 1 | 1 |
| Crimean‐Congo Haemorrhagic Fever | 0 | 0 | 0 | 0 | 4 | 0 | 3 | 1 | 4 | 1 | 1 |
| Cysticercosis | 2 | 0 | 0 | 0 | 0 | 3 | 0 | 4 | 0 | 1 | 1 |
| Dermatophytosis /Mycosis | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Echinococcosis | 1 | 0 | 0 | 0 | 4 | 1 | 4 | 1 | 4 | 1 | 1 |
| Enterotoxaemia | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 |
| Foot and mouth disease | 1 | 0 | 0 | 0 | 4 | 0 | 4 | 4 | 3 | 1 | 1 |
| Infectious bovine keratoconjunctivitis | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| (Inter)digital dermatitis | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mastitis | 4 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 7 | 0 | 1 |
| Metritis | 0 | 1 | 1 | 0 | 0 | 0 | 1 | ||||
| Necrobacillosis (laryngitis) | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Neosporosis | 4 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Rabies | 1 | 0 | 0 | 0 | 4 | 0 | 4 | 2 | 7 | 1 | 1 |
| Scabies | 4 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 |
| Secondary infections | 0 | 1 | 1 | 0 | 0 | 0 | 1 | ||||
| Tuberculosis (bovine) | 1 | 0 | 0 | 0 | 4 | 4 | 4 | 4 | 8 | 1 | 1 |
| Winter haemorragic enteritis | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Anaplasmosis/ Ehrlichiosis | 2 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 5 | 1 | 1 |
| Anthrax | 1 | 0 | 0 | 0 | 4 | 4 | 4 | 3 | 4 | 1 | 1 |
| Aujeszky's Disease | 1 | 0 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 1 | 1 |
| Babesiosis (bovine) | 2 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 1 | 1 | 1 |
| Botulism | 2 | 0 | 0 | 0 | 3 | 2 | 4 | 0 | 6 | 1 | 1 |
DS: Data source; RT: Regression Tree; OS: overall score.
In bold: positive selection criteria for each DS.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.6. Synthesis of biosecurity measures related to the most important diseases identified
For the most important diseases identified (those ranked as important by the three data sources), a literature review was performed in PubMed to identify their related risk factors and biosecurity measures. The keywords used for the search were as follow: “name(s) of the disease” or “name(s) of the pathogen” and “cattle or bovine or cow or beef or calves or dairy” (if disease affecting multiple species only) and “epidemiology” or “pathogenesis” or “control” or “risk”.
Among the articles selected, only those articles mentioning an analysis and/or the identification of disease‐specific risk factors or BSM were fully read.
3. RESULTS
3.1. Veterinary survey
The first two questions were answered by 107 RVP, while 93 of them answered the third question. The Chi square test performed on Walloon survey showed no significant differences regarding the ratio of respondents per province and the distribution of the RVP per province (Chi square(4 df; α = 0.05) = 4.98; p‐value = 0.29).
A regression tree analysis, based on the GS, classified the diseases according to their importance. Out of the 74 diseases listed, 13 diseases were classified as being of high importance (mean = 5.157, STD = 0.345), 11 of significant importance (mean = 3.975, STD = 0.320), 19 of moderate importance (mean = 2.946, STD = 0.270), and 21 of low importance (mean = 2.118, STD = 0.228) (Table 1, Figures 2 and 3).
Figure 2.
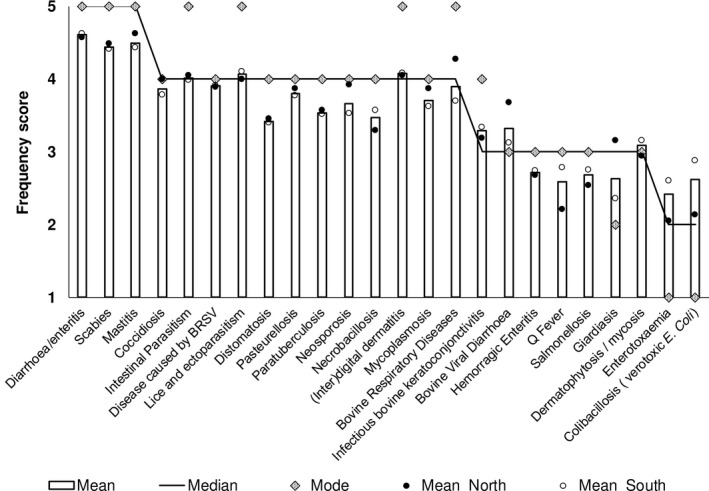
Reported frequency score of the 24 diseases of high or significant global sore (N = 107)
Figure 3.
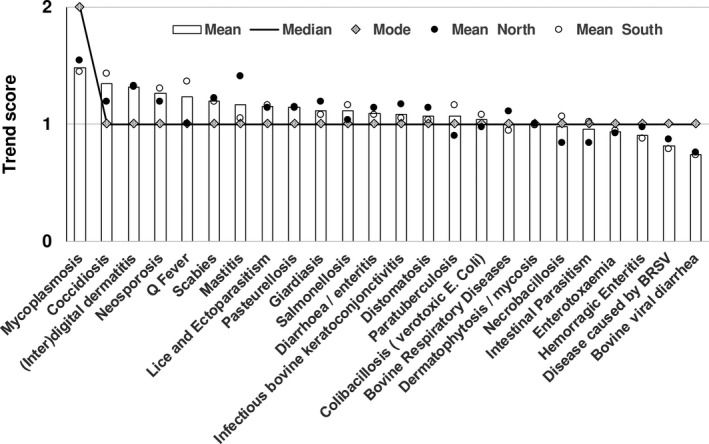
Reported trend score for the 24 diseases with a high or significant global score (N = 107). Y‐axis: 0 = decreasing; 1 = constant; 2 = increasing
Two of the most frequent diseases (bovine viral diarrhoea [BVD] and disease caused by the bovine respiratory syncytial virus [BRSV]) presented a moderate trend, the majority of veterinary practitioners considering them as constant. Eleven diseases were perceived as increasing over the last three years, but with a low or moderate frequency: anaplasmosis, babesiosis, botulism, cryptococcosis, colibacillosis (verotoxic Escherichia coli), enterotoxaemia (Clostridium spp.), giardiasis, leptospirosis, Lyme disease, Q fever, and salmonellosis (Table 4).
Table 4.
Transmission pathways and biosecurity measures related to the six most important diseases (a) Transmission pathways. (b) Biosecurity measures related to the diseases
| (a) | |||||
|---|---|---|---|---|---|
| Transmission pathways | Q fever/coxiellosis | Bovine respiratory diseasesa | Bovine Viral diarrhoea | Infectious bovine rhinotracheitis | Salmonellosis (nontyphoidal) |
| Direct and indirect contact | Yes | Yes | Yes | Yes | Yes |
| Inhalation | Yes | Yes | Yes | Yes | Yes |
| Ingestion | Yes | Yes | Yes | No | Yes |
| Transplacental and venereal | No | No | Yes | No | No |
| Vector | Yes (ticks) | No | No | No | No |
| (b) | |||||||
|---|---|---|---|---|---|---|---|
| Biosecurity measures | Q Fever / Coxiellosis | Bovine respiratory diseasesa | Bovine Viral Diarrhoea | Infectious Bovine Rhinotracheitis | Salmonellosis (nontyphoidal) | Total number of diseases addressed | |
| 1 | Animal movements | ||||||
| 1.1 | Closed herd / No movements | 2 | 2 | 2 | 2 | 2 | 5 |
| 1.2 | All in/all out system of each age group and each separate stable | 2 | 2 | 2 | 2 | 2 | 5 |
| 1.3 | Divide calves in high and low risk groups based on veal calves risk classification | 0 | 2 | 1 | 2 | 1 | 4 |
| 1.4 | Purchase from a single source and collect history on the farm of origin (status, disease history, health management history) | 2 | 2 | 2 | 2 | 2 | 5 |
| 1.5 | Premovement testing (against specific diseases) | 2 | 2 | 2 | 2 | 1 | 5 |
| 1.6 | Quarantine (3 weeks, separate area, or building (3 m distances) and testing for entering or re‐entering animals | 2 | 2 | 2 | 2 | 2 | 5 |
| 1.6 | Good transport conditions: safely, in a clean truck, decent loading ramp, no overcrowding / commingling, calm handling, as short as possible, not passing through a sorting centre | 2 | 2 | 1 | 2 | 2 | 5 |
| 2 | Vertical or venereal transmissions | ||||||
| 2.1 | No breeding animals shared with other farms | 1 | 0 | 2 | 1 | 0 | 3 |
| 2.2 | Artificial insemination | 0 | 0 | 2 | 2 | 0 | 2 |
| 3 | Direct contact with external shedders/carriers and vectors | ||||||
| 3.1 | Prevent contact in pastures with animals of neighbouring farms and wildlife (pigs and ruminants) (simple or doubles fences) | 0 | 1 | 2 | 2 | 1 | 4 |
| 3.2 | Closed housing / locked doors (prevent contact with pets, carnivores, rodents,… in stables) | 0 | 2 | 2 | 1 | 1 | 4 |
| 3.3 | Prevent access of pets in stables/ food storage facilities, manure/litter disposal facilities,… | 0 | 0 | 2 | 1 | 2 | 3 |
| 3.4 | Avoid piling manure | 0 | 2 | 2 | 0 | 0 | 2 |
| 3.5 | Preventive measures against ticks (acaricides and environmental measures) | 2 | 0 | 0 | 0 | 0 | 1 |
| 3.6 | Rodents control program | 2 | 2 | 2 | 0 | 2 | 4 |
| 4 | Food and water contamination from external shedders/carriers | ||||||
| 4.1 | Storage of feed in clean and closed structures to prevent their contamination | 0 | 2 | 0 | 0 | 2 | 2 |
| 4.2 | Clean water and feed troughs regularly | 0 | 2 | 2 | 1 | 2 | 4 |
| 4.1 | No access to surface water/ Prevent access to running or stagnant water in pastures | 0 | 0 | 0 | 0 | 2 | 1 |
| 4.2 | Cleaning and disinfection of feeding utensils / Not using them for handling manure | 0 | 2 | 2 | 2 | 2 | 4 |
| 4.1 | pH drinking water under 8,0 | 0 | 0 | 0 | 0 | 2 | 1 |
| 5 | Contamination through fomites | ||||||
| 5.1 | Prevent contact of farmer or worker with animals from other farms | 2 | 0 | 2 | 0 | 1 | 3 |
| 5.2 | Access restriction for visitors + Visitors control and register | 2 | 2 | 2 | 2 | 1 | 5 |
| 5.3 | Vehicle access restriction / no vehicles in areas where animals are kept/ passing by, separate access routes | 2 | 2 | 2 | 2 | 2 | 5 |
| 5.4 | In‐house or clean boots and clothes for visitors (availed by farmer) / | 2 | 2 | 2 | 2 | 2 | 5 |
| 5.5 | Footbaths usage and hand washing facilities | 2 | 2 | 2 | 2 | 2 | 5 |
| 5.6 | No equipment or vehicles shared with other farms | 2 | 2 | 2 | 2 | 2 | 5 |
| 5.7 | Animal transport vehicle and other vehicles leak proof and cleaned and disinfected before entry, through separate access routes. | 2 | 2 | 2 | 2 | 1 | 5 |
| 6 | General management | ||||||
| 6.01 | Animal health regular monitoring and recording. | 1 | 2 | 2 | 2 | 1 | 5 |
| 6.02 | Identification and elimination/segregation of carriers/ infected animals by regular testing | 2 | 2 | 2 | 2 | 2 | 5 |
| 6.03 | Working from young to old animals | 0 | 2 | 2 | 2 | 2 | 4 |
| 6.04 | Avoid excessive stress or accumulation of stressful events (especially for calves) | 0 | 2 | 0 | 2 | 2 | 3 |
| 6.05 | Bedding/ litter removal; keeping fresh and clean beddings; no recycling of bedding | 2 | 2 | 1 | 2 | 2 | 5 |
| 6.06 | Cemented floors / concrete flooring | 0 | 2 | 0 | 0 | 0 | 1 |
| 6.07 | Tie stall or stanchion facilities | 2 | 2 | 0 | 2 | 2 | 4 |
| 6.08 | Housing density | 2 | 2 | 2 | 2 | 2 | 5 |
| 6.09 | Good ventilation and air quality (positive pressure ventilation of >15 cubic ft. per minute per calf); maintaining a dry environment | 2 | 2 | 1 | 2 | 1 | 5 |
| 6.10 | House the animals per sex, no mixed groups | 0 | 2 | 0 | 1 | 0 | 2 |
| 6.11 | Proper feeding | 0 | 2 | 1 | 0 | 2 | 3 |
| 6.12 | Experience, training and awareness raising of handlers | 1 | 2 | 2 | 1 | 1 | 5 |
| 7 | General hygiene practices | ||||||
| 7.1 | Cleaning and disinfection of equipment after each usage (calving, milking, …) | 2 | 2 | 2 | 2 | 2 | 5 |
| 7.2 | Proper cleaning and disinfection of surgical instruments and needles between animals | 2 | 0 | 2 | 2 | 0 | 3 |
| 7.3 | Hygiene stables: sanitary vacancies, cleaning stables before introduction of new calves, steam or hot water, thorough drying of multiple days, | 2 | 2 | 2 | 2 | 2 | 5 |
| 7.4 | Personal working hygiene of workers / farmer (boots, clothes, hands,…), especially between age groups | 2 | 2 | 2 | 2 | 2 | 5 |
| 8 | Management of sick or quarantined animals | ||||||
| 8.1 | Quick recognition, isolation and treatment of sick animals | 2 | 2 | 2 | 1 | 2 | 5 |
| 8.2 | Sick animals treated last | 1 | 2 | 2 | 1 | 2 | 5 |
| 8.3 | Quarantine facilities and work organization (capacity = 2% total herds size, separate building, specific clothes and equipments, hands washing facilities, usage of gloves) | 1 | 2 | 2 | 2 | 2 | 5 |
| 8.4 | Separate housing of relapses and chronic cases | 1 | 2 | 2 | 0 | 0 | 3 |
| 9 | Parturition | ||||||
| 9.1 | Testing all cases of abortion | 2 | 0 | 1 | 0 | 0 | 2 |
| 9.2 | Maternity pen existent and separated from other areas, easy to clean and drain | 2 | 2 | 2 | 2 | 2 | 5 |
| 9.3 | Not using maternity pens for sick animals | 2 | 2 | 2 | 1 | 2 | 5 |
| 9.4 | Cleaning and disinfection (handler, animal and calving areas) | 2 | 2 | 2 | 1 | 2 | 5 |
| 9.5 | Immediate clearing of airways / Navel dipping and disinfection | 0 | 2 | 0 | 1 | 0 | 2 |
| 9.6 | Immediate separation of the calf from the mother <–> Keep the calf with cow for 24 hours (oldest) | 0 | 2 | 0 | 1 | 2 | 3 |
| 9.7 | Proper disposal of foetal membranes and tissues after abortion and/or calving | 2 | 0 | 1 | 0 | 0 | 2 |
| 10 | Calves management | ||||||
| 10.1 | Proper colostrum intake (delay, quality, and quantity) | 0 | 2 | 1 | 0 | 2 | 3 |
| 10.2 | Sufficient supply of milk + proper quality (not infected / pasteurized, proper temperature) Milk quality control and proper quantity | 0 | 2 | 1 | 0 | 0 | 2 |
| 10.3 | Gradual supply of concentrates/hay for better adaptation to new diet | 0 | 2 | 0 | 0 | 2 | 2 |
| 10.4 | Individual hutches adapted (warm, dry, well bedded, and ventilated) without possible contact between calves (>1.25 m apart) | 0 | 2 | 1 | 0 | 2 | 3 |
| 10.5 | Daily cleaning of bedding and housing of calves (stress‐free, dust‐free) | 0 | 2 | 1 | 1 | 2 | 4 |
| 10.6 | Hutches cleaned, disinfected and thoroughly dried before housing new calves (also underneath) | 0 | 2 | 1 | 0 | 2 | 3 |
| 10.7 | Use of one bucket per calf with a teat / Cleaning the buckets after each feeding | 0 | 0 | 1 | 0 | 1 | 2 |
| 10.8 | Regrouping calves from individual hutches to group pens only after vaccination, with calves of same age and in small groups (7‐10) | 0 | 2 | 2 | 2 | 1 | 4 |
| 10.9 | Calves and young stock separated from older animals and other age groups | 0 | 2 | 2 | 2 | 2 | 4 |
| 11 | Prevent human and environmental contamination | ||||||
| 11.1 | Prevent human contamination (zoonosis) | 2 | 0 | 0 | 0 | 2 | 2 |
| 11.2 | Proper disposal of manure from other farms within 500 metres | 0 | 0 | 2 | 0 | 1 | 2 |
| 11.3 | Manure spreading in the absence of wind only | 2 | 0 | 0 | 0 | 0 | 1 |
| 11.4 | Manure treatment before spreading on soils (lime or calcium cyanide 0.4%). | 2 | 0 | 0 | 0 | 0 | 1 |
Includes BRSV, mycoplasmosis, pasteurellosis, para influenza virus 3, and other respiratory diseases.
Coding: “2” for measure listed in literature review either as addressing a specific risk factor or BSM; “1” for measure not found as such during the review, but should have an effect on the disease prevention and management due to its different transmission pathway; “0” for measure without influence on the disease.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The diseases with the lower trend scores, and therefore globally perceived as decreasing or stable in Belgium, were: foot and mouth disease (FMD), hypodermosis, rabies, actinobacillosis, actinomycosis, Aujeszky's disease, bovine spongiform encephalopathy (BSE), bluetongue, brucellosis, infectious bovine rhinotracheitis (IBR), and Schmallenberg disease.
From the analysis of the disease indexes, two diseases mentioned by the RVP and not listed initially were identified: metritis/endometritis and different infectious diseases grouped as secondary infections (septicaemia, umbilical infections, peritonitis/reticulitis, (peri‐), and (poly)arthritis) (Figure 4). These two diseases were then added to the initial list of 90 diseases. Mastitis and (inter)digital dermatitis were, by far, the two most important diseases in terms of disease index and GS.
Figure 4.
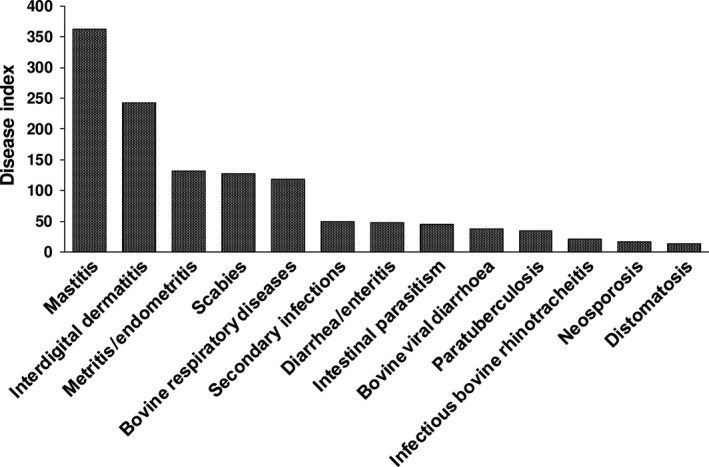
Disease index of the most important diseases affecting adult cattle, i.e., disease index above 0.66 centile (N = 93). Bovine respiratory diseases (BRD) include: pasteurellosis (Mannheimia haemolytica), pneumonia, bronchopneumonia, tracheitis, respiratory infections, and respiratory complex; diarrhoea/enteritis gathers: cryptosporidiosis, colibacillosis (E. coli), as well as diseases associated with rotavirus and coronavirus
After analysing Q3, three diseases showed a high disease index (>66th centile) but without a high or significant GS: the two diseases not listed in the initial list of diseases, i.e., metritis/endometritis and secondary infections, and IBR, not classified as important by the regression tree analysis. These three diseases were thus classified as important.
3.2. Laboratory databases
Analysis of laboratory databases revealed that an increasing number of tests or a high proportion of positive results was observed for 19 diseases. Six of them were not classified as important by the veterinary survey. A significantly increased number of tests was requested (>66th centile) for bluetongue, leptospirosis, and bovine enzootic leucosis (BEL) over the last 3 years. In addition, three diseases showed >25% positive results over the same period, i.e., cryptosporidiosis, Schmallenberg disease (>90% positive results), and bovine herpes virus 4 (Bo‐HV4; not included in the initial list of diseases but added afterwards). For Bo‐HV4, proportions of positive results reached 31% in ARSIA database versus 58% in DGZ database.
Fifteen diseases out of the 27 selected from the veterinary survey analysis are not showing an increased number of tests or percentage of positive tests over the last 3 years in DS 2 (Table 3).
3.3. Diseases prioritization exercises
In addition to the 34 diseases selected as important based on veterinary survey and laboratory databases, 14 diseases were selected from this data source. Most of them were major zoonoses, OIE/FASFC notifiable diseases and/or important cattle diseases which prevalence has been widely reduced by control programs: anaplasmosis, anthrax, Aujeszky's disease, babesiosis, bluetongue, botulism, BSE, brucellosis, campylobacteriosis, Crimean‐Congo haemorragic fever (CCHF), cryptosporidiosis, cysticercosis, echinococcosis, FMD, leptospirosis, listeriosis, rabies, Schmallenberg disease, and bovine tuberculosis (boTB).
3.4. Classification tool for adult cattle diseases in Belgium
Results of the final classification after application of the different filters are summarized in Table 3 for the 48 diseases considered as important. Six of them were identified as important by the three DS: BRD, BRSV, BVD, IBR, Q fever, and salmonellosis.
Fourteen diseases came out as important from at least two DS. Finally, 28 diseases were revealed by only one DS: 15 by prioritization exercises, 11 through the veterinary survey, and two based on laboratory databases (Bo‐HV4 and BEL).
As a reminder, the initial list of diseases included 77 items (74 diseases initially listed and three diseases added during data analysis), thus 29 of them were not classified as important at the end of the process. They are listed in Supporting Information Table S2, along with the diseases with no occurrence in European countries.
3.5. Synthesis of the biosecurity measures related to the six most important diseases
A total of 76 articles were reviewed: 6 for BRSV, 17 for BRD, 11 for BVD, 13 for IBR, 15 for Q fever, and 14 for salmonellosis (Supporting Information Table S3). A synthetic table of the six most important diseases‐related BSM (Table 4) was developed (i.e., diseases identified by the three data sources). Due to similarities and frequent co‐infections, BRSV was included in the BRD.
All six diseases can be transmitted by five possible pathways: direct and indirect contact, inhalation, ingestion, trans‐placental/ venereal, and vector‐borne. The BSM listed could be grouped into six different risk categories (animal movements, vertical and venereal transmission, direct contact with external shedders/carriers and vectors, feed and water contamination, indirect contamination through fomites, human and environmental contamination) as well as five different practices (general management, general hygiene practices, management of sick animals, calves, and calving management). Seventy‐five percent of the 67 BSM listed contribute to the prevention and control of at least three diseases, while 27 BSM contribute controlling and preventing six of them. These measures mainly fall into the following categories: animal movements, contamination through fomites, and general management (e.g., housing density, proper ventilation, clean and dry bedding). Six preventive measures are disease‐specific, i.e., (a) cemented floors/concrete floor for BRD, (b) tick control measures, manure treatment before spreading and/or spreading in the absence of wind for Q fever, and (c) preventing access to surface water and pH of drinking water below 8 for salmonellosis.
4. DISCUSSION
The most important diseases from different perspectives (farm, animal health, economical, environmental, and public health impacts) were identified using an original prioritization methodology based on the outcomes of three data sources and after correction of biases related to each of them. In particular, nineteen diseases not listed in the previous diseases prioritization exercises have been identified and represent a major concern for cattle holders while not necessarily addressed by a national control program for now.
Discrepancies between the vet survey (DS1) and laboratory data (DS2) for 13 diseases are mainly explained by the fact that they are usually diagnosed and treated in the field, on the basis of clinical signs. It is the case for the two diseases showing the most significant disease index but not coming out from DS 2 analysis (mastitis and interdigital dermatitis).
Neosporosis and paratuberculosis did not show an increasing trend but are frequently suspected by the RVP. Furthermore, they are both part of a national control program with a mean of, respectively, 155,379 and 290,057 annual test requests (DS2). A real increase was therefore unlikely.
Six diseases showed a significant increase through analysis of laboratory databases, but not from the RVP’ point of view. Indeed, the number of tests requested over the last 3 years increased for BEL, leptospirosis, and bluetongue. Even though Belgium was declared as BEL‐officially free in 1997 (AFSCA, 2017a,b), it is still tested in parallel with brucellosis to maintain this status (CODA‐CERVA, Riocreux Flavien personal communication) and do not represent a real increase of suspicions. Its classification as “important” could therefore be reconsidered. For both leptospirosis and bluetongue, the number of test requests increased significantly in 2015, despite a low frequency reported by RVP. This could be related to increased surveillance motivated by bluetongue outbreaks in France and suspected outbreaks of leptospirosis in Belgium. Indeed, for leptospirosis, a peak of abortions, with icteric syndrome, was observed during the first half of 2014, which led to increased testing (Delooz et al., 2015). In addition, subclinical infections are frequently reported with bluetongue (Brenner et al., 2010).
We observed more than 25% of positive results for Bo‐HV4 disease, Schmallenberg disease and cryptosporidiosis. These diseases were not listed as frequent, increasing or important by the RVP. Previous studies have confirmed the endemic status of Bo‐HV4 in southern Belgium, along with a high seroconversion rate of cows (Delooz, Czaplicki, Houtain, Dal Pozzo, & Saegerman, 2016). Nevertheless, the relationship between Bo‐HV4 and abortion is still subject to controversy and the disease might be underranked by the RVP due to the nonpathognomonic character of clinical signs. In order to further assess the role of Bo‐HV4 in abortions, a recent study included the search of the virus in the abortion protocol already implemented in southern Belgium (Delooz et al., 2016; Delooz, Czaplicki, Houtain, Mullender, & Saegerman, 2012) and highlighted a possible association; specific awareness raising messages were already sent in that region (Delooz et al., 2012). Regarding Schmallenberg disease, such high proportion of positive results is probably related to the confirmatory character of the test, within a herd management program or policy, as clinical signs are quite pathognomonic; it does probably not reflect a high disease prevalence nor the current circulation rate of the virus. At last, a high proportion of positive tests was noticed for cryptosporitiosis. Cryptosporidium sp. is a coccidium causing clinical signs mostly in young animals; adult cattle is resistant and, thus, does not show any clinical signs (Geurden, 2007).
The review of previous disease prioritization exercises led to include additional diseases, also considered as important, in the list. These were: (a) major zoonoses with little or no impact on animal health, i.e., campylobacteriosis, CCHF, cysticercosis and echinococcosis, (b) diseases eradicated from Belgium or targeted by an effective national control program (anthrax, Aujeszky's disease, BSE, boTB, brucellosis, FMD, and rabies), and (c) low‐incidence diseases such as anaplasmosis, babesiosis, and botulism.
The list of 48 important diseases compiled in this study is coherent with the Belgian context and includes all the OIE notifiable diseases present in Belgium with the exception of trichomonosis. The disease is rarely diagnosed by RVP or tested in the laboratories; in addition, zoonotic strains do not seem to be incriminated in cattle abortions (Shaapan, 2016). Its inclusion in the list depends on the objectives and foreseen usage of the disease classification exercise.
Out of the 48 diseases, 25 are nonnotifiable but of major importance in Belgium due to their economic impact and/or high occurrence. Nineteen of them were not considered as important by the previous prioritization exercises while relevant in Belgian adult cattle. This additional list could guide the decision makers for future control programs as these diseases are a major concern for cattle holders. The six diseases identified as important by the three data sources are covering the different diseases transmission pathways, therefore the proper implementation of their related BSM (Table 4) should improve the prevention and control of the majority of other cattle diseases. Based on the transtheoretical model of behaviour change, as well as other theories and existing models (Armitage, 2009; Mase, Gramig, & Prokopy, 2017; Morris, Marzano, Danady, & O'Brien, 2012; Prochaska & Diclemente, 1983), the “possible personal benefits” is a constant key factor motivating the adoption of new behaviour. Therefore, identifying the risk factors and associated biosecurity measures related to the six diseases with a high or significant disease index could be used to improve the technical guidance for farmers and better answer their main concerns. Once the farmer has engaged into a behaviour change and is convinced of the efficiency and relevance of biosecurity, the introduction of additional measures will be accepted easily. As the six most important diseases to consider cover all the possible transmission pathways, future researches should focus on the BSM prioritization based on their level of implementation and acceptation by the herders, their feasibility and their cost‐effectiveness in terms of disease(s) prevention. In order to ensure the acceptability of the BSM to be prioritized by the farmers a participative approach in recommended in order to take into account the farmers opinions, perceptions, and expertise on the topic.
5. CONCLUSION
Due to their possible impact on the economy, it is important to raise the level of awareness of the herders regarding emerging and exotic diseases. Nevertheless, starting by addressing the farmer's priority issues is a key strategy for them to adopt the biosecurity measures on a long‐term perspective. Identifying the most important diseases affecting cattle farms is therefore necessary in order to initiate the process of change. Specific measures related to public health purposes could be introduced easily afterwards. Future researches should focus on the assessment of the level of implementation of the BSM related to the most important diseases to be targeted (six in the case of Belgian cattle herds), as well as the possible constraints and factors affecting their adoption by the farmers in order to be able to prioritize the most effective BSM to be promoted.
The methodology proposed and relying on the outcomes of a veterinary survey, the analysis of the laboratory databases over the past 3 years and the review of previous prioritization exercises, allowed identifying the diseases of major concern for cattle holders. The proposed methodology represents a practical tool for other users who could easily adjust the selection criteria to their specific objectives, needs, and context. That makes possible the future development of a biosecurity tool useable at the national level.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
This study was supported by the Belgian Federal Public Service for Health, Food Safety, and Environment (Contract RT 15/4 BOBIOSEC1). The implementation of the survey and further analysis were possible; thanks to the collaboration with ARSIA, DGZ and CODA‐CERVA who supported the veterinary survey process and shared their 3‐year databases. We would also like to thank all the rural veterinary practitioners and the heads of Pathology Departments of both Gent and Liege universities who took the time to answer the online survey. We do hope that the outcomes of this study and further studies will be helpful to their practices.
Renault V, Damiaans B, Sarrazin S, et al. Classification of adult cattle infectious diseases: A first step towards prioritization of biosecurity measures. Transbound Emerg Dis. 2018;65:1991–2005. 10.1111/tbed.12982
REFERENCES
- AFSCA . (2017a). Notification obligatoire. Retrieved from http://www.afsca.be/notificationobligatoire/
- AFSCA . (2017b). Situation zoosanitaire en Belgique. Retrieved from http://www.afsca.be/santeanimale/zoosanitaire-belgique/default.asp
- Andrews, A. H. , Blowey, R. W. , Boyd, H. , & Roger, G. E. (2008). Bovine medecine: Diseases and husbandry of cattle (2nd ed.). West Sussex, UK: Wiley‐Blackwell. [Google Scholar]
- ANSES . (2012). Avis de l'ANSES relatif à “la hiérarchisation de 103 maladies animales présentes dans les filières ruminants, équidés, porcs, volailles et lapins en France métropolitaine.” Saisine n° « 2010‐SA‐0280 », Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail, Maisons‐Alfort, France.
- Armitage, C. J. (2009). Is there utility in the transtheoretical model? British Journal of Health Psychology, 14(2), 195–210. 10.1348/135910708X368991 [DOI] [PubMed] [Google Scholar]
- Brennan, M. L. , & Christley, R. M. (2013). Cattle producers’ perceptions of biosecurity. BMC Veterinary Research, 9, 71 10.1186/1746-6148-9-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, J. , Oura, C. , Asis, I. , Maan, S. , Elad, D. , Maan, N. , … Batten, C. (2010). Multiple serotypes of bluetongue virus in sheep and cattle, Israel. Emerging Infectious Diseases, 16(12), 2003–2004. 10.3201/eid1612.100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberti, A. , Gavier‐Widén, D. , Yon, L. , Hutchings, M. R. , & Artois, M. (2015). Prioritisation of wildlife pathogens to be targeted in European surveillance programmes: Expert‐based risk analysis focus on ruminants. Preventive Veterinary Medicine, 118(4), 271–284. 10.1016/j.prevetmed.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Delooz, L. , Czaplicki, G. , Houtain, J. Y. , Dal Pozzo, F. , & Saegerman, C. (2016). Laboratory findings suggesting an association between BoHV‐4 and bovine abortions in Southern Belgium. Transboundary and Emerging Diseases, 64, 1100–1109. 10.1111/tbed.12469 [DOI] [PubMed] [Google Scholar]
- Delooz, L. , Czaplicki, G. , Houtain, J. Y. , Mullender, C. , & Saegerman, C. (2012). Implication du BoHV‐4 comme agent étiologique d'avortements chez les bovins In Symposium AESA, University of Liège, Liège, 30 November 2012 (p. 1). [Google Scholar]
- Delooz, L. , Mori, M. , Petitjean, T. , Evrard, J. , Czaplicki, G. , & Saegerman, C. (2015). Congenital jaundice in bovine aborted foetuses: An emerging syndrome in southern Belgium. Transboundary and Emerging Diseases, 62(2), 124–126. 10.1111/tbed.12326 [DOI] [PubMed] [Google Scholar]
- DISCONTOOLS . (2016). DISEASES DATABASE. Retrieved from http://www.discontools.eu/Diseases
- ECDC . (2017). European Centre for Disease Prevention and Control. Retrieved from http://ecdc.europa.eu/en/Pages/home.aspx
- EFSA‐ECDC . (2015). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2013. European Food Safety Authority ‐ European Centre for Disease Prevention and Control (ECDC). EFSA Journal, 13, 1–191. [Google Scholar]
- European Comission . (2007). A new Animal Health Strategy for the European Union (2007–2013) where “Prevention is better than cure.” European Communities, 2007, 28. Retrieved from http://ec.europa.eu/food/animal/diseases/strategy/index_%0Aen.htm
- FAAV/WIV/CODA‐CERVA . (2015). Trends and sources 2012‐2013, report on zoonotic agents in Belgium. Bruxelles, Belgium: FAVV‐AFSCA. [Google Scholar]
- Francoz, D. , & Yvon, C. (2014). Manuel de médecine des bovins. Paris, France: MED'COM. [Google Scholar]
- Geurden, T. (2007). Cryptosporidium and Giardia in calves in Belgium. Doctoral thesis. Gent University, 186 pp. [Google Scholar]
- Gunn, G. J. , Heffernan, C. , Hall, M. , McLeod, A. , & Hovi, M. (2008). Measuring and comparing constraints to improved biosecurity amongst GB farmers, veterinarians and the auxiliary industries. Preventive Veterinary Medicine, 84(3–4), 310–323. 10.1016/j.prevetmed.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Havelaar, A. H. , van Rosse, F. , Bucura, C. , Toetenel, M. A. , Haagsma, J. A. , Kurowicka, D. , … Braks, M. A. H. (2010). Prioritizing emerging zoonoses in the Netherlands. PLoS One, 5(11), e13965 10.1371/journal.pone.0013965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe, F. G. H. , & Ruegg, P. L. (2006). Opinions and Practices of Wisconsin Dairy Producers About Biosecurity and Animal Well‐Being. Journal of Dairy Science, 89(6), 2297–2308. 10.3168/jds.S0022-0302(06)72301-3 [DOI] [PubMed] [Google Scholar]
- Humblet, M.‐F. , Vandeputte, S. , Albert, A. , Gosset, C. , Kirschvink, N. , Haubruge, E. , … Saegerman, C. (2012). Multidisciplinary and evidence‐based method for prioritizing diseases of food‐producing animals and zoonoses. Emerging Infectious Diseases, 18(4). 10.3201/eid1804.111151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut de l'Elevage . (2000). Maladies des bovins (3ème Editi). Paris, France: Editions France Agricole. [Google Scholar]
- Kahrs, R. F. (2001). Viral diseases of cattle (2nd ed., 336 pp, I. S. U. Press (Ed.)). Ames, IA: Iowa State University Press. [Google Scholar]
- Kristensen, E. , & Jakobsen, E. B. (2011). Danish dairy farmers’ perception of biosecurity. Preventive Veterinary Medicine, 99(2), 122–129. 10.1016/j.prevetmed.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Lemon, S. C. , Roy, J. , Clark, M. A. , Friedmann, P. D. , & Rakowski, W. (2003). Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Annals of Behavioral Medicine, 26(3), 172–181. 10.1207/S15324796ABM2603_02 [DOI] [PubMed] [Google Scholar]
- Mase, A. S. , Gramig, B. M. , & Prokopy, L. S. (2017). Climate change beliefs, risk perceptions, and adaptation behavior among Midwestern U.S. crop farmers. Climate Risk Management, 15, 8–17. [Google Scholar]
- McIntyre, K. M. , Setzkorn, C. , Hepworth, P. J. , Morand, S. , Morse, A. P. , & Baylis, M. (2014). A quantitative prioritisation of human and domestic animal pathogens in europe. PLoS One, 9(8), e103529 10.1371/journal.pone.0103529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. , Marzano, M. , Danady, N. , & O'Brien, L. (2012). Theories and models of behaviour and behaviour change. Forest research, (27 pp).
- Nöremark, M. , Frössling, J. , & Lewerin, S. S. (2010). Application of routines that contribute to on‐farm biosecurity as reported by Swedish livestock farmers. Transboundary and Emerging Diseases, 57(4), 225–236. 10.1111/j.1865-1682.2010.01140.x [DOI] [PubMed] [Google Scholar]
- Phylum . (2010). Listing and categorisation of priority animal diseases, including those transmissible to humans – Mission report. Colomiers, France: World Organisation for Animal Health (OIE). [Google Scholar]
- Prochaska, J. Q. , & Diclemente, C. C. (1983). Stages and processes of self‐change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology, 51(3), 390–395. 10.1037/0022-006X.51.3.390 [DOI] [PubMed] [Google Scholar]
- Saegerman, C. , Porter, S. R. , & Humblet, M. F. (2011). The use of modelling to evaluate and adapt strategies for animal disease control. Revue Scientifique et Technique (International Office of Epizootics), 30(2), 555–569. [DOI] [PubMed] [Google Scholar]
- Salford Systems . (2001). CART: Tree‐structured non‐parametric data analysis. San Diego, CA: Salford Systems. [Google Scholar]
- Sarrazin, S. , Cay, A. B. , Laureyns, J. , & Dewulf, J. (2014). A survey on biosecurity and management practices in selected Belgian cattle farms. Preventive Veterinary Medicine, 117(1), 129–139. 10.1016/j.prevetmed.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Sayers, R. G. , Sayers, G. P. , Mee, J. F. , Good, M. , Bermingham, M. L. , Grant, J. , & Dillon, P. G. (2013). Implementing biosecurity measures on dairy farms in Ireland. The Veterinary Journal, 197(2), 259–267. 10.1016/j.tvjl.2012.11.017 [DOI] [PubMed] [Google Scholar]
- Scott, P. R. , Penny, C. D. , & Macrae, A. (2011). Cattle medecine. London, UK: Manson publishing; 10.1201/b15179 [DOI] [Google Scholar]
- Shaapan, R. M. (2016). The common zoonotic protozoal diseases causing abortion. Journal of Parasitic Diseases, 40(4), 1116–1129. 10.1007/s12639-015-0661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2006). Setting priorities in communicable disease surveillance. Retrieved from http://www.who.int/csr/resources/publications/surveillance/WHO_CDS_EPR_LYO_2006_3/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


