Abstract
Porcine Epidemic Diarrhoea Virus (PEDV) causes watery diarrhoea, dehydration, and a high mortality rate among suckling pigs. Recently, PEDV had a large negative economic impact on the swine industries in Asia and North America. In 2014, PEDV re‐emerged in many European countries, but most countries only reported a few sporadic cases. Here, we report the epidemic wave that occurred in Italy from 2015 to 2017. During this time, PEDV was detected by real‐time PCR in 438 farms located mainly in the high‐density pig production area in Northern Italy. Most of the outbreaks were in farrow‐to‐finish, farrow‐to‐wean and finisher farms. Clinical signs were observed mainly in suckling and fattening animals, while mortality rates were higher in piglets, reaching 50%. A sequence analysis showed that a PEDV strain, similar to the OH851 S‐INDEL strain isolated in the USA in January 2014, was responsible for the outbreaks in Italy in 2015 and 2016. However, from January 2017, a recombinant variant strain, containing a portion of the Swine Enteric Coronavirus in the S1 gene, spread and almost completely outcompeted the previous nonrecombinant strain. In total, 14.1% of the environmental swabs collected from trucks at slaughterhouses after animals were unloaded tested positive for PEDV before the trucks were cleaned and disinfected, and 46% remained positive after cleaning and disinfection processes were performed. Moreover, environmental swabs indicated that 17.3% of the empty trucks arriving at the farms to load animals were PEDV‐positive. This study indicates that trucks can have an important role in the spread of PEDV in Italy.
Keywords: enteritis, Italy, PEDV, S‐INDEL, swine, transport
1. INTRODUCTION
Porcine Epidemic Diarrhoea (PED) is a viral disease that causes acute diarrhoea, dehydration, and a high mortality rate in suckling piglets. The virus responsible for PED (PEDV) belongs to the genus Alphacoronavirus, family Coronaviridae, order Nidovirales (Carstens, 2010). Porcine Epidemic Diarrhoea was first described in Europe (UK) in 1971 (Wood, 1977) and subsequently spread to other European countries (Pensaert & de Bouck, 1978). In the 80's and 90's, PED epidemics became infrequent in Europe (Nagy, Nagy, Meder, & Mocsari, 1996; Pijpers, van Nieuwstadt, Terpstra, & Verheijden, 1993; Pritchard, Paton, Wibberley, & Ibata, 1999), while severe epidemics were reported in some Asian countries, such as Japan and Korea (Kweon et al., 1993; Takahashi, Okada, & Ohshima, 1983). In Thailand in 2007–2008 and in China in 2010–2012, severe PED outbreaks were reported (Li et al., 2012; Puranaveja et al., 2009; Sun et al., 2012; Wang et al., 2013). Since May 2013, PED has become an epidemic in the USA (Stevenson et al., 2013), causing huge economic losses in swine production. Distinct viral strains have been identified in the USA. The highly virulent PEDV strain associated with severe outbreaks (Stevenson et al., 2013) is called “US original” or “non‐S‐INDEL”, in reference to the S‐INDEL, which shows minimal to no clinical signs (Wang, Byrum, & Zhang, 2014b) and is characterized by insertions and deletions in the spike (S) gene. Additionally, another coronavirus, genetically distinct from PEDV and similar to that detected in Hong Kong in 2011 (Woo et al., 2012), has been identified. This virus, called Porcine Delta Coronavirus (PDCoV), (Marthaler et al., 2014) has been found with a relatively high viral load, often in association with PEDV in farms experiencing acute diarrhoea in sows and piglets (Wang, Byrum, & Zhang, 2014a).
In Italy, PED has been present since the early 90s, and its spread has been increasing as infections caused by the coronavirus transmissible gastroenteritis of pigs (Transmissible Gastroenteritis Virus, TGEV) decreases. After the severe epidemic of 2005–2006 (Martelli et al., 2008), sporadic outbreaks, characterized by mild symptoms and mortality rates slightly above the average, have occurred from 2007 to 2014 (Boniotti et al., 2016). A high level of genetic variability was observed among the strains circulating in Italy during that period (Boniotti et al., 2016). In particular, a novel coronavirus generated by recombination between PEDV and TGEV, called Swine Enteric Coronavirus (SeCoV), was identified. SeCoV was circulating in Italy from 2009 to 2012, was found in Germany (Akimkin et al., 2016) in 2012, and has recently been detected in Central Eastern Europe (Belsham et al., 2016). In July 2014, two new cases of PED, with mild clinical signs, were reported (Boniotti et al., 2016). Based on the S1 gene sequence, these new strains have a high identity with the American strain S‐INDEL OH851, which is defined as being less virulent. Since the end of 2014, PED has also been reported in Belgium (Theuns et al., 2015), Germany (Hanke et al., 2015), the Netherlands (Van Wolf et al., 2015), France (Grasland et al., 2015), Spain (EFSA, 2016), Slovenia (Toplak et al., 2016), Austria (Steinrigl et al., 2015), and Portugal (Mesquita et al., 2015), and strains genetically related to the American S‐INDEL strain have been identified in all of these countries. However, the virulent American prototype strain has only been identified in the Ukraine (Dastjerdi, Carr, Ellis, Steinbach, & Williamson, 2015).
In the USA, the S‐INDEL variant appears less virulent than the non‐S‐INDEL strain (Wang et al., 2014b), and experimental infections appear to confirm these observations (Chen et al., 2016; Leidenberger et al., 2017; Lin et al., 2015). However, occasional description of outbreaks, with high mortality rates in suckling piglets, has been reported in Europe (Bertasio et al., 2016; Mesquita et al., 2015; Stadler et al., 2015).
Many factors can contribute to the rapid spread of an infectious agent, even in a vast geographical area (Jung & Saif, 2015; Lee, 2015), and only strict control measures can contain the economic losses sustained by pig producers. Pig transportation is often the main suspected source of PEDV infection, and thus, it can play a role in the transmission of PEDV to different herds (EFSA, 2016; Lowe et al., 2014). For the control and prevention of the disease, management, biosecurity and/or cleaning and disinfection measures should be adopted. Indeed, particular attention should be paid to the cleaning of transportation trucks, and the efficacy of decontamination procedures needs to be periodically monitored and verified.
The aim of this study is to describe the PED epidemic wave that hit Italy in 2015–2017, to provide data about mortality levels registered during outbreaks caused by the S‐INDEL strains, and to evaluate the role of transportation in the spread of PEDV, with particular attention to the efficacy of cleaning and disinfection procedures.
2. MATERIALS AND METHODS
2.1. Samples
Between January 2015 and June 2017, 3,005 stool and 777 pig tissue samples were voluntarily submitted by veterinarians or farmers to determine the causative agent(s) of enteritis cases from 840 farms. Additionally, 2,182 environmental swabs were collected at seven slaughterhouses from the low‐floors of trucks after animals were unloaded. The samples represented 1,091 swabs taken before and after, respectively, cleaning using high‐pressure washing systems and disinfecting by glutaraldehyde, quaternary ammonium salts, chloramine T, and Virkon®‐based products. We also collected 126 environmental swabs from the floors of 81 trucks upon arrival at 23 farms, prior to animal loading. In 36 cases, the samples were only taken from the tractor, while in 45 cases they were taken from both the tractor and trailer.
Each environmental sample was collected by swabbing the four corners and the perimeter of the low‐floors of the trucks, using a home‐made multistratified pad formed by an absorbent sterile bandage. After the sampling, pads were placed in sterile bags until taken to the laboratory. There, swab samples were washed with 50 ml of saline solution, and then transferred into sterile tubes.
2.2. Clinical data
The mortality and clinical data that were collected from 105 farms confirmed PEDV‐positive by PCR (Supporting Information Table S1). In particular, data were obtained from the following farm production types: 46 finishers, 40 farrow‐to‐wean, five farrow‐to‐finish and 14 wean‐to‐finish. Clinical evaluations were determined by recording the percentages of diarrhoeic animals in each age group (suckling, weaned, fattener, and breeder), and these values were divided into four classes, low (0%–5%), medium (6%–20%), high (21%–50%), and very high (>50%). The mortality rate was calculated for the duration of the outbreak as the percentage of dead animals over the total animals of each category. Because the different age categories were not present at all 105 farms, percentages of diarrhoeic animals and mortality rates were recorded from 40, 39, 46, and 79 farms for breeders, suckling, weaned, and finishers, respectively. For breeders, data were recorded from the different areas within the herd in which they were present: insemination area, farrowing unit, gestation box, and gestation cage. For fattener, data were recorded both from growing‐finishing (30–60 kg) and fattener (60–170 kg).
2.3. PEDV detection and identification
Stool and tissue samples from the herds and swabs collected before truck cleaning and disinfection processes were diluted 1:10 (w/v) in minimum essential media. Suspensions were clarified by centrifugation for 10 min at 4,000 g to eliminate debris. Swabs collected after the cleaning and disinfection operations were submitted directly for extraction, without any preprocessing. In total, 200 μl of the supernatant was subjected to RNA extraction and analysed by real‐time PCR in which a fragment of the S1 gene from PEDV was amplified using a method described by Bertasio et al., 2016. The samples with Ct values >40 were considered as negative. Ct values were considered to compare PEDV titres.
Additionally, between January and June 2015, 1,112 samples (878 stools and 234 tissues) from 200 farms were analysed using a commercial kit (EZ‐PED/TGE/PDCoV; Tetracore, Rockville, MD, USA) for the detection of TGEV, PDCoV, and PEDV.
To confirm the presence of the S‐INDEL variant, the sequence of a portion (564 nt) of the S1 gene, 20,570–21,134 nt, which is referred to as the CV777 sequence (Accession number AF353511) was obtained from each new PEDV‐positive herd and from herds with a PEDV reinfection if more than 5 months had elapsed from when PEDV was first detected. RT‐PCR was performed by adding 5 μl of extracted RNA to 20 μl of the OneStep RT‐PCR kit (QIAGEN, Hilden, Germany) reaction mixture containing 0.4 μM of each primer (PEDV_S_1F: GGTAAGTTGCTAGTGCGTAA and PEDV_S_1R: TCCCATGTTATGCCGACAA). The amplification was carried out at 50°C for 30 min for the RT reaction, followed by the activation of the Hot‐start DNA polymerase at 95°C for 15 min and by 45 cycles in three steps: 94°C for 30 s, 51°C for 30 s, and 72°C for 1 min. An additional extension for 10 min at 72°C was added at the end of the run. The PCR products were purified using NucleoSpin® Gel and PCR Clean‐up reagents (Macherey‐Nagel, Düren, Germany) and were subjected to nucleotide sequence analyses. Cycle sequencing reactions were performed using the BigDye® Terminator Cycle Sequencing kit, version 1.1 (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. Reactions were purified using a BigDye XTerminator™ purification kit (Applied Biosystems) and sequenced on a 3500xL genetic analyser (Applied Biosystems) according to the manufacturer's instructions. Nucleotide sequences were assembled using the SeqMan module of the DNASTAR software package (Lasergene, Madison, WI, USA). Sequences were deposited in GenBank under accession nos. MH028493–MH028607. The nucleotide sequences were aligned to 32 selected PEDV sequences available from the GenBank database using ClustalW software implemented in BioEdit, version 7.2.5 (Hall, 1999). A phylogenetic analysis was performed using the neighbour‐joining method and the maximum composite‐likelihood model, with a 1,000 bootstrap replicates in MEGA6 (Tamura, Dudley, Nei, & Kumar, 2007).
3. RESULTS
3.1. PEDV outbreaks
After two initial sporadic outbreaks in mid‐2014, PED started rapidly spread beginning in January 2015 in a high‐density pig production area of North Italy. Porcine Epidemic Diarrhoea Virus was detected by real‐time PCR in 438 farms located mainly in the Po Valley with only a few in the rest of Italy (Figure 1). Porcine Epidemic Diarrhoea outbreaks occurred mainly during the winter months and three epidemic peaks were observed based on the samples received: February–April 2015, December 2015–March 2016, and December 2016–March 2017 (Figure 2). Between January and June 2015, 200 farms were also tested for the presence TGEV and PDCoV, but no samples tested positive for these viruses.
Figure 1.
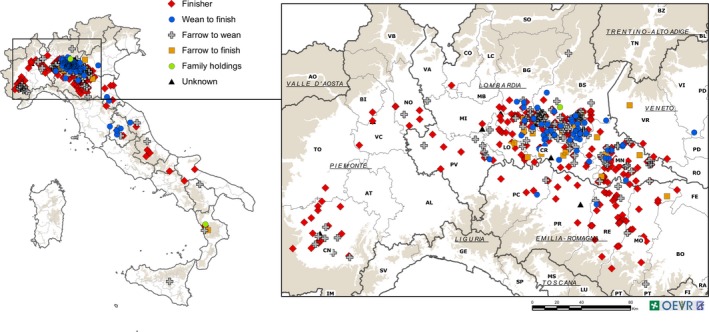
Geographical distribution of the Porcine Epidemic Diarrhoea outbreaks. Farms that tested positive were located mainly in the Po Valley, with only a few in the rest of Italy. Finisher, red diamond; wean‐to‐finish, blue circle; farrow‐to‐wean, grey cross; farrow‐to‐finish, orange square; family holding, green circle; and unknown, black triangle
Figure 2.
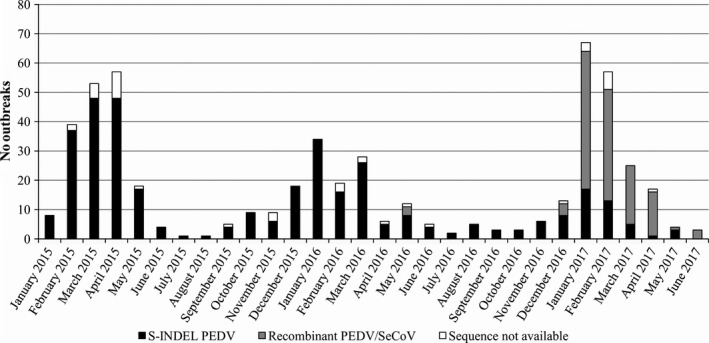
Number of outbreaks per month separated into the causal genetic variant strains of Porcine Epidemic Diarrhoea Virus (PEDV). From January 2015 to June 2017, three PED epidemic peaks occurred during the winter months. From the beginning of 2017, a recombinant variant (PEDV/SeCoV) appeared and became prevalent. The S‐INDEL PEDV strains, recombinant PEDV/SeCoV strains and unavailable sequences are shown with black, grey, and white, respectively. SeCoV, Swine Enteric Coronavirus
Porcine Epidemic Diarrhoea Virus reinfection cases, which were registered even in fattening farms where the all‐in‐all‐out system is applied, occurred in 24.31% and 22.96% of the previously infected herds during the second and third epidemics, respectively. Of the 531 positive holdings, 268 were finisher, 23 farrow‐to‐finish, 142 farrow‐to‐wean, 87 wean‐to‐finish, two family holdings, and nine unknowns. Clinical symptoms were observed in animals of all ages, but the most severe signs and the highest percentages of diarrhoeal animals (more than 50%) were observed in the fattening and piglet groups (Figure 3). The mortality rate was higher in piglets, with peaks of up to 50% (Figure 4).
Figure 3.
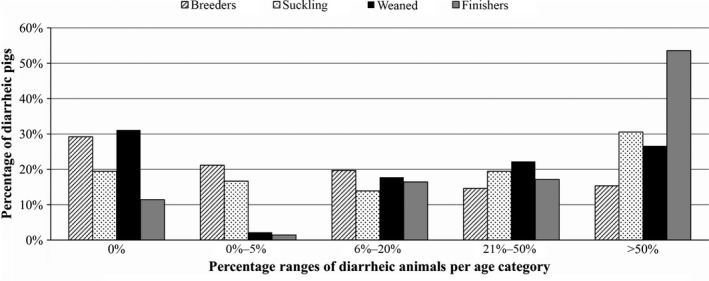
Percentages of diarrhoeic pigs per age category in 105 herds. Clinical evaluations were performed on 148 breeders, 39 suckling, 46 weaned, and 141 finishers. Diarrhoea was observed in animals of all ages, but greater percentages were observed in the fattening and piglet groups. Breeders, bars with lines; suckling, bars with dots; weaned, black bars; and finishers, grey bars. Percentage ranges of diarrhoeic animals are fixed on the x‐axis
Figure 4.
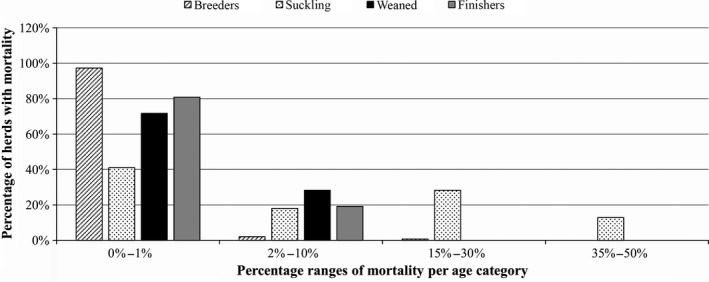
The Porcine Epidemic Diarrhoea Virus (PEDV)‐associated mortality rate per age category in 105 herds. The mortality rate was higher in piglets than in the other categories. Percentage ranges of mortality rates are fixed on the x‐axis
To detect the PEDV variants circulating in Italy, the N‐terminal region of the S1 gene, containing insertions and deletions that distinguish S‐INDEL from non‐S‐INDEL strains, was analysed in each new positive herd and from herds with PEDV reinfection (if more than 5 months had elapsed since PEDV was first detected). The PEDV strain type was identified in 491 samples (434 stools, 15 rectal swabs, and 42 intestines/intestinal contents) out of 531 positive PEDV samples, which came from 403 of the 438 farms. Because of technical problems, such as low titre or degraded sample, the S1 gene could not be sequenced in 40 samples. A phylogenetic tree was constructed based on 115 strains (excluding identical and/or partial nucleotide sequences) found in Italy and 32 sequences available in the NCBI database (Supporting Information Figure S1). The strains from Italy were divided into two clusters. In total, 360 of 491 (73.3%) showed a high degree of similarity (97.6%–100%) with the European S‐INDEL strains circulating in 2014–2015 (Figure 2). The other 131 sequences (26.7%) exhibited a high degree of identity (98.6%–99.8%) with the PEDV SLOreBAS‐1 strain identified in Slovenia in 2015 (KY019623) and in Hungary in 2016 (Valko et al., 2017). This strain harbours a recombinant S1 gene containing a fragment of ~400 nt that has a high identity with SeCoV, an already recombinant strain. In this study, the recombinant PEDV/SeCoV strain was first detected in May 2016 (three out of 131), but was mostly detected in samples procured after December 2016 (Figure 2). The proportions of PED cases caused by the recombinant PEDV/SeCoV variant that occurred in January, February, March, and April 2017 were 73.4%, 74,5%, 80%, and 93.75%, respectively, which made it the most widespread PEDV strain in Italy.
3.2. Truck contamination
In total, 154 out of 1,091 (14.1%) environmental swabs collected at slaughterhouses from the low‐floors of trucks after animals were unloaded tested positive for PEDV before the cleaning and disinfection operations were performed. In addition, 81 out of 1,091 (7.4%) environmental swabs of the same trucks, collected after routine cleaning and disinfection operations, tested positive for the virus. Of the 154 trucks that had originally tested positive, 71 (46%) remained so even after the cleaning and disinfection operations. In addition, swabs corresponding to 12 of the 937 (1.3%) PEDV‐negative swabs taken before cleaning, were detected as PEDV‐positive after the cleaning and disinfection procedures, probably owing to different areas being selected in the two samplings. Thus, the cleaning and disinfection procedures succeeded in eliminating the virus in only 54% of the trucks that initially tested PEDV‐positive.
Even though the collection of environmental swabs did not occur equally during the study period, the percentage of positive swabs from trucks increased in the winter months, as expected (Supporting Information Figure S2).
We also evaluated the PEDV titres, in terms of the Ct values obtained from real‐time PCR analyses, in truck samples taken before and after the cleaning procedures (Table 1 and Supporting Information Figure S3a,b). While 77.5% of the positive precleaning swabs showed high Ct values (Ct ≥ 30), 12.7% had high viral titres, with Ct values <25. In the postcleaning swabs, all of the samples showed Ct values ≥25 (Table 1).
Table 1.
Ct value distributions for Porcine Epidemic Diarrhoea Virus‐positive swabs collected before and after the cleaning and disinfection procedures at pig slaughterhouses in Italy
| Ct values of real‐time PCR, % | Total | |||||
|---|---|---|---|---|---|---|
| <20 | 20–24.99 | 25–29.99 | 30–34.99 | 35–39.99 | ||
| Before cleaning | 4.0 | 8.7 | 9.8 | 37.6 | 39.9 | 173 |
| After cleaning | 0.0 | 0.0 | 6.2 | 25.9 | 67.9 | 81 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The analysis performed on the environmental swabs collected from empty trucks arriving at the farms to load animals, revealed that 17 out of 126 samples, corresponding to 14 out of 81 trucks (17.3%), tested PEDV‐positive. In particular, PEDV was detected in 13 out of 81 tractors (16%), and four out of 45 trailers (8.9%).
4. DISCUSSION
Unlike other European countries, PED has remained present in Italy since its first appearance in the 1980s. After the 1990s, when PED disappeared from the other European countries, endemic waves, like the one that occurred in 2006 (Martelli et al., 2008), were followed by periods of sporadic outbreaks (2007–2014) (Boniotti et al., 2016). We cannot exclude that single cases could have occurred in Europe before the current US outbreak but were, most likely, neither investigated nor diagnosed as PED. In addition, seroprevalence, at both the animal and herd level, was high in farms from Northern Italy, even when only sporadic PED cases were detected (Alborali, Boniotti, & Lavazza, 2014). Unfortunately, very few serosurveys have been carried out in other European countries, but the limited data available showed an absent or very low seroprevalance (EFSA, 2016).
Following the recent epidemic in the USA, PEDV was detected in Europe in 2014, and a few cases were observed in Belgium, Holland, France, Germany, and Portugal. In Italy, PEDV was first reported in July 2014 (Boniotti et al., 2016) and, since the beginning of 2015, a large number of PED outbreaks have occurred (531 out of 1,210 cases of enteritis), with peaks in the winter months. Outbreaks were observed in all of the farm production types but mainly in the fattening and farrow‐to‐wean farms. The typical clinical signs of PED were observed mainly in suckling and fattening animals, but patterns and severity levels differed. Watery diarrhoea with the presence of mucus and blood was observed in piglets, as well as in gestating and farrowing sows (Bertasio et al., 2016). In this study, 31% of herds with suckling pigs and 54% of herds with fattening pigs showed outbreaks in which more than 50% of the animals were diarrhoeic. The durations of disease's clinical signs were variable, ranging from a few days to up to 9 weeks (Bertasio et al., 2016). The mortality rates were higher in piglets, varying from 0% to 50%. These figures are similar to those reported in other European countries in the same period (Mesquita et al., 2015; Stadler et al., 2015; Steinrigl et al., 2015) and are in agreement with the EFSA Report of 2016, which indicated that the mortality was higher in the suckling piglet age group, with a mean of 18%, ranging from 0% to 84%.
The impacts of infection and the severity of the clinical signs may have resulted from the concurrent effects of coinfections with other pathogens (Bertasio et al., 2016; Jung, Kang, Lee, & Song, 2008; Zhao et al., 2016) and strain pathogenicity (Chen et al., 2016; Lin et al., 2015), or from other factors, such as biosecurity, herd size, farm management, sanitary status, and herd immune status (EFSA, 2016).
The strains that arrived in Italy and in the other European countries in 2014 showed a 98.5%–99.5% identity with the OH851 strain isolated in the USA in January 2014 (Boniotti et al., 2016; Grasland et al., 2015; Hanke et al., 2015; Mesquita et al., 2015; Steinrigl et al., 2015; Theuns et al., 2015; Toplak et al., 2016; Van Wolf et al., 2015). However, after January 2017, a recombinant variant, containing a portion of the SeCoV S1 gene, began to spread, resulting in 93.7% of the outbreaks in April 2017, almost completely outcompeting the nonrecombinant variant. This strain was also observed in Slovenia in 2015 (KY019623) and in Hungary in 2016 (Valko et al., 2017).
The emergence of new PEDV variants with potentially different pathogenic features is of great concern for swine health. In conjunction with a systematic laboratory confirmation of the presence of PEDV during outbreaks, continuous molecular surveillance is necessary to understand strain circulation and infection sources, and to allow the implementation of efficient control measures.
In this study, we also investigated whether animal transportation could be a risk factor in the spread of PEDV. As a consequence of the swine vesicular disease outbreaks, which occurred in Italy in 2010, strict biosecurity measures, in particular the cleaning and disinfection of vehicles at slaughterhouse after animal unloading, were adopted. However, this study indicates that these measures are not effective enough to completely eliminate PED contamination from trucks. Porcine Epidemic Diarrhoea Virus can still be detected by RT‐PCR even after disinfection with several commercially available disinfectants (Bowman et al., 2015). In particular, during the winter months, when the virus titres in the faeces from infected animals are high (Ct < 25), these procedures reduced but did not completely eliminate the virus in 46% of the examined trucks that had tested positive prior to cleaning. The presence of the PEDV genome may not necessarily indicate the presence of infectious viral particles. However, Tun, Cai, & Khafipour (2016) demonstrated that PEDV has high rate of survival outside its swine host, being infective in manure for up to 9 months after the initial outbreaks on farms.
On the basis of this study, two main measures need to be implemented in Italy: (a) education programs for farmers and drivers to stress the importance of truck cleaning and disinfection after each transport, as well as of using disposable material and defining the separation truck loading areas and farm/slaughter premises; (b) control of the correct application of the cleaning and disinfection procedure based on the volumes of water and disinfectant used by each driver at the truck‐wash stations of the slaughterhouses.
To avoid the spread of infection, constant and scrupulous attention needs to be paid to the cleaning and disinfection procedures and to all of the other biosecurity measures, including restricting human traffic between fattening and farrowing units and limiting contact between trailers or drivers and the farm interior during the loading process at the pig farms or between drivers and the slaughter facilities during the unloading process (Lee, 2015; Lowe et al., 2014). Otherwise, their application becomes useless, especially for a high‐morbidity viral disease such as PEDV.
CONFLICT OF INTEREST
The authors declare that they have neither financial nor personal relationships with other people or organizations that may compromise or inappropriately influence their work.
Supporting information
ACKNOWLEDGEMENTS
We thank Anna Mangeli and Veronica Papini for their skilled technical assistance. Thanks also to Science Docs for the English language editing of this manuscript. This work was funded by the Italian Ministry of Health (project PED_SURV‐E52I14001210001 and project PRC2014005).
Boniotti MB, Papetti A, Bertasio C, et al. Porcine Epidemic Diarrhoea Virus in Italy: Disease spread and the role of transportation. Transbound Emerg Dis. 2018;65:1935–1942. 10.1111/tbed.12974
REFERENCES
- Akimkin, V. , Beer, M. , Blome, S. , Hanke, D. , Hoper, D. , Jenckel, M. , & Pohlmann, A. (2016). New chimeric porcine coronavirus in swine feces, Germany, 2012. Emerging Infectious Diseases, 22(7), 1314–1315. 10.3201/eid2207.160179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborali, G. L. , Boniotti, M. B. , & Lavazza, A. (2014). Surveillance and control of PED coronavirus in pigs in Italy. Paper presented at the SECD International Meeting, Chicago, Illinois, September 23–25, 2014, Chicago, Illinois, USA. 7. Retrieved from http://www.aphis.usda.gov/animal_health/animal_dis_spec/swine/downloads/meeting/SpeakerAbtracts_and_Bios.pdf
- Belsham, G. J. , Rasmussen, T. B. , Normann, P. , Vaclavek, P. , Strandbygaard, B. , & Botner, A. (2016). Characterization of a novel chimeric swine enteric coronavirus from diseased pigs in central eastern Europe in 2016. Transboundary and Emerging Diseases, 63(6), 595–601. 10.1111/tbed.12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertasio, C. , Giacomini, E. , Lazzaro, M. , Perulli, S. , Papetti, A. , Lavazza, A. , & Boniotti, M. B. (2016). Porcine epidemic diarrhea virus shedding and antibody response in swine farms: A longitudinal study. Frontiers in Microbiology, 7, 2009 10.3389/fmicb.2016.02009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniotti, M. B. , Papetti, A. , Lavazza, A. , Alborali, G. , Sozzi, E. , Chiapponi, C. , & Marthaler, D. (2016). Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerging Infectious Diseases, 22, 83–87. 10.3201/eid2201.150544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, A. S. , Nolting, J. M. , Nelson, S. W. , Bliss, N. , Stull, J. W. , Wang, Q. , & Premanandan, C. (2015). Effects of disinfection on the molecular detection of porcine epidemic diarrhea virus. Veterinary Microbiology, 179(3–4), 213–218. 10.1016/j.vetmic.2015.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens, E. B. (2010). Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2009). Archives of Virology, 155(1), 133–146. 10.1007/s00705-009-0547-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Gauger, P. , Stafne, M. , Thomas, J. , Madson, D. , Huang, H. , & Zhang, J. (2016). Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S‐INDEL‐variant strains in conventional neonatal piglets. Journal of General Virology, 97, 1107–1121. 10.1099/jgv.0.000419 [DOI] [PubMed] [Google Scholar]
- Dastjerdi, A. , Carr, J. , Ellis, R. , Steinbach, F. , & Williamson, S. (2015). Porcine epidemic diarrhea virus among farmed pigs, Ukraine. Emerging Infectious Diseases, 21, 2235–2237. 10.3201/eid2112.150272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2016). Collection and review of updated epidemiological data on porcine epidemic diarrhoea. EFSA Journal, 14(2), 4375 10.2903/j.efsa.2016.4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasland, B. , Bigault, L. , Bernard, C. , Quenault, H. , Toulouse, O. , Fablet, C. , & Blanchard, F. (2015). Complete genome sequence of a porcine epidemic diarrhea S gene indel strain isolated in france in December 2014. Genome Announcements, 3(3), e00535‐15 10.1128/genomeA.00535-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T. A. (1999). BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Hanke, D. , Jenckel, M. , Petrov, A. , Ritzmann, M. , Stadler, J. , Akimkin, V. , & Höper, D. (2015). Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerging Infectious Diseases, 21, 493–496. 10.3201/eid2103.141165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Kang, B. K. , Lee, C. S. , & Song, D. S. (2008). Impact of porcine group A rotavirus co‐infection on porcine epidemic diarrhea virus pathogenicity in piglets. Research in Veterinary Science, 84(3), 502–506. 10.1016/j.rvsc.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , & Saif, L. J. (2015). Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. The Veterinary Journal, 204(2), 134–143. 10.1016/j.tvjl.2015.02.017 10.1016/j.tvjl.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon, C. , Kwon, B. , Jung, T. , Kee, Y. , Hur, D. , Hwang, E. , & An, S. (1993). Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean Journal of Veterinary Research, 33(2), 249–254. [Google Scholar]
- Lee, C. (2015). Porcine epidemic diarrhea virus: An emerging and re‐emerging epizootic swine virus. Virology Journal, 12, 193 10.1186/s12985-015-0421-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidenberger, S. , Schroder, C. , Zani, L. , Auste, A. , Pinette, M. , Ambagala, A. , & Blome, S. (2017). Virulence of current german PEDV strains in suckling pigs and investigation of protective effects of maternally derived antibodies. Scientific Reports, 7(1), 10825 10.1038/s41598-017-11160-w 10.1038/s41598-017-11160-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Li, H. , Liu, Y. , Pan, Y. , Deng, F. , Song, Y. , & He, Q. (2012). New variants of porcine epidemic diarrhea virus, china, 2011. Emerging Infectious Diseases, 18(8), 1350–1353. 10.3201/eid1808.120002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. M. , Annamalai, T. , Liu, X. , Gao, X. , Lu, Z. , El‐Tholoth, M. , & Wang, Q. (2015). Experimental infection of a US spike‐insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross‐protection to the original US PEDV infection. Veterinary Research, 46, 134 10.1186/s13567-015-0278-9 10.1186/s13567-015-0278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, J. , Gauger, P. , Harmon, K. , Zhang, J. , Connor, J. , Yeske, P. , & Main, R. (2014). Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerging Infectious Diseases, 20(5), 872–874. 10.3201/eid2005.131628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli, P. , Lavazza, A. , Nigrelli, A. , Merialdi, G. , Alborali, L. , & Pensaert, M. (2008). Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Veterinary Record, 162, 307–310. 10.1136/vr.162.10.307 [DOI] [PubMed] [Google Scholar]
- Marthaler, D. , Raymond, L. , Jiang, Y. , Collins, J. , Rossow, K. , & Rovira, A. (2014). Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerging Infectious Diseases, 20, 1347–1350. 10.3201/eid2008.140526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita, J. R. , Hakze‐van der Honing, R. , Almeida, A. , Lourenco, M. , van der Poel, W. H. , & Nascimento, M. S. (2015). Outbreak of porcine epidemic diarrhea virus in portugal, 2015. Transboundary and Emerging Diseases, 62(6), 586–588. 10.1111/tbed.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, B. , Nagy, G. , Meder, M. , & Mocsari, E. (1996). Enterotoxigenic escherichia coli, rotavirus, porcine epidemic diarrhoea virus, adenovirus and calici‐like virus in porcine postweaning diarrhoea in Hungary. Acta Veterinaria Hungarica, 44(1), 9–19. [PubMed] [Google Scholar]
- Pensaert, M. B. , & de Bouck, P. (1978). A new coronavirus‐like particle associated with diarrhea in swine. Archives of Virology, 58(3), 243–247. 10.1007/BF01317606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers, A. , van Nieuwstadt, A. P. , Terpstra, C. , & Verheijden, J. H. (1993). Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. Veterinary Record, 132(6), 129–131. 10.1136/vr.132.6.129 [DOI] [PubMed] [Google Scholar]
- Pritchard, G. C. , Paton, D. J. , Wibberley, G. , & Ibata, G. (1999). Transmissible gastroenteritis and porcine epidemic diarrhoea in britain. Veterinary Record, 144(22), 616–618. 10.1136/vr.144.22.616 [DOI] [PubMed] [Google Scholar]
- Puranaveja, S. , Poolperm, P. , Lertwatcharasarakul, P. , Kesdaengsakonwut, S. , Boonsoongnern, A. , Urairong, K. , & Thanawongnuwech, R. (2009). Chinese‐like strain of porcine epidemic diarrhea virus, Thailand. Emerging Infectious Diseases, 15(7), 1112–1115. 10.3201/eid1507.081256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler, J. , Zoels, S. , Fux, R. , Hanke, D. , Pohlmann, A. , Blome, S. , & Ladinig, A. (2015). Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Veterinary Research, 11, 142 10.1186/s12917-015-0454-1 10.1186/s12917-015-0454-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrigl, A. , Fernandez, S. R. , Stoiber, F. , Pikalo, J. , Sattler, T. , & Schmoll, F. (2015). First detection, clinical presentation and phylogenetic characterization of porcine epidemic diarrhea virus in Austria. BMC Veterinary Research, 11, 310 10.1186/s12917-015-0624-1 10.1186/s12917-015-0624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, G. W. , Hoang, H. , Schwartz, K. J. , Burrough, E. R. , Sun, D. , Madson, D. , … Yoon, K. (2013). Emergence of porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. Journal of Veterinary Diagnostic Investigation, 25, 649–654. 10.1177/1040638713501675 [DOI] [PubMed] [Google Scholar]
- Sun, R. Q. , Cai, R. J. , Chen, Y. Q. , Liang, P. S. , Chen, D. K. , & Song, C. X. (2012). Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerging Infectious Diseases, 18(1), 161–163. 10.3201/eid1801.111259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Okada, K. , & Ohshima, K. (1983). An outbreak of swine diarrhea of a new‐type associated with coronavirus‐like particles in Japan. Nihon Juigaku Zasshi: The Japanese Journal of Veterinary Science, 45(6), 829–832. 10.1292/jvms1939.45.829 [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Dudley, J. , Nei, M. , & Kumar, S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24(8), 1596–1599. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Theuns, S. , Conceição‐Neto, N. , Christiaens, I. , Zeller, M. , Desmarets, L. M. B. , Roukaerts, I. D. M. , & Nauwynck, H. J. (2015). Complete genome sequence of a porcine epidemic diarrhea virus from a novel outbreak in Belgium, January 2015. Genome Announcements, 3(3), pii: e00506‐15 10.1128/genomeA.00506-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak, I. , Ipavec, M. , Kuhar, U. , Kusar, D. , Papic, B. , Koren, S. , & Toplak, N. (2016). Complete genome sequence of the porcine epidemic diarrhea virus strain SLO/JH‐11/2015. Genome Announcements, 4(2), e01725‐15. 10.1128/genomeA.01725-15.10.1128/genomeA.01725-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun, H. M. , Cai, Z. , & Khafipour, E. (2016). Monitoring survivability and infectivity of porcine epidemic diarrhea virus (PEDv) in the infected on‐farm earthen manure storages (EMS). Frontiers in Microbiology, 7, 265 10.3389/fmicb.2016.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko, A. , Biksi, I. , Csagola, A. , Tuboly, T. , Kiss, K. , Ursu, K. , & Dan, A. (2017). Porcine epidemic diarrhoea virus with a recombinant S gene detected in Hungary, 2016. Acta Veterinaria Hungarica, 65(2), 253–261. 10.1556/004.2017.025 [DOI] [PubMed] [Google Scholar]
- derVan Wolf, P. J. , Van Walderveen, A. , Meertens, M. , Van Hout, A. , Duinhof, T. , Geudeke, M. , … Dikman, R. (2015). First case of porcine epidemic diarrhea (PED) caused by a new variant of PED virus in the Netherlands. [Abstract]. In: Proceedings of the 7th European Symposium of Porcine Health Management, 2015 Apr 22–24, Nantes, France.
- Wang, L. , Byrum, B. , & Zhang, Y. (2014a). Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerging Infectious Diseases, 20(7), 1227–1230. 10.3201/eid2007.140296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Byrum, B. , & Zhang, Y. (2014b). New variant of porcine epidemic diarrhea virus, United States, 2014. Emerging Infectious Diseases, 20(5), 917–919. 10.3201/eid2005.140195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhao, P. , Guo, L. , Liu, Y. , Du, Y. , Ren, S. , & Wu, J. (2013). Porcine epidemic diarrhea virus variants with high pathogenicity, China. Emerging Infectious Diseases, 19(12), 2048–2049. 10.3201/eid1912.121088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P. C. , Lau, S. K. , Lam, C. S. , Lau, C. C. , Tsang, A. K. , Lau, J. H. , & Yuen, K. Y. (2012). Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. Journal of Virology, 86(7), 3995–4008. 10.1128/JVI.06540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, E. N. (1977). An apparently new syndrome of porcine epidemic diarrhoea. Veterinary Record, 100(12), 243–244. 10.1136/vr.100.12.243 [DOI] [PubMed] [Google Scholar]
- Zhao, Z. P. , Yang, Z. , Lin, W. D. , Wang, W. Y. , Yang, J. , Jin, W. J. , & Qin, A. J. (2016). The rate of co‐infection for piglet diarrhea viruses in china and the genetic characterization of porcine epidemic diarrhea virus and porcine kobuvirus. Acta Virologica, 60(1), 55–61. 10.4149/av_2016_01_55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


