Summary
A domestic ferret from Lima, Peru, died after ten days of non‐specific clinical signs. Based on pathology, immunohistochemistry and molecular analysis, ferret systemic coronavirus (FRSCV)‐associated disease was diagnosed for the first time in South America. This report highlights the potential spread of pathogens by the international pet trade.
Keywords: ferrets, Mustelidae, coronavirus, polymerase chain reaction
Introduction
Since the beginning of the 21st century, ferrets have become increasingly popular as pets in many countries (Johnson‐Delaney, 2010; Zaffarano, 2010). In 2004, a disease of ferrets characterized by clinical signs and lesions similar to those of feline infectious peritonitis (FIP) was first recognized in Spain and has since been documented in the United States, Europe and Asia (Martínez et al., 2006; Garner et al., 2008; Perpiñán and López, 2008; Laprie et al., 2009; Michimae et al., 2010; Murray et al., 2010; Terada et al., 2010; Wise et al., 2010). The disease was initially called ferret infectious peritonitis (Johnson‐Delaney, 2010). A novel Alphacoronavirus, subsequently named ferret systemic coronavirus (FSCV or FRSCV), has been confirmed to be the aetiologic agent (Garner et al., 2008; Terada et al., 2010). Neither FRSCV nor FRSCV‐associated disease has been previously reported in South America.
Materials and Methods
In February 2013, a seven‐month‐old male ferret was received at the Wild and Exotic Animals’ Clinic at the School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos (UNMSM), Lima, Peru. Five months prior to illness, the ferret had been legally imported from Spain via Chile with seven other ferrets. The animal underwent a 30‐day quarantine period once in Chile and again in Peru. The ferret was reportedly vaccinated for canine distemper virus and rabies virus. Prior to submission, the ferret reportedly experienced eight days of hypothermia and diarrhoea before the owners hospitalized it in a private veterinary clinic. Although antibiotic and supportive treatment was administered for 2 days at the private clinic, the animal became anorexic and was referred to UNMSM.
At the time of referral, the ferret weighed 360 g and had a rectal temperature of 37.8°C. The mucous membranes were pale yellow. The ferret was in poor nutritional condition, with a body score of 2/5, and it was severely dehydrated. It was lethargic and the perineum was stained by dark faeces. The ferret had hind limb paresis and a nervous tic in forelimbs and eyelids. A haematological evaluation revealed severe anaemia and leukocytosis. Therapy with enrofloxacin, sodium chloride 0.9%, vitamin B complex, vitamin C and hospitalization in a warm room (28–30°C) was initiated, but the animal died the next morning.
Post‐mortem examination was performed and paired tissue samples duodenum, brain, cerebellum, liver, kidney, spleen, jejunum, large intestine, mesenteric lymph nodules and lungs were collected and stored in both 10% buffered formalin and frozen at −20°C, respectively. Formalin‐fixed tissues were sectioned at 5 μm, stained with haematoxylin and eosin and examined by light microscopy. Immunohistochemistry was performed with a monoclonal antibody that targets the conserved region of the N gene of feline coronavirus. Domestic cat tissues infected by FIP virus were used as a positive control. RNA extractions from tissue samples were conducted using TRIzol ReagentTM (Invitrogen, Carlsbad, CA, USA), following the manufacturer's instructions. Reverse transcription was performed with M‐MLV Reverse Transcriptase (Invitrogen) and random primers. Nested PCR was performed using generic primers, which amplify a 440‐bp segment in the open reading frame (ORF) 1b region, encoding RNA‐dependent RNA polymerase (RdRp) of viruses in the family Coronaviridae. The external primers were 5′GGKTGGGAYTAYCCKAARTG and 5′TGYTGTSWRCARAAYTCRTG (Chu et al., 2011) and the internal primers were 5′GGTTGGGACTATCCTAAGTGTGA and 5′ CCATCATCAGATAGAATCATCATA (Woo et al., 2005). Phylogenetic analysis was performed using a 397‐bp fragment.
Results
The main gross findings at necropsy were multiple white, soft, 1‐ to 4‐mm‐diameter nodules that were scattered throughout the mesentery, omentum, peritoneum, diaphragm, serosa surface of oesophagus, small intestine and gall bladder, and capsule of liver, kidneys and spleen (Fig. 1a). Lymph nodes were generally enlarged. The cerebrum was moderately congested.
Figure 1.
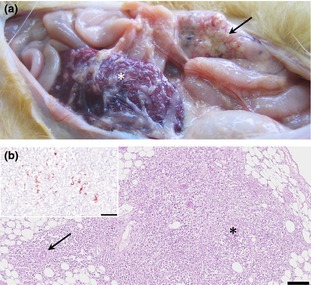
Domestic ferret, abdominal cavity. (a) In situ observation of abdominal cavity, note the pale‐yellowish nodules in the mesentery (arrow) and in the opaque omentum surrounding the spleen (asterisk). (b) Microscopy of the mesenteric nodules, observe the marked cellular infiltration in the mesenteric fat tissue (asterisk) with the special patter surrounding blood vessels (arrow). HE, Bar = 100 μm. Inset, mesenteric nodules, positive cytoplasmic reaction for anti‐feline coronavirus in macrophages. IHC, Bar = 50 μm.
Microscopic findings included dense, primarily perivascular infiltrates of macrophages, lymphocytes and neutrophils beneath the peritoneum and within the mesentery. Multifocal to coalescing pyogranulomatous peritonitis was evident along serosal surfaces of the gastrointestinal tract, gall bladder, kidneys, spleen, liver, peritoneum and mesentery (Fig. 1b). Additional lesions included moderate multifocal histiocytic meningoencephalitis, severe multifocal to coalescing pyogranulomatous hepatitis with bile duct hyperplasia, moderate multifocal pyogranulomatous interstitial nephritis, moderate diffuse lymphoplasmacytic interstitial pneumonia, moderate focally extensive granulomatous enteritis and mild focal histiocytic myocarditis.
Immunohistochemistry revealed macrophages within inflammatory foci of meninges, lung, liver and kidney, and serosa of gastrointestinal tract, mesenteric lymph nodes, spleen and mesentery (Fig. 1b) had specific cytoplasmic labelling for coronavirus antigen. Brain and cerebellum were positive for coronavirus by PCR, but viral nucleic acid was not detected in small and large intestine, liver, kidney, spleen, mesenteric lymph nodes and lungs. The coronavirus sequence detected in this study belonged to the genus Alphacoronavirus, lineage 1a. The samples of the brain (GenBank accession no. KR758767) and cerebellum (GenBank accession no. KR758768) had one SNP (single nucleotide polymorphism) in the amplified sequence. Based on BLAST analysis (Altschul et al., 1990), the sequence from the Peruvian ferret was 93–95% similar to sequences from ferrets in Japan, 62% similar to feline coronavirus, 62% similar to transmissible gastroenteritis virus (TGEV) and 78% similar to mink coronavirus (Fig. 2).
Figure 2.
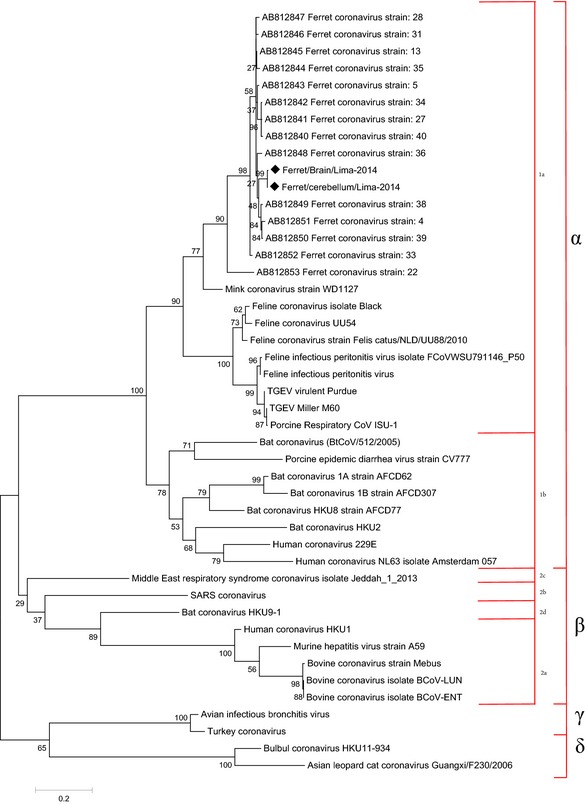
Phylogenetic tree constructed based on the nucleotide sequences of the partial RNA‐dependent RNA polymerase (RdRp). The tree was constructed by the maximum‐likelihood method in MEGA 6.0 software. GenBank accession numbers for the nucleotides sequences are KR758767 (Ferret/Brain/Lima 2014) and KR758768 (Ferret/Cerebellum/Lima 2014). The sequences of this study are shown in  .
.
Discussion
Based on immunohistochemical and molecular findings, FRSCV infection was diagnosed in a ferret imported from Spain to Peru. This is the first time systemic coronavirus infection of ferrets has been described in Latin America. The ferret had haematological values and clinical signs similar to those described for FRSCV‐associated disease. However, these signs are relatively non‐specific, and many other clinical signs associated with FRCSV were not observed, including palpable intra‐abdominal masses, splenomegaly, nephromegaly, bruxism, sneezing/nasal discharge, systolic heart murmur, urine discoloration, dyspnoea, peripheral lymphadenomegaly, rectal mucosal erythema, seizures, opisthotonus, vomiting and pyrexia (Garner et al., 2008; Martínez et al., 2008; Johnson‐Delaney, 2010; Murray et al., 2010).
Gross post‐mortem findings described for FRSCV‐associated disease include circumscribed to coalescing, white, tan or slightly pink, irregularly shaped nodules on surfaces and within the parenchyma of multiple organs (e.g. spleen, liver, kidney, lung, mesentery and lymph nodes), in addition to protein‐rich effusions in body cavities (Martínez et al., 2006; Garner et al., 2008; Johnson‐Delaney, 2010; Murray et al., 2010 ). Histologically, FRSCV‐associated disease is characterized by pyogranulomatous inflammation particularly of the visceral peritoneum, mesenteric adipose tissue, liver, lungs, kidneys, lymph nodes, spleen, pancreas, adrenal glands, stomach, brain and/or blood vessels (Garner et al., 2008; Martínez et al., 2008; Johnson‐Delaney, 2010). This is consistent with the findings in our case, in which the most striking gross lesions were widely scattered white nodules over abdominal and thoracic organs, which corresponded to pyogranulomatous inflammation, including pyogranulomatous vasculitis. In addition, histopathology of the brain revealed histiocytic meningoencephalitis.
FRSCV has been widely reported in Spain (Martínez et al., 2006; Garner et al., 2008; Murray et al., 2010) but never in South America. In Peru, ferrets have been imported as pets since late 2012, and the current population is believed to be <250 individuals (J. Lescano, personal communication). The ferret involved in our case was born in Spain and had a cumulative quarantine period (in Chile and Peru) of about 60 days. Thereafter, it lived in Lima city for about 90 additional days without showing any clinical signs. There was reportedly no contact with other ferrets than the imported group. This suggests the infection occurred in Spain, and the animal stayed clinically asymptomatic for approximately 150 days. Current knowledge about the pathogenesis of FRSCV‐associated disease is scarce (Murray et al., 2010), and there are no data on its incubation period. However, comparing with domestic cats, the development of FIP disease may occur during any stage after initial viraemia (Kipar and Meli, 2014). Although this virus shared an identity of 93–95% with isolates from Japan, no sequences are available for comparison from Spain, and this similarity may not have any bearing on the origin of the virus.
In summary, this is the first reported case of FRSCV‐associated disease in South America. This report aims to highlight the possible role played by legal international animal trade in the dissemination of pathogens between continents, even when the standards established by animal health authorities are met.
Acknowledgements
We thank Barbara C. Shock for reviewing the manuscript.
References
- Altschul, S. F. , Gish W., Miller W., Myers E. W., and Lipman D. J., 1990: Basic local alignment search tool. J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Chu, D. K. W. , Leung C. Y. H., Gilbert M., Joyner P. H., Ng E. M., Tse T. M., Peiris J. S. M., and Poon L. M., 2011: Avian Coronavirus in wild aquatic birds. J. Virol. 85, 12815–12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner, M. M. , Ramsell K., Morera N., Juan‐Sallés C., Jiménez J., Ardiaca M., Montesinos A., Teifke J. P., Löhr C. V., Evermann J. F., Baszler T. V., Nordhausen R. W., Wise A. G., Maes R. K., and Kiupel M., 2008: Clinicopathologic features of a systemic coronavirus‐associated disease resembling feline infectious peritonitis in a domestic ferret (Mustela putorius). Vet. Pathol. 45, 236–246. [DOI] [PubMed] [Google Scholar]
- Johnson‐Delaney, C. A. , 2010: Emerging ferret diseases. J. Exot. Pet Med. 19, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar, A. , and Meli M. L., 2014: Feline infectious peritonitis: still an enigma? Vet. Pathol. 51, 505–526. [DOI] [PubMed] [Google Scholar]
- Laprie, C. , Duboy J., and Martinez J., 2009: Systemic Coronavirus‐associated disease in the domestic ferret (Mustela putorius): histopathologic and immunohistochemical characterization in three ferrets [in French]. PMCAC 44, 111–115. [Google Scholar]
- Martínez, J. , Ramis A. J., Reinacher M., and Perpiñán D., 2006: Detection of feline infectious peritonitis virus‐like antigen in ferrets. Vet. Rec. 158, 523. [DOI] [PubMed] [Google Scholar]
- Martínez, J. , Reinacher M., Perpiñán D., and Ramis A., 2008: Identification of Group 1 Coronavirus antigen in multisystemic granulomatous lesions in ferrets (Mustela putorius furo). J. Comp. Pathol. 138, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michimae, Y. , Mikami S.‐I., Okimoto K., Toyosawa K., Matsumoto I., Kouchi M., Koujitani T., Inoue T., and Seki T., 2010: The first case of feline infectious peritonitis‐like pyogranuloma in a ferret infected by coronavirus in Japan. J. Toxicol. Pathol. 23, 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. , Kiupel M., and Maes R. K., 2010: Ferret coronavirus‐associated diseases. Vet. Clin. North. Am. Exot. Anim. Pract. 13, 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perpiñán, D. , and López C., 2008: Clinical aspects of systemic granulomatous inflammatory syndrome in ferrets (Mustela putorius furo). Vet. Rec. 162, 180–184. [DOI] [PubMed] [Google Scholar]
- Terada, Y. , Minami S., Noguchi K., Mahmoud H. Y. A. H., Shimoda H., Mochizuki M., Une Y., and Maeda K., 2010: Genetic characterization of coronaviruses from domestic ferrets, Japan. Emerg. Infect. Dis. 20, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, A. G. , Kiupel M., Garner M. M., Clarck A. K., and Maes R. K., 2010: Comparative sequence analysis of the distal one‐third of the genomes of a systemic and enteric ferret coronavirus. Virus Res. 149, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P. C. , Lau S. K., Chu C. M., Chan K. H., Tsoi H. W., Huang Y., Wong B. H., Poon R. W., Cai J. J., Luk W. K., Poon L. L., Wong S. S., Guan Y., Peiris J. S., and Yuen K. Y., 2005: Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79, 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffarano, B. , 2010: Ferrets: examination and standards of care. J. Exot. Pet Med. 19, 73–81. [Google Scholar]


