Abstract
From the severe porcine epidemic diarrhoea (PED) epidemics that struck in 2013 in the United States of America and other countries of North and South America, two types of porcine epidemic diarrhoea virus (PEDV) were isolated, namely the InDel and the non‐InDel strains. They are differentiated by insertions/deletions in the S1 nucleotide sequence of the S gene, and differences in virulence were observed from the clinical cases. In 2014, a PED outbreak occurred in a pig farm in France, from which an InDel strain was isolated. This study aimed at comparing, under experimental conditions, the pathogenicity and the direct and indirect transmissions between a non‐InDel strain isolated from a PED‐affected piglet in 2014 in the USA and the French InDel strain. All infected pigs showed clinical signs with the non‐InDel strain although only the inoculated and direct contact pigs showed clinical signs in the InDel strain group. Although viral RNA was detected in air samples with both strains, the indirect contact pigs remained free from infection with the InDel strain in contrast to the non‐InDel group in which airborne transmission occurred in the indirect contact pigs. All infected pigs shed virus in faeces regardless of PEDV strain with 9 of 30 pigs showing intermittent faecal shedding. The transmission rate by direct contact was found to be 2.17‐fold higher than the non‐InDel strain compared with the InDel. In conclusion, the InDel strain was less pathogenic than the non‐InDel strain in our experimental conditions. The transmission route differed between the two strains. Direct contact was the main transmission route for the InDel strain, although the non‐InDel strain was transmitted through direct contact and indirectly through the air.
Keywords: epidemiology, InDel strain, non‐InDel strain, pathology, porcine epidemic diarrhoea virus, transmission
1. INTRODUCTION
Porcine epidemic diarrhoea (PED) was described for the first time in England in 1971, and some PED cases have been identified thereafter in other European countries (Jung & Saif, 2015; Song & Park, 2012). This disease, characterized by a severe, profuse watery diarrhoea with or without vomiting and dehydration, is caused by a positive sense, single‐stranded RNA enveloped coronavirus of 28 kb called the porcine epidemic diarrhoea virus (PEDV) (Jung & Saif, 2015). PEDV reference strain, CV777, was isolated in Belgium in 1977 (Pensaert & de Bouk, 1978). After several outbreaks in the 70s, PED has been persisting in Europe with sporadic cases until the late 1990s (Jung & Saif, 2015). Outbreaks of PED were also described in Asia in the 1980s and then between 2010 and 2012 (Jung & Saif, 2015; Wang et al., 2013). A new type of strain, genetically different from the reference strain, was isolated during those recent outbreaks, inducing a strong mortality despite the frequent uses of vaccines based on CV777 strain in Asia (Li et al., 2012; Wang et al., 2013). In 2013, swine producers in the United States of America were hit by PED although the country had previously been free from the disease. The PED disease spread rapidly throughout the country and in neighbouring countries of North and South America such as Canada and Mexico. Two genetically distant PEDV strains were isolated in the USA, namely the non‐InDel strains, genetically similar to the aforementioned Asian strains, and the InDel strains, showing insertions–deletions in the S1 part of the S gene (Jung & Saif, 2015; Vlasova et al., 2014). From clinical reports, these two strains seemed to behave differently in terms of morbidity and mortality: The non‐InDel strains were associated with more severe clinical cases and higher case fatality rate compared with the InDel strains (Vlasova et al., 2014; Wang, Byrum, & Zhang, 2014). In contrast with non‐InDel strains, exclusively reported in America and Asia, InDel strains were also identified in Europe since 2014 (EFSA, 2016; Stadler et al., 2015, Vlasova et al., 2014). To date, PEDV circulates in America, Asia and Europe with different patterns in terms of epidemiological behaviour and persistence of the virus in the pig population.
Several studies focusing on the different types of PEDV strains allowed for a better understanding of PEDV epidemiology and pathogenicity. Because of a very low infectious dose (Thomas et al., 2015), PEDV can be transmitted by contact with contaminated equipment, the staff or contaminated vehicles used for animal transport (Bowman, Krogwold, Price, Davis, & Moeller, 2015; Jung & Saif, 2015; Lowe et al., 2014), but oro‐faecal transmission is the main transmission route. PEDV is shed in faeces favouring a rapid transmission to susceptible pigs sharing the same environment as infected animals (Jung & Saif, 2015). Airborne transmission was evidenced over relatively long distances for non‐InDel strains (Alonso et al., 2014), but still needs to be investigated as a potential transmission route for InDel PEDV strains.
Although rapid transmission is commonly recognized, the transmission was not quantitatively assessed for any PEDV strain and uncertainties remain about the pathogenicity, transmission efficiency and further consequences of a potential introduction of non‐InDel PEDV strains into European pig populations. Therefore, the objective of this study was to compare the pathogenicity and quantify the level of PEDV during transmission through different routes of strains that represent the two types of PEDV isolated in 2014, an American non‐InDel strain (PEDV/USA/2014/IOWA) and a French InDel strain (PEDV/FR/001/2014) under experimental conditions in a naïve pig population.
2. MATERIALS AND METHODS
2.1. Inocula
Two inocula were used during the experimental trials. They resulted from the homogenization of a jejunum sample (20%w/v) collected from pigs affected by PED. The PEDV InDel strain, PEDV/FR/001/2014 strain (GB No: KR011756), was amplified on a 3‐week‐old SPF pig inoculated with homogenate of jejunum collected from PED‐affected pigs belonging to a farm located in the north of France in 2014. The inoculum was prepared from the jejunum of the SPF PED‐affected pig. For the PEDV non‐InDel strain, PEDV/USA/2014/IOWA strain (GB No: MF37363), jejunum samples were collected from pigs from one herd affected by a non‐InDel PEDV strain in the state of Iowa (USA) in 2014 and homogenized in phosphate‐buffered saline. The two homogenates were centrifuged at 10,000× g for 10 min at 4°C, and supernatants were passed through a 0.45‐μm filter. Next‐generation sequencing (NGS) was performed on each inoculum to obtain the PEDV complete genome sequence and to ensure the absence of other RNA viral sequences. The inocula were also negative for porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV). These absences were assessed by the absence of seroconversion against PCV2 and PRRSV after inoculation.
2.2. Animals and experimental design
The experiments were carried out in the level‐3 biosecurity, air‐filtered facilities of the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) in accordance with European and French regulations on animal experiments. The experimental design was approved by the ethics committee on animal experiments registered under number #16 by the French Ministry of Research (referral no. 16‐032). For each strain, twenty large‐white specific pathogen‐free (SPF) weaned pigs, 28 days old, were equally distributed into two separate rooms (A and B) containing two separate pens (10 pigs per room) (Figure 1). Three additional SPF weaned piglets were housed in a third room C and used as negative control. In each room A and B, a seeder pig was placed in pen A in direct contact with four other pigs. In pen B, five naïve pigs were placed in indirect contact with pen A. The pens A and B were 40 cm apart and separated by a solid partition between the two pens to prevent any direct transfer of faecal material. The same design was used for the American non‐InDel strain. The control animals were sampled first. Strict biosecurity measures were used during this two experiments to avoid any sample contamination. The outside portion of pen A and pen B was washed before every stage of sampling, and a footbath was set up before the entry of each pen. Individuals entering pens washed their boots and wading boots before passing through the footbath. The animals in pen B were also sampled before those of pen A. The manipulator also showered and changed clothes between each room of the two repetitions.
Figure 1.
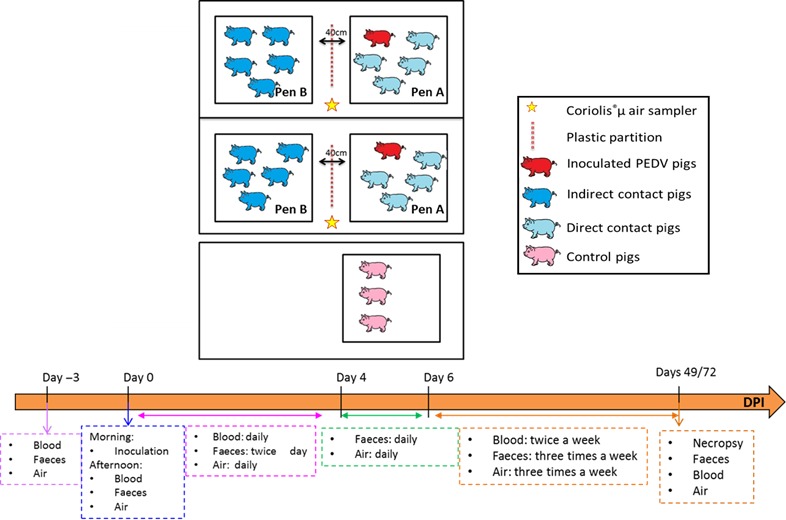
Design of the experimental trials [Colour figure can be viewed at http://wileyonlinelibrary.com]
On day 0 (D0), each seeder pig of the infected groups was orally inoculated with 5 ml of a PEDV inoculum titrating 108 copies of viral genome/ml (estimated to ≈105 TCID50/ml) for each strain. The InDel and the non‐InDel trials lasted 49 and 72 days postinoculation (dpi), respectively, when pigs were euthanized including anaesthesia (Zoletil®, Virbac, Carros, France, 15 mg/kg) followed by bleeding before necropsy.
2.3. Clinical assessment
Individual body weights were recorded on a weekly basis from D0, prior to inoculation, until the end of the experiment to assess the average daily weight gain (ADWG). Rectal temperatures were monitored daily for each pig from D0 until the end of the trial. The pigs were also scored daily for faecal consistency using the following criteria: (0) absence of faeces, (1) normal, (2) semi‐liquid without a formed consistency and (3) liquid/watery contents. In addition, because of the severe impact of the non‐InDel strain, the appearance, behaviour, vivacity and respiration of every pig were recorded the first week of the experiment.
2.4. Biological samples and necropsy
Faecal samples were collected from all the pigs prior to inoculation and then the afternoon of the inoculation (0.5 dpi), twice a day from 1 to 4 dpi, daily at 5 and 6 dpi and three times a week at 7 dpi until the end of the trial (Figure 1). One gram of faeces (or one ml of liquid faeces in case of diarrhoea) was homogenized with 9 ml of Dulbecco's phosphate‐buffered saline (Sigma, Saint Louis, United States of America) and centrifuged at 15,000× g for 10 min at 4°C. Supernatants were collected and stored at −80°C until use.
Blood was sampled prior to inoculation (0 dpi), the afternoon after inoculation (0.5 dpi) and then once a day the first week after inoculation (1‐4 dpi) and twice a week until the end of the trial. Blood samples were centrifuged at 1,200× g for 10 min at 4°C, and sera were stored at −80°C until use.
After euthanasia, at 49 and 72 dpi for the InDel and the non‐InDel trial, respectively, necropsies were performed and macroscopic lesions were evaluated. During necropsy, the following organs were collected and stored at −80°C in RNA later tissue storage reagent (Sigma, Saint Louis, USA): duodenum, jejunum, ileum, colon, spleen, liver, mesenteric and inguinal lymph nodes, psoas muscle and lungs.
Air samples were also collected using a Coriolis®μ air sampler (Bertin, Montigny‐le‐Bretonneux, France) placed between the two pens (Figure 1) prior to inoculation (0 dpi) and then the afternoon after inoculation (0.5 dpi), once a day the first week after inoculation (1–6 dpi) and three times a week until the end of the trial. The Coriolis®μ air sampler technology is based on a cyclonic technology, which is combined with a high air flow rate. The collection of the virus was performed in 0.005% Triton X‐100 milliQ water solution with a flow rate of 300 L/min for 10 min. After collection, the samples were concentrated with the Amicon® Ultra‐2 30K device (Merck, Darmstadt, Germany) and stored at −80°C until use.
2.5. PEDV genome quantification
Viral RNAs were extracted from the faecal homogenates, the sera and the air samples using the Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Five μl of eluted RNA was used as templates for PEDV RT‐qPCR. RNA extraction controls were performed, every five samples to check for any PEDV contamination, by replacing sample with RNAse‐free water. RNA extracted from faeces with the RNeasy Mini kit was diluted to 1:10 to avoid any PCR inhibition.
The number of PEDV genome copies was assessed by real‐time PCR using Power SYBR® Green RNA‐to‐Ct™ 1‐step kit (Thermo Fisher Scientific, Waltham, United States of America) on an Applied Biosystems® 7500 real‐time PCR system (Thermo Fisher Scientific, Waltham, United States of America). The PCR conditions were a holding stage of two steps, one‐first step at 48°C for 30 min and a second one at 95°C for 10 min, followed by 40 repeated cycles with two steps, a first step of 15 s at 95°C and a second of 1 min at 60°C. At last, a melt curve stage composed of four steps, a first step of 1 min at 95°C, a second step of 1 min at 60°C, a third step with a gradual increase in temperature with 0.35°C for 0.3 s to obtain a temperature of 95°C and a fourth step of 15 s at 60°C. The primers used were described in Gallien et al. (2018) and were designed from the conserved regions of the PEDV nucleocapsid gene for universal detection of both strains (forward, 5′‐CGCAAAGACTGAACCCACTAA‐3′; reverse, 5′‐TTGCCTCTGTTGTTACTTGGAGAT‐3′). The copy numbers were quantified using a range of PEDV N RNA from 101 to 108 copies/5 μl.
For each PCR run, a positive control containing PEDV RNA extract from a PEDV cell culture supernatant was included. Two negative controls were also included in the plate; the RNA samples were replaced by RNAse‐free water. One negative control was placed close to the positive control and the second one at the end of the plate. All samples were processed in duplicate.
2.6. PEDV serology
Sera were tested for PEDV antibodies using a commercial ELISA test, ID Screen® PEDV Indirect (ID Vet, Grabels, France). The ELISA test is validated if the mean value of the positive control optical density (OD) is greater than 0.350 and if the ratio of the mean values of the positive and negative controls is greater than 3. Then, for each sample, the S/P (sample‐to‐positive) ratio was calculated. Samples with S/P ratio equal or higher than 60% were considered as positive for PEDV antibodies.
2.7. Estimation of epidemiological parameters
2.7.1. Shedding periods and time to seroconversion
The duration of the shedding period was characterized as the time interval between the first and the last PCR‐positive faecal sample for each animal. Survival analysis was used to assess whether differences exist between the two challenge strains (Cox proportional hazard model; R software: function coxph) and to estimate the distributions of the shedding period (parametric survival model assuming a Weibull distribution; R software: function survreg).
2.7.2. Transmission parameters and latency period
PEDV transmission process was fitted to a SEIR model. Each individual was classified as susceptible, exposed, infectious or recovered on the basis of their virological results. Pigs were considered as susceptible (S) during the time window from exposure (day 0 postinoculation) and the time they were effectively infected (t inf). At that time, the animals turn into the exposed class (E) for a duration δE, during which they are infected, but do not shed viral particles, being unable to infect other pigs. t sh = t inf + δE represents the actual first shedding time, from which time the animals are infectious (I), and stands between the times of the last PCR‐negative (t neg) and the first PCR‐positive faecal samples (t pos) for each animal (tneg < t sh < t pos). Furthermore, the infectious process is initiated by the inoculated seeder pig in each room, and all infection events in contact individuals occurred only once the seeders became infectious, that is, for each contact pig. One can notice here that for seeder pigs, inoculated at t 0, the shedding time is equivalent to the duration of the latency period (). At last, pigs were considered as recovered (R) after their last PCR‐positive faecal sample, when they did not play any role in the infectious process anymore.
Two transmission routes were considered to represent the observed infectious process: transmission by direct contact between penmates and by indirect contact with animals in neighbouring pens. Let βw and βb denote the within‐ and between‐pen transmission rates. The force of infection exerted on a typical susceptible individual (i) at time t is defined by , where I w and I b represent the number of infected animals in the same pen as individual i and in the neighbouring pen, respectively; n is the total number of pigs in each pen. With these notations, the probability p i for an individual i to get infected at time is given by while having escaped infection on the time interval with the probability .
Assuming a gamma distribution for the duration of the latency period δE, with shape and scale parameters α and κ, the likelihood of the data is expressed by:
The first term of the likelihood corresponds to the probability of occurrence of observed infections; the second term represents the probability of observed infection failure whenever some individual would remain susceptible throughout the experiment; and the third term accounts for the distribution of latency period in seeder pigs. Bayesian inference was performed using Monte Carlo Markov Chains. The individual values for the shedding time and latency duration were, respectively, drawn within their plausible range, that is, and . Uniform prior distributions were assumed for the parameters governing the latency duration distribution with a relatively large variation range (α ∼ U(1,15), κ ∼ U(0,5)), and normal distributions were used as prior for the log‐transformed transmission rates (log (βw) ∼ N(0,2) and (log (βb) ∼ N(0,5)). Parameters were sequentially updated using Metropolis–Hastings algorithm. Three independent chains were run with initial values of parameters randomly drawn in the prior distribution, and 30000 iterations were run for each chain (10% were discarded as burn‐in iterations). Convergence was assessed through visual inspection of parameter outputs and classical diagnostic tests (Heidelberger, Geweke and Gelman‐Rubin diagnostics) (Hu, Gonzales, & Gubbins, 2017).
2.8. Statistical analysis
The normality of all the data was checked with a Shapiro test. If the data followed a normal law, the homogeneity of the variances was checked with a Levene test. Then, if the variances were homogeneous, an ANOVA test was carried out to compare the mean between groups. If the data did not follow a normal law or if the variances were not homogeneous, a Kruskal–Wallis test was used to compare the mean between groups. p‐values less than 0.05 were considered significant. Statistical analyses of data were carried out with the statistical software R version 3.2.5. (Team, 2014).
3. RESULTS
3.1. Clinical assessment and zootechnical data
No clinical signs were observed in any pigs prior to the beginning of the trials and in control pigs throughout the experiment.
In the groups challenged with the InDel strain, only the inoculated and the direct contacts pigs showed clinical signs. However, diarrhoea was observed in only 25% of these direct contact pigs simultaneously. In fact, diarrhoea was occasionally observed between 4 and 25 dpi. Vomiting was sporadically observed between 1 and 49 dpi (at 1, 7, 21, 37, 42, 44, 46 and 49 dpi). The inoculated and direct contact pigs also showed signs of lethargy and anorexia the first week after inoculation.
In the non‐InDel strain groups, all animals involved in the experiment showed clinical signs (including indirect contact pigs), with more severe clinical signs compared to the InDel inoculated groups. Diarrhoea started on days 1 and 2 postinoculation for the inoculated and contact pigs (direct and indirect contacts), respectively, with 80% of the indirect contact pigs and 75% of the direct contact pigs with diarrhoea on 2 dpi. After 2 dpi, occasional diarrhoea was noticed in the direct contact pigs (5 dpi) and indirect contact pigs (at 3 and 6 dpi). Occasional vomiting was also noticed for the inoculated and the direct contact pigs at 1 and 31 dpi and for the indirect contact pigs at 11, 31 and 46 dpi. All the pigs were lethargic from 2.5 to 3 dpi and showed signs of dehydration and anorexia, the first week of the trial. No hyperthermia was observed during the experiments.
A reduction in growth performance was also observed in the InDel groups during the first 3 weeks of the trial for the inoculated pigs and the second and the third week of the trial for the direct contact pigs (Figure 2a). Growth performance was not impacted after 22 dpi. A significant reduction in growth performance was observed during the first week postinoculation (direct contacts, indirect contacts and inoculated pigs) of the non‐InDel groups, reaching the control group level thereafter (Figure 2B). The non‐InDel strain showed a greater impact on the ADWG the first week after inoculation than the InDel strain. After this first week, a greater impact on ADWG was observed in the InDel groups until 22 dpi. Indeed, the InDel direct contact pigs gained on average 205.4 g more per day than the non‐InDel direct contact pigs, the first week postinoculation. After this first week, the non‐InDel direct contact pigs gained on average 150 g more per day than the InDel direct contact pigs until 22 dpi (Figure 2).
Figure 2.
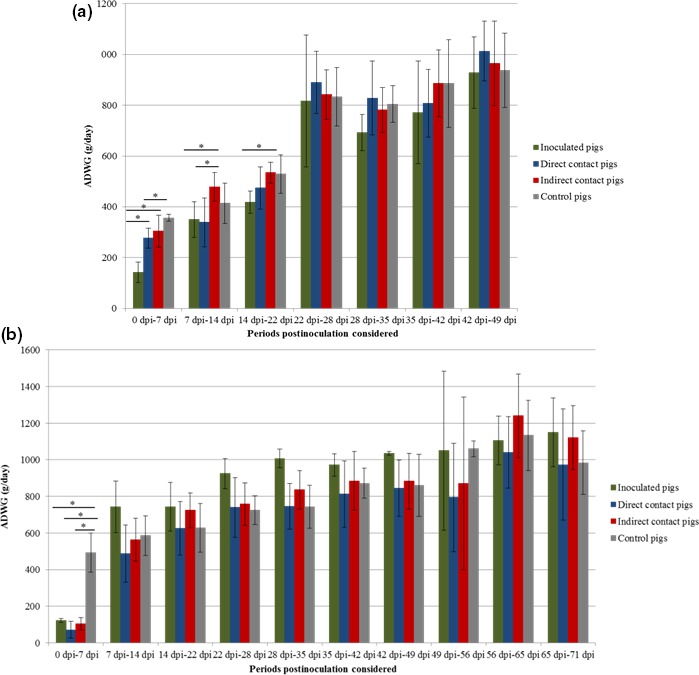
Average daily weight gain (ADWG) (gram per day) according to the status for InDel strain (a) and non‐InDel strain (b). Differences between strains were considered significant when p < 0.05 (*), p < 0.01 (**) or p < 0.001 (***) [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.2. PEDV faecal shedding
Prior to inoculation, all the pigs were negative for PEDV RNA in faeces. All the control pigs remained negative for PEDV in faeces until the end of the trials (49 and 72 dpi, respectively).
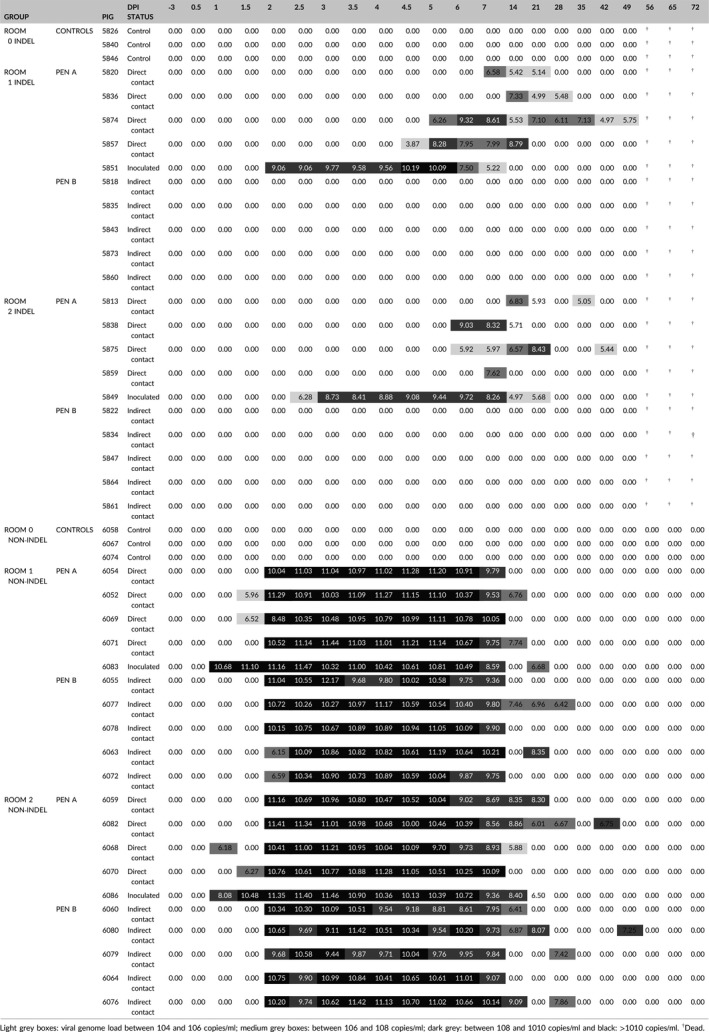
In the InDel groups, the inoculated pigs began to shed virus in their faeces 1 ± 0.5 days after the non‐InDel inoculated pigs (Table 1). For direct contact pigs, faecal samples from InDel groups were found positive 4 days later at 5 dpi for 2 pigs, compared with the non‐InDel pigs (Table 1). None of the InDel indirect contact pigs shed PEDV in their faeces (Table 1).
Table 1.
Individual virological data: PEDV RNA in faeces (log(PEDV genome copies/ml)) in controls, inoculated, direct and indirect contact pigs
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In the non‐InDel groups, PEDV RNA was first detected in the faeces of the inoculated pigs and one of the contact group pigs at 1 dpi. The two inoculated pigs shed 4.80 × 1010 and 1.20 × 108 genomic copies/ml of faeces, respectively. Faecal samples collected from pigs in the direct contact group were RT‐qPCR positive at 1.5 and 2 dpi, in two and five pigs, respectively. The faeces of the 10 indirect contact pigs tested positive for PEDV at 2 dpi (Table 1).
3.3. Viremia and seroconversion
Prior to inoculation, no PEDV RNA was detected in serum and no pig tested seropositive for PEDV antibody. For the two trials, viremia was highly transient and barely detectable (Table 2). All non‐InDel inoculated and direct contact pigs seroconverted except one of the direct contact pigs. All non‐InDel indirect contact pigs showed also a serological response. However, 7 of 19 animals of the non‐InDel group, which exhibited a serological response, demonstrated PEDV antibodies for a short period of time and were seronegative by the end of the experiment. The delays between infection and seroconversion for the two strains were significantly different. The average delay between infection and seroconversion was twice as long for the non‐InDel strain than for the InDel strain (24.8 vs 12.5 days on average) (Figure 3).
Table 2.
Average genome load (number of copies/ml of serum) and number of viremic pigs during the InDel and non‐InDel trials for the inoculated, direct contact and indirect contact pigs
| Status | Strain | Days postinoculation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −3 | 0 | 1 | 2 | 3 | 4 | 7 | 11 | |||
| Inoculated pigs | InDel | Number of PEDV viremic pigs | 0/2 | 0/2 | * 1/2 | 1/2 | 0/2 | * 1/2 | 0/2 | 0/2 |
| Average genome load | 4.12 × 10 3 | 8.83 × 10 2 | 3.34 × 10 3 | |||||||
| Inoculated pigs | Non‐InDel | Number of PEDV viremic pigs | 0/2 | 0/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Average genome load | 1.79 × 10 3 | |||||||||
| Direct contact pigs | InDel | Number of PEDV viremic pigs | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 1/8 | 0/8 |
| Average genome load | 3.94 × 10 3 | |||||||||
| Direct contact pigs | Non‐InDel | Number of PEDV viremic pigs | 0/8 | 0/8 | 0/8 | 0/8 | 1/8 | * 1/8 | * 1/8 | 0/8 |
| Average genome load | 1.07 × 10 2 | 1.36 × 10 3 | 2,61 × 10 2 | |||||||
| Indirect contact pigs | InDel | Number of PEDV viremic pigs | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Average genome load | ||||||||||
| Indirect contact pigs | Non‐InDel | Number of PEDV viremic pigs | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 | 2/10 | 1/10 | 0/10 |
| Average genome load | 3.48 × 10 3 | 5.05 × 10 2 | 7.72 × 10 1 | |||||||
Bold values indicated that PEDV equivalent genome copies were detected. The grey boxes indicated that no PEDV equivalent genome copies were detected. *Same pig.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
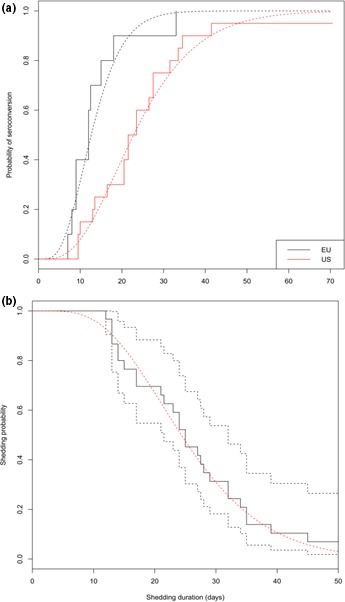
Delays between infection and seroconversion (a) and the probability of shedding (b) for the InDel strain indicated in black and the non‐InDel strain indicated in red. In (a), the solid lines represent the raw data and the dotted lines represent the probability of seroconversion after shedding. In (b), the black solid line represents the raw data, the black dotted lines represent the confidence interval, and the red dotted line represents the probability of shedding [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.4. PEDV detection in air samples
No PEDV RNA was detected in the air before inoculation of pigs either with the InDel or the non‐InDel strain.
In the InDel groups, PEDV genome was detected in the air at low levels at 2 dpi and then from 5 to 35 dpi (Figure 4). The onset of the PEDV RNA detection in the air sample matched or overlapped with the first detection of the virus in faeces. The viral load detected in the air reached a maximum of 1.78 × 104 genomic copies/L of air on day 14 postinoculation. In spite of the presence of PEDV RNA in the air, no indirect contact pigs were infected by the virus.
Figure 4.
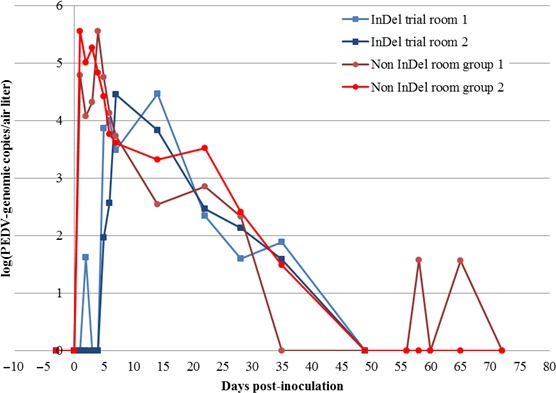
Average PEDV viral genome load in air expressed in log(PEDV genome copies per L of air) for the InDel and non‐InDel trials [Colour figure can be viewed at http://wileyonlinelibrary.com]
In the non‐InDel groups, PEDV genome was detected in the air earlier compared with the InDel trial. Indeed, PEDV RNA was detected from 1 to 35 dpi. Low quantity of PEDV RNA was further recovered at 65 dpi. The number of genomic copies in the air reached a value of 2.11 × 105 genomic copies/L of air at 4 dpi. From 2 dpi, the number of genomic copies in the air was already higher (5.74 × 104 genomic copies/L of air) than the maximum genomic copies detected in air collected from the InDel groups (Figure 4). For the InDel strain, a correlation of 0.83 was found between the number of pigs which shed virus and the number of copies of PEDV genome in the air.
3.5. Estimation of epidemiological parameters
No significant difference in the duration of shedding period according to the challenge strains could be evidenced in our experimental settings (Cox proportional hazard model, p‐value). Based on this result, shedding periods for both groups were aggregated and analysed using a parametric survival model assuming a Weibull distribution. The average shedding duration was estimated to 26 days [21; 32] (Figure 3). During this period, intermittent shedding was observed for three and for six animals infected with the InDel and non‐InDel strains, respectively (Table 1). In contrast, transmission parameters were strongly related to the challenge strain. The estimated transmission rate by direct contact was twice as high for the non‐InDel strain (βw = 2.96; 95% CI: [1.33; 5.23]) compared to the InDel strain (βw = 1.36; 95% CI: [0.6; 5.6]) (Table 3). Furthermore, the spread of the non‐InDel strain by indirect contact was quantified with a transmission rate estimated to 0.5 ([0.1; 1.3]), whereas the InDel strain was not transmitted to the pigs in the neighbouring pen. The average latency period was estimated to 2 days [1.2; 3.6] for the InDel strain but was almost negligible for the non‐InDel strain (estimated to 0 [0.0; 0.2]) (Table 3).
Table 3.
Estimation of epidemiological parameters for the InDel and the non‐InDel strain
| Epidemiological parameters | InDel strain | Non‐InDel strain |
|---|---|---|
| βw | 1.36 (0.6; 5.6) | 2.95 (1.33; 5.23) |
| βb | 0 (0.0; 0.2) | 0.5 (0.1;1.3) |
| Average duration of the latency period (days) | 2.0 (1.2; 3.6) | 0.003 (0.001; 0.15) |
| Average shedding duration (days) | 26 (21; 32) | |
Numbers between brackets correspond to the confidence interval of 95%.
βw: direct transmission rate; βb: indirect transmission rate.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
The severity of porcine epidemic diarrhoea can be linked to several parameters such as the animal age, the infectious dose, the susceptibility of pigs, the environmental conditions or the viral strain (Shibata et al., 2000). The objective of this study was to compare the pathogenicity and transmissibility of an American non‐InDel strain considered highly pathogenic and an InDel strain isolated in France in 2014 in experimental conditions. In case of infection with a non‐InDel and an InDel strain, 100% of morbidity (except for the InDel indirect contact pigs) was observed, but clinical signs were not apparent at the same time among the two types of PEDV‐infected groups. As early as 1 dpi, the first clinical signs were observed for the non‐InDel strain, although the InDel strain induced the first clinical signs only at 5 dpi. These results are in agreement with previous experimental studies involving the non‐InDel strain reporting the occurrence of diarrhoea and occasional vomiting from 2 to 10 days after inoculation (Crawford et al., 2015; Madson et al., 2014; Niederwerder et al., 2016; Shibata et al., 2000) and also with previous studies involving InDel strains reporting the appearance of clinical signs within 1 week after infection (Hanke et al., 2015; Mesquita et al., 2015; Stadler et al., 2015). Intermittent clinical signs were also noticed with the two strains after the occurrence of the first clinical signs; InDel‐infected pigs showed clinical signs (occasional diarrhoea, vomiting and despondency) from 8 to 49 dpi, and non‐InDel‐infected pigs showed clinical signs from 3 to 46 dpi. PEDV replicates in the enterocytes and leads to a significant reduction in both the intestinal epithelial cells, as well as the digestive enzyme activity (Jung, Ahn, & Chae, 2006). However, the regeneration of intestinal enterocytes was shown possible between 2 and 4 days after damage (Moon, 1971), which might explain the intermittent clinical signs observed in the InDel and non‐InDel trials. The clinical signs were more pronounced for the non‐InDel trial pigs in our study. It has already been shown that differences in severity of clinical signs could be linked to the different strains; the InDel strain leads to less severe clinical signs compared with the non‐InDel strain (Chen et al., 2016). The development of the disease was responsible for weight loss, which depended on the strain used. All the non‐InDel pigs had more severe weight loss during the first week after inoculation compared to the InDel pigs. As in our study, Madson et al. (2014) detected a significant alteration in ADWG during the first week postinoculation for non‐InDel‐infected pigs (Madson et al., 2014). Regarding the InDel pigs, growth retardation was observed the second and the third week after inoculation. After the first 3 weeks of the trial, no difference in ADWG was observed between controls and infected pigs within the non‐InDel group.
A trend between viral shedding and growth impact was observed for all the non‐InDel trial pigs. As seen previously, all the inoculated, direct and indirect contact pigs showed a significant difference of ADWG compared with the control pigs, the first week postinoculation. This period corresponded to the highest levels of virus shedding in this group. After that, the average amount of genomic copies of PEDV declined for the inoculated, the direct and indirect contact pigs and the pigs recovered normal growth performance.
PEDV RNA was detected in the faeces of all pigs of the InDel and non‐InDel trial except for the InDel indirect contact pigs. The PEDV RNA was detected earlier in faeces of the non‐InDel trial pigs at 1 dpi compared to 2 ± 0.5 dpi in faeces of InDel inoculated pigs, that is, 1 ± 0.5 days earlier for the non‐InDel pigs. Similar results were observed from faeces of direct contact pigs where PEDV RNA was detected in faeces of non‐InDel direct contact pigs between 1 and 2 dpi and between 4.5 and 14 dpi in faeces of InDel direct contact pigs, that is, on average 7 days earlier for the non‐InDel direct contact pigs. These results suggest the non‐InDel PEDV strain replicates more quickly in the target cells and/or demonstrates the ability to propagate to higher levels more quickly in a pig population. The shedding periods were similar for the InDel (except for the indirect contact pigs) and non‐InDel strains. The shedding periods for the non‐InDel and InDel strains were close to previous observations from other studies which reported shedding periods of 17–23 or 18–20 days on average for the non‐InDel (Madson et al., 2014; Thomas et al., 2015) and for the InDel strain, respectively (Leidenberger et al., 2017; Lohse et al., 2017). Two others studies on PEDV InDel strains revealed that pigs inoculated with an InDel strain began to shed virus earlier than in our experimental conditions, that is, shedding began at 1 dpi, and the presence of PEDV RNA in faeces was detected until the pigs were necropsied at 7 dpi (Chen et al., 2016; Yamamoto, Soma, Nakanishi, Yamaguchi, & Niinuma, 2015). In accordance with the results of other studies (Chen et al., 2016), the amount of genomic copies in faeces was more important in infected pigs by the non‐InDel strain than for those that were infected by the InDel strain whatever the pig status (inoculated, direct contact, indirect contact) in our experimental conditions.
Two phases of PEDV shedding in faeces were noticed for some pigs in our study without the presence of any clinical signs. The same phenomenon had already been described recently (Crawford et al., 2015). During the second phase of PEDV shedding in faeces, no clinical signs were observed. The presence of PEDV RNA in faeces in spite of the absence of clinical signs could raise problems in terms of occasional transmission of the disease by asymptomatic pigs that remain unknown to the producer.
Several studies revealed the occurrence of PEDV RNA in air samples and their infectivity (Alonso, Raynor, Davies, & Torremorell, 2015; Alonso et al., 2014; Beam et al., 2015). Results of our study confirmed the detection of non‐InDel and InDel PEDV RNA in air samples. The duration of detection of PEDV RNA in the air was similar for the InDel and for the non‐InDel strains (30 vs 34 days on average). Although the viral genome was detected in the air, airborne transmission to pigs in indirect contact was only effective for the non‐InDel strain under our experimental conditions. The amount of PEDV genomic copies in air was higher for the non‐InDel than for the InDel strain. Thus, we could hypothesize that the amount of PEDV genomic copies in air with the InDel strain was too low to infect pigs by airborne transmission (104 genome copies/L). The infectious capacity of PEDV by the air could also be linked to the strain. In fact, in a recent study using a different non‐InDel strain from ours, airborne transmission to pigs in indirect contact was not effective even when PEDV RNA was detected in nasal swabs of infected pigs but at low levels (Niederwerder et al., 2016).
The transmission characteristics of the two strains were significantly different in our experimental conditions. Indeed, the direct transmission rate quantified in our experimental conditions was more than twofold higher for the non‐InDel strain than for the InDel strain. An indirect transmission rate could only be calculated for the non‐InDel strain (βw = 0.5). A lower duration of latency for the non‐InDel strain was also observed which is in agreement with the faster replication observed from the virological data. From our experimental results, a faster transmission in case of non‐InDel strain should be expected in a pig population. This faster transmission could be linked to higher virulence and faster replication of the PEDV non‐InDel strain in the epithelial cells. The comparison of the transmission parameters calculated for the two PEDV strains with those estimated for other porcine viruses revealed a transmission rate sevenfold higher than those obtained for porcine reproductive and respiratory syndrome virus (βw PRRS = 0.24) (Rose et al., 2015) or the porcine circovirus type 2b (βw PCV2b = 0.28) (Andraud et al., 2009; Rose et al., 2015). However, these parameters were comparable to those obtained recently for swine influenza viruses (Cador, Andraud, Willem, & Rose, 2017). Considering the high transmission rate for both strains and the duration of shedding of approximately 20 days, those viruses are expected to persist easily within the population. Differences in terms of indirect transmission suggest that InDel strains of PEDV could be more easily controlled by strict segregation of infected animals.
All pigs seroconverted during the trials except some pigs infected by the non‐InDel strain. The seroconversion appeared for the first time at 14 dpi for both strains. A seroconversion at 14 dpi was already obtained with non‐InDel strain in other studies (de Arriba, Carvajal, Pozo, & Rubio, 2002; Thomas et al., 2015). The absence of seroconversion for some pigs could be linked to the sensitivity of the ID Vet ELISA test which could be too low (Tignon et al., 2017). The ELISA test used in our study is based on the PEDV N protein as an antigen, and the PEDV N antibodies might not persist as long as the antibodies directed against the PEDV S protein.
To conclude, in our experimental settings, the InDel strain was less pathogenic than the non‐InDel strain. The InDel strain could only be transmitted to direct contact pigs although the non‐InDel strain could be transmitted to direct contact and indirect contact pigs with a faster direct transmission. These data should be considered when developing control strategies for InDel or non‐InDel PEDV to reduce the probability of introduction in a pig population.
CONFLICT OF INTEREST
The authors declare that they have noncompeting interests.
ACKNOWLEDGEMENTS
The authors are grateful to ANSES, INRA and INAPORC for their financial support. We also would like to thank Phillip Gauger from Iowa State University who provided the US non‐InDel strain, all the team members at the experimental laboratory for their contribution and the members of the unit of viral genetic of ANSES.
Gallien S, Andraud M, Moro A, et al. Better horizontal transmission of a US non‐InDel strain compared with a French InDel strain of porcine epidemic diarrhoea virus. Transbound Emerg Dis. 2018;65:1720–1732. 10.1111/tbed.12945
REFERENCES
- Alonso, C. , Goede, D. P. , Morrison, R. B. , Davies, P. R. , Rovira, A. , Marthaler, D. G. , & Torremorell, M. (2014). Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Veterinary research, 45, 73 10.1186/s13567-014-0073-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, C. , Raynor, P. C. , Davies, P. R. , & Torremorell, M. (2015). Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS ONE, 10, e0135675 10.1371/journal.pone.0135675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andraud, M. , Rose, N. , Grasland, B. , Pierre, J. S. , Jestin, A. , & Madec, F. (2009). Influence of husbandry and control measures on porcine circovirus type 2 (PCV‐2) dynamics within a farrow‐to‐finish pig farm: A modelling approach. Preventive Veterinary Medicine, 92, 38–51. 10.1016/j.prevetmed.2009.07.009 [DOI] [PubMed] [Google Scholar]
- de Arriba, M. L. , Carvajal, A. , Pozo, J. , & Rubio, P. (2002). Isotype‐specific antibody‐secreting cells in systemic and mucosal associated lymphoid tissues and antibody responses in serum of conventional pigs inoculated with PEDV. Veterinary immunology and immunopathology, 84, 1–16. 10.1016/S0165-2427(01)00386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam, A. , Goede, D. , Fox, A. , McCool, M. J. , Wall, G. , Haley, C. , & Morrison, R. (2015). A porcine epidemic diarrhea virus outbreak in one geographic region of the United States: Descriptive epidemiology and investigation of the possibility of airborne virus spread. PLoS ONE, 10, e0144818 10.1371/journal.pone.0144818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, A. S. , Krogwold, R. A. , Price, T. , Davis, M. , & Moeller, S. J. (2015). Investigating the introduction of porcine epidemic diarrhea virus into an Ohio swine operation. BMC Veterinary Research, 11, 38 10.1186/s12917-015-0348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador, C. , Andraud, M. , Willem, L. , & Rose, N. (2017). Control of endemic swine flu persistence in farrow‐to‐finish pig farms: A stochastic metapopulation modeling assessment. Veterinary research, 48, 58 10.1186/s13567-017-0462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Gauger, P. C. , Stafne, M. R. , Thomas, J. T. , Madson, D. M. , Huang, H. , … Zhang, J. (2016). Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S‐INDEL‐variant strains in conventional neonatal piglets. Journal of General Virology, 97, 1107–1121. 10.1099/jgv.0.000419 [DOI] [PubMed] [Google Scholar]
- Crawford, K. , Lager, K. , Miller, L. , Opriessnig, T. , Gerber, P. , & Hesse, R. (2015). Evaluation of porcine epidemic diarrhea virus transmission and the immune response in growing pigs. Veterinary research, 46, 49 10.1186/s13567-015-0180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA . (2016). Collection and review of updated scientific epidemiological data on porcine epidemic diarrhoea. EFSA Journal, 14, 4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallien, S. , Moro, A. , Lediguerher, G. , Catinot, V. , Paboeuf, F. , Bigault, L. , … Grasland, B. (2018). Evidence of porcine epidemic diarrhea virus (PEDV) shedding in semen from infected specific pathogen‐free boars. Veterinary Research, 49, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke, D. , Jenckel, M. , Petrov, A. , Ritzmann, M. , Stadler, J. , Akimkin, V. , … Hoper, D. (2015). Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerging Infectious Diseases, 21, 493–496. 10.3201/eid2103.141165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, B. , Gonzales, J. L. , & Gubbins, S. (2017). Bayesian inference of epidemiological parameters from transmission experiments. Scientific Reports, 7, 16774 10.1038/s41598-017-17174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Ahn, K. , & Chae, C. (2006). Decreased activity of brush border membrane‐bound digestive enzymes in small intestines from pigs experimentally infected with porcine epidemic diarrhea virus. Research in Veterinary Science, 81, 310–315. 10.1016/j.rvsc.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Jung, K. , & Saif, L. J. (2015). Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Veterinary Journal, 204, 134–143. 10.1016/j.tvjl.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidenberger, S. , Schroder, C. , Zani, L. , Auste, A. , Pinette, M. , Ambagala, A. , … Blome, S. (2017). Virulence of current German PEDV strains in suckling pigs and investigation of protective effects of maternally derived antibodies. Scientific Reports, 7, 10825 10.1038/s41598-017-11160-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Li, H. , Liu, Y. , Pan, Y. , Deng, F. , Song, Y. , … He, Q. (2012). New variants of porcine epidemic diarrhea virus, China, 2011. Emerging Infectious Diseases, 18, 1350–1353. 10.3201/eid1803.120002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse, L. , Krog, J. S. , Strandbygaard, B. , Rasmussen, T. B. , Kjaer, J. , Belsham, G. J. , & Botner, A. (2017). Experimental infection of young pigs with an early european strain of porcine epidemic diarrhoea virus and a recent US strain. Transboundary and Emerging Diseases, 64, 1380–1386. 10.1111/tbed.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, J. , Gauger, P. , Harmon, K. , Zhang, J. , Connor, J. , Yeske, P. , … Main, R. (2014). Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerging Infectious Diseases, 20, 872–874. 10.3201/eid2005.131628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson, D. M. , Magstadt, D. R. , Arruda, P. H. , Hoang, H. , Sun, D. , Bower, L. P. , … Yoon, K. J. (2014). Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3‐week‐old weaned pigs. Veterinary microbiology, 174, 60–68. 10.1016/j.vetmic.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Mesquita, J. R. , Hakze‐van der Honing, R. , Almeida, A. , Lourenço, M. , van der Poel, W. H. , & Nascimento, M. S. (2015). Outbreak of porcine epidemic diarrhea virus in Portugal, 2015. Transboundary and Emerging Diseases, 62, 586–588. 10.1111/tbed.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, H. W. (1971). Epithelial cell migration in the alimentary mucosa of the suckling pig. Proceedings of the Society for Experimental Biology and Medicine, 137, 151–154. 10.3181/00379727-137-35533 [DOI] [PubMed] [Google Scholar]
- Niederwerder, M. C. , Nietfeld, J. C. , Bai, J. , Peddireddi, L. , Breazeale, B. , Anderson, J. , … Hesse, R. A. (2016). Tissue localization, shedding, virus carriage, antibody response, and aerosol transmission of Porcine epidemic diarrhea virus following inoculation of 4‐week‐old feeder pigs. Journal of Veterinary Diagnostic Investigation, 28, 671–678. 10.1177/1040638716663251 [DOI] [PubMed] [Google Scholar]
- Pensaert, M. B. , & de Bouk, P. (1978). A new corona virus‐like particle associated with diarrhea in swine. Archives of virology, 58, 243–247. 10.1007/BF01317606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, N. , Renson, P. , Andraud, M. , Paboeuf, F. , Le Potier, M. F. , & Bourry, O. (2015). Porcine reproductive and respiratory syndrome virus (PRRSv) modified‐live vaccine reduces virus transmission in experimental conditions. Vaccine, 33, 2493–2499. 10.1016/j.vaccine.2015.03.040 [DOI] [PubMed] [Google Scholar]
- Shibata, I. , Tsuda, T. , Mori, M. , Ono, M. , Sueyoshi, M. , & Uruno, K. (2000). Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Veterinary microbiology, 72, 173–182. 10.1016/S0378-1135(99)00199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, D. , & Park, B. (2012). Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes, 44, 167–175. 10.1007/s11262-012-0713-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler, J. , Zoels, S. , Fux, R. , Hanke, D. , Pohlmann, A. , Blome, S. , … Ladinig, A. (2015). Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Veterinary Research, 11, 142 10.1186/s12917-015-0454-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R. C. (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Thomas, J. T. , Chen, Q. , Gauger, P. C. , Giménez‐Lirola, L. G. , Sinha, A. , Harmon, K. M. , … Zhang, J. (2015). Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in Naïve conventional neonatal and weaned pigs. PLoS ONE, 10, e0139266 10.1371/journal.pone.0139266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tignon, M. , Christiaens, I. , Redant, V. , Nauwynck, H. , Ojkic, D. , Nelson, E. , & Cay, A. B. (2017). Comparative study of serological methods for diagnosis of Porcine Epidemic Diarrhea Virus (PEDV) infection. European Symposium Of Porcine Health Management. Prague, Czech Republic.
- Vlasova, A. N. , Marthaler, D. , Wang, Q. , Culhane, M. R. , Rossow, K. D. , Rovira, A. , … Saif, L. J. (2014). Distinct characteristics and complex evolution of PEDV strains, North America, May 2013‐February 2014. Emerging Infectious Diseases, 20, 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Byrum, B. , & Zhang, Y. (2014). New variant of porcine epidemic diarrhea virus, United States, 2014. Emerging Infectious Diseases, 20, 917–919. 10.3201/eid2005.140195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhao, P. , Guo, L. , Liu, Y. , Du, Y. , Ren, S. , … Wu, J. (2013). Porcine epidemic diarrhea virus variants with high pathogenicity, China. Emerging Infectious Diseases, 19, 2048–2049. 10.3201/eid1912.121088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, R. , Soma, J. , Nakanishi, M. , Yamaguchi, R. , & Niinuma, S. (2015). Isolation and experimental inoculation of an S INDEL strain of porcine epidemic diarrhea virus in Japan. Research in Veterinary Science, 103, 103–106. 10.1016/j.rvsc.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]


