Abstract
Background
There is growing evidence that community‐acquired respiratory virus (CARV) increases the risk of pulmonary invasive fungal disease (IFD) in the allogeneic hematopoietic stem cell transplantation (allo‐HSCT) setting. To date, there is a lack of knowledge regarding the risk factors (RFs), as well as the most critical period for subsequent onset of IFD after CARV infections in allo‐HSCT recipients.
Methods
In this prospective longitudinal observational CARV survey, we analyzed the effect of CARV on subsequent IFD development in 287 adult allo‐HSCT recipients diagnosed with 597 CARV episodes from December 2013 to December 2018. Multiplex PCR panel assays were used to test CARVs in respiratory specimens.
Findings
Twenty‐nine out of 287 allo‐HSCT recipients (10%) developed IFD after a CARV episode. The median time of IFD onset was 21 days (range, 0‐158 days) from day of the first CARV detection. Generalized estimating equation model identified 4 risk factors for IFD: ATG‐based conditioning regimen [odds ratio (OR) 2.34, 95% confidence interval (CI) 1.05‐5.2, P = .038], CARV lower respiratory tract disease (OR 10.6, 95% CI 3.7‐30.8, P < .0001), CARV infection during the first year after transplant (OR 5.34, 95% CI 1.3‐21.8, P = .014), and corticosteroids during CARV (OR 2.6, 95% CI 1.1‐6.3, P = .03).
Conclusion
Allo‐HSCT recipients conditioned with ATG and under corticosteroid therapy at the time of CARV LRTD during the first year after transplant may require close monitoring for subsequent IFD.
Keywords: allogeneic hematopoietic stem cell transplantation, community‐acquired respiratory virus, immunodeficiency score index, invasive Aspergillosis, invasive pulmonary fungal disease
1. INTRODUCTION
Pulmonary invasive fungal disease (IFD) is a relevant cause of morbidity and mortality in the allogeneic hematopoietic stem cell transplantation (allo‐HSCT) setting.1, 2 Established risk factors (RFs) for IFDs in allo‐HSCT [mainly for invasive aspergillosis (IA)] include factors associated with a profound immunosuppressed status, such as duration of neutropenia before engraftment, use and duration of glucocorticosteroid therapy, type of donor, baseline disease, recipient's age, and graft‐versus‐host disease (GVHD).2, 3, 4, 5, 6, 7, 8 However, there is growing awareness and evidence that community‐acquired respiratory virus (CARV) also increases the risk of IA in immunocompromised and healthy patients, primarily but not limited to influenza infections.5, 9, 10, 11, 12, 13, 14, 15 Allo‐HSCT recipients suffer annual and lifelong exposure to CARV infections; in fact, these infections after allo‐HSCT are as common as around 30%.8, 16 Obviously, not every allo‐HSCT recipient with CARV infection has the same risk of developing IFD. To date, there are limited data regarding the true rate of IFD, the RFs for specific respiratory virus infection, and the most critical period for IFD development after CARV infections in allo‐HSCT recipients.
The current study comes from a 5‐year prospective longitudinal observational epidemiologic survey of CARV infections and complications in allo‐HSCT recipients with upper (URTD) and/or lower respiratory tract disease (LRTD) symptoms after allo‐HSCT. We report herein the incidence rate, characteristics, and RFs of IFD in a series of consecutive allo‐HSCT recipients after a molecular‐proven CARV infection.
2. PATIENTS AND METHODS
2.1. Study cohort and respiratory virus survey protocol
We conducted a prospective longitudinal study of CARV infections in adult (>18 years) allo‐HSCT recipients at two transplant centers in Valencia (at the Hospital Clinic Universitari of Valencia [HCUV] and at the Hospital Universitari i Politècnic la Fe in Valencia [HLF]), Spain. The cohort was comprised entirely of allo‐HSCT recipients with molecular‐proven CARV infection who were screened for CARV when they developed respiratory symptoms. This survey was carried out at the HCUV between December 2013 and May 2016 and at HLF from June 2016 to December 2018. The current study analyzed all consecutive CARV infectious episodes in allo‐HSCT recipients who were relapse‐free of baseline disease at the time of CARV detection from December 1, 2013 to December 1, 2018. Data have been updated on January 28, 2019.
The CARV survey methodology has previously been described in detail elsewhere.17, 18 Briefly, at both centers, we developed a prospective CARV survey in allo‐HSCT recipients with symptoms suggesting a respiratory virus infection. Irrespective of their transplant date, all recipients with URTD and/or LRTD symptoms were encouraged to undergo a detailed respiratory virus screening through molecular testing. We do not monitor viral shedding negativity after a first detection, except for recipients with active and persistent respiratory symptoms for longer than one month. In such cases, we repeated the PCR test to rule out the acquisition of a new CARV. If the same subtype of CARV was found, we considered as a long‐lasting CARV episode.
Clinical and biological variables at the time of CARV PCR testing were prospectively recorded. Variables included transplant characteristics, conditions included in the immunodeficiency scoring index (ISI),19 hospital admission, immunosuppressant drugs, presence of acute or chronic GvHD, use of antifungal prophylaxis at the time of CARV infection, and subsequent onset of IFD. All allo‐HSCT recipients were followed on a regular basis at each transplant center for lifelong. The study protocol was approved by the ethics committee of the Hospital universitari i politècnic La Fe with reference code number 2019/0041.
2.2. Antifungal prophylaxis policy
At HCUV, from January 2013 to February 2014, antifungal prophylaxis was based on voriconazole 200 mg twice daily (BID) from day + 1 to day + 100 and thereafter in cases with active GvHD requiring steroid therapy. From March 2014, antifungal prophylaxis consisted of micafungin (50 mg/day iv) from the start of the conditioning regimen until neutrophil recovery and switched to posaconazole 300mg daily in tablets until day + 100 or while on steroids to treat moderate‐to‐severe GvHD. At HLF, from January 2010 to December 2016 antifungal prophylaxis was based on fluconazole (100 mg/day) from the start of conditioning until the day of stem cell infusion (day 0) and voriconazole 100 mg/BID a day thereafter until day + 100 or while on steroids.20 In January 2017, antifungal prophylaxis was changed and consisted of fluconazole (100 mg/day) during conditioning until day + 7 and posaconazole (300 mg/day) thereafter until day + 100 or while on steroids.
2.3. Definitions
URTD was considered in recipients with upper respiratory symptoms (rhinorrhea, sore through, sinusitis, otitis, or pharyngitis) and a positive identification of CARV by PCR test in respiratory samples and the lack of LRTD symptoms without any sign of pulmonary infiltrates in chest X‐ray or CT scan. We classified LRTD as possible or confirmed according to previous definitions.21 There were no probable episodes since we do not perform bronchoscopies in patients without radiological evidence of pulmonary involvement. We defined CARV URTD and/or LRTD episodes following ECIL‐4 guidelines.22 According to the ECIL‐4 recommendations, an episode was considered to be resolved when complete remission of respiratory symptoms was observed. In cases with a new onset of respiratory symptoms in which we detected the same virus from the prior CARV episode along with another newly emerging CARV, we considered it as a new co‐viral infectious disease episode. In contrast, cases in which we detected a CARV and the recipient had long‐lasting respiratory symptoms that were re‐screened during follow‐up testing positive for a new emerging CARV along with the initial CARV were considered as a unique co‐viral CARV infectious disease episode.
2.4. CARV infections management
As per protocol, we started oseltamivir in allo‐HSCT recipients with influenza infection provided the respiratory symptoms have not been resolved at the time of microbiological results. Annual influenza vaccination was also recommended to all patients at both transplant centers after the third month following allo‐HSCT. For patients with moderate‐to‐severe GVHD at the time of vaccination program who had received gammaglobulin, antithymocytic globulin (ATG), or rituximab within the three months before the flu vaccine period, vaccine administration remained at physician discretion.17 Respiratory syncytial virus (RSV) and human parainfluenza virus (HPIV) were managed according to our interventional protocol.23 Briefly, oral ribavirin was given at a loading dose (maximum daily dose of 30 mg/kg) until resolution of respiratory symptoms in recipients with LRTD caused by RSV or HPIV, whereas patients with URTD must had 3 or more ISI points and/or 2 or more risk factor according to the ECIL guidelines,22 and/or presenting one or more co‐infective virus(es) before starting ribavirin. Routine prophylactic intravenous immunoglobulins (0.4 g/kg) were given when IgG serum levels were below 300 mg/dL.
IFD diagnosis and classification was performed agreeing with definitions of the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG).24 Possible IFD episodes were considered, but finally, they were not included in the analyses. The IFD date was taken as the date the test yielded the diagnosis. IFD was considered to be directly related to CARV infection when it occurred in the 2 months following either the date of CARV detection or date of most recent CARV detection (in cases of long‐lasting active respiratory symptoms and viral shedding with several consecutive PCR tests detecting the same CARV). Recipients with probable‐proven IFD prior to the development of a CARV episode were not considered as a CARV‐related IFD, and they were not included in the analyses. Acute graft‐versus‐host disease (aGvHD) was defined according to standard criteria.25
2.5. Technical and diagnostic considerations
CARV testing was performed with three RT‐PCR multiplex platforms, two of which have been described in detail elsewhere.17, 18 Briefly, the Luminex xTAG RVP Fast v1 assay (Luminex Molecular Diagnostics) and the CLART® PneumoVir DNA array assay (Genomica) were used at the HCUV and at HLF, respectively. At HLF from July 2018 onwards, CARV screening in allo‐HSCT recipients was performed by BioFire FilmArray® Respiratory Panel (BioFire Diagnostics [a bioMerieux company]), which is able to detect 15 respiratory viruses: influenza (Influ) virus types A, B, and C (with influenza A subtyping), HPIV types 1‐4, adenovirus (AdV), coronaviruses (HCoV) HKU1, NL63, 229E and OC43, human metapneumovirus (hMPV), human rhinovirus/enterovirus (EvRh), and RSV, and also detects three bacteria: Mycoplasma pneumoniae, Chlamydia pneumoniae, and Bordetella pertussis. At both centers, Aspergillus galactomannan antigen (GM) monitoring by Platelia™ Aspergillus Ag Kit (Bio‐Rad) was performed in all recipients. GM monitoring was done in serum samples twice a week from day of stem cell infusion until hospital discharge and on a weekly basis thereafter until day + 100 and at each outpatient visit while receiving steroids to treat GvHD afterward. GM was also screened in bronchoalveolar lavage fluid.
2.6. Endpoints and statistics
The primary endpoint of the current study was to determine the IFD incidence and its RFs in allo‐HSCT recipients with prior documented CARV, URTD, and/or LRTD. Secondary endpoints included the analysis of CARV‐related IFD characteristics and its effect on overall survival (OS).
Categorical variable frequencies were compared using the χ2 test (Fisher's exact test), whereas the Mann‐Whitney U test was used to compare differences between medians. Generalized estimating equation (GEE)26 was used to assess the association of clinical and biological RFs with CARV‐related IFD. This method is used when analyzing clustered data, as ours, where episodes can be grouped within the same patient. GEE fits marginal mean models with asymptotically normal and consistent estimators if there is a correct specification of marginal means. Two different models were carried out in the current study: one to evaluate RFs for IFD after overall CARV (1) and those limited to the lower respiratory tract (2).
| (1) |
where response variable is IFD (Y = 0, 1) and factors are as follows: ATG as a part of conditioning (x 1 = 0, 1), CARV LRTD (x 2 = 0, 1), Allo‐HSCT 1 year (x 3 = 0, 1), corticosteroids (x 4 = 0, 1), and patient CMV status (x 5 = 0, 1). Moreover, bj is the random effect associated with each individual j and sij the experimental error, both are considered random and independent.
Analogously, the model associated with the lower respiratory tract (2) was:
| (2) |
where response variable is IFD (Y = 0, 1) and factors are as follows: ATG as a part of conditioning (x 1 = 0,), active GvHD at the time RVI (x 2 = 0, 1), Allo‐HSCT 1 year (x 3 = 0, 1), corticosteroids (x 4 = 0, 1), and patient CMV status (x 5 = 0, 1). Moreover, bj is the random effect associated with each individual j and sij the experimental error, both are considered random and independent.
The method for best model selection was “quasi‐likelihood under the independence model criterion” (QIC). This method was proposed by Pan27 for quasi‐likelihood theory such as GEE. Typical model selection criteria such as Akaike's information criterion (AIC) cannot be used in GEE as they are based on maximum likelihood theory. Tested variables included type of donor, HLA mismatch, ATG as a part of conditioning, GVHD prophylaxis, CARV LRTD, triazole antifungal prophylaxis, on immunosuppression drugs, absolute lymphocyte count (ALC) < 0.5 × 109/L, ALC < 0.2 × 109/L, ALC < 0.1 × 109/L, absolute neutrophil count < 0.5 × 109/L, age ≥40 years, active GvHD at the time CARV, allo‐HSCT ≤ 6 months, allo‐HSCT ≤ 1 year, myeloablative conditioning, corticosteroids, viral co‐infection, ISI score (low, moderate, and high), patient CMV serostatus, and having received antiviral therapy. All these analyses were carried out with R28 and the package for GEE29 and Barton and Barton30 for QIC calculations.
The probability of OS according to subsequent presence or absence of IFD was considered from the time of CARV detection using Kaplan‐Meier curves31 performing univariate comparisons with log‐rank test.32 Descriptive and survival analyses were carried out with the SPSS (version 20.0) statistical package.
3. RESULTS
We accounted for 326 allo‐HSCT recipients developing 804 episodes of URTD and/or LRTD symptoms that were screened for CARVs. Two‐hundred and ninety‐eight allo‐HSCT recipients (91%) developed as a minimum of one episode of molecularly proven CARV with 633 CARV episodes (78%) overall. Thirty‐six episodes (6%) were excluded since baseline disease relapse occurred before the CARV detection. Finally, 287 allo‐HSCT recipients with 597 consecutive molecular‐documented CARV infectious episodes met inclusion criteria. Clinical and transplant characteristics are detailed in Table 1. This series included a high‐risk cohort, since 63% of the recipients were allografted from alternative donors [adult unrelated donor (URD), single cord blood units (CBU), and haplo‐identical family donors]. Donor‐recipient human leukocyte antigen (HLA) mismatch (considering high‐resolution typing of HLA A, B, C, DRB1, and DQB1) represented 42% of the cohort. Sixty‐six recipients (23%) received antithymocyte globulin (ATG) because of URD‐recipient HLA mismatch (n = 8), cord blood transplant (n = 52), or HLA‐identical sibling in the context of a clinical trial (n = 6).
Table 1.
Patient characteristics
| Characteristics | (n = 287) |
|---|---|
| Age (y), median (range) | 46 (18‐71) |
| Male, n (%) | 167 (58) |
| Baseline disease, n (%) | |
| AL/MDS/MPD | 189 (66) |
| Lymphoid disorders | 92 (32) |
| Others | 6 (2) |
| Disease status at transplant, n (%) | |
| CR | 203(71) |
| PR | 34 (12) |
| Refractory/active disease | 50 (17) |
| Prior ASCT, n (%) | 67 (23) |
| Period of transplant, n (%) | |
| 2017‐2018 | 89 (31) |
| 2015‐2016 | 75 (26) |
| 2013‐2014 | 79 (28) |
| 2011‐2012 | 23 (8) |
| 2008‐2010 | 21 (7) |
| Conditioning regimen, n (%) | |
| RIC | 135 (47) |
| Type of donor, n (%) | |
| HLA‐identical sibling donor | 105 (37) |
| Unrelated donor | 69 (24) |
| Umbilical cord blood | 52 (18) |
| Haplo‐identical family donor | 61 (21) |
| PB stem cell source, n (% | 228 (79) |
| HLA fully matched, n (%) | 167 (58) |
| ATG as a part of the conditioning regimen, n (%) | 66 (23) |
| GvHD prophylaxis, n (%) | |
| Sir‐Tac | 31 (11) |
| CsA + MTX | 73 (25) |
| Post‐Cy | 116 (40) |
| CsA + PDN and Others | 67 (23) |
| Number of recipients with CARVs per seasons, n (%) | |
| 2018‐2019 | 13 (5) |
| 2017‐2018 | 90 (31) |
| 2016‐2017 | 39 (14) |
| 2015‐2016 | 30 (10) |
| 2014‐2015 | 59 (20) |
| 2013‐2014 | 56 (20) |
| Number of CARV episodes | 597 |
| Number of LRTD CARV episodes | 203 |
| Median time from allo‐HSCT to CARV, days (range) | 198 (−7 to 6177) |
| Number of recipients with IFD after CARVs, n (%) | 29 (10) |
| Median time from CARV to IFD, days (range) | 21 (0‐158) |
| Median time from allo‐HSCT to IFD, days (range) | 211 (20‐989) |
| Median F‐up after CARV, days (range) | 285 (1‐3106) |
Abbreviations: AL, acute leukemia; allo‐HSCT, allogeneic hematopoietic stem cell transplantation; ASCT, autologous stem cell transplantation; ATG, antithymocyte globulin; CARV, community‐acquired respiratory virus; CR, complete remission; CsA, cyclosporine A; F‐up, follow‐up; IFD, invasive pulmonary infectious fungal disease; LRTD, lower respiratory tract disease; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; MTX, methotrexate; PDN, prednisone; Post‐Cy, post‐transplant cyclophosphamide; PR, partial remission; RIC, reduced intensity conditioning; Sir, sirolimus; Tac, tacrolimus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.1. CARV respiratory virus infection characteristics
One hundred and forty‐two (49%) out of 287 recipients had 1 CARV episode, 66 recipients (23%) had 2 CARV episodes, 38 (13%) had 3 episodes, 21 (7%) had 4, 8 (3%) had 5 and 6, 4 (1%) had 7, and 1 (0.5%) had 9 CARV episodes. CARV episodes were diagnosed at median of 198 days after transplant (range, day‐7 to day + 6177). Three‐hundred and ninety‐four CARV episodes (66%) were limited to the URTD, whereas 203 (34%) had LRTD involvement (106 possible LRTD and 97 proven LRTD). Antiviral therapy with oseltamivir or ribavirin was given in 169 (28%) out of 597 CARV episodes. Table 2 summarizes the most common CARV types and rates of URTD and LRTD.
Table 2.
Type of CARV episodes and later‐occurring IFD according to CARV type and respiratory location
| EvRh | RSV | Influ | HPiV | hMPV | AdV | HCoV | HBoV | |
|---|---|---|---|---|---|---|---|---|
| Number of Episodes, n (%)a | 238 (40) | 136 (23) | 118 (20) | 97 (16) | 60 (10) | 12 (2) | 36 (6) | 17 (3) |
| Overall IFD, n (%)a | 10 (4) | 7 (5) | 5 (4) | 5 (5) | 4 (7) | 1 (8) | 0 | 0 |
| CARV URTD, n (%)a | 170 (72) | 79 (58) | 77 (65) | 61 (63) | 34 (57) | 4 (31) | 26 (72) | 12 (71) |
| IFD after URTD, n (%)a | 2 (1) | 1 (1) | 0 | 0 | 1 (3) | 0 | 0 | 0 |
| CARV LRTD, n (%)a | 68 (28) | 57 (42) | 41 (35) | 36 (37) | 26 (43) | 8 (66) | 10 (28) | 5 (29) |
| IFD after LRTD, n (%)a | 8 (12) | 6 (11) | 5 (12) | 5 (14) | 3 (11) | 1 (13) | 0 | 0 |
Abbreviation: ADV, adenovirus; AdV, adenovirus; CARV, community‐acquired respiratory virus; EvRh, Enterovirus/rhinovirus; HCoV, human coronavirus; hMPV, human metapneumovirus; HPiV, human parainfluenza virus; IFD, invasive pulmonary fungal disease; Influ, human influenza virus; LRTD, lower respiratory tract disease; RSV, respiratory syncytial virus; URTD, upper respiratory tract disease.
The sum of the episodes does not match the overall number of episodes (n = 597) since multiple CARVs were detected in the same respiratory sample in 116 (19%) out of 597 CARV episodes. IFD after CARV co‐infection was 8% (9 out of 116)
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. CARV episodes characteristics according to IFD development
Out of 287 allo‐HSCT recipients, 29 (10%) developed IFD within two months after a CARV episode at a median of 21 days (range 0‐158 days) from the day of CARV diagnosis. In five out of 29 cases (17%), IFD was diagnosed at median of 109 days (range 66 to 158 days) after the initial CARV diagnosis. However, these cases had long‐lasting respiratory symptoms and repeated PCR test detected the same CARV subtype in the two months before the diagnosis of IFD. All the IFDs involved the lungs, and in 26 (89%) cases, the diagnostic was IA, meeting criteria of probable (n = 24) or proven (n = 2). Probable IA cases had GM index in blood (n = 18) and/or BAL (n = 11) above the threshold values. The remaining 3 patients developed proven pulmonary invasive fungal disease caused by Cladophialopora carrionii (isolated from 2 consecutive BAL cultures and confirmed in the autopsy), scedosporium apiospermum (BAL culture), and mucor racemosus (diagnosed in autopsy). Patients’ clinical and biological characteristics according to whether they developed IFD are shown in Table 3. The IFD group had significantly higher rates of factors included in the ISI score (lymphopenia, active GVHD, steroid therapy) (P ≤ .05 for all comparisons). Of note, 26 out of 29 IFD (91%) occurred within the first year after transplantation. The overall rate of IFD after CARV was 5%, whereas this rate was higher in recipients developing CARV during the first year of transplant (7%). IFD was diagnosed in 25 out of 203 LRTD episodes (12%) compared to 4 out of 394 URTD episodes (1%) (P = .0001). Out of 133 CARV episodes involving the LRTD within the first year after transplant, 23 (17%) developed IFD. We found no differences in IFD rates according to CARV type (Table 2).
Table 3.
Clinical and biological characteristics of CARV episodes in allo‐HSCT recipients according to the development of a later invasive pulmonary infectious disease
| CARV Without IFD (n = 568) | CARV With IFD (n = 29) | P value | |
|---|---|---|---|
| Immunodeficiency Scoring Index, n (%)a | |||
| ANC < 0.5 × 109/L (3pts) | 42 (7) | 5 (17) | .07 |
| ALC< 0.2 × 109/L (3pts) | 72 (13) | 10 (34) | .003 |
| Age ≥ 40 y (2pts) | 401 (71) | 23 (79) | .6 |
| Myeloablative conditioning regimen (1pt) | 284 (50) | 17 (59) | .4 |
| GvHD (acute or chronic; 1pt) | 287 (51) | 20 (69) | .05 |
| Corticosteroids (1pt) | 190 (33) | 20 (69) | .0001 |
| Recent or pre‐engraftment allo‐HSCT (1pt) | 54 (10) | 4 (14) | .5 |
| ISI, n (%) | |||
| Low risk (0‐2) | 198 (35) | 2 (7) | |
| Moderate risk (3‐6) | 308 (54) | 21 (72) | .005 |
| High risk (7‐12) | 61 (11) | 6 (21) | |
| Other characteristicsa | |||
| On IS, n (%) | 426 (75) | 26 (90) | .07 |
| ALC< 0.1 × 109/L, n (%) | 48 (8) | 4 (14) | .23 |
| ALC < 0.5 × 109/L, n (%) | 135 (23) | 15 (52) | .002 |
| Allo‐HSCT ≤ 6 mo, n (%) | 197 (35) | 15 (52) | .07 |
| Allo‐HSCT ≤ 1 y, n (%) | 337 (59) | 26 (90) | .0001 |
| IFD prophylaxis during CARV infection, n (%) | |||
| Triazole | 159 (28) | 15 (52) | .001 |
| No prophylaxis | 409 (73) | 14 (48) | |
| CARV characteristics and clinical consequences | |||
| CARV LRTD, n (%) | 178 (31) | 25 (86) | .0001 |
| Possible | 95 (16) | 11 (38) | .5 |
| Proven | 83 (14) | 14 (48) | .5 |
| Hospital admission, n (%) | 171 (30) | 23 (79) | .0001 |
| Fever during CARV, n (%) | 320 (57) | 23 (79) | .005 |
| Overall mortality rate, n (%) | 92 (16) | 17 (58) | .0001 |
| Day + 30 | 15 (3) | 3 (10) | .05 |
| Day + 60 | 23 (5) | 6 (21) | .002 |
| Day + 90 | 36 (6) | 6 (21) | .01 |
Abbreviations: ALC, absolute lymphocyte count; Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; ANC, absolute neutrophil count; ATG, antithymocyte globulin; CARV, community‐acquired respiratory virus; GvHD, graft‐versus‐host disease; IFD, invasive pulmonary infectious fungal disease; IS, immunosuppressants; LRTD, lower respiratory tract disease.
All variables were captured at the time of CARV diagnosis.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.3. Risk factors for IFD after CARV and outcome
GEE models of RFs for IFD after overall CARV episodes and those with LRTD are shown in Table 4.
Table 4.
Generalized estimating equation models of risk factors for infectious fungal disease after overall CARV and those limited to the lower respiratory tract
| Factors | GEE IFD (n = 597) | GEE IFD in recipients with CARV LRTD (n = 203) | ||
|---|---|---|---|---|
| OR (95% CI) | Pr(>|W|) | OR (95% CI) | Pr(>|W|) | |
| Intercept | 0.00392 (0.000896‐0.0172) | <0.0001 | 0.00755 (0.000879‐0.0648) | <0.0001 |
| ATG as a part of conditioning | 2.34076 (1.046525‐5.2356) | 0.038 | 3.09494 (1.327891‐7.2134) | 0.009 |
| CARV LRTD | 10.63917 (3.668696‐30.8534) | <0.0001 | nt | nt |
| Allo‐HSCT ≤ 1 y corticosteroids |
5.34718 (1.311639‐21.7989) 2.62746 (1.098765‐6.2830) |
0.014 0.030 |
5.34718 (1.311639‐21.7989) nt |
0.019 nt |
| CARV co‐infection | 0.27566 (0.058127‐1.3073) | 0.105 | 0.27389 (0.055030‐1.3632) | 0.114 |
| Patient CMV status | 2.88806 (0.847759‐9.8387) | 0.090 | 2.31278 (0.644803‐8.2955) | 0.198 |
| Active GvHD at the time RVI | nt | 2.97987 (1.016354‐8.7367) | 0.047 | |
| Estimated scale parameter | 1.21 | 1.15 | ||
| QIC | 178.21 | 134.83 | ||
Abbreviations: Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; ATG, antithymocytic globulin; CARV LRTD, community‐acquired respiratory virus lower respiratory tract disease; CI, confidence interval; GEE, Generalized estimating equation models; GvHD, graft‐versus‐host disease; IFD, infectious fungal disease; nt, not tested; OR, odds ratio; Pr(>|W|), P value of the Wald test; QIC, quasi‐likelihood under the independence model criterion.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In order to identify transplant and CARV circumstances related to IFD, we included the 597 evaluable recipient/episode pairs. The GEE model with lower QIC calculations included 6 variables (ATG as a part of conditioning, CARV LRTD, allo‐HSCT ≤1 year, corticosteroids, viral co‐infection, and patient CMV serostatus). Of these, four were statistically significant RFs: ATG‐based conditioning regimen [odds ratio (OR) 2.34, 95% confidence interval (CI) 1.05‐5.2, P = .038], CARV LRTD (OR 10.6, 95% CI 3.7‐30.8, P < .0001), CARV infection during the first year after transplant (OR 5.34, 95% CI 1.3‐21.8, P = .014), and corticosteroids during CARV (OR 2.6, 95% CI 1.1‐6.3, P = .03). Based on these 4 RFs, we elaborated a risk score with 3 groups according to the presence of 0, 1‐2, and 3‐4 RFs (Figure 1). This risk model was predictive (c‐statistics 0.62) and differentiated 3 groups with different probabilities of developing later IFD.
Figure 1.
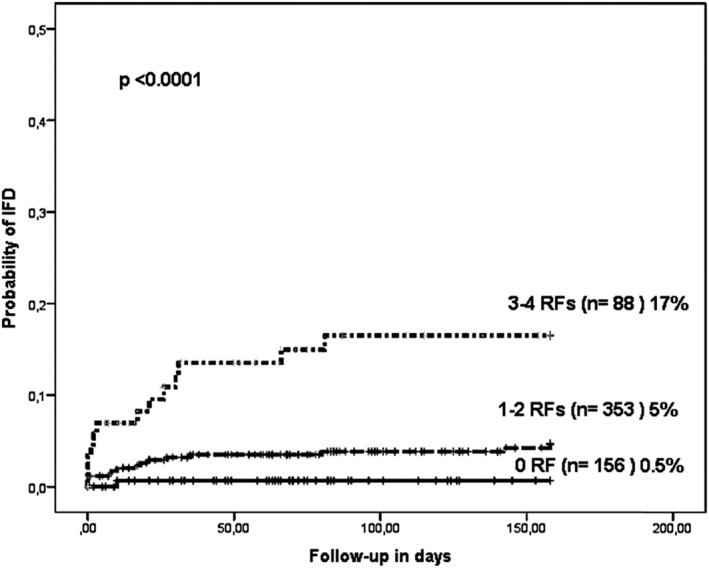
Cumulative incidence of developing invasive fungal disease according to the the presence of risk factors
We analyzed episodes of CARV involving LRTD to determine RFs for IFD in these cases (n = 203). The GEE model with lower QIC calculations included 5 variables (ATG as a part of conditioning, active GvHD, allo‐HSCT ≤1 year, viral co‐infection, and patient CMV serostatus). Of these, three were statistically significant RFs: Once more, ATG was associated with higher probability of IFD (OR 3.09, 95% CI 1.3‐7.2, P = .009) as was CARV LRTD during the first year after transplant (OR 5.3, 95% CI 1.3‐21.8, P = .019). Finally, active GvHD was also associated with IFD (OR 2.9, 95% CI 1.01‐8.7, P = .047).
Developing IFD after CARV was associated with worse OS at three months after CARV diagnosis. OS was 93% for CARV episodes not developing IFD vs 79% for those with IFD (P = .003). However, the OS in allo‐HSCT recipients with LRTD developing IFD was similar to those without IFD (76% vs 83%, respectively, P = .3; Figure 2A,B).
Figure 2.
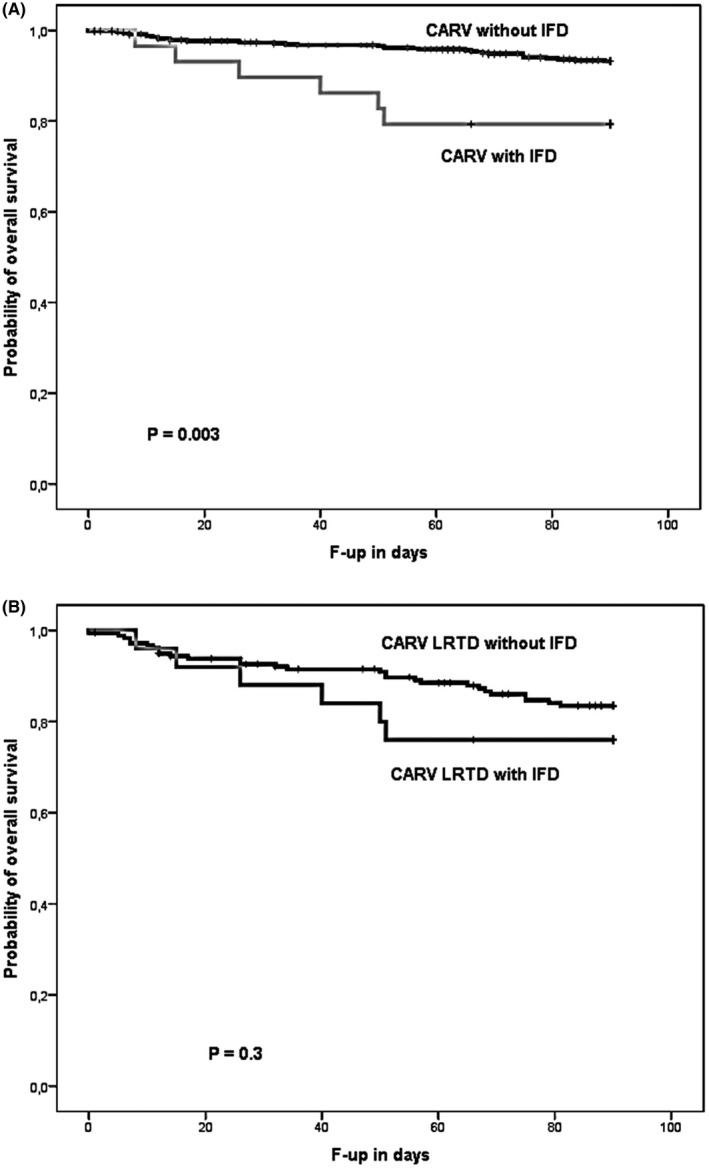
Overall survival according to development of fungal infectious disease after A, communityacquired respiratory virus (CARV) infection and B, CARV lower respiratory tract disease (LRTD)
4. DISCUSSION
This study shows an overall IFD rate of 10% (29 out of 287 allo‐HSCT recipients), whereas the rate was 5% according to overall CARV infectious episodes (29 out of 597 CARV episodes). GEE analysis identified ATG, CARV LRTD, having a CARV episode within the first year after transplant and corticosteroids during CARV as the main RFs for IFD. These RFs led to the stratification of 3 risk groups with statistically significant differences in IFD rates. Remarkably, patients at low risk (none of the RFs) had a very low IFD rate (0.5%). Although the IFD rate was still low (5%) in recipients with 1 or 2 RFs, the recipients with 3 or 4 RFs showed a higher rate of IFD (17%). Additionally, we found ATG, active GvHD, and a CARV episode during the first year of transplant as the main RFs for IFD in recipients with CARV LRTD.
Although the relationship between CARVs and invasive mold infections and/or IA has been consistently established in the allo‐HSCT setting,5, 7, 9, 33 the true rate of later‐occurring IFD in recipients developing CARV infections after allo‐HSCT is not known. Data from retrospective series reported rates of IFD after CARV in allo‐HSCT recipients as ranging from 18% to 41%.5, 33 However, our IFD rates vary according to CARV location (URTD or LRTD), time of CARV diagnosis, and presence of concurrent RFs. In this sense, the IFD rate in recipients with URTD was 1%, whereas it was 12% in those with LRTD. The IFD rate was even higher (17%) in recipients developing a CARV LRTD during the first year after stem cell infusion. Our data show that IFD rates were lower than previously reported ones. These differences may be explained by potential selection bias introduced in retrospective studies, since it is likely that mostly severe cases were included. In contrast, our data came from a prospective CARV survey that made it possible to detect a high number of CARV episodes (in particular mild and moderate cases) leading to the more representative rates reported herein. Of note, this study was conducted in the setting of antifungal prophylaxis during the period at higher risk (first 3 months after transplant and/or while on corticosteroids therapy). This strategy is not universally applied worldwide.33 Thus, it is likely that the use of triazole prophylaxis may have reduced our IFD rate.
The current study provides significant insights into RFs for CARV‐related IFD in this particular scenario. Our first observation was that most IFD cases (92%) occurred during the first year after transplant, particularly in recipients with LRTD. This was an important observation, suggesting that prophylactic and diagnostic effort should be focused in this context. The current study confirmed that CARV LRTD was an independent RF for IFD, pointing to a direct CARV involvement in alterations to the bronchial mucosa or pulmonary defensive mechanisms.34, 35
The fact that IFD was a later event in our series [23 (79%) of 29 recipients developed IFD after day + 100] might explain why neutropenia did not enter as a potential RF in the GEE model. In contrast, the use of ATG was an important RF for IFD. This finding is not surprising, since ATG produces lymphopenia and a significant deferral in T‐cell immune reconstitution for longer than a year after stem cell infusion.36, 37 ATG has been previously identified as a RF for IA.5 In fact, Aspergillus‐specific CD4+ T‐cell activity has been recognized as significant in regulating active pulmonary inflammation and likely adding antifungal effector activity.38, 39, 40 Another potential explanation could be that ATG in our series was mainly used in the context of single CBU transplantation (78% of overall ATG use) and this procedure has been consistently associated with higher incidence of IFD than other sources of hematopoietic stem cells.41
As per protocol, in the current series, the recipients on corticosteroids therapy received triazole antifungal prophylaxis. However, corticosteroids use was still associated with increased risk of IFD during CARV infections in our series. This finding is not completely unexpected since it is well established that corticosteroids increased the risk of IFD in allo‐HSCT recipients.2, 3, 4, 5, 6, 7, 8 In addition, the use corticosteroids in the context of CARV infection in allo‐HSCT recipients may have favored CARV progression from the URTD to the LRTD.19, 21 Both conditions together may explain the higher risk of IFD of corticosteroids use in spite of an adequate antifungal prophylaxis. Although our data support the negative effect of corticosteroids therapy in the host immune response, it may also reflect the biologic influence of the underlying disease by itself.
Regarding the type of CARV, previous reports have focused the attention in influenza infection increasing the risk of IA in critically ill and immunosuppressed patients.9, 10, 11, 12, 13, 14, 15 However, our data indicated that in the allo‐HSCT setting most CARVs, except HCoV and HBoV, were as likely to facilitate IFD. Our observations pointed out that the risk of IFD should be considered in all type of CARVs.
Finally, the development of invasive pulmonary IFD complicating CARV infections after allo‐HSCT had a negative effect on outcome. The dismal prognosis of IFD after allo‐HSCT is well characterized.1 In the current series, three‐month all‐cause mortality was significantly higher in recipients with IFD. This fact highlights the need for effective diagnostic and prevention strategies for detecting and reducing IFD in these severely immunosuppressed patients.
We acknowledge that this study has some limitations, such as the use of different antifungal prophylaxis strategies over time and the use of three different PCR methods differing (minimally) in analytical performance. In spite of this, our study has strengths that merit consideration, notably our use of molecular testing and our data collection from a prospective CARV survey.
In conclusion, we provide evidence that IFD after CARV infection is significant, showing a negative association on outcomes. Allo‐HSCT recipients with 3 or 4 RFs may require close monitoring for the future development of IFD.
AUTHORS CONTRIBUTION
JLP and DN were responsible for analyzing, writing, and supervising the writing of the manuscript. MDG, JM, IL, AP, EG, EMGB, CC, MG, MS, GS, JCHB, CS, and JS were responsible for reviewing the analysis interpretation, suggesting modifications to the text, critically reviewing the manuscript, and for final approval of the manuscript.
ACKNOWLEDGEMENTS
We would like to thank Juan A. Carbonell‐Asins from the Instituto de Investigación Sanitaria INCLIVA for his statistical support performing the Generalized estimating equation model analyses.
CONFLICT OF INTEREST
Jose Luis Piñana has received both advisory for preclinical/clinical research and financial support to assist to the Spanish society of hematology annual meeting 2018 from Merck and Sharp Dohme Pharmaceutics. The other authors report no potential conflicts of interest.
Piñana JL, Gómez MD, Montoro J, et al. Incidence, risk factors, and outcome of pulmonary invasive fungal disease after respiratory virus infection in allogeneic hematopoietic stem cell transplantation recipients. Transpl Infect Dis. 2019;21:e13158 10.1111/tid.13158
REFERENCES
- 1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531‐540. [DOI] [PubMed] [Google Scholar]
- 2. Kuster S, Stampf S, Gerber B, et al. Incidence and outcome of invasive fungal diseases after allogeneic hematopoietic stem cell transplantation: a Swiss transplant cohort study. Transpl Infect Dis. 2018;20(6):e12981. [DOI] [PubMed] [Google Scholar]
- 3. Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139:519‐531. [DOI] [PubMed] [Google Scholar]
- 4. Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827‐833. [DOI] [PubMed] [Google Scholar]
- 5. Garcia‐Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jantunen E, Nihtinen A, Anttila VJ. Changing landscape of invasive aspergillosis in allogeneic stem cell transplant recipients. Transpl Infect Dis. 2008;10:156‐161. [DOI] [PubMed] [Google Scholar]
- 7. Mihu CN, King E, Yossepovitch O, et al. Risk factors and attributable mortality of late aspergillosis after T‐cell depleted hematopoietic stem cell transplantation. Transpl Infect Dis. 2008;10:162‐167. [DOI] [PubMed] [Google Scholar]
- 8. Martino R, Subira M, Rovira M, et al. Invasive fungal infections after allogeneic peripheral blood stem cell transplantation: incidence and risk factors in 395 patients. Br J Haematol. 2002;116:475‐482. [DOI] [PubMed] [Google Scholar]
- 9. Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358‐4366. [DOI] [PubMed] [Google Scholar]
- 10. Hasejima N, Yamato K, Takezawa S, Kobayashi H, Kadoyama C. Invasive pulmonary aspergillosis associated with influenza B. Respirology. 2005;10:116‐119. [DOI] [PubMed] [Google Scholar]
- 11. Vandenbos F, Mondain‐Miton V, Roger PM, Saint‐Paul MC, Dellamonica P. Invasive pulmonary aspergillosis during influenza: a fortuitous association? Presse Med. 1999;28:1755. [PubMed] [Google Scholar]
- 12. Alba D, Gomez‐Cerezo J, Cobo J, Ripoll MM, Molina F, Vazquez JJ. Invasive pulmonary aspergillosis associated with influenza virus. An Med Interna. 1996;13:34‐36. [PubMed] [Google Scholar]
- 13. Shapiro D, Ferriss J. Influenza A and aspergillosis. Chest. 1986;89:318‐319. [DOI] [PubMed] [Google Scholar]
- 14. Lewis M, Kallenbach J, Ruff P, Zaltzman M, Abramowitz J, Zwi S. Invasive pulmonary aspergillosis complicating influenza A pneumonia in a previously healthy patient. Chest. 1985;87:691‐693. [DOI] [PubMed] [Google Scholar]
- 15. Mezger M, Steffens M, Beyer M, et al. Polymorphisms in the chemokine (C‐X‐C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem‐cell transplantation and influence CXCL10 expression in monocyte‐derived dendritic cells. Blood. 2008;111:534‐536. [DOI] [PubMed] [Google Scholar]
- 16. Milano F, Campbell AP, Guthrie KA, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115(10):2088‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piñana JL, Pérez A, Montoro J, et al. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross‐sectional prospective observational study. Clin Infect Dis. 2019;68(11):1894‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piñana JL, Madrid S, Pérez A, et al. Epidemiologic and clinical characteristics of coronavirus and bocavirus respiratory infections after allogeneic stem cell transplantation: a prospective single‐center study. Biol Blood Marrow Transplant. 2018;24(3):563‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah DP, Ghantoji SS, Ariza‐Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123:3263‐3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montoro J, Sanz J, Lorenzo JI, et al. Invasive fungal disease in patients undergoing umbilical cord blood transplantation after myeloablative conditioning regimen. Eur J Haematol. 2019;102(4):331‐340. [DOI] [PubMed] [Google Scholar]
- 21. Seo S, Xie HU, Campbell AP, et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014;58:1357‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL‐4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piñana JL, Hernández‐Boluda JC, Calabuig M, et al. A risk‐adapted approach to treating respiratory syncytial virus and human parainfluenza virus in allogeneic stem cell transplantation recipients with oral ribavirin therapy: a pilot study. Transpl Infect Dis. 2017;19(4):e12729. [DOI] [PubMed] [Google Scholar]
- 24. De Pauw B, Walsh T, Donnelly J, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft‐versus‐host disease in human recipients of marrow from HL‐A‐matched sibling donors. Transplantation. 1974;18:295‐304. [DOI] [PubMed] [Google Scholar]
- 26. Liang K‐Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13‐22. [Google Scholar]
- 27. Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120‐125. [DOI] [PubMed] [Google Scholar]
- 28. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 29. Halekoh U, Højsgaard S, Yan J, et al. The r package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1‐11. [Google Scholar]
- 30. Barton K, Barton MK.Package ‘mumin’. Version, 1:18; 2015.
- 31. Klein JP, Rizzo JD, Zhang M‐J, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909‐915. [DOI] [PubMed] [Google Scholar]
- 32. Klein JP, Rizzo JD, Zhang M‐J, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001;28:1001‐1011. [DOI] [PubMed] [Google Scholar]
- 33. Martino R, Piñana JL, Parody R, et al. Lower respiratory tract respiratory virus infections increase the risk of invasive aspergillosis after a reduced‐intensity allogeneic hematopoietic SCT. Bone Marrow Transplant. 2009;44(11):749‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alonso JM. Immunity and pathophysiology of respiratory tract infections. Med Mal Infect. 2008;38:433‐437. [DOI] [PubMed] [Google Scholar]
- 35. Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft‐versus‐host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96(8):2703‐2711. [PubMed] [Google Scholar]
- 37. Mohty M, Bay JO, Faucher C, et al. Graft‐versus‐host disease following allogeneic transplantation from HLA‐identical sibling with antithymocyte globulin‐based reduced‐intensity preparative regimen. Blood. 2003;102(2):470‐476. [DOI] [PubMed] [Google Scholar]
- 38. Rivera A, Epps HV, Hohl T, Rizzuto G, Pamer E. Distinct CD4+ T cell responses to live and heat‐inactivated Aspergillus fumigatus conidia. Infect Immun. 2005;73:7170‐7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rivera A, Hohl T, Pamer EG. Immune responses to Aspergillus fumigatus infections. Biol Blood Marrow Transplant. 2006;12(Suppl 1):47‐49. [DOI] [PubMed] [Google Scholar]
- 40. Beck O, Topp MS, Koehl U, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus . Blood. 2006;107:2562‐2569. [DOI] [PubMed] [Google Scholar]
- 41. Fukuda T, Boeckh M, Guthrie KA, et al. Invasive aspergillosis before allogeneic hematopoietic stem cell transplantation: 10‐year experience at a single transplant center. Biol Blood Marrow Transplant. 2004;10(7):494‐503. [DOI] [PubMed] [Google Scholar]


