Summary
Porcine deltacoronavirus (PDCoV) is a recently identified coronavirus in the genus Deltacoronavirus that can cause enteric disease with clinical signs including diarrhoea, vomiting, dehydration and mortality in neonatal piglets. Although evidence of the prevalence of PDCoV in China is accumulating, little published information about Chinese PDCoV isolates is available. In this study, we investigated the presence of PDCoV in 49 faecal/intestinal samples from piglets with diarrhoea on different farms in Hebei province. Five samples (10.2%) were positive for PDCoV, but no coinfection of PDCoV with other enteropathogens was observed. A PDCoV strain named HB‐BD was successfully isolated from the intestinal contents of a diarrhoeic piglet and serially propagated in swine testicular (ST) cells for >40 passages. The complete genome of the HB‐BD strain was sequenced and analysed. Genomic analysis showed that the HB‐BD strain had a closer relationship with Chinese strains than those from other countries and was grouped within the Chinese PDCoV cluster. The results of this study will be valuable for further research of PDCoV genetic evolution and development of effective diagnostic reagents, assays and potential vaccines against newly emerged PDCoV strains.
Keywords: Hebei province, isolation, phylogenetic analysis, porcine deltacoronavirus
1. INTRODUCTION
Porcine deltacoronavirus (PDCoV) is a novel member of the genus Deltacoronavirus of the family Coronaviridae. The disease caused by this virus is characterized by severe watery diarrhoea and vomiting in neonatal piglets. It is symptomatically indistinguishable from diarrhoea caused by porcine epidemic diarrhoea virus (PEDV) and transmissible gastroenteritis virus (TGEV) (Li et al., 2014; Song et al., 2015; Wang, Byrum, & Zhang, 2014), but presents more mildly and with lower mortality in piglets (30%–40%) than typical PEDV infection (Hu, Jung, et al., 2015). Since its initial isolation in the United States in February 2014 (Li et al., 2014; McCluskey et al., 2016; Marthaler et al., 2014), PDCoV has been identified in Canada, South Korea, China and Thailand. Molecular surveillance of diarrhoeal samples of pigs from the United States indicated a 30% infection of PDCoV, which is considered a common viral pathogen of pigs (Marthaler et al., 2014; Zhang, 2016; Jung, Hu, & Saif, 2016). However, owing to the fact that the PDCoV is a recently emerging viral pathogen, there is limited knowledge about the virus (Thachil, Gerber, Xiao, Huang, & Opriessnig, 2015). There are presently no effective treatments or vaccines available to control PDCoV (Hu, Jung, et al., 2015).
The PDCoV is an enveloped, positive‐sense, single‐stranded RNA virus. The full genome of PDCoV is approximately 25.4 kb in length (Woo et al., 2012), encodes two polymerase proteins (ORF1a/b) gene, four major structural proteins for the spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins, as well as non‐structural protein 6 (NS6) and non‐structural protein 7 (NS7) (Lee & Lee, 2014; Li et al., 2014). The functions of PDCoV individual proteins have not been elucidated. However, among the structural proteins of the other coronaviruses, the S protein is known to play pivotal roles in interacting with the cellular receptor to mediate viral entry and is an antigenic target for neutralizing antibodies (Park, Song, Ha, & Park, 2007; Sato et al., 2011). The M protein is a transmembrane protein that is responsible for the transport of nutrients across the membrane. In addition, the M protein plays an important role in viral assembly, as well as the induction of virus neutralization antibodies (Fan, Zuo, Li, & Pei, 2012). The coronavirus N protein binds viral RNA and highly conserved. It is involved in several of the biological activities of the virus (Chang et al., 2006; Molenkamp & Spaan, 1997). The S, M and N protein genes have been targeted for the development of virological and serological diagnostic assays for PDCoV (Chen, Gauger, et al., 2015; Ma et al., 2015; Marthaler et al., 2014; Song et al., 2015; Su et al., 2016; Wang et al., 2014). Therefore, it is important to understand the molecular characteristics of the prevalent strains and its relevance to the development of diagnostic reagents, assays and potential vaccines against emergent PDCoV strains. Although some publications have reported the detection of PDCoV RNA in domestic pigs in mainland China (Chen, Zhu, et al., 2015; Song et al., 2015; Wang, Yue, Fang, & Huang, 2015), a few have reported on the isolation of PDCoV or the characteristics of those isolates.
In this study, five of 49 samples collected from different swine farms reporting cases of diarrhoea in Hebei province were PDCoV positive. A PDCoV strain, HB‐BD, was successfully isolated and serially propagated in swine testicular (ST) cells. The complete genome of the HB‐BD strain was sequenced and analysed.
2. MATERIALS AND METHODS
2.1. Sample collection and testing
From October 2015 to March 2016, faecal/intestinal samples (n = 49) from piglets with diarrhoea on different farms in Hebei province of China were submitted to the Veterinary Preventive Medicine Laboratory, College of Veterinary Medicine, Hebei Agricultural University, Baoding, China. To detect the PDCoV genome in the collected samples, viral RNAs were extracted from samples using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcription was performed using the PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa Biotechnology, Dalian, China) with an oligo dT primer. RT‐PCR primers (F: 5′‐TAACTCCGCCATCAAAC‐3′ and R: 5′‐CCACTTCCACGCTCCT‐3′) targeting the N gene were used with the following reaction conditions: 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 50°C for 30 s and 72°C for 30 s, with a final extension at 72°C for 10 min. In addition, molecular detection of the other three diarrhoea‐related enteric viruses, PEDV, TGEV and porcine rotavirus (PRoV), was performed using previously reported methods for further evaluation of possible coinfection status with PDCoV (Hu, Li, et al., 2015; Jeong et al., 2009; Sun, Leng, Zhai, Chen, & Song, 2014).
2.2. Virus isolation, propagation and titration
Isolation of PDCoV was attempted on samples that were positive for PDCoV alone. The isolated strain was used for sequence analysis and phylogenetic analysis, the details of which are presented in Table 1. To get the inoculum for virus isolation, 1 ml of the original intestinal sample was diluted with phosphate‐buffered saline (0.01M, pH7.4), vortexed and centrifuged at 4,200 g for 10 min at 4°C. The supernatants were separated and filtered through a 0.22 μm filter and then used as the inoculum for virus isolation in cell culture.
Table 1.
Oligonucleotide primers used for amplification of the complete genome of PDCoV strain HB‐BD by RT‐PCR
| Primer | Sequence(5′‐3′) | Nucleotide positiona | Product size (bp) |
|---|---|---|---|
| PDCoV1F | ACATGGGGACTAAAGATAAAAATTATAG | 1‐28 | 1580 |
| PDCoV1R | GATACTTCATAACTCTGGCAATC | 1,558‐1,580 | |
| PDCoV2F | TGATGTTCTGCTAGCCTT | 1,485‐1,502 | 1862 |
| PDCoV2R | CGAGTGTCAGAGGTGTGT | 3,329‐3,346 | |
| PDCoV3F | CACTGATGTAGGCGATGA | 3,282‐3,299 | 1581 |
| PDCoV3R | CACGACTTTACGAGGATGA | 4,844‐4,862 | |
| PDCoV4F | TCCATTTGGACCCACCTC | 4,727‐4,744 | 1686 |
| PDCoV4R | TAGCCTGCTGACTAAGACG | 6,394‐6,412 | |
| PDCoV5F | AGTCAGCAGGCTATACGTGTGA | 6,400‐6,421 | 1785 |
| PDCoV5R | GAATGTTGTCTACTGCCCACGC | 8,163‐8,184 | |
| PDCoV6F | GGAGGCGGTTCACAGTTGTA | 7,996‐8,015 | 1807 |
| PDCoV6R | GGTGGAAACCGTAACATTGCTG | 9,781‐9,802 | |
| PDCoV7F | TGGCAGTTAAGATGTCCC | 9,710‐9,727 | 1956 |
| PDCoV7R | TGTAGCATTCCTCCTCGA | 11,647‐11,664 | |
| PDCoV8F | CTAACTGCGCTCGGTTTA | 11,539‐11,556 | 1812 |
| PDCoV8R | GGTAGAATCGCTGGCTTT | 13,333‐13,350 | |
| PDCoV9F | CTGTGGCAGGAGTGTCTA | 13,060‐13,077 | 1620 |
| PDCoV9R | GAAGTTTAATGAAGCGTTG | 14,661‐14,679 | |
| PDCoV10F | AGTCATACGCTACCGCAACC | 14,623‐14,642 | 1502 |
| PDCoV10R | GCCATCGGCAACTCCTACT | 16,106‐16,124 | |
| PDCoV11F | TGGCATGATTGTGGTGCAG | 16,056‐16,074 | 1682 |
| PDCoV11R | AACAGCTGTGTAGTTGGCAG | 17,718‐17,737 | |
| PDCoV12F | CAACCGCACTAACTTACCTGT | 17,654‐17,674 | 1672 |
| PDCoV12R | TTCTGGTGGCCTCACAAGAA | 19,306‐19,325 | |
| SF1 | TATTATCTCGGCTCGTGAG | 19,241‐19,259 | 827 |
| SR1 | AGTGTTATGAGTGTATCGG | 20,049–20,067 | |
| SF2 | TACTTTACCTATTACGGT | 19,963–19,980 | 867 |
| SR2 | TGTGATAGCACCGACAACG | 20,809–20,827 | |
| SF3 | CACAGGTGAGCTTTATGC | 20,710–20,727 | 817 |
| SR3 | AGAGCCAGTATACATTGCC | 21,508–21,526 | |
| SF4 | TCTAGAGACATGGCCATCG | 21,419–21,437 | 808 |
| SR4 | ACTGGTAGAGTATAAGTTG | 22,208–22,226 | |
| SF5 | TTTTCATGCATGCAGTGCT | 22,110–22,128 | 804 |
| SR5 | TTAACACAAGCTAAGCAAG | 22,895–22,913 | |
| PDCoV13F | TGTCATCTGCATTGGTGTGGC | 22857‐22877 | 264 |
| PDCoV13R | AGCCTCCTTGGAGGATGACG | 23101‐23120 | |
| MF | TACTCCAAGCCGAACCCCGT | 22,972–22,991 | 744 |
| MR | GCTGCAAGTGGCAGTTGCA | 23,698–23,716 | |
| PDCoV14F | CACTTGCAGCTGCGAGATTTA | 23707‐23727 | 312 |
| PDCoV14R | GGACTACTGGTGCAGCCATG | 23999‐24018 | |
| NF | CATCGCTCCAAGTCATTCTT | 23,942–23,961 | 1110 |
| NR | CATAGGTTGATGTCTACGCT | 25,022–25,041 | |
| PDCoV15F | GAGATAAAGCAGGAATCAGCAGC | 25002‐25024 | 419 |
| PDCoV15R | GCTCCATCCCCCCTATAAGCC | 25400‐25420 |
PDCoV, porcine deltacoronavirus.
Nucleotide position is numbered based on the PDCoV/NH strain (KU981059).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The PDCoV was isolated in the ST cell line (American Type Culture Collection No. CRL1746). The growth medium for ST cells was advanced Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat‐inactivated foetal bovine serum (Zhejiang Tianhang Biotechnology Co. Ltd. Hangzhou, China) and 1% penicillin–streptomycin solution, and the maintenance medium for PDCoV propagation was DMEM supplemented with 1% penicillin–streptomycin solution.
For the first inoculation, cells were cultured in a T25 flask. When the ST cell reached 80% confluence in the flask, cells were washed twice with maintenance medium and were used for virus inoculation. A volume of 900 μl of maintenance medium together with 100 μl of the filtered sample was added to the flask. After incubation of the virus for 60 min at 37°C in 5% CO2 incubator, the cells were washed twice, and 10 ml maintenance medium with 1% pancreatin was added to each flask. The cells were then cultured continuously at 37°C in 5% CO2 incubator and were observed daily for the cytopathic effect (CPE). When an obvious CPE was observed in around 90% of the cell monolayers 2–3 days after inoculation, the flasks were frozen at −80°C and thawed three times. The supernatants and cells were harvested together and stored at −80°C. These samples were used as seed stocks for subsequent passage and for the detection of PDCoV and other enteric viruses. The isolated PDCoV strain, HB‐BD, was plaque purified as previously described (Hu, Jung, et al., 2015).
For PDCoV serial propagation, 80% confluent ST cells in a T25 flask were washed twice with maintenance medium, and then, 1 ml of the virus inoculum containing 1% pancreatin was added to the flask. The cells were cultured at 37°C in 5% CO2 incubator. After 2–3 days, 80% of the virus‐infected cells showed CPE, and the cultures were collected after three frozen‐thawed cycles. The supernatants were stored at −80°C and used for PDCoV propagation.
Viral titre was measured using 50% tissue culture infectious dose (TCID50) assays on ST cells in a 96‐well plate, as previously described (Hu, Jung, et al., 2015).
2.3. Immuno fluorescence assay
PDCoV‐infected ST cells were fixed with 4% paraformaldehyde at 4°C for 30 min then washed three times with PBS and permeabilized with 0.2% Triton X‐100 for 10 min at room temperature. After blocked with 5% bovine serum albumin (BSA) at 37°C for 1 hr, the cells were incubated with PDCoV polyclonal antibodies which were produced from PDCoV HB‐BD‐immunized BALB/c mice. After 1 hr incubation at 37°C, the cells were washed three times with PBS and incubated with FITC‐labelled goat anti‐mouse secondary antibody for 1 hr. The stained cells were examined using a fluorescence microscope.
2.4. Sequencing and phylogenetic analysis of the complete genome of the HB‐BD strain
The 5th passage of PDCoV HB‐BD cell culture sample was centrifuged at 4,000 g for 5 min. A volume of 250 μl of the clarified supernatants was used to extract viral RNA using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The RNA samples were stored at −80°C and used as the template for RT‐PCR.
To characterize PDCoV HB‐BD genome, a set of overlapping primers were designed based on the PDCoV strain, NH (GenBank accession no. KU981059), to amplify the nearly complete PDCoV genome (Table 1). The primers were synthesized by Beijing Sunbiotech Co. Ltd. (Beijing, China). Reverse transcription was performed using the PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa Biotechnology, Dalian, China) and carried out using an oligo dT primer. The cDNA was amplified by PCR using Taq DNA Polymerase (TaKaRa, Dalian, China). The PCR mixture (20 μl) contained 4 μl cDNA, 2 μl of 10 × PCR Buffer, 3 μl of 2.5 mM dNTP, 1 μl forward primer and reverse primer, respectively, 0.5 μl of Taq polymerase (5 U/μl) and 8.5 μl of H2O. Fragments of the HB‐BD strain were amplified under the following conditions: denaturation at 94°C for 5 min, 35 cycles of 94°C × 30 s, 55°C × 30 s and 72°C × 2 min, and a final extension of 72°C for 10 min.
The PCR product was cloned into the pMD19‐T cloning vector (TaKaRa). The positive recombinant plasmids were verified by restriction enzyme digestion and were submitted to a commercial company (Sangon Biotech, Shanghai, China) for sequencing. The raw genomic sequence fragments were imported to SeqMan in DNASTAR (DNASTAR, Inc., Madison, WI, USA) for assembly and annotation.
Sequence alignment analysis was processed using the Clustal W program in the DNAStar software, and the phylogenetic tree was constructed by the neighbour‐joining method with 1000 bootstrap replications using the MEGA 7.0.14 software. The PDCoV/HB‐BD strain and the reference strains used for the sequence analysis are presented in Table 2.
Table 2.
The HB‐BD isolate strain and other reference strains of PDCoV and other coronaviruses used for sequence alignment and phylogenetic analysis
| Strain | Location and temporal information | Accession no. |
|---|---|---|
| PDCoV/HB‐BD‐complete genome | China | MF948005 |
| PDCoV/HB‐BD‐M gene | China | KY129985 |
| PDCoV/HB‐BD‐N gene | China | KY129986 |
| PDCoV/HB‐BD‐S gene | China | MF037204 |
| CHN‐JS‐2014 | China | KP757892 |
| CH‐HB‐2014 | China | KP757891 |
| CH/SXD1/2015 | China | KT021234 |
| PDCoV/NH | China | KU981059 |
| CHN‐Tianjin | China | KY065120 |
| CH/Sichuan/S27/2012 | China | KT266822 |
| CHN‐AH‐2004 | China | KP757890 |
| HKU15‐155 | China | JQ065043 |
| HKU15‐44 | China | JQ065042 |
| PDCoV/CHJXNI2/2015 | China | KR131621 |
| OH11846 | USA | KT381613 |
| OH1978 | USA | KJ462462 |
| PA3148 | USA | KJ584358 |
| IL2768 | USA | KJ584355 |
| NE3579 | USA | KJ584359 |
| MI6148 | USA | KJ620016 |
| IN2847 | USA | KJ569769 |
| SD3424 | USA | KJ584356 |
| KY4813 | USA | KJ584357 |
| OhioCVM1/2014 | USA | KJ769231 |
| 8734/USA‐IA/2014 | USA | KJ567050 |
| PDCoV/USA/lllinois133/2014 | USA | KJ601777 |
| PDCoV/USA/lllinois136/2014 | USA | KJ601779 |
| PDCoV/USA/lllinois134/2014 | USA | KJ601778 |
| PDCoV/USA/lllinois121/2014 | USA | KJ481931 |
| KNU14‐04 | Korea | KM820765 |
| PDCoV/Swine/Thailand/S5011/2015 | Thailand | KU051641 |
| PDCoV/Swine/Thailand/S5015L/2015 | Thailand | KU051649 |
| PEDV/CH/JX‐1/2013 | China | KF760557 |
| PEDV/CV777 | Belgium | AF353511 |
| PEDV/OH1414 | USA | KJ408801 |
| TGEV H16 | China | FJ755618 |
| TGEV‐virulent‐Purdue | USA | DQ811789 |
PDCoV, porcine deltacoronavirus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.5. Nucleotide sequence accession number
The nucleotide sequences of the complete M, N, S gene and complete genome of the PDCoV strain, HB‐BD, were deposited in the GenBank database with the accession nos. KY129985, KY129986, MF037204 and MF948005, respectively.
3. RESULTS
3.1. PDCoV detection
Clinical samples collected from diarrhoeic piglets from different farms in Hebei, China, between October 2015 and March 2016 were submitted for pathogen detection and isolation. Of the 49 porcine faecal/intestinal samples examined, five (10.2%) were PDCoV positive; 31 (63.3%) were PEDV positive; two (4.1%) were PRoV positive; and none of the samples were positive for TGEV. Although previous studies report coinfection of pigs with PDCoV and PEDV (Song et al., 2015; Zhai et al., 2016; Zhang, 2016), none of the samples in the present study showed dual infection with PDCoV and PEDV. Furthermore, the five PDCoV‐positive samples all showed single infection with PDCoV.
3.2. Viral isolation and propagation in ST cell monolayers
The five PDCoV‐positive samples were inoculated on ST cell monolayers, respectively. However, only PDCoV strain HB‐BD‐inoculated cell monolayers showed a visible CPE that consisted of enlarged, rounded, densely granular cells that occurred either singly or in clusters, with evidence of cell shrinkage and detachment (Figure 1). The purification of PDCoV HB‐BD was performed as previously described (Hu, Jung, et al., 2015; Hu, Li, et al., 2015). To confirm whether PDCoV alone was replicated in ST cells, the cultures were evaluated for PDCoV, PEDV, TGEV and PRoV, respectively. Results showed that only PDCoV was detected from the HB‐BD inoculated cell cultures that were all negative for other enteric viruses. This demonstrated that the PDCoV HB‐BD strain was successfully isolated from the ST cells. The propagation of PDCoV HB‐BD in ST cells was also confirmed by IFA staining using mouse anti‐PDCoV antibodies (Figure 2). To date, the isolate has been serially propagated in ST cells for >40 passages.
Figure 1.
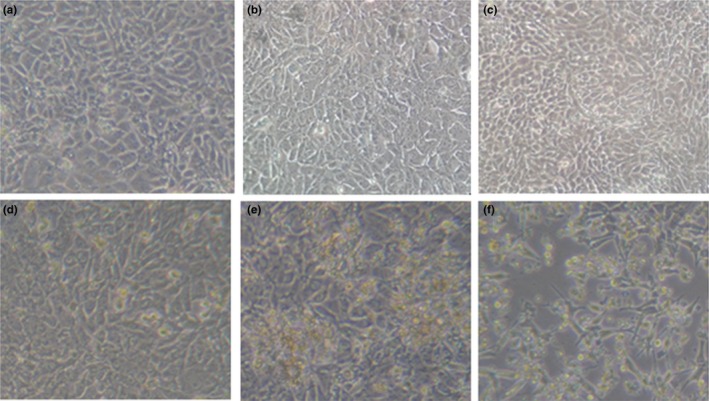
Cytopathic effects of PDCoV isolate in incubated ST cells. ST cells were incubated with the 28th passage of PDCoV HB‐BD. (a), (b) and (c): mock‐inoculated ST cells at p.i. day 1, 2 and 3, respectively. (d), (e) and (f) :HB‐BD‐inoculated ST cells at p.i. day 1, day 2 and day 3, respectively
Figure 2.
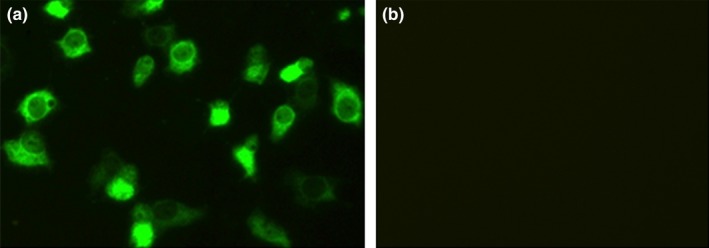
Detection of PDCoV isolate HB‐BD (28th passages) in ST cells by IF straining using mouse anti‐PDCoV antiserum. (a) IF straining of HB‐BD‐infected ST cells; (b) IF straining of mock‐incubated ST cells. ST cells were fixed at p.i. day 1
3.3. Virus titration performed with the TCID50 protocol
During serial passages, the PDCoV infectious titres were determined using a TCID50 assay on ST cells. Four passages of the isolate (P5, P9, P13 and P19) were detected. The results showed that the virus titres for these four passages were 105.3 TCID50/ml, 106.2 TCID50/ml, 106.9 TCID50/ml and 106.5 TCID50/ml, respectively.
3.4. Sequence alignment and Phylogenetic analysis of PDCoV strain HB‐BD genome
The complete genome sequence of PDCoV HB‐BD has been sequenced and deposited in GenBank under the accession number of MF948005. Sequencing results showed that the complete genome sequence of PDCoV HB‐BD was 25,420 nucleotides (nt) in length excluding the 3′ poly (A) tail. The gene order of its genomic structure was 5′‐UTR‐ORF1‐S‐E‐M‐NSP6‐N‐NSP7‐3′UTR, and the nucleotides numbers of these parts were 540, 18803, 3480, 252, 654, 285, 1029, 603 and 392, respectively.
The complete genome sequence of PDCoV HB‐BD shared 97.2%‐99.5% nucleotide identity with the other 28 PDCoV reference strains (including American and individual Asian strains) available in GenBank and had the highest nucleotide identity (99.5%) with PDCoV/NH. Both PDCoV HB‐BD and PDCoV/NH had a unique base insertion in the 5′‐UTR (150C151). By comparing S gene with those PDCoV reference strains, a 3‐nt (AAT, from 154 to 156) deletion was observed in the S gene of the HB‐BD strain, which was also present in other Chinese PDCoV strains, with the exception of PDCoV strains, CHN‐AH‐2004 and HKU15‐44. This unique feature in the S gene can be used as a genetic marker to discriminate PDCoV strains in China from those in the United States, South Korea and Thailand (Chen, Zhu, et al., 2015). Further analysis of the S gene showed that the HB‐BD strain reported in the present study shared 95.8%–99.1% nt identity with the other 28 PDCoV reference strains. When compared with the Chinese PDCoV strains, the HB‐BD strain showed 97.9%–99.1% nt identity and 97.2%–99.2% deduced amino acid (aa) identity. The HB‐BD strain shared the highest nt identity (99.1%) and deduced aa identity (99.2%) with PDCoV/NH, which was isolated from Heilongjiang province. Both HB‐BD and PDCoV/NH isolates had two amino acid mutations (L45H and Y123H). Furthermore, HB‐BD had four further amino acid mutations (H149Y, R888T, A 894T and G910C).
There were no nucleotide insertions or deletions in the M gene ORF of the HB‐BD isolate, and the sequence was deposited in GenBank under the accession number KY129985. In comparison with the foreign strains, we discovered that all Chinese strains, including the HB‐BD strain, had three nucleotide mutations (G72A, C174T and C459T) that were similar to those of the strains from Thailand. In addition, the HB‐BD strain had a unique nucleotide mutation (C63T). However, these nucleotide mutations did not lead to changes in the predicted amino acid sequences of the PDCoV isolates.
The multiple sequence alignment results of the M gene showed that the HB‐BD isolate shared high nucleotide homology (99.1%–99.4%) with other Chinese strains and had the highest nucleotide homology (99.4%) with four Chinese PDCoV strains (HKU15‐155, JS‐2014, SXD1‐2015 and NH) and the lowest nucleotide identity (98.6%) with two strains from Thailand, S5011 and S5015L. Furthermore, the HB‐BD strain shared 98.9%–99.2% and 99.1% nucleotide identities with reference U.S. strains and Korean strains, respectively. Moreover, the HB‐BD isolate exhibited 100% aa identities with all reference sequences except that for the HKU15‐155 strain, because that strain had one aa substitution (A83P).
The N gene sequence of all 29 PDCoV strains was identified as 1029 nt in size that encoded a protein of 342 aa. Sequence alignment results suggested that there was no deletion or insertion in the N gene regions. The PDCoV isolate, HB‐BD, shared 97.8%–99.4% nt identity and 98.8%–99.4% aa identity with reference PDCoV strains, respectively, and shared 98.4%–99.4% nt identity and 98.8%–99.4% deduced aa identity with the other Chinese strains. The HB‐BD strain had the highest nt identity (99.4%) and aa identity (99.4%) with HKU15‐155 and had the lowest nt homology (97.8%) with two strains from Thailand, S5011 and S5015L. Further analysis showed that the HB‐BD strain had one aa substitution (V 43 A), which is similar to observations in the HKU15‐155 and CHN‐AH‐2004 strains. In addition, HB‐BD had one further aa mutation (A 308 V).
To analyse the phylogenetic relationships between the HB‐BD isolate and the reference strains, a phylogenetic tree was constructed. The representative tree is shown in Figure 3. In the context of the complete genome phylogenetic tree, all 29 PDCoV strains were separated into the genus Deltacoronavirus, a cluster that was distinct from the PEDV and TGEV strains.
Figure 3.
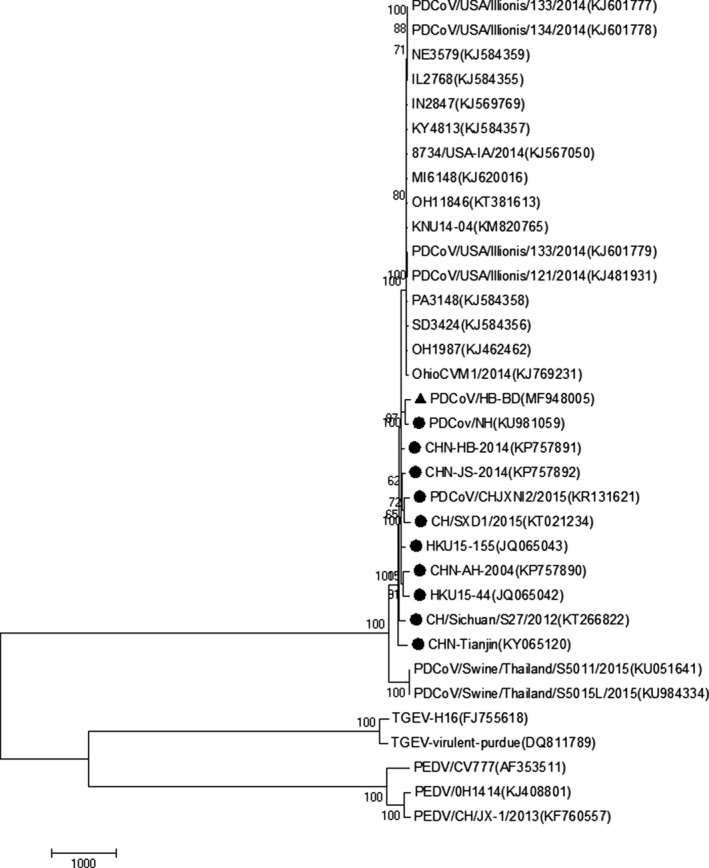
Phylogenetic tree of the nucleotide sequences of PDCoV isolates based on the complete genome. All the reference sequences used in this study were obtained from the GenBank database. The names of the strains, places of isolation and GenBank accession numbers are shown in Table 1. The Tree was constructed by the neighbour‐joining method with 1,000 bootstrap replications using MEGA 7.0.14 software. The isolate identified in this study is indicated by black triangle, and the Chinese reference strains are indicated by black dots
Further analysis demonstrated that the PDCoV isolate, HB‐BD, was more closely related to other Chinese PDCoV isolates than to those isolated from the United States, South Korea and Thailand (Figure 3).
4. DISCUSSION
PDCoV is novel swine enteropathogenic coronaviruses that have emerged in several pig breeding countries in recent years (Homwong et al., 2016; Madapong et al., 2016; Zhai et al., 2016; Zhang, 2016). In China, as PDCoV HKU15‐44 and HKU15‐155 strains were first identified in Hong Kong in 2012 (Woo et al., 2012), PDCoV has been reported in some local provinces and the prevalence of PDCoV has been investigated (Chen, Zhu, et al., 2015; Dong et al., 2015; Song et al., 2015; Wang et al., 2015). In one study, 64 faecal and intestinal samples collected from six pig farms in the Shanxi, Guangdong and Hubei provinces in China, were examined for enteropathogens using RT‐PCR (Chen, Zhu, et al., 2015). The prevalence of PDCoV infection was 23.4% (15/64). In one survey performed by Song et al. (2015), 356 porcine faecal/intestinal samples that were collected between November 2012 and March 2015 from diarrhoeic pigs on 51 farms in Jiangxi province, China, were examined using nested RT‐PCR. The result showed that monoinfection with PDCoV was 33.71% (120/356) and coinfection with PDCoV and PEDV was 19.66% (70/356), thus 58.33% (70/120) of the PDCoV‐positive samples were co‐infected with PEDV. In the study performed by Dong et al. (2015), 215 intestinal or faecal samples were collected from piglets with clinical diarrhoea at various times during 2004–2014 in Anhui, Guangxi, Hubei and Jiangsu provinces, of mainland China, for the detection of enteropathogens. Among these samples, 14 (6.51%) were positive for PDCoV and 50% (7/14) of the 14 PDCoV‐positive samples were also positive for PEDV. These results showed that PDCoV coinfections with other pathogens, especially PEDV, are more common in these provinces. However, little is known about the prevalence of PDCoV in Hebei province, a major pig‐rearing province in China. In the present study, 49 faecal and intestinal samples from piglets with diarrhoea on different farms in Hebei province were collected, from which PDCoV was detected using RT‐PCR. Other general enteropathogens, such as PEDV, TGEV and PRoV, were also examined. Five (10.2%) of the 49 samples were positive for PDCoV, 31 (63.3%) were positive for PEDV, and two (4.1%) were positive for PRoV. Although the prevalence of PDCoV infection was lower than that of PEDV, the result indicates that PDCOV infection exists in the Hebei pig population. Interestingly, no coinfection with PDCoV and PEDV was detected. In addition, all five PDCoV‐positive samples were negative for all other enteropathogens detected in the present study. These findings are somewhat different from observations of infection with PDCoV in other provinces and other countries. If a greater number of samples were collected, some coinfection might have been detected.
Although the prevalence of PDCoV in some provinces of China has been confirmed and the genetic characteristics of some Chinese PDCoV strains and those from other countries have been analysed, there is little published information about Chinese PDCoV isolates. In the present study, using ST cells, the PDCoV HB‐BD strain was successfully isolated from the intestinal contents of a piglet with diarrhoea. After several passages, propagation of PDCoV HB‐BD was confirmed by the detection of a typical CPE and infectious virus titration. The M, N and S gene sequences of HB‐BD were also determined. To confirm whether the replication of any other swine enteric viruses occurred on ST cells, the cell culture‐passaged samples were detected by RT‐PCR and tested negative for PDCoV, PEDV, TGEV and PRoV. These results all indicate that the PDCoV HB‐BD isolate was highly adapted to ST cells. The findings of Hu, Jung, et al. (2015) and Dong et al. (2016) indicate that the success rate of PDCoV isolation from samples of intestinal contents is relatively higher than that from faecal samples. However, even if the PDCoV virus is isolated from intestinal contents, the success rate is also influenced by other factors, such as virus viability, infectious virus titres and other substances in the intestinal contents (Hu, Jung, et al., 2015; Dong et al., 2016). In the present study, we attempted to isolate the virus from two intestinal content samples and three faecal samples that were positive for PDCoV. However, in the ST cells, only the PDCoV HB‐BD strain was successfully isolated from intestinal contents. The success rate of virus isolation is relatively low. These results are consistent with those of previous reports (Dong et al., 2016; Hu, Jung, et al., 2015).
To understand the genetic characteristics of the HB‐BD isolate, the complete genome of the isolate was sequenced, and phylogenetic analysis was performed with known sequences selected from the GenBank database. The results showed that all PDCoV strains were distinct from PEDV and TGEV, and clustered within the genus Deltacoronavirus. The HB‐BD strain had a closer relationship with other Chinese strains than those from other countries and was grouped within the Chinese PDCoV cluster in the phylogenetic trees. When compared with the Chinese strains, HB‐BD strain showed most closely related to the NH strain (emerged in Heilongjiang province) and the HKU15‐155 strain (isolated in Hong Kong). Based on the S gene, HB‐BD shared the highest aa identity (99.2%) with the NH strain. In addition, both strains have two aa mutations (L45H and Y123H). However, based on the N gene, the HB‐BD strain had the highest aa identity (99.4%) with HKU15‐155 and shared a similar aa substitution (V43A). Further analysis showed that HB‐BD had four further aa mutations (H149Y, R888T, A894T and G910C) in the S gene, one further aa mutation (A308V) in the N gene and a unique nucleotide mutation (C63T) in the M gene. Studies have shown that the S protein of coronaviruses is associated with receptor binding and host adaptation (Graham & Baric, 2010; Sato et al., 2011) and the N protein participates in viral transcription and replication (Brian & Baric, 2005). Further research in HB‐BD should be conducted to understand whether these mutations can lead to changes in virulence, pathogenicity and antigenicity of PDCoV.
In conclusion, the presence of PDCoV in Hebei province was confirmed, and the PDCoV strain, HB‐BD, was successfully isolated and serially passaged in ST cells. The complete genome of HB‐BD was sequenced and characterized. These results will be valuable for further research of PDCoV genetic evolution. The immunogenicity and pathogenicity of the HB‐BD strain require further study for the development of effective diagnostic reagents, assays and potential vaccines against newly emerged PDCoV strains.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by the Program of One Hundred Young Academic Leaders Training of Agricultural University of Hebei, China (No. 0318011), Science and technology innovation program of Hebei Province for graduate student (CXZZBS2017065) and Natural Science Foundation of Hebei Province of China (C2015204121).
Liu B.‐J., Zuo Y.‐Z., Gu W.‐Y., et al. Isolation and phylogenetic analysis of porcine deltacoronavirus from pigs with diarrhoea in Hebei province, China. Transbound Emerg Dis. 2018;65:874–882. 10.1111/tbed.12821
B.‐J. Liu and Y.‐Z. Zuo contributed equally to this work.
REFERENCES
- Brian, D. , & Baric, R. (2005). Coronavirus genome structure and replication. Current Topics in Microbiology Immunology, 287, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. K. , Sue, S. C. , Yu, T. H. , Hsieh, C. M. , Tsai, C. K. , Chiang, Y. C. , … Huang, T. H. (2006). Modular organization of SARS coronavirus nucleocapsid protein. Journal of Biomedical Science, 13, 59–72. 10.1007/s11373-005-9035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Gauger, P. , Stafne, M. , Thomas, J. , Arruda, P. , Burrough, E. , … Zhang, J. (2015). Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5‐day‐old neonatal piglets. Virology, 482, 51–59. 10.1016/j.virol.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. , Zhu, Y. , Wu, M. , Ku, X. , Yao, L. , & He, Q. (2015). Full‐length genome characterization of Chinese porcine deltacoronavirus strain CH/SXD1/2015. Genome Announcements, 3(5), e01284–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, N. , Fang, L. , Yang, H. , Liu, H. , Du, T. , Fang, P. , … Xiao, S. (2016). Isolation, genomic characterization, and pathogenicity of a Chinese porcine deltacoronavirus strain CHN‐HN‐2014. Veterinary Microbiology, 196, 98–106. 10.1016/j.vetmic.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, N. , Fang, L. , Zeng, S. , Sun, Q. , Chen, H. , & Xiao, S. (2015). Porcine deltacoronavirus in Mainland China. Emerging Infectious Diseases, 21, 2254–2255. 10.3201/eid2112.150283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. H. , Zuo, Y. Z. , Li, J. H. , & Pei, L. H. (2012). Heterogeneity in membrane protein genes of porcine epidemic diarrhea viruses isolated in China. Virus Genes, 45, 113–117. 10.1007/s11262-012-0755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, R. L. , & Baric, R. S. (2010). Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross‐species transmission. Journal of Virology, 84, 3134–3146. 10.1128/JVI.01394-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homwong, N. , Jarvis, M. C. , Lam, H. C. , Diaz, A. , Rovira, A. , Nelson, M. , & Marthaler, D. (2016). Characterization and evolution of porcine deltacoronavirus in the United States. Preventive Veterinary Medicine, 123, 168–174. 10.1016/j.prevetmed.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H. , Jung, K. , Vlasova, A. N. , Chepngeno, J. , Lu, Z. , Wang, Q. , & Saif, L. J. (2015). Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. Journal of Clinical Microbiology, 53, 1537–1548. 10.1128/JCM.00031-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Jr, X. , Li Jr, N. , Tian Jr, Z. , Yin Jr, X. , Qu, L. , & Qu, J. (2015). Molecular characterization and phylogenetic analysis of transmissible gastroenteritis virus HX strain isolated from China. BMC Veterinary Research, 11, 72 10.1186/s12917-015-0387-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, Y. J. , Park, S. I. , Hosmillo, M. , Shin, D. J. , Chun, Y. H. , Kim, H. J. , … Cho, K. O. (2009). Detection and molecular characterization of porcine group C rotaviruses in South Korea. Veterinary Microbiology, 138, 217–224. 10.1016/j.vetmic.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Hu, H. , & Saif, L. J. (2016). Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Research, 226, 50–59. 10.1016/j.virusres.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Chung, H. C. , Nguyen, V. G. , Moon, H. J. , Kim, H. K. , Park, S. J. , … Park, B. K. (2016). Detection and phylogenetic analysis of porcine deltacoronavirus in Korean Swine Farms, 2015. Transboundary and Emerging Diseases, 63, 248–252. 10.1111/tbed.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , & Lee, C. (2014). Complete genome characterization of Korean porcine deltacoronavirus strain KOR/KNU14‐04/2014. Genome Announcements, 2(6), e01191–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Chen, Q. , Harmon, K. M. , Yoon, K. J. , Schwartz, K. J. , Hoogland, M. J. , … Zhang, J. (2014). Full‐length genome sequence of porcine deltacoronavirus strain USA/IA/2014/8734. Genome Announcements, 2(2), e00278–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Zhang, Y. , Liang, X. , Lou, F. , Oglesbee, M. , Krakowka, S. , & Li, J. (2015). Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. MBio, 6, e00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Zhang, Y. , Liang, X. , Oglesbee, M. , Krakowka, S. , Niehaus, A. , … Li, J. (2016). Two‐way antigenic cross‐reactivity between porcine epidemic diarrhea virus and porcine deltacoronavirus. Veterinary Microbiology, 186, 90–96. 10.1016/j.vetmic.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madapong, A. , Saeng‐Chuto, K. , Lorsirigool, A. , Temeeyasen, G. , Srijangwad, A. , Tripipat, T. , … Nilubol, D. (2016). Complete genome sequence of porcine deltacoronavirus isolated in Thailand in 2015. Genome Announcements, 4, e00408–e00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler, D. , Raymond, L. , Jiang, Y. , Collins, J. , Rossow, K. , & Rovira, A. (2014). Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerging Infectious Diseases, 20, 1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey, B. J. , Haley, C. , Rovira, A. , Main, R. , Zhang, Y. , & Barder, S. (2016). Retrospective testing and case series study of porcine delta coronavirus in U.S. Swine herds. Preventive Veterinary Medicine, 123, 185–191. 10.1016/j.prevetmed.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenkamp, R. , & Spaan, W. J. M. (1997). Identification of a specific interaction between the coronavirus mouse hepatitis virus A59 nucleocapsid protein and packaging signal. Virology, 239, 78–86. 10.1006/viro.1997.8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Dong, L. F. , Zeng, S. , Qianqian Sun, H. C. , & Xiao, S. (2015). Porcine deltacoronavirus in Mainland China. Emerging Infectious Diseases, 21, 2254–2255. 10.3201/eid2112.150283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. J. , Song, D. S. , Ha, G. W. , & Park, B. K. (2007). Cloning and further sequence analysis of the spike gene of attenuated porcine epidemic diarrhea virus DR13. Virus Genes, 35, 55–64. 10.1007/s11262-006-0036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T. , Takeyama, N. , Katsumata, A. , Tuchiya, K. , Kodama, T. , & Kusanagi, K. (2011). Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes, 43, 72–78. 10.1007/s11262-011-0617-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, D. , Zhou, X. , Peng, Q. , Chen, Y. , Zhang, F. , Huang, T. , … Tang, Y. (2015). Newly emerged porcine deltacoronavirus associated With diarrhoea in swine in China: Identification, prevalence and full‐length genome sequence analysis. Transboundary and Emerging Diseases, 62, 575–580. 10.1111/tbed.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, M. , Li, C. , Guo, D. , Wei, S. , Wang, X. , Geng, Y. , … Sun, D. (2016). A recombinant nucleocapsid protein‐based indirect enzyme‐linked immunosorbent assay to detect antibodies against porcine deltacoronavirus. The Journal of Veterinary Medical Science, 78, 601–606. 10.1292/jvms.15-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, R. , Leng, Z. , Zhai, S.‐L. , Chen, D. , & Song, C. (2014). Genetic variability and phylogeny of current Chinese porcine epidemic diarrhea virus strains based on Spike, ORF3, and membrane genes. The Scientific World Journal, 2014, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil, A. , Gerber, P. F. , Xiao, C. T. , Huang, Y. W. , & Opriessnig, T. (2015). Development and application of an ELISA for the detection of porcine deltacoronavirus IgG antibodies. PLoS ONE, 10, e0124363 10.1371/journal.pone.0124363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Byrum, B. , & Zhang, Y. (2014). Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerging Infectious Diseases, 20, 1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. W. , Yue, H. , Fang, W. , & Huang, Y. W. (2015). Complete genome sequence of porcine deltacoronavirus strain CH/Sichuan/S27/2012 from Mainland China. Genome Announcements, 3, e00945–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P. C. , Lau, S. K. , Lam, C. S. , Lau, C. C. , Tsang, A. K. , Lau, J. H. , … Yuen, K. Y. (2012). Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. Journal of Virology, 86, 3995–4008. 10.1128/JVI.06540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, S. L. , Wei, W. K. , Li, X. P. , Wen, X. H. , Zhou, X. , Zhang, H. , … Wang, D. (2016). Occurrence and sequence analysis of porcine deltacoronaviruses in southern China. Virology Journal, 13, 136 10.1186/s12985-016-0591-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. (2016). Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Research, 226, 71–84. 10.1016/j.virusres.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Tsai, Y. L. , Lee, P. Y. , Chen, Q. , Zhang, Y. , Chiang, C. J. , … Wang, H. T. (2016). Evaluation of two singleplex reverse transcription‐Insulated isothermal PCR tests and a duplex real‐time RT‐PCR test for the detection of porcine epidemic diarrhea virus and porcine deltacoronavirus. Journal of Virological Methods, 234, 34–42. 10.1016/j.jviromet.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


