Abstract
Background
Recently, a platform of T‐cell replete haploidentical hematopoietic stem cell transplantation (haplo‐HSCT) using post‐transplant cyclophosphamide (Cy) has shown high reproducibility and acceptable safety profile.
Method
This prospective cohort analysis allowed us to collect data on infections among 70 consecutive recipients of haplo‐HSCT affected by various hematologic malignancies.
Results
After a median follow‐up of 23 months, cumulative incidence of viral infections was 70% (95% confidence interval [CI] 59–81) at 100 days and 77% (95% CI 67–87) at 1 year; 35 of 65 patients at risk had CMV reactivation (54%) and the rate of polyomavirus‐virus‐associated cystitis was 19% (13/70). Cumulative incidence of bacterial and fungal infections at 1 year were 63% (95% CI 51–75) and 12% (95% CI 4–19), respectively. Of note, only 1 invasive fungal infection occurred beyond 1 year after transplant (day +739).
Conclusion
In conclusion, despite a high rate of viral infections in the early period, present data suggest a satisfactory infectious profile after T‐cell replete haplo‐HSCT using post‐transplant Cy. These results may help clinicians to improve both prophylactic and therapeutic antimicrobial strategies in this emerging haploidentical setting.
Keywords: infections, haploidentical stem cell transplantation, T‐cell replete, antimicrobial prophylaxis, hematologic malignancies, cyclophosphamide
One of the major limitations of hematopoietic stem cell transplantation from haploidentical donor (haplo‐HSCT) is the impaired immune reconstitution owing to extensive immunosuppression necessary to overcome human leukocyte antigen disparity. Despite important advances over the last decades, infections are still mostly responsible for toxicity and non‐relapse mortality among transplanted patients, owing to prolonged immunosuppression related, or not, to chronic graft‐versus‐host disease (GVHD) 1, 2. A platform for T‐cell replete haplo‐HSCT using post‐transplant cyclophosphamide (Cy) 3 showed a low treatment‐related mortality (TRM) and a high feasibility with an acceptable safety profile. This type of haplo‐HSCT seems to compare favorably with T‐cell depleted methods, in terms of infectious complications 3, 4. To further explore this topic, we report herein the incidence of infections in a single‐center cohort of 70 consecutive patients receiving T‐cell replete haplo‐HSCT with post‐transplant Cy at our center, in order to provide useful information about the post‐transplant period after this type of emerging transplant platform.
Patients and methods
Data on patients with hematologic malignancies who underwent haplo‐HSCT between April 2009 and April 2014 at Humanitas Cancer Center (Milan, Italy) were prospectively collected using electronic patients' charts.
Conditioning regimens were myeloablative, reduced‐intensity, or non‐myeloablative. Non‐myeloablative conditioning consisted of Cy 14.5 mg/kg/day intravenously (i.v.) on days −6 and −5, fludarabine 30 mg/m2/day i.v. on days −6 to −2, and total body irradiation (TBI) 200 cGy on day −1. Two reduced‐intensity regimens were administered: (i) thiotepa 10 mg/kg/day i.v. on day −6, fludarabine 30 mg/m2/day i.v. on days −5 to −2, Cy 30 mg/kg/day i.v. on days −5 and −4, and TBI 200 cGy on day −1; or (ii) thiotepa 5 mg/kg/day i.v. on days −6 and −5, fludarabine 50 mg/m2/day i.v. on days −4 to −2, and busulfan 3.2 mg/kg/day i.v. on days −4 and −3, as modified from Sanz et al. 5. The myeloablative regimen consisted of thiotepa 5 mg/kg/day i.v. on days −6 and −5, fludarabine 50 mg/m2/day i.v. on days −4 to −2, and busulfan 3.2 mg/kg/day i.v. on days −4 to −2.
All patients received unmanipulated bone marrow or mobilized peripheral stem cells. GVHD prophylaxis was performed with Cy 50 mg/kg/day on days +3 and +4; tacrolimus 1 mg/day i.v. from day +5 (to reach a concentration of 5–15 ng/mL), or cyclosporine 3 mg/kg/day i.v. from day +5 (to reach a concentration of 100–200 ng/mL), both up to day +100 and then tapered up to day +180, unless GVHD occurred; and mycophenolate mofetil 15 mg/kg 3× a day orally from day +5 to +35.
Infections were defined according to European Society for Blood and Marrow Transplantation (available at: http://www.ebmt.org/Contents/About-EBMT/Who-We-Are/ScientificCouncil/Documents/IDWPdefinitions.pdf), including microbiologically documented viral, bacterial, or fungal infections with or without laboratory and/or radiologic features consistent with organ involvement. Cytomegalovirus (CMV) reactivation (or de novo infection) and disease were diagnosed as reported elsewhere 6, and invasive fungal infections (IFIs) were classified according to the definitions of the EORTC/MSG consensus group 7; only proven or probable IFIs were recorded. Data were recorded as of June 3, 2014 for all patients. The study was approved by the local Institutional Review Board.
Supportive care
Antimicrobial prophylaxis was started during the conditioning regimen and consisted of acyclovir 500 mg/m² 3 times in a day; levofloxacin 500 mg/day; and cotrimoxazole 2 tablets per day until day −2, and then 1 tablet every other day was resumed after hematologic reconstitution. Antifungal prophylaxis was performed with an echinocandin (either caspofungin or micafungin 50 mg/day) until day +5, when itraconazole (200 mg/day i.v.) was administered, unless contraindicated; otherwise the echinocandin was maintained. After September 2013, the echinocandin was maintained for all patients. Acyclovir, levofloxacin, and antifungal prophylaxis were administered until engraftment occurred.
Twice weekly blood polymerase chain reaction (PCR) CMV monitoring was started at day +15 until day +100 and weekly until day +180, or when clinically indicated. Weekly Epstein–Barr virus (EBV) monitoring by PCR was started at day +15 up to day +100, or when clinically indicated. All other tests were performed whenever indicated.
Piperacillin‐tazobactam alone or in combination with an aminoglycoside was administered as empirical therapy for febrile neutropenia, unless previous colonization for resistant bacteria was documented; in this case, an appropriate antibacterial agent was delivered. The same was true when a suspected bacterial infection occurred, with the exception of pneumonia for which linezolid was added, in combination with the above‐cited antibacterial drug(s). First‐line preemptive therapy for CMV infection/reactivation was with intravenous ganciclovir, whereas foscarnet was administered if the patient was in aplasia. EBV reactivation and polyomavirus‐related hemorrhagic cystitis were treated by rituximab and cidofovir, respectively. Threshold of CMV viremia for the initiation of therapy was 3300 copies/mL; threshold of EBV viremia was 10,000 copies/mL.
Immunophenotypic analysis
Analysis of circulating lymphocytes was performed at regular intervals whenever available, at days +7, +14, +21, and +28, and every month afterward. The following monoclonal antibodies and combinations were used: CD45, CD3/CD4, CD3/CD8, CD19, CD16/CD56 (Beckman Coulter, Fullerton, California, USA), to quantify T, B, and NK cell compartments at the different time points studied.
Statistical analysis
Categorical variables were expressed as absolute numbers with respective percentage and continuous variables as the median with the respective range. Cumulative incidence of viral, bacterial, or fungal infections was calculated using competing risk analysis 8 starting from the day 0 to the day of the first infection; death was considered as the competing event. Infection incidence was also expressed as the events/1000 patient‐days (pt‐days) for each time period within 1 year after transplant, with intervals defined as follows: days 0–30, 31–100, 101–180, and 181–365. Owing to the paucity of late events, data beyond day +365 were collected singularly and classified according to pathogen group. Neutrophil engraftment was defined as the first of 3 consecutive days with a persistent count >0.5 × 109/L; platelet engraftment was defined as the first of 3 consecutive days with a persistent count >20 × 109/L (https://portal.ebmt.org/sites/clint2/clint/Documents/StatGuidelines_oct2003.pdf).
The Kaplan–Meier method was used to compute overall survival 9; cumulative incidence of TRM and acute and chronic GVHD were calculated using competing risk analysis 8. Death because of documented infection was defined as infection‐related death. Log‐rank test was used to compare the incidence of infections with flow cytometry results.
Results
Seventy consecutive adult patients undergoing haplo‐HSCT were identified. The main patient and transplant characteristics are shown in Table 1. Fifty‐five patients (79%) were affected by lymphoma, and 81% of patients underwent haplo‐HSCT in partial or complete remission. Only 5 patient/donor pairs were CMV D−/R−. As concerns antifungal prophylaxis, 5 patients did not receive itraconazole owing to moderate increase in pre‐transplant liver function tests (n = 1) or reduction in left ventricular ejection fraction (n = 4); 11 more patients did not receive itraconazole because haplo‐HSCT was performed after September 2013. Three patients received secondary antifungal prophylaxis with voriconazole because of previous pulmonary aspergillosis. Median follow‐up of living patients was 23 months (range 1–60) from day of stem cell infusion.
Table 1.
Main patients' and haplo‐HSCT characteristics
| Characteristics | HLA‐haploidentical (n = 70) |
|---|---|
| Age, median, in years (range) | 45 (19–72) |
| Diagnosis, n (%) | |
| Hodgkin's lymphoma | 35 (50) |
| Non‐Hodgkin's lymphoma | 20 (29) |
| Multiple myeloma | 2 (3) |
| Acute leukemia | 11 (15) |
| Chronic lymphoid leukemia | 2 (3) |
| CMV serostatus, n (%) | |
| Donor (−)/Recipient (−) | 5 (7) |
| Donor (−)/Recipient (+) | 11 (15) |
| Donor (+)/Recipient (−) | 6 (8) |
| Donor (+)/Recipient (+) | 48 (70) |
| Graft type, n (%) | |
| BM | 66 (94) |
| PBSC | 4 (6) |
| Time to diagnosis—haplo‐HSCT median, in months (range) | 26 (3–195) |
| Disease status at haplo‐HSCT, n (%) | |
| CR | 38 (54) |
| PR | 19 (27) |
| SD | 3 (4) |
| PD | 10 (15) |
| Conditioning regimen, n (%) | |
| NMA | 48 (68) |
| RIC | 16 (23) |
| MA | 6 (9) |
| Year of haplo‐HSCT, median (range) | 2012 (2009–2014) |
Haplo‐HSCT, haploidentical hematopoietic stem cell transplantation; HLA, human leukocyte antigen; CMV, cytomegalovirus; BM, bone marrow; PBSC, peripheral blood stem cells; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; NMA, non‐myeloablative; RIC, reduced‐intensity conditioning; MA, myeloablative.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
At last follow‐up, a total of 224 documented infectious events occurred among 67 of 70 patients, with a median of 3 events/patient (range 1–10); 55% were of viral origin (n = 123), 40% bacterial (n = 89), 5% fungal (n = 11).
Cumulative incidence of first viral infection was 70% (95% confidence interval [CI] 59–81) and 77% (95% CI 67–87) at day +100 and +365, respectively; at day +365, the incidence of bacterial infections was 63% (95% CI 51–75), and that of IFIs was 12% (95% CI 4–19) (Fig. 1). In 54% (35 of 65 patients at risk) at least 1 CMV reactivation developed; of these, 26 patients had 1 CMV reactivation, and 5, 2, 1, and 1 patients had a total of 2, 3, 4, and 7 CMV reactivations, respectively. Two non‐fatal (1 colitis, 1 pneumonia) and 1 fatal (pneumonia) CMV diseases occurred. No primary CMV infections occurred in the 5 CMV D−/R− patient/donor pairs. Polyomavirus‐related hemorrhagic cystitis was observed in 13 patients (19%): 10 were caused by BK virus and 3 by JC virus. Importantly, no EBV‐related lymphoproliferative disorders occurred so far.
Figure 1.
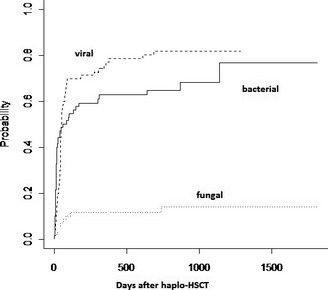
Cumulative incidence of infections after haploidentical hematopoietic stem cell transplantation (haplo‐HSCT). Cumulative incidence of first bacterial, fungal, and viral infections is shown here according to cumulative incidence method and competing risks (see text).
Forty‐five patients (64%) presented with at least 1 documented bacterial infection: 10 (14%), 21 (30%), and 14 (20%) patients had an infection by gram‐positive, gram‐negative, or both types of bacteria, respectively.
Eleven IFIs were detected in 9 patients: n = 6 probable invasive aspergillosis (pneumonia in 5 patients and sinusitis in 1), n = 5 invasive candidiasis, all by non‐albicans Candida (2 candidemias, 2 colitis, and 1 hepatosplenic candidiasis); median of occurrence was 62 days from haplo‐HSCT (range 0–739). Among the 3 patients receiving secondary antifungal prophylaxis, only 1 non‐albicans Candida colitis was observed at day +77 in 1 patient. No IFIs occurred under active GVHD. Notably, only 2 IFIs occurred beyond day +180: 1 pulmonary aspergillosis at day +191, and 1 candidemia at day +739, this latter in a patient who was under salvage treatment for post‐transplant relapse. Details of etiologies are reported in Table 2.
Table 2.
Etiologies of the 224 documented infections
| Type | Pathogen | Number of events |
|---|---|---|
| Virus | CMV | 53 |
| VZV | 7 | |
| HSV‐1/2 | 6 | |
| EBV | 24 | |
| HHV‐6 | 8 | |
| Rotavirus | 2 | |
| BK virus | 10 | |
| JC virus | 3 | |
| RSV | 2 | |
| Parainfluenza virus | 1 | |
| H1N1 influenza | 3 | |
| Coronavirus | 1 | |
| Rhinovirus | 1 | |
| Adenovirus | 1 | |
| HBV | 1 | |
| Bacteria | Escherichia coli | 25 |
| Pseudomonas aeruginosa | 8 | |
| Stenotrophomonas maltophila | 2 | |
| Enterococcus faecalis | 5 | |
| Enterococcus faecium | 4 | |
| Staphylococcus coagulase‐negative | 18 | |
| Streptococcus pneumoniae | 6 | |
| Staphylococcus aureus | 8 | |
| Clostridium difficile | 7 | |
| Mycoplasma pneumoniae | 1 | |
| Acinetobacter baumanii | 2 | |
| Corynebacterium species | 2 | |
| Enterobacter cloacae | 1 | |
| Fungi | Aspergillus | 6 |
| Candida, non‐albicans | 5 |
CMV, cytomegalovirus; VZV, varicella zoster virus; HSV, herpes simplex virus; EBV, Epstein–Barr virus; HHV‐6, human herpesvirus type 6; RSV, respiratory syncytial virus; HBV, hepatitis B virus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
We did not observe significant differences of infectious events according to conditioning regimen administered (data not shown). When considering the timing of all episodes, bacterial infections occurred mostly between day 0 and +30, whereas viral infections/reactivations between days +31 and +100, with 11.08 bacterial events/1000 pt‐days between day 0 and +30, and 15.15 viral events/1000 pt‐days between days +31 and +100 (Fig. 2). The overall incidence of viral events between day 0 and day +180 was 8.8 events/1000 pt‐days. A total of 13 bacterial and 13 viral infections were observed after 1 year from transplant.
Figure 2.
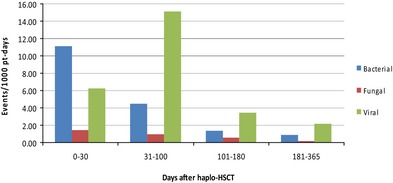
Bacterial, fungal, and viral infections at different post‐transplant intervals. Incidence of infections is here expressed as number of events per 1000 patient (pt)‐days at 4 post‐transplant intervals: from day 0 to +30, from +31 to +100, from +101 to +180, from +181 to +365. Haplo‐HSCT, haploidentical hematopoietic stem cell transplantation.
Transplant outcomes
Engraftment rate was 91% (64 of 70 patients), with a median of 20 days (range 14–49) and 27 days (range 16–115) for neutrophil and platelet recovery, respectively. Four patients died before engraftment (on days +13, +22, +33, and +48 because of gram‐negative sepsis, multiorgan failure, progressive disease, and bacterial pneumonia, respectively) and 2 presented primary antibody‐linked graft failure, and are alive at last follow‐up, after autologous reconstitution (days +530 and +834). Cumulative incidence of acute grade 2–4 and 3–4 GVHD was 23% (95% CI 10–40) and 4% (95% CI 0–9) respectively; chronic GVHD was 8% (95% CI 1–27). Two‐year overall survival was 48% (95% CI 35–58) and TRM was 26% (95% CI 12–38); 18 patients (26%) relapsed or progressed after haplo‐HSCT. Infection‐related deaths were 9% (6/70, occurring between days +13 and +113; bacterial pneumonia = 2, gram‐negative sepsis = 1, CMV pneumonia = 1, H1N1 pneumonia = 1, and JC virus‐related progressive multifocal leukoencephalopathy = 1). The other, non‐infectious, causes of TRM were heart failure (n = 4), secondary malignancy (n = 2 myelodysplastic syndrome, n = 1 esophageal cancer), multiorgan failure (n = 3), thrombotic microangiopathy (n = 1), and acute hepatitis (n = 1). At last follow‐up, 34 patients are alive and 28 of them are in complete remission.
Immunophenotype and infections
Immunophenotypic analysis reveals a progressive increase in all lymphocyte subset counts from day +7 through later post‐transplantation time points. We found a trend toward less viral infection incidence among those patients who have a total lymphocyte count >2/mm3 at day +28: hazard ratio 0.67, P = 0.10. No other associations were observed. Lymphocyte subsets and number of patients analyzed are shown in Figure 3.
Figure 3.
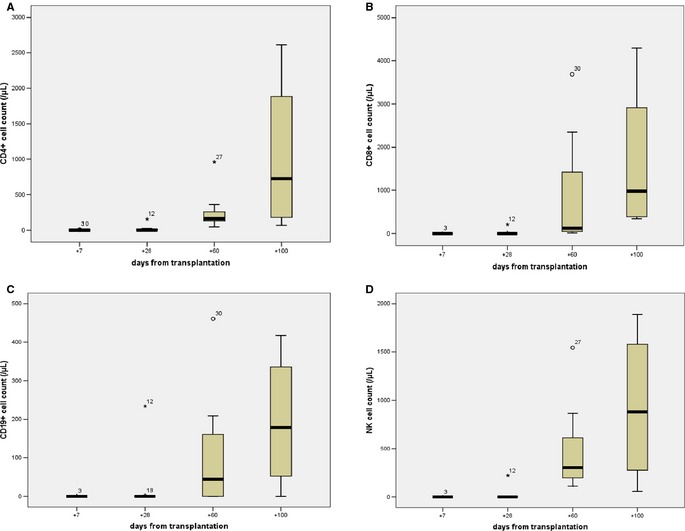
Lymphocyte subset counts by flow cytometry analysis. Subsets are graphically presented as boxplots; (A) CD3+/CD4+ cells; (B) CD3+/CD8+ cells; (C) CD19+ cells; (D) CD16+/CD56+ cells. Blood samples were collected on the planned day ±3 days according to logistical and/or clinical reasons: day +7 (n = 11), +28 (n = 12), +60 (n = 10), +100 (n = 4). Time points at days +14 and +21 are not reported in the Figure, because of close similarity to the very low values measured at day +7.
Discussion
In the present analysis, we described infectious complications after unmanipulated, T‐cell replete haplo‐HSCT using post‐transplant Cy in 70 consecutive patients and found, aside from a high incidence of viral infections/reactivations, especially in the early post‐transplant period, a quite low incidence of late bacterial infections, together with a very low incidence of IFIs after day +180 (2 events in the overall 11 observed). Present findings confirm that the infectious profile is better in T‐cell replete vs. T‐cell depleted haplo‐transplantation 10; the lower incidence of infections observed after day +100 may reflect a partial and quite effective restoration of antimicrobial immunity during the post‐transplant period in this type of haplo‐HSCT. Importantly, the low rate of chronic GVHD seen in our cohort is likely to contribute to this phenomenon, as chronic GVHD is known to be a major risk factor of late morbidity and mortality 11. Nevertheless, we found an unexpected 26% TRM incidence, higher than that originally reported with post‐HSCT Cy 3; this may be a result of the inclusion of patients with more advanced disease in the haplo‐HSCT program at our center.
As concerns viral infections, our results are in line with previous publications in the setting of T‐cell replete haploidentical transplants. Ciurea et al. 12 reported 10.8 events/1000 pt‐days within the first 6 months from transplant (vs. 8.8 events/1000 pt‐days between day +0 and +180 in our hands), and Raiola et al. 13 found that 62% of patients presented with a viral infection in the first year. The 54% of CMV reactivations found here was comparable to the 38–50% reported in similar haploidentical settings 3, 12, 13; the slightly higher incidence that we found here may be explained by the longer follow‐up in our series. The polyomavirus‐associated cystitis rate of 19%, which is lower than that reported in the myeloablative setting 14, is likely because of the different conditioning regimens in our cohort, although a role played by the different GVHD prophylaxis cannot be excluded; indeed, BK virus nephropathy was found to be more frequently associated with tacrolimus than with cyclosporine in recipients of kidney allografts 15. Here, a quarter of the patients (18/70) received cyclosporine as GVHD prophylaxis. Interestingly, we confirm the lack of EBV‐related lymphoproliferative disorders, as recently reported also by Kanakry et al. 16.
We found a 12% incidence of fungal infections, none of them fatal; it is important to note that fatal episodes of fungal infections are among the major limiting toxicities associated with T‐cell depletion in haplo‐HSCT 17, 18; we cannot exclude a role played by the use of anti‐mold prophylaxis during marrow aplasia, although it is difficult to draw definitive conclusions owing to the lack of a true control arm in our study, and to the low number of IFIs. Of note, we observed only 2 IFIs 6 months after transplant.
Concerning bacterial infections, we may explain the low prevalence of late bacterial infections (i.e., beyond 1 year) by the surprising 8% incidence of chronic GVHD; in fact, the risk of bacterial events remained low in the absence of late immunosuppressive therapy 11. With a median follow‐up of 23 months, we observed 13 late bacterial events in a total of 31 patients having at least 1 year of observation (last observation day is 60 months after haplo‐HSCT).
All this information argues in favor of the fact that giving a T‐cell replete graft without deep in vivo T‐depletion (i.e., with anti‐thymocyte globulin or alemtuzumab) and with post‐transplant Cy allows a satisfactory infectious profile after transplant. The post‐transplant high‐dose Cy permits naive and non‐activated memory cells to reconstitute the immune system later on 19, 20, enabling patients to be protected from late infectious events. The same mechanism probably also explains the high viral reactivation incidence found in the first 6 months, owing to the low number of adoptively transferred memory T cells in the early phase after transplantation (Lugli E. et al., unpublished data).
We acknowledge that potential selection bias may be present in the study, as we cannot exclude the possibility that some non‐severe or very late infections were not captured because of incomplete reporting. However, all patients were followed at the same institution; therefore, it is unlike that clinically relevant infectious complications were missed; moreover, diagnostic procedures and prophylactic measures were similar for all patients, thus contributing to the accuracy of diagnosis of the infectious events.
In conclusion, the present single‐center data on 70 consecutive patients receiving T‐cell replete haplo‐HSCT with post‐transplant Cy confirm a high rate of viral infections before day +100 and a lower incidence of infections afterward, suggesting a satisfactory although non‐optimal immune reconstitution after this type of transplantation. Future comparisons with other haploidentical platforms and/or other alternative stem cell sources (i.e., cord blood), as well as investigations of novel strategies of transfer of immunity are warranted. Furthermore, the present data may provide useful information in an attempt to improve control of infections by adequate prophylaxis and/or antimicrobial therapy in the early post‐transplant period, after use of the emerging transplant platform of haplo‐HSCT.
Acknowledgements
Thanks: We thank all personnel working in the Hematology and Transplantation Unit at Humanitas Cancer Center for their remarkable contribution in patients' care and assistance to their families.
Author contributions: R.C. designed the study, performed data analysis, and wrote the manuscript; S.B., B.S., F.T., and E.M. provided clinical care; A.V. collected data and performed statistical analysis; R.M., E.C., and I.T. provided laboratory and microbiological data; E.L. and D.M. critically revised the manuscript; C.C‐S., A.S., and L.C. provided clinical care and critically revised the manuscript.
Conflict of interest: All authors declare no financial conflict of interest.
Crocchiolo R., Bramanti S., Vai A., Sarina B., Mineri R., Casari E., Tordato F., Mauro E., Timofeeva I., Lugli E., Mavilio D., Carlo‐Stella C., Santoro A., Castagna L.. Infections after T‐replete haploidentical transplantation and high‐dose cyclophosphamide as graft‐versus‐host disease prophylaxis. Transpl Infect Dis 2015: 17: 242–249. All rights reserved
References
- 1. Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic cell transplantation. N Engl J Med 2010; 363: 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wingard JR, Majhail NS, Brazauskas R, et al. Long‐term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 2011; 29: 2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luznik L, O'Donnell PV, Symons HJ, et al. HLA‐haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high‐dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanakry JA, Kasamon YL, Gocke CD, et al. Outcomes of related donor HLA‐identical or HLA‐haploidentical allogeneic blood or marrow transplantation for peripheral T cell lymphoma. Biol Blood Marrow Transplant 2013; 19: 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanz J, Sanz MA, Saavedra S, et al. Cord blood transplantation from unrelated donors in adult with high‐risk acute myeloid leukemia. Biol Blood Marrow Transplant 2010; 16: 86–94. [DOI] [PubMed] [Google Scholar]
- 6. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097. [DOI] [PubMed] [Google Scholar]
- 7. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 10. Mulanovich VE, Jiang Y, de Lima M, Shpall EJ, Champlin RE, Ciurea SO. Infectious complications in cord blood and T‐cell depleted haploidentical stem cell transplantation. Am J Blood Res 2011; 1: 98–105. [PMC free article] [PubMed] [Google Scholar]
- 11. Robin M, Porcher R, De Castro Araujo R, et al. Risk factors for late infections after allogeneic hematopoietic stem cell transplantation from a matched related donor. Biol Blood Marrow Transplant 2007; 13: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 12. Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2013; 19: 117–122. [DOI] [PubMed] [Google Scholar]
- 14. Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high‐risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse‐free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant 2012; 18: 1859–1866. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch HH, Randhawa P, AST Infectious Diseases Community of Practice . BK polyomavirus in solid organ transplantation. Am J Transplant 2013; 13: 179–188. [DOI] [PubMed] [Google Scholar]
- 16. Kanakry JA, Kasamon YL, Bolaños‐Meade J, et al. Absence of post‐transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post‐transplantation cyclophosphamide as graft‐versus‐host disease prophylaxis. Biol Blood Marrow Transplant 2013; 19: 1514–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aversa F, Terenzi A, Tabilio A, et al. Full haplotype‐mismatched hematopoietic stem‐cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23: 3447–3454. [DOI] [PubMed] [Google Scholar]
- 18. Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide‐gene‐engineered donor lymphocytes after family haploidentical haemopoietic stem‐cell transplantation for leukaemia (the TK007 trial): a non‐randomised phase I‐II study. Lancet Oncol 2009; 10: 489–500. [DOI] [PubMed] [Google Scholar]
- 19. Luznik L, Jones RJ, Fuchs EJ. High‐dose cyclophosphamide for graft‐versus‐host disease prevention. Curr Opin Hematol 2010; 17: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krenger W, Blazar BR, Hollander GA. Thymic T‐cell development in allogeneic stem cell transplantation. Blood 2011; 117: 6768–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]


