Summary
An outbreak of intravascular catheter‐related infections by extended‐spectrum β‐lactamase (ESBL)‐producing Escherichia coli in calves in an animal teaching hospital is reported. Pulsed‐field gel electrophoresis was used for strain typing to determine the origin and dissemination of these strains. All 19 strains harboured the bla CTX ‐M‐14, and six strains also overexpressed their chromosomal AmpC gene. Evidence on the introduction of the strain from a beef herd, experiencing neonatal diarrhoea and increased mortality, to the clinic through admission of diarrhoeic calves was provided. Strains isolated from phlebitis cases from other herds up to 5 months later showed a high similarity with the initial strain, suggesting that the strain had become nosocomial. The catheter infections with ESBL/AmpC‐producing E. coli resulted in a prolonged hospitalization, increased anti‐microbial use and mortality. This report points towards the potential dangers of the emergence of ESBL/AmpC‐producing bacteria in susceptible food animals and warns farmers and veterinarians for the facility by which they are introduced into another environment.
Keywords: ESBLs, phlebitis, intravascular catheter, PFGE, cattle, Escherichia coli
Introduction
Broad‐spectrum β‐lactamases are enzymes which render Gram‐negative bacteria, resistant to β‐lactam antibiotics with extended spectrum (3rd‐ and 4th‐generation cephalosporins in veterinary medicine) (Brolund, 2014). In cattle, β‐lactam antibiotics are frequently used for the treatment of common infections, such as mastitis or omphalitis, but also for life‐threatening conditions such as pneumonia or blood stream infection (BSI) and for surgical prophylaxis (Chicoine et al., 2008; Pardon et al., 2012). Scientific discussion on β‐lactamase nomenclature is still ongoing, but at present, broad‐spectrum β‐lactamases are divided into three categories (Giske et al., 2009). The first group are the extended‐spectrum beta‐lactamases (ESBLs), consisting of CTX‐M enzymes, which are most frequently found, SHV and TEM enzymes. These β‐lactamases are horizontally transferable through plasmids and sensitive to clavulanic acid. A second group holds the AmpC gene, which confers resistance to clavulanic acid as well. The third group consists of the carbapenemases (e.g. metallo β‐lactamases). Carbapenems are not registered for veterinary use, because they are used as last resort molecules for bacteria which show ESBL or AmpC resistance in humans. Important is the fact that broad‐spectrum β‐lactamase‐producing Enterobacteriaceae often display multidrug‐resistant phenotypes, further jeopardizing treatment options (Brolund, 2014).
In recent years, in the footsteps of a growing concern of the spread of ESBL/AmpC genes in the human population, ESBL genes have been found, with highly variable prevalence, in companion animals and in most food animals and derived products (Dierikx et al., 2012, 2013). In cattle, ESBL‐producing Escherichia coli strains are commonly found, predominantly in dairy cattle (Schmid et al., 2013). Also in veal calves, an industry renowned for its intensive antimicrobial use, a worrisome increase in ESBL gene prevalence from 4% in 1998 to 39% in 2010 was observed (Pardon et al., 2012; Hordijk et al., 2013a,b). A similarity in ESBL resistance genes and plasmids carrying these genes among isolates from animals and humans may suggest an exchange of resistance between different reservoirs (Dierikx et al., 2012; Madec et al., 2012). Whereas in human medicine, numerous reports on clinical infections [mainly urinary tract infections (UTI) and BSI] exist; evidence on the clinical relevance of veterinary ESBL‐producing isolates is limited to single papers on UTI in cats and dogs (Huber et al., 2013) or wound infections in horses (Smet et al., 2012). In cattle, ESBL genes have been detected in mastitis pathogens (E. coli and Klebsiella pneumonia) (Ghatak et al., 2013; Ohnishi et al., 2013; Timofte et al., 2014), but involvement in other diseases has not been documented.
This article documents on the introduction and dissemination of an ESBL/AmpC‐producing E. coli (blaCTX‐M‐14/AmpC) involved in intravascular catheter infections in calves in an animal teaching hospital.
Materials and Methods
Study animals and case history
Animals sampled were 1–3 weeks old Belgian Blue (BB) calves, hospitalized in a veterinary teaching hospital (n = 6), of which three developed phlebitis and a jugular abscess following long‐term intravascular catheterization. Additionally, at the herd of origin of the initial case, six calves of the same age were sampled. Samples were taken from jugular abscesses in cases and from faeces in healthy animals.
The first case animals came from a 200 head, purebred BB beef farm, located in the south of Belgium (province of Hainaut). The herd had been sanitized for bovine viral diarrhoea virus, and at birth, every calf was still tested by PCR on an ear biopsy. Calves received 2–4 l of colostrum, enriched with 146 g of a colostrum supplement (Colostrival, Ecuphar, Oostkamp, Belgium). For 6 months, the farm experienced neonatal diarrhoea and sepsis problems, with a mortality rate of 20% in the first month of life. Empiric treatment with paromomycin, cefquinome, marbofloxacine and gentamicin did not resolve morbidity and mortality. Five diarrhoeic calves were admitted at the clinic for treatment and further diagnosis.
Enteric pathogens diagnostics
As part of the routine approach of diarrhoea cases in calves at the clinic, a commercially available lateral immunochromatography test for bovine coronavirus, rotavirus, Escherichia coli F5 and Cryptosporidium parvum was performed on faecal samples (Tetrastrips BIO K 156, Bio‐X, Jemelle, Belgium). A faecal sample was inoculated on brilliant green agar for Salmonella spp. and on Columbia blood agar for Clostridium perfringens (anaerobic incubation).
Bacteriology, virulence factors and anti‐microbial susceptibility testing
Initially, samples from the jugular abscesses were inoculated on Columbia blood agar plates with 5% sheep blood (Oxoid Ltd., Basingstoke, Hampshire, UK) and aerobically incubated overnight at 37°C (Quinn et al., 1999). After phenotypical identification of E. coli, one colony per plate was taken and purified for further testing.
All E. coli isolates were tested by PCR for the presence of known virulence factors, namely verotoxin (VT1 & 2), EAE gen (E. coli attaching and effacing), fimbrial adhesins (F41, F5), thermostable enterotoxin (STA) and cytotoxic necrotizing factors (CNF1 and CNF2).
The E. coli isolates were tested for resistance against β‐lactams to confirm the presence of an ESBL or AmpC β‐lactamase, as described previously (Smet et al., 2008). Minimum inhibitory concentration (MIC) tests were carried out for 13 anti‐microbials (Fig. 3) on Mueller–Hinton agar plates (Oxoid, Erembodegem, Belgium) containing double dilutions of the anti‐microbials. Concentrations of the anti‐microbial compounds ranged from 0.03 to 1024 μg/ml. Antibiotic‐free agar plates were included as a control for normal growth. E. coli ATCC 25922 and Staphylococcus aureus ATCC 29213 were used as a reference strain.
Figure 3.
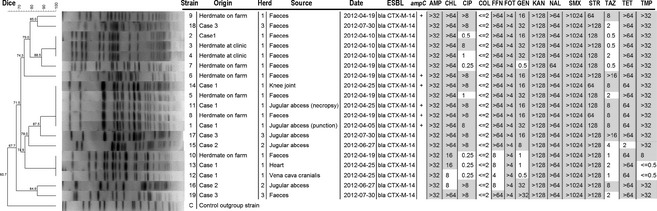
Dendrogram showing the relatedness between macrorestriction and pulsed‐field gel electrophoresis (PFGE) patterns generated from E. coli isolates. Isolate identification, corresponding clinical case or origin, herd of origin, source of the isolate, date of first isolation, the presence of ESBL genes, mutations (+) in the promoter region of the chromosomal AmpC and the minimal inhibitory concentration (mg/ml) of different antibiotics are showed at the right of the dendogram. Minimum inhibitory concentration (MIC) results in grey corresponded to resistance according to the clinical break points European Committee on Anti‐microbial Susceptibility Testing (EUCAST, 2014). When no clinical break points were available, wild‐type populations (susceptibility) were based on the MIC distributions ECOFF (EUCAST, 2014). AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; COL, colistin; FFN, florfenicol; FOT, cefotaxime; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; SMX, sulphonamide; STR, streptomycin; TAZ, ceftazidime; TET, tetracycline (NCLS, 2008).
Inocula were prepared by suspending overnight cultures in phosphate‐buffered saline (PBS) to a density of 0.5 on the McFarland scale and further diluted to 1/10. Approximately 1 × 105 colony‐forming units of the strains were then inoculated on plates by means of a Densley multipoint inoculater (Mast, Sussex, UK). Plates were incubated aerobically at 35°C for 24 h. The MIC was defined as the lowest concentration of the anti‐microbial agent that allowed no visible growth. Minimum inhibitory concentration (MIC) results were interpreted according to the clinical break points European Committee on Anti‐microbial Susceptibility Testing (EUCAST, 2014). When no clinical breakpoints were available, wild‐type populations (susceptibility) were based on the MIC distributions ECOFF (EUCAST, 2014).
Characterization of β‐lactamases
Genes encoding an extended‐spectrum β‐lactamase (ESBL) or AmpC β‐lactamase were analysed by PCR and sequencing as previously described (Smet et al., 2008). Briefly, DNA was extracted by inoculating a single colony of each isolate from a blood agar plate into 1 ml of Luria broth. After overnight incubation at 37°C, cells were harvested after centrifugation at 16 000 g for 5 min. The pellet was resuspended in 1 ml of distilled water, and cells were lysed by heating at 95°C for 5 min. Cellular debris was removed by centrifugation at 16 000 g for 5 min. PCR for the detection of genes encoding TEM‐, SHV‐, CTX‐M‐ and CMY‐type enzymes was performed. All PCR amplicons were sequenced, and the obtained nucleotide sequences were compared with those previously described for bla genes (BLAST database: http://www.ncbi.nlm.nih.gov/BLAST/).
Strain identification
To obtain further insights into the epidemiology (origin and spread) of broad‐spectrum β‐lactamase‐producing E. coli strains at the hospital, strains were identified by XbaI macrorestriction followed by pulsed‐field gel electrophoresis (PFGE) (Bertrand et al., 2006; Ribot et al., 2006).
Results
Clinical management and outcome
In February–March 2012, five Belgian Blue calves, aged between 8 and 10 days and with an average weight of 52 kg [standard deviation (SD) = 2, 3], were admitted to a veterinary teaching clinic. At arrival, calves were markedly dehydrated, showed recumbency, depression, tachycardia and marked, profuse diarrhoea. Rectal temperature varied between 38.3°C and 39.5°C. One calf (case 1) had marked telogen effluvium, resulting in large zones of hair loss all over the body.
A permanent intravenous catheter (Cavafix Certo G14; B. Braun, Oss, the Netherlands) was placed in the jugular vein after clipping and catheter site skin preparation with 0.5% chlorhexidine and 90% alcohol. Dehydration and metabolic acidosis were corrected by continuous perfusion with NaCl 0.9% and 1 l of NaHCO3 4.0%. All calves showed failure of passive transfer, meaning a lack of colostrum uptake, as evidenced by a total immunoglobulin level under 10 g/l on electrophoresis. Three of five calves were C. parvum positive.
Cefquinome (2 mg/kg IV, b.i.d., Cobactan 4.5%; MSD, Boxmeer, the Netherlands) was administered to prevent septicaemia. Flunixin meglumine (1.1 mg/kg, IV, emdofluxin, Emdoka, Hoogstraten, Belgium) was given for 3 days. A week later, 1 day after removal of the catheter, one calf demonstrated recurrent fever. A phlebitis of the left jugular vein was confirmed by ultrasound (5.4 mHz rectal transducer; Mylab Gold, Esaote, The Netherlands) (Fig. 1). Therapy was changed to doxycycline (2.5 mg/kg PO, b.i.d, 3 days, Doxyral 50%; Emdoka). Fever persisted, and therapy was changed to penicillin (10 000 IU/kg IM) and neomycin (5 mg/kg IM, Neopen; MSD A.H.) for 7 days. Fever remained and the general condition of the calf deteriorated. An abscess developed on the jugular vein, and a bacterial culture on an aspiration of the abscess was performed (Case 1) (Fig. 2). The calf died and necropsy showed a marked phlebitis of the left jugular vein with abscess formation and large thrombi in the vena cava cranialis, the heart and several pulmonary arteries. A purulent arthritis was present in both knee joints. Samples for bacteriology were taken from the jugular abscess, vena cava cranialis, heart and knee joint.
Figure 1.
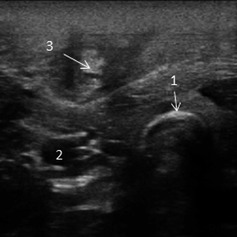
Ultrasonographic image of a phlebitis of the left jugular vein of case 2 caused by an extended‐spectrum β‐lactamase (ESBL)‐producing Escherichia coli. 1. Tracheal rings; 2. a. carotis communis; 3. Total obstruction of the lumen of the v. jugularis due to phlebitis and thrombus formation.
Figure 2.
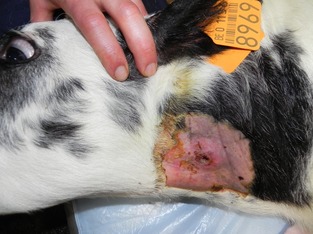
Jugular abscess formation in association with a phlebitis caused by an ESBL‐producing E. coli in a 28 days old Belgian Blue (BB) calf (Case 2). [Colour figure can be viewed at http://wileyonlinelibrary.com].
In June and July, three other hospitalized calves from other owners developed phlebitis between 8 and 18 days after catheter placement. In two of these calves (Cases 2 and 3), there was abscess formation on the jugular vein, which was sampled for bacteriological examination. All calves completely recovered after treatment with 13.13 mg/kg amoxicillin clavulanic acid for 15–21 days (Rodriguez‐Bano et al., 2012). Extra hygienic measures were taken at the clinic; cephalosporin use was limited and special attention was paid to aseptic placement of catheters according to published guidelines for human medicine (O'Grady et al., 2011). No new cases occurred since.
Bacteriology, β‐lactamases and strain identification
An E. coli with an ESBL phenotype was retrieved in the jugular abscess of all three cases. Extended‐spectrum β‐lactamase‐producing E. coli were also detected in the faeces of case 1 and in three other hospitalized calves tested at the same time. At necropsy, a pure culture of E. coli with an ESBL phenotype was isolated from all sampled body sites, suggesting a BSI originating from the catheter site (Fig. 3). Subsequently, at the farm of origin, faecal samples were taken from six calves of the same age class, and ESBL‐producing E. coli were found in all samples.
In total, 19 E. coli isolates with an ESBL phenotype were further characterized, all of which tested negative by PCR for genes encoding virulence factors. All strains were multiresistant and carried the bla CTX‐M14 gene (Fig. 3). Six of the 19 E. coli isolates showing a combined ESBL/AmpC phenotype and isolates from the jugular abscess of case 1 had mutations in the promoter region of their chromosomal AmpC gene (‐88 (C→T), ‐82 (A→G), ‐42 (C→T), ‐18 (G→A), ‐1 (C→T), +58 (C→T)).
Eleven unique [Dice similarity (DS) < 100%] PFGE subtypes existed among the 19 E. coli isolates. A dendrogram describing their relatedness is presented in Fig. 3. The strain isolated from the jugular abscess of case 1 was also isolated from faecal samples from the calves at the herd of origin, evidencing introduction of the strain at the clinic (DS = 100%). A very similar strain (DS = 87.5%) was isolated months later. In case 1, two different strains were isolated at necropsy. One, identical to the first isolation from the abscess, was still found in the abscess and in the knee joint, whereas another one was found in the vena cava cranialis and the heart, suggestive for plasmid transfer. Similarly, two different strains were also isolated from the jugular abscess of case 2.
Discussion
As in humans and other animal species, ESBL‐producing Enterobacteriaceae have become widespread in cattle (Dierikx et al., 2012, 2013). In particular, dairy cattle and veal calves are regarded as a reservoir (Hordijk et al., 2013a; Schmid et al., 2013; Haenni et al., 2014). In human medicine, BSIs with ESBL‐producing Enterobacteriaceae have been associated with treatment failure, prolonged hospitalization and an increased mortality risk (Brolund, 2014). Isolation of ESBL‐producing E. coli from a herd with increased mortality is reported in dairy cattle, but no direct evidence on the clinical relevance of this isolate could be provided (Liebana et al., 2006). The present report demonstrates that ESBL‐producing E. coli can cause treatment failure and mortality in calves as a consequence of septicaemic spread. Particularly important is that this infection occurred in calves with failure of passive transfer. Also in humans, especially immunocompromised persons such as infants are more susceptible to clinical infections with ESBL‐producing Enterobacteriaceae (Rettedal et al., 2012).
The case strains did not have any of the tested virulence factors and were likely opportunists colonizing the catheter site. All isolated strains harboured the bla CTX‐M14 gene, which is among the more frequent ESBL genes in cattle (Hordijk et al., 2013a,b). Additionally, six strains also showed an AmpC phenotype, of which two were isolated from the herd of origin. These isolates overexpressed their chromosomal AmpC gene due to mutations in the promotor and attenuator region (Caroff et al., 2000). The strains found in the faeces of case 1 and calves hospitalized at that time differed from the strain isolated from the abscess. Most likely, different strains had been introduced into the clinic by the calves from the problem herd, and cephalosporin use at the clinic further selected for different ESBL‐producing strains. The presence of two different strains at necropsy in case 1 is suggestive for plasmid transfer.
To the authors' knowledge, this is the first report on a nosocomial infection with ESBL/AmpC‐producing E. coli in animals, with evidence on the route of introduction. The presence of this bacteria resulted in a prolonged hospitalization, increased anti‐microbial use and mortality. Particularly interesting is that clear evidence is provided on the source of the strain, namely a beef farm experiencing diarrhoea and high neonatal mortality. Cephalosporins were used on this particular farm and possibly selected for ESBLs and AmpC. These findings underscore the risk of introducing an ESBL strain into the own herd when purchasing animals, admitting them to a clinic or even just visiting other herds without substantial biosecurity measures. In spite of speculative, it is not unlikely that the increased mortality in the herd of origin is related to the ESBL‐producing E. coli, as over 30% of diarrhoea cases in calves are septicaemic and failure of passive transfer is the main risk factor (Fecteau et al., 1997). Possibly, the economic and ethical consequences of infections with ESBL‐producing E. coli might be worse and more widespread than currently perceived in practice.
The strains isolated from the first case and the following cases were very similar, suggesting that the strain became nosocomial. Extended‐spectrum β‐lactamase epidemiology is very complex. Over time different strains come and go in a population as a consequence of horizontal gene transfer and clonal spread, particularly when anti‐microbial use is continued (Liebana et al., 2006). Under these conditions, a multiresistant plasmid might have become endemic, rather than one particular E. coli strain.
The presence of the ESBL‐producing E. coli could have been confirmed earlier by culturing the insertion site and the hub of the catheter after removal. For economic reasons, the same catheter remained in place relatively long, which is contraindicated in case of phlebitis. Revision of the catheterization protocol and adaptation according to published guidelines (O' Grady et al., 2011) together with a limitation of cephalosporin use was sufficient to control the problem. Despite the absence of new cases, persistence of the initial strain or its plasmid cannot be excluded.
In summary, this report documents on intravascular catheter infections by an ESBL/AmpC‐producing E. coli in calves, which resulted in prolonged hospitalization, treatment failure and mortality. Evidence that the strain originated from a beef farm experiencing increased mortality and using cephalosporins is provided. Based on the similarity of the strains between subsequent cases, the infection became nosocomial at an animal teaching hospital. This article points towards the potential dangers of the emergence of broad‐spectrum β‐lactamases in susceptible food animals and warns farmers and veterinarians for the facility by which they are introduced into another environment.
Conflict of interest
None to declare.
Acknowledgements
This work was supported by internal funding. M. A. Argudín is supported by a research grant from the Fundación Alfonso Martín Escudero.
References
- Bertrand, S. , Weill F. X., Cloeckaert A., Vrints M., Mairiaux E., Praud K., Dierick K., Wildemauve C., Godard C., Butaye P., Imbrechts H., Grimont P. A., and Collard J. M., 2006: Clonal emergence of extended‐spectrum beta‐lactamase (CTX‐M‐2)‐producing Salmonella enterica serovar Virchow isolates with reduced susceptibilities to ciprofloxacin among poultry and humans in Belgium and France (2000 to 2003). J. Clin. Microbiol. 44, 2897–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolund, A. , 2014: Overview of ESBL‐producing Enterobacteriaceae from a Nordic perspective. Infect. Ecol. Epidemiol. 4, 24555 Availabel at 10.3402/ee.v4.24555 (accessed April 5, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff, N. , Espaze E., Gautreau D., Richet H., and Reynaud A., 2000: Analysis of the effects of ‐42 and ‐32 ampC promoter mutations in clinical isolates of Escherichia coli hyperproducing AmpC. J. Antimicrob. Chemother. 45, 783–788. [DOI] [PubMed] [Google Scholar]
- Chicoine, A. L. , Dowling P. M., Boison J. O., and Parker S., 2008: A survey of antimicrobial use during bovine abdominal surgery by western Canadian veterinarians. Can. Vet. J. 49, 1105–1109. [PMC free article] [PubMed] [Google Scholar]
- Dierikx, C. M. , van Duijkeren E., Schoormans A. H., van Essen‐Zandbergen A., Veldman K., Kant A., Hujsdens X. W., van der Zwaluw K., Wagenaar J. A., and Mevius D. J., 2012: Occurrence and characteristics of extended‐spectrum‐beta‐lactamase‐ and AmpC‐producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 67, 1368–1374. [DOI] [PubMed] [Google Scholar]
- Dierikx, C. , van der Goot J., Fabri T., van Essen‐Zandbergen A., Smith H., and Mevius D., 2013: Extended‐spectrum‐beta‐lactamase‐ and AmpC‐beta‐lactamase‐producing Escherichia coli in Dutch broilers and broiler farmers. J. Antimicrob. Chemother. 68, 60–67. [DOI] [PubMed] [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2014: Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0. Available at http://www.eucast.org (accessed April 5, 2015).
- Fecteau, G. , Paré J., Van Metre D. C., Smith B. P., Holmberg C. A., Guterbock W., and Jang S., 1997: Use of a clinical sepsis score for predicting bacteremia in neonatal dairy calves on a rearing farm. Can. Vet. J. 38, 101–104. [PMC free article] [PubMed] [Google Scholar]
- Ghatak, S. , Singha A., Sen A., Guha C., Ahuja A., Bhattacharjee U., Das S., Pradhan N. R., Puro K., Jana C., Dey T. K., Prashantkumar K. L., Das A., Shakuntala I., Biswas U., and Jana P. S., 2013: Detection of New Dehli metallo‐beta‐lactamase and extended‐spectrum beta‐lactamase genes in Escherichia coli isolated from mastitic milk samples. Transbound. Emerg. Dis. 60, 385–389. [DOI] [PubMed] [Google Scholar]
- Giske, C. G. , Sundsfjord A. S., Kahlmeter G., Woodford N., Nordmann P., Paterson D. L., Canton R., and Walsh T. R., 2009: Redefining extended‐spectrum beta‐lactamases: balancing science and clinical need. J. Antimicrob. Chemother. 63, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni, M. , Châtre P., Métayer V., Bour M., Signol E., Madec J. Y., and Gay E., 2014: Comparative prevalence and characterization of ESBL‐producing Enterobacteriaceae in dominant versus subdominant enteritc flora in veal calves at slaughterhouse, France. Vet. Microbiol. 171, 321–327. [DOI] [PubMed] [Google Scholar]
- Hordijk, J. , Wagenaar J. A., van de Giessen A., Dierikx C., van Essen‐Zandbergen A., Veldman K., Kant A., and Mevius D., 2013a: Increasing prevalence and diversity of ESBL/AmpC‐type beta‐lactamase genes in Escherichia coli isolated from veal calves from 1997 to 2010. J. Antimicrob. Chemother. 68, 1970–1973. [DOI] [PubMed] [Google Scholar]
- Hordijk, J. , Wagenaar J. A., Kant A., van Essen‐Zandbergen A., Dierikx C., Veldman K., Wit B., and Mevius D., 2013b: Cross‐sectional study on prevalence and molecular characteristics of plasmid mediated ESBL/AmpC‐producing Escherichia coli isolated from veal calves at slaughter. PLoS One 8, e65681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, H. , Zweifel C., Wittenbrink M. M., and Stephan R., 2013: ESBL‐producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 162, 992–996. [DOI] [PubMed] [Google Scholar]
- Liebana, E. , Batchelor M., Hopkins K. L., Clifton‐Hadley F. A., Teale C. J., Foster A., Barker L., Threlfall E. J., and Davies R. H., 2006: Longitudinal farm study of extended spectrum beta‐lactamase‐mediated resistance. J. Clin. Microbiol. 44, 1630–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec, J. Y. , Poirel L., Saras E., Gourquechon A., Girlich D., Nordmann P., and Haenni M., 2012: Non‐ST131 Escherichia coli from cattle harbouring human‐like bla(CTX‐M‐15)‐carrying plasmids. J. Antimicrob. Chemother. 67, 578–581. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards 2008: Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals‐ Third Edition: Approved Standard M31‐A3. CLSI, Wayne, PA, USA. [Google Scholar]
- O'Grady, N. P. , Alexander M., Burns L. A., Dellinger E. P., Garland J., Heard S. O., Lipsett P. A., Masur H., Mermel L. A., Pearson M. L., Raad I. I., Randolph A., Rupp M. E., Saint S., and the Healthcare Infection Control Practices Advisory Committee (HICPAC) 2011: Guidelines for the prevention of intravascular catheter‐related infections. Available at http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf (accessed April 5, 2015). [Google Scholar]
- Ohnishi, M. , Okatani A. T., Harada K., Sawada T., Marumo K., Murakami M., Sato R., Esaki H., Shimura K., Kato H., Uchida N., and Takahashi T., 2013: Genetic characteristics of CTX‐M‐type extended‐spectrum‐β‐lactamase (ESBL)‐producing enterobacteriaceae involved in mastitis cases on Japanese dairy farms, 2007 to 2011. J. Clin. Microbiol. 51, 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon, B. , Catry B., Dewulf J., Persoons D., Hostens M., De Bleecker K., and Deprez P., 2012: Prospective study on quantitative and qualitative antimicrobial and anti‐inflammatory drug use in white veal calves. J. Antimicrob. Chemotherap. 67, 1027–1038. [DOI] [PubMed] [Google Scholar]
- Quinn, P. J. , Carter M. E., Markey B. K., and Carter G. R., 1999: Enterobacteriaceae In: Quinn P. J., Carter M. E., Markey B. K. and Carter G. R. (eds), Clinical Veterinary Microbiology, pp. 209–236. Harcourt Publishers Ltd., London, UK. [Google Scholar]
- Rettedal, S. , Löhr I. H., Natas O., Giske C. G., Sundsfjord A., and Oymar K., 2012: First outbreak of extended‐spectrum‐β‐lactamase‐producing Klebsiella pneumoniae in a Norwegian neonatal intensive care unit; associated with contaminated breast milk and resolved by strict cohorting. APMIS 120, 612–621. [DOI] [PubMed] [Google Scholar]
- Ribot, E. M. , Fair M. A., Gautom R., Cameron D. N., Hunter S. B., Swaminathan B., and Barrett T. J., 2006: Standardization of pulsed‐field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3, 59–67. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Bano, J. , Navarro M. D., Retamar P., Picon E., and Pascual A., 2012: Beta‐Lactam/beta‐lactam inhibitor combinations for the treatment of bacteremia due to extended‐spectrum beta‐lactamase‐producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin. Infect. Dis. 54, 167–174. [DOI] [PubMed] [Google Scholar]
- Schmid, A. , Hormansdorfer S., Messelhausser U., Käsbohrer A., Sauter‐Louis C., and Mansfeld R., 2013: Prevalence of extended‐spectrum beta‐lactamase‐producing Escherichia coli on bavarian dairy and beef cattle farms. Appl. Environ. Microbiol. 79, 3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet, A. , Martel A., Persoons D., Dewulf J., Heyndrickx M., Catry B., Herman L., Haesebrouck F., and Butaye P., 2008: Diversity of extended‐spectrum β‐lactamases and class c β‐lactamases among cloacal Escherichia coli isolates in Belgian broiler farms. Antimicrob. Agents Chemother. 52, 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet, A. , Boyen F., Flahou B., Doublet B., Praud K., Martens A., Butaye P., Cloeckaert A., and Haesebrouck F., 2012: Emergence of CTX‐M‐2‐producing Escherichia coli in diseased horses: evidence of exchanges of bla(CTX‐M‐2) linked to ISCR1. J. Antimicrob. Chemother. 67, 1289–1291. [DOI] [PubMed] [Google Scholar]
- Timofte, D. , Maciuca I. E., Evans N. J., Williams H., Walttret A., Fick J. C., and Williams N. J., 2014: Detection and molecular characterization of Escherichia coli CTX‐M‐15 and Klebsiella pneumonia SHV‐12 β‐lactamases from bovine mastitis isolates in the United Kingdom. Antimicrob. Agents Chemother. 58, 789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]


