Abstract
An understanding of the prevalence of diseases in free‐ranging populations of felids is limited, and there is even less known about the overall health and diseases of wild felids that inhabit or utilize urban areas. We collected serum samples from 9 radiocollared mountain lions (Puma concolor) in the mountains surrounding Tucson, Arizona, USA, from August 2005 to August 2008. We tested serum samples for evidence of exposure to 10 feline viruses: Feline Calicivirus (FCV), Feline Herpesvirus, Feline Enteric Coronavirus, Feline Syncytial Virus–Feline Foamy Virus, Feline Infectious Peritonitis, Feline Immunodeficiency Virus, Feline Panleukopenia Virus (FPLV), Feline Leukemia Virus, Canine Distemper Virus (CDV), and Toxoplasma gondii. The highest prevalences of exposure were: T. gondii (8/9), FPLV (7/9), and FCV (6/9). One male was seropositive for CDV, T. gondii, and FPLV. Mountain lions inhabiting smaller fragmented landscapes and urban areas have more contact with other felids and domesticated animals. Frequent contact among mountain lions, other felids, and domesticated animals can lead to higher risk of exposure and facilitate the spread of the disease from animal to animal. © 2012 The Wildlife Society.
Keywords: canine distemper, disease ecology, Feline Calicivirus, Feline Panleukopenia, Puma concolor, Toxoplasma gondii
When populations become isolated because of increased fragmentation and loss of habitat, chances of disease epizootics increase (Bradley and Altizer 2007). Anthropogenic activities can influence the role of diseases by facilitating a transmission zone for disease from domestic animals into the carnivore population (Deem et al. 2001). As increases in human population and habitat fragmentation continue, wildlife populations become isolated and there is an increased proximity of humans and their domesticated animals to wildlife (Deem et al. 2001). Several studies have examined strictly urban carnivores, particularly those that are hosts for zoonotic diseases including coyote (Canis latrans; Grinder and Krausman 2001); raccoon (Procyon lotor; Junge et al. 2007), and hooded skunk (Mephitis macroura; Hass and Dragoo 2006). A few researchers examined disease exposure across an urban–rural gradient in species such as red foxes (Vulpes vulpes; Truyen et al. 1998), bobcats (Lynx rufus), and gray foxes (Urocyon cinereoargenteus; Riley et al. 2004). Studies of the effects of an urban environment on disease transmission are lacking for mountain lions. This is of particular concern for areas in Arizona that have high rates of urban expansion and that are adjacent to mountain lion habitat. Arizona is the fifth‐fastest‐growing state in the United States (U.S. Census Bureau; http://www.census.gov/). Due to the species' solitary nature, intraspecific interactions among mountain lions are typically limited to territorial fights, mating, and family‐group contacts (Logan and Sweanor 2001). However, mountain lions that inhabit fragmented landscapes have an increased likelihood of contact with other species and domesticated animals and therefore may have an increased prevalence of disease exposure (Riley et al. 2004). The long‐distance dispersal capabilities of this species (Launder 2007) increase opportunities for contact between domesticated and wild animals.
We collected serologic data from 9 lions in the mountain ranges surrounding Tucson, southern Arizona, USA (32.189°N, 110.881°E) to establish baseline knowledge of enzootic pathogens at the urban–wildland interface. We tested for antibody or antigens to Feline Calicivirus (FCV); Feline Immunodeficiency Virus (FIV); Feline Infectious Peritonitis (FIP); Feline Enteric Coronavirus (FECV); Feline Panleukopenia Virus (FPLV; also known as Feline Parvovirus); Feline Syncytial Virus–Feline Foamy Virus (FSyV–FFV); Feline Herpesvirus (FHV); Feline Leukemia Virus (FeLV); Canine Distemper Virus (CDV); and Toxoplasma gondii.
We choose these diseases because their prevalence is common among domestic cats within the urban environment, and because interspecies transmission might occur or are of concern to humans. Feline Calicivirus is a highly infectious pathogen and, although all felids are susceptible, natural infection has only been reported in domestic cats and cheetahs (Acinonyx jubatus; Riley et al. 2004, Nams 2006). Feline Immunodeficiency Virus is endemic in cats throughout the world; although symptoms are well‐documented in domestic cats and cheetahs, the virus has no historical association of pathogenicity in wild felids (Nellemann et al. 2003). Feline Infectious Peritonitis is believed to be caused by FECV that has mutated slightly. Feline Enteric Coronavirus causes mild‐to‐severe enteritis (i.e., inflammation of the intestine) in kittens, and fever, vomiting, and diarrhea and can persist in the animal for years (Nellemann et al. 2001). Feline Infectious Peritonitis, on the other hand, is severe and almost always fatal. A cat can carry the Coronavirus and never get FIP, but if there is some sort of immune suppression (e.g., stress or co‐infection with another virus) that lowers the immune response, then the FIP virus begins replicating (Nellemann et al. 2001). Feline Panleukopenia Virus is potentially a population‐limiting viral disease of Felidae (Anderson 1983) and can cause disease in some members of related families (e.g., raccoon, mink [Mustela vison], and coatimundi [Nasua nasua; Nams 2006]). Feline Syncytial Virus and FHV were selected because they are ubiquitous throughout the cat world. The incidence of FeLV infection is directly related to the population density of cats. Despite vaccinations in captive wild and domestic cats, FeLV remains the most important causes of morbidity and mortality in cats worldwide (Nellemann et al. 2001). Canine distemper was selected because it is common among dogs and collared peccary (Pecari tajacu), which are both potential prey items for mountain lions. Toxoplasmosis (i.e., litter‐box disease) is caused by the coccidian protozoa T. gondii (Cronin et al. 2000). Cats do not show signs of illness while passing oocysts and an adult cat will not pass oocysts again after recovering from an initial exposure. Toxoplasmosis is a zoonotic hazard for humans and may cause abortion, stillbirth, or pre‐term delivery in both humans and cats.
STUDY AREA AND METHODS
During 2005–2008, as part of a larger study, we used hounds and leg‐hold snares to capture and radio‐collar mountain lions [see Nicholson (2009) for further details]. We immobilized each lion using Ketamine (Ketamine HCL; Wildlife Pharmaceutical, Ft. Collins, CO; 2.0–7.0 mg/kg) and medetomidine hydrochloride (Domitor; Wildlife Pharmaceutical; 0.2–0.7 mg/kg). Medetomidine was reversed using antisedan (Atipamezole hydrochloride; Pfizer Inc., New York, NY) at a dose of 3 mg of atipamezole for every 1 mg of medetomidine. We collected blood via saphenous venipuncture into serum separator tubes, placed the samples on ice, and (within 1–4 hr after collection) had them centrifuged (15 min at approx. 3,000 rpm). We froze the serum samples (−12°C) until they were tested.
The Animal Health Diagnostic Center, College of Veterinary Medicine of Cornell University, Ithaca, NY, USA, performed the serology (accession no. 93055‐06 and no. 88136‐07). The following testing procedures were used: serum neutralization for CDV, FCV, FHV, FECV, FSyV–FFV; kinetic enzyme‐linked immunosorbent assay (ELISA) for T. gondii, FIP, and FIV using PetChek (Idexx Laboratories, Westbrook, ME); hemagglutiation inhibition for FPLV; and p27 antigen by ELISA for FeLV (ViraChek; Synbiotics Corporation, Kansas City, MO).
As part of the larger mountain lion study (Nicholson 2009), we created a 95% fixed‐kernel home range (Worton 1989) within ArcGIS v9.3 by using Home Range Tools v. 1.1 extension (Rodgers et al. 2007). We also calculated the percent of each home range that was in an urban area (Fig. 1). We created an urban boundary layer by combining high‐resolution satellite imagery (1‐m pixel resolution) with an Arizona Department of Transportation (ADOT) urban boundary layer and a road‐density layer. The ADOT city layer already accounted for incorporated cities in Arizona and we used this layer as the base map. To find towns not accounted for, or to modify the existing boundary of an ADOT city boundary, we used the road‐density layer to locate high‐density areas (>3 km of road/0.5 km2) and overlaid them on satellite imagery. We then used heads‐up digitizing to outline urban areas (for more details see Nicholson 2009).
Figure 1.
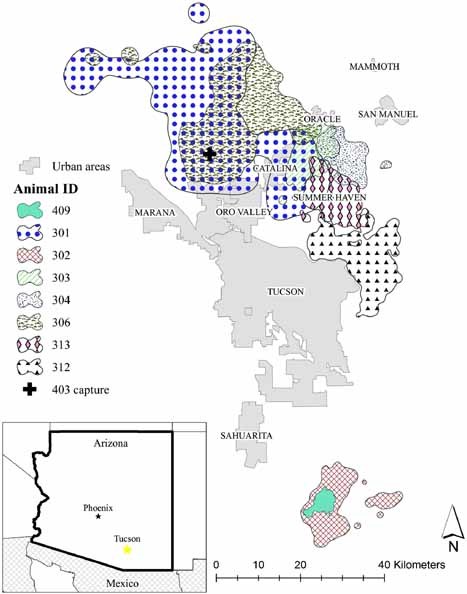
Mountain lion home ranges (fixed‐kernel home range; 95% home range) in relation to urban areas in southwestern Arizona, USA, 2005–2008. +Initial capture of lion 403 (not radio‐collared due to young age and small size).
We attempted a series of binary linear‐regression models using a logit‐link function for each disease. We assumed exposure to each virus was independent. We used a natural‐log transformation to normalize home range size data and hypothesized that the larger the home range (essentially, the more area used by an individual), the more likely the individual would test positive for a disease. Similarly, we assumed that the greater the percentage of a mountain lion's home range that overlapped an urban area, the more likely it would test positive for a disease because of the increased chance of exposure to, and transfer of disease from, domesticated animals.
RESULTS
The disease agent detected with the highest prevalence in our study was T. gondii (Table 1), which is enzootic in Felidae. Prevalence of antibody to FPLV in Arizona (n = 7/9 [78%]) was similar to other studies (Table 2). We had higher prevalence of antibody to FCV (n = 6/9 [67%]) in this study than was found elsewhere (Table 2). The presence of antibodies to T. gondii and FPLV were detected in 8 and 7 of the mountain lions, respectively (Table 1). Mountain lions 409 and 302 had overlapping home ranges (Fig. 1) and had similar antibody exposure (Table 1). In this study, there were no mountain lions that tested positive for FHV, FIP, or FeLV (Table 1).
Table 1.
Mountain lion home‐range size (km2), percent of home range consisting of urban area, and the diseases for which the lion tested seriopositive (data collected in southwestern AZ, USA, 2005–2008).
| ID | Home range (km2) | % Urban | Diseasesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCV | FHV | FPLV | FECV | FIP | FeLV | FIV | FSyV | T. gondii | CDV | |||
| 301 | 1,213.56 | 5.53 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| 302 | 183.08 | 0.00 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| 303 | 135.52 | 1.51 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 304 | 125.56 | 0.04 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 306 | 619.59 | 0.49 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 312 | 272.99 | 0.11 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| 313 | 168.18 | 0.59 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 403b | 0.00 | NA | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 409 | 28.61 | 0.00 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Total positive | 6 | 0 | 7 | 2 | 0 | 0 | 6 | 1 | 8 | 1 | ||
Diseases: FCV, Feline Calcivirus; FHV, Feline Herpes; FPLV, Feline Panleukopenia Virus; FECV, Feline Enteric Coronavirus; FIP, Feline Infectious Peritonitis; FeLV, Feline Leukemia Virus; FIV, Feline Immunodeficiency Virus; FSyV, Feline Syncytial Virus; T. gondii, Feline Toxoplasmosis; CDV, Canine Distemper Virus.
Mountain lion 403 was not radiocollared due to age and small size.
Table 2.
Comparison of seropositive individuals from various studies in the United States that tested mountain lions and urban and rural bobcats for 11 diseases. Sample size (n) and percent positive.
| Study | Location | Speciesa | n | Diseasesb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCV | FHV | FPLV | FECV | FIP | FeLV | FIV | FSyV | T. gondii | CDV | ||||
|
Nicholson (2009) |
SW AZ | P.c. | 9 | 67 | 0 | 78 | 22 | 0 | 0 | 33 | 11 | 89 | 11 |
|
Biek et al. (2006) |
Rocky Mt. | P.c. | 207 | 18 | 0 | 69 | 28 | 50 | 11 | ||||
|
Paul‐Murphy et al. (1994) |
CA | P.c. | 58 | 17 | 19 | 93 | 28 | 4 | 0 | 58 | |||
|
Riley et al. (2004 ; urban) |
CA | L.r. | 12 | 17 | 0 | 0 | 0 | 0 | 0 | 100 | |||
|
Riley et al. (2004 ; rural) |
CA | L.r. | 13 | 67 | 0 | 0 | 8 | 0 | 0 | 77 | |||
|
Roelke et al. (1993) |
FL | P.c. | 38 | 56 | 0 | 78 | 19 | 0 | 37 | 9 | |||
|
Olmsted et al. (1992) |
AZ | P.c. | 10 | 80 | |||||||||
|
Olmsted et al. (1992) |
NM | P.c. | 2 | 50 | |||||||||
Species: P.c., Puma concolor; L. r., Lynx rufus.
Diseases: FCV, Feline Calcivirus; FHV, Feline Herpes; FPLV, Feline Panleukopenia Virus; FECV, Feline Enteric Coronavirus; FIP, Feline Infectious Peritonitis; FeLV, Feline Leukemia Virus; FIV, Feline Immunodeficiency Virus; FSyV, Feline Syncytial Virus; T. gondii, Feline Toxoplasmosis; CDV, Canine Distemper Virus.
One mountain lion (M301) was seropositive to CDV, T. gondii, FPLV, and FECV and was subsequently euthanized due to recapture‐related injuries. The carcass was frozen and then necropsied at the Arizona Veterinary Diagnostic Lab (accession #06‐6615; AZVDL, Tucson, Arizona, USA). Tissue samples were collected at necropsy, fixed in formalin, and mounted in paraffin blocks for sectioning for histology. Microscopic examination of brain, liver, spleen, heart, lungs, kidney, adrenal glands, trachea, stomach, intestine, pancreas, urinary bladder, and foot pad was not revealing although freezing artifact severely degraded tissue morphology. Polymerase chain reaction (PCR) testing of unfixed brain tissue was reported as negative for CDV. Fluorescent antibody testing of brain tissue for rabies virus was also reported as negative by the Arizona Department of Health Services rabies laboratory.
Retrospectively, de‐paraffinized formalin‐fixed sections of stomach, urinary bladder, lung, and spleen were used for testing for antigens of CDV using immunohistochemistry (IHC [accession #09‐1973; Washington Animal Disease Diagnostic Laboratory Pullman, WA, USA]) as unfixed tissue was not available. In addition, tissue from paraffin blocks containing spleen and small intestine was tested for amplicons of FPLV and tissue from paraffin blocks containing skeletal muscle and small intestine was tested for amplicons of T. gondii using PCR (accession #089‐51144; Veterinary Diagnostic Laboratory at Colorado State University Ft. Collins, CO, USA). Tests were negative for CDV, FPLV, and T. gondii.
DISCUSSION AND MANAGEMENT IMPLICATIONS
There were some trends to indicate that individual lions with larger home ranges, and those with home ranges that overlapped with urban areas, had greater percent of disease exposure (Fig. 2); however, due to our sample size, these trends are not statistically significant. These hypotheses need to be investigated with additional samples. Apart from interspecific contact, another explanation for our findings would be intraspecific transmission among mountain lions of some of the viral agents.
Figure 2.
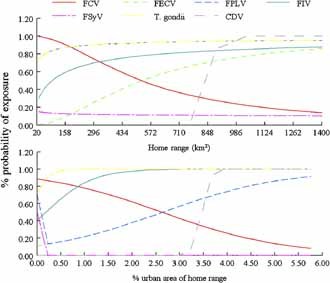
Linear regression trends of percent probability of exposure of disease tested against the home range size (km2) and percent of the home range size that overlaps with an urban area for mountain lions in southwestern Arizona, USA, 2005–2008. FCV, Feline Calcivirus; FECV, Feline Enteric Coronavirus; FPLV, Feline Panleukopenia Virus; FIV, Feline Immunodeficiency Virus; FSyV, Feline Syncytial Virus; T. gondii, Feline Toxoplasmosis; CDV, Canine Distemper Virus.
Feline herpes and FIV are endemic in African lions (Panthera leo) in the Serengeti and FCV, FPLV, FECV, and CDV showed patterns of disease prevalence indicative of discrete disease epidemics over a 30‐year study (Packer et al. 1999). Our (n = 3/9 [33%]) seropositive results for FIV antibodies are reasonable because FIV is not uncommon in mountain lions. Chronic infection is asymptomatic in this host in Montana, Washington, Texas, and Florida in the United States (Evermann et al. 1997, Biek and Poss 2002, Biek et al. 2006, Miller et al. 2006), or in Brazilian felids (Filoni et al. 2006) and African lions (Roelke et al. 2006). Seroprevalence of antibody to FCV was high in bobcats (Table 2) in California, USA, in rural zones where they potentially came into contact with domestic cats (Riley et al. 2004).
Members of the cat family are the only known definitive hosts for T. gondii (Aiello 1998). After being shed in feces, the sporulated oocysts of T. gondii can persist in the environment for up to a year (Aiello 1998). Antibodies to T. gondii were detected in 22% of mountain lions sampled in North, Central, and South America (Table 2; Kikuchi et al. 2004). Mountain lions in the southwestern United States (i.e., AZ, CA, NM) were reported to be more likely to be seropositive for T. gondii than those from northwestern and mountain states because of climatic differences or survival of the tachyzoites in the prey of mountain lions (Kikuchi et al. 2004).
The puma‐lentivirus (PLV) is closely related to FIV and antibodies are common in wild felid populations (Olmsted et al. 1992). Commercially available ELISA kits based on domestic cat FIV do not have an acceptable ability to recognize seropositive samples from mountain lions (Franklin et al. 2007). Because of this, our results (n = 3/9 [33%]) may not completely depict actual exposure to FIV. Using PLV‐specific ELISA test to screen wild felid populations for lentivirus exposure may provide more reliable results.
We speculate that the presence of high levels of antibody to CDV, FPLV, FECV, and T. gondii in serum from mountain lion M301 may indicate prior infection with those agents. Infection by CDV might have resulted from contact with an actively infected wild or domestic canid, collared peccary, or raccoon. The collared peccary is a common prey of mountain lions in the Southwest and has been reported as infected with, or seropositive to, CDV (Murphy et al. 1999, Noon et al. 2003). In a 4‐year study on CDV exposure in peccary in and around Tucson, 58% of samples collected were positive for virus‐neutralizing antibody (Noon et al. 2003). Contact between mountain lions and infected feral or domesticated felids or other infected free‐ranging wild felids present in their habitat might explain some of our findings.
Continuation of long‐term serologic studies of mountain lions over several years would allow a better evaluation of the possibility of intraspecific transmission. As humans encroach into habitats of large predators, managers and biologists should take advantage of opportunities to develop more complete understanding of the disease ecology of wild carnivores to enhance their management.
ACKNOWLEDGMENTS
We thank the hounds‐men, B.R. Buckley, B. Jansen, B. Kluever, and R. Thompson, who participated in trapping and the acquisition of blood samples. We thank W.B. Ballard, S.L. Wesche, and J. Heffelfinger for comments on earlier drafts of this manuscript and B. Rickert and E. Kerr of the Arizona Veterinary Diagnostic Laboratory for sample handling and preparation. Funding was provided by Arizona Game and Fish Department and the University of Arizona. Capture and handling procedures were approved by the Animal Care and Use Committee at the University of Arizona (protocol no. 05‐184).
Associate Editor: Rominger
LITERATURE CITED
- Aiello, S. E. , editor. 1998. The Merck veterinary manual. Eighth edition. Merck, Whitehouse Station, New Jersey, USA. [Google Scholar]
- Anderson, A. E. 1983. A critical review of the literature on puma (Felis concolor). Colorado Division of Wildlife Special Report no. 54, Fort Collins, USA.
- Biek, R. , and Poss M.. 2002. Large‐scale sampling of cougar populations and one of their pathogens: working with cougar hunters in Montana. Intermountain Journal of Sciences 8:247. [Google Scholar]
- Biek, R. , Ruth T. K., Murphy K. M., C. R. Anderson, Jr. , Johnson M., DeSimone R., Gray R., Hornocker M. G., Gillin C. M., and Poss M.. 2006. Factors associated with pathogen seroprevalence and infection in Rocky Mountain cougars. Journal of Wildlife Diseases 42:606–615. [DOI] [PubMed] [Google Scholar]
- Bradley, C. A. , and Altizer S.. 2007. Urbanization and the ecology of wildlife diseases. Trends in Ecology & Evolution 22:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin, M. A. , Whitlaw H. A., and Ballard W. B.. 2000. Northern Alaska oil fields and caribou. Wildlife Society Bulletin 28:919–922. [Google Scholar]
- Deem, S. L. , Karesh W. B., and Weisman W.. 2001. Putting theory into practice: wildlife health in conservation. Conservation Biology 15:1224–1233. [Google Scholar]
- Evermann, J. F. , Foreyt W. J., Hall B., and McKeirnan A. J.. 1997. Occurrence of puma lentivirus infection in cougars from Washington. Journal of Wildlife Diseases 33:316–320. [DOI] [PubMed] [Google Scholar]
- Filoni, C. , Catao‐Dias J. L., Bay G., Durigon E. L., Jorge R. S. P., Lutz H., and Hofmann‐Lehmann R.. 2006. First evidence of feline herpesvirus, calicivirus, parvovirus, and Ehrlichia exposure in Brazilian free‐ranging felids. Journal of Wildlife Diseases 42:470–477. [DOI] [PubMed] [Google Scholar]
- Franklin, S. P. , Troyer J. L., TerWee J. A., Lyren L. M., Kays R. W., Riley S. P. D., Boyce W. M., Crooks K. R., and Vandewoude S.. 2007. Variability in assays used for detection of lentiviral infection in bobcats (Lynx rufus), pumas (Puma concolor) and ocelots (Leopardus pardalis). Journal of Wildlife Diseases 43:700–710. [DOI] [PubMed] [Google Scholar]
- Grinder, M. , and Krausman P. R.. 2001. Morbidity‐mortality factors and survival of an urban coyote population in Arizona. Journal of Wildlife Diseases 37:312–317. [DOI] [PubMed] [Google Scholar]
- Hass, C. C. , and Dragoo J. W.. 2006. Rabies in hooded and striped skunks in Arizona. Journal of Wildlife Diseases 42:825–829. [DOI] [PubMed] [Google Scholar]
- Junge, R. E. , Bauman K., King M., and Gompper M. E.. 2007. A serologic assessment of exposure to viral pathogens and leptospira in an urban raccoon (Procyon lotor) population inhabiting a large zoological park. Journal of Zoo and Wildlife Medicine 38:18–26. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y. , Chomel B. B., Kasten R. W., Martenson J. S., Swift P. K., and O'Brien S. J.. 2004. Seroprevalence of Toxoplasma gondii in American free‐ranging or captive pumas (Felis concolor) and bobcats (Lynx rufus). Veterinary Parasitology 120:1–9. [DOI] [PubMed] [Google Scholar]
- Launder, J. W. 2007. Use of dispersal distance to assess the long term conservation of mountain lions. Pages 69 in Sixth Mountain Lion Workshop. San Antonio, TX, USA.
- Logan, K. A. , and Sweanor L. L.. 2001. Desert puma: evolutionary ecology and conservation of an endangered carnivore. Island Press, Washington, D.C., USA. [Google Scholar]
- Miller, D. L. , Taylor S. K., Rotstein D. S., Pough M. B., Barr M. C., Baldwin C. A., Cunningham M., Roelke M., and Ingram D.. 2006. Feline immunodeficiency virus and puma lentivirus in Florida panthers (Puma concolor coryi): epidemiology and diagnostic issues. Veterinary Research Communications 30:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, F. A. , Gibbs E. P. J., Horzinek M. C., and Studdert M. J.. 1999. Veterinary virology. Third edition. Academic Press, San Diego, California, USA. [Google Scholar]
- Nams, V. O. 2006. Animal movement rates as behavioural bouts. Journal of Animal Ecology 75:298–302. [DOI] [PubMed] [Google Scholar]
- Nellemann, C. , Vistnes I., Jordhoy P., and Strand O.. 2001. Winter distribution of wild reindeer in relation to power lines, roads and resorts. Biological Conservation 101:351–360. [Google Scholar]
- Nellemann, C. , Vistnes I., Jordhoy P., Strand O., and Newton A.. 2003. Progressive impact of piecemeal infrastructure development on wild reindeer. Biological Conservation 113:307–317. [Google Scholar]
- Nicholson, K. L. 2009. Spatial movements and ecology of mountain lions in Arizona. Dissertation, University of Arizona, Tucson, USA.
- Noon, T. H. , Heffelfinger J. R., Olding R. J., Wesche S. L., and Reggiardo C.. 2003. Serologic survey for antibodies to canine distemper virus in collared peccary (Tayassu tajacu) populations in Arizona. Journal of Wildlife Diseases 39:221–223. [DOI] [PubMed] [Google Scholar]
- Olmsted, R. A. , Langley R., Roelke M. E., Goeken R. M., Adger‐Johnson D., Goff J. P., Albert J. P., Packer C., Laurenson M. K., and Caro T. M.. 1992. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. Journal of Virology 66:6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, C. , Altizer S., Appel M., Brown E., Martenson J., O'Brien S. J., Roelke‐Parker M., Hofmann‐Lehmann R., and Lutz H.. 1999. Viruses of the Serengeti: patterns of infection and mortality in African lions. Journal of Animal Ecology 68:1161–1178. [Google Scholar]
- Paul‐Murphy, J. , Work T., Hunter D., McFie E., and Fjelline D.. 1994. Serologic survey and serum biochemical reference ranges of the free‐ranging mountain lion (Felis concolor) in California. Journal of Wildlife Diseases 30:205–215. [DOI] [PubMed] [Google Scholar]
- Riley, S. P. D. , Foley J., and Chomel B.. 2004. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a National Park in California. Journal of Wildlife Diseases 40:11–22. [DOI] [PubMed] [Google Scholar]
- Rodgers, A. R. , Carr A. P., Beyer H. L., Smith L., and Kie J. G.. 2007. Home Range Tools. Version 1.1. Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research, Thunder Bay, Canada.
- Roelke, M. E. , Forrester D. J., Jacobson E. R., Kollias G. V., Scott F. W., Barr M. C., Evermann J. F., and Pirtie E. C.. 1993. Seroprevalence of infectious disease agents in free‐ranging Florida panthers (Felis concolor coryi). Journal of Wildlife Diseases 29:36–49. [DOI] [PubMed] [Google Scholar]
- Roelke, M. E. , Pecon‐Slattery J., Taylor S., Citino S., Brown E., Packer C., VandeWoude S., and O'Brien S. J.. 2006. T‐lymphocyte profiles in FIV‐infected wild lions and pumas reveal CD4 depletion. Journal of Wildlife Diseases 42:234–248. [DOI] [PubMed] [Google Scholar]
- Truyen, U. , Muller T., Heidrich R., Tackmann K., and Carmichael L. E.. 1998. Survey on viral pathogens in wild red foxes (Vulpes vulpes) in Germany with emphasis on parvoviruses and analysis of a DNA sequence from a red fox parvovirus. Epidemiology and Infection 121:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton, B. J. 1989. Kernel methods for estimating the utilization distribution in home range studies. Ecology 70:164–168. [Google Scholar]


