Abstract
The new outbreak of coronavirus from December 2019 has brought attention to an old viral enemy and has raised concerns as to the ability of current protection measures and the healthcare system to handle such a threat. It has been known since the 1960s that coronaviruses can cause respiratory infections in humans; however, their epidemic potential was understood only during the past two decades. In the present review, we address current knowledge on coronaviruses from a short history to epidemiology, pathogenesis, clinical manifestation of the disease, as well as treatment and prevention strategies. Although a great amount of research and efforts have been made worldwide to prevent further outbreaks of coronavirus-associated disease, the spread and lethality of the 2019 outbreak (COVID-19) is proving to be higher than previous epidemics on account of international travel density and immune naivety of the population. Only strong, joint and coordinated efforts of worldwide healthcare systems, researchers, and pharmaceutical companies and receptive national leaders will succeed in suppressing an outbreak of this scale.
Keywords: coronavirus, SARS-CoV, MERS-CoV, COVID-19, epidemiology, virulence strains, pathogeny, diagnostics
1. Introduction
Respiratory infections are common in the cold seasons world-wide, and consequently considered trivial and mild. Affected individuals rarely consult medical professionals, instead treating themselves with symptomatic medications. Droplet and aerosol transmission further facilitates rapid dissemination to numerous individuals at once, which amplifies socioeconomic impact even with minimal increases to fatality rate, particularly among patients with comorbidities.
The latest and contemporary outbreak of a respiratory pathogen, namely severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for coronavirus disease (COVID-19), has brought to attention a hidden threat from an old enemy. This is the third major coronavirus outbreak over the past 20 years that has had substantial socioeconomic impact, but the first in the 21st century to affect countries across all continents except Antarctica. The general panic and insecurity expressed across all sociopolitical and economic tiers has dramatically disrupted day-to-day life, international travel and trade. Notwithstanding severe lifestyle disruptions, depression-associated disease has been reported due to extreme measures of isolation.
Here we emphasize current knowledge regarding coronaviruses from a short history to epidemiology, diagnosis, pathogenesis, clinical manifestation, as well as treatment and prevention strategies. Forward lessons informing future strategies to improve surveillance and prevent recurrence are highlighted.
2. A brief overview of coronavirus infections in human history
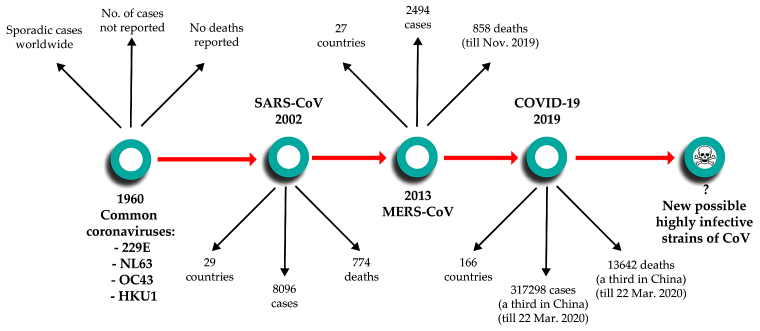
SARS-CoV-2 belongs to the Orthocoronavirinae subfamily, family Coronaviridae, order Nidovirales (1). It comprises of four subtypes, α- and β-coronaviruses that can infect humans, and gamma- and delta-coronaviruses, which are found only in animals. It is a zoonotic virus that can be transmitted from animals to humans and, once adapted to do so, between humans via airborne droplets and aerosols (2). The most involved carrier animal species for human infections is the bat (3), though animal reservoirs extend to cattle, pigs, turkeys, camels, mice, dogs, cats, ferrets and mink (4,5). The first coronavirus infections were reported in 1960 as a cause for the common cold. Since then, until 2002, four subtypes of coronaviruses were reported to infect humans, two α coronaviruses - 229E and NL63 and 2 β coronaviruses - OC43 and HKU1, which routinely produce noncomplicated infections of the upper and/or lower respiratory tract (3). Then, 2002 marked a key moment in our understanding of coronavirus-induced disease with the emergence of the first lethal severe acute respiratory syndrome (SARS)-causing coronavirus. Similarly to SARS-CoV-2, the original SARS-CoV emerged in Guangdong Province in China, spreading through human transmission chains to infect at least 8,096 individuals in 29 countries and
succumb 774 patients (6). In 2012, a novel β-coronavirus that had not previously been observed in humans was detected for the first time in a patient in Saudi Arabia. Since then, the new coronavirus, which causes Middle Eastern Respiratory Syndrome and is now known as MERS-CoV, has infected >2,494 individuals across 27 countries and led to the death of at least 858 individuals as of November 2019, through a series of emergences and re-emergences from camelid hosts (7). On December 8, 2019, in Wuhan, Hubei Province, China, the first case was reported of a new coronavirus that produces pneumonia. Since then, the new virus first named 2019-nCoV and subsequently renamed SAS-CoV-2 was identified as a member of the β-coronavirus subtype, spread rapidly via human-to-human transmission. At the time of authoring (March 23, 2020), over 317,298 cases have been recorded worldwide across 166 countries, with over 13,642 deaths attributed to the virus (8) (Fig. 1).
Figure 1.
History of coronavirus infections in humans.
3. Epidemiology of coronavirus infections
The emergence of virulence strains of coronavirus over the past 20 years has increased the interest of scientists regarding coronavirus strain variability, distribution, and the ability to be transmitted between animals to humans and further from humans-to-humans. The strains have slightly different distributions across sex, age and other demographic attributes. For example, whilst SARS-CoV-2 affects males more than females with substantial age-associated mortality increases in frail elderly subjects, the four societally non-disruptive coronaviruses affect more females and children between 5 and 14 years (9).
In contrast, SARS-CoV-1 affected more females, and relatively young people with the median age of SARS infected patients in Guangzhou province, China at 28.6 years) (10), and mortality rate at ~12% in people over 65 years (11). In comparison, the MERS coronavirus was more frequently detected among males and middle-aged adults aged 50-59 years (12). Emerging data from hospitalized COVID-19 cases in Wuhan (Hubei province, China) indicate males (68%) and middle-age adults in the age group of 50-59 years to be predominantly affected, with a prevalence under 10% in people aged <39 years. Among these cases, 51% had chronic diseases such as cardiovascular and cerebrovascular diseases (40%), endocrine system disease (13%), digestive system disease (11%), respiratory system disease (1%), malignant tumor (1%), and nervous system disease (1%) (13). Likewise, data from Italy as of 15/3/20 indicate a median age of 64 years with middle aged (51-70 years old) and elderly (75.7%), predominantly male (59.8%) patients experiencing mild (46.1%) to severe (24.9%) disease and the burden of mortality (70-79 years old: case fatality ratio 12.5%; 80-89 years old: 19.7%; >90 years old: 22.7%) (14). Whilst the older population demographics of Italy is believed to drive the higher case fatality ratio differences (7.9%) vs. China (4.0%) (15), the Italian healthcare system is experiencing increased requirement for intensive care unit (ICU) admission (~10% of cases) (16). Anecdotal reports from frontline clinicians in the principally affected regions in Italy indicate all ICU beds dedicated to COVID-19 and patient admission to ICU/mechanic ventilation restricted to age groups with a higher likelihood of survival. Of note, these reports indicate 20% of ICU cases to involve individuals under the age of 65 and as young as 20 years of age. The reader is strongly advised to consider carefully the territory-specific diversity of case severity definition as well as the diagnostic triage/definition criteria before making direct epidemiological comparisons.
Table I.
The comparative clinical evolution of coronavirus infections.
| Coronavirus strain | Incubation period | Death period | Symptoms |
|---|---|---|---|
| SARS CoV | 4-10 days | 20-25 days | Fever, dry cough, myalgia, dyspnea, headache, sore throat, sputum production, rhinorrhea watery diarrhea confusion, poor appetite |
| MERS CoV | 5-6 days | 11-13 days | Myalgia, fever, chills, malaise associated with confusion, cough, shortness of breath, dyspnea pneumonia |
| COVID-19 | 3-7 days | 17-24 days | Fever, cough, dyspnea, muscle ache confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea, vomiting, anosmia, dysgeusia |
One additional aspect complicating case epidemiological oversight pertains to the highly mutagenic nature of coronaviruses, which is common across RNA viruses, and has led to inappropriate comparative evolution interpretations and even the incorrect assertion of mild and sever COVID-19 arising from separate strains of SARS-CoV-2. By comparing genomes of sequenced COVID-19 strains, it was shown that synonymous mutations in spike genes between COVID-19 and rat or bat coronaviruses (RaTG13 and Bat-SL-CoVZC45) were quite different. The ratio of nucleotide substitutions to amino acid substitutions was 9.07 in COVID-19 compared with RaTG13, which was significantly higher than the 3.91 ratio from COVID-19 to Bat-SL-CoVZC45 (17). Additionally, there were numerous concerns that COVID-19 was an engineered bioweapon, hypotheses fed by speculation that sequences from COVID-19 were identical to those in HIV. Alignments showed that they are not present in any other coronavirus strains, but show identity/similarity with sequences in HIV-1 gp120 and Gag, the former being also a cellular receptor recognition protein (18). Considering that these inserts appear in hyper-variable regions of the protein and are as short as 6 residues in length, it is most probable that they arose naturally: as a consequence the article describing these similarities was recently withdrawn from publication. To date, no evidence supports that SARS-CoV-2 is man-made: COVID-19 closely resembles two other coronaviruses that have triggered outbreaks in recent decades, SARS-CoV-1 (79% sequence identity) and MERS-CoV (51.8% identity) (19), and all three viruses are most likely to have originated in bats (17,20): SARS-CoV-2 has a 96% sequence identity with a recently sequenced bat coronavirus recovered from the wild by random sampling (21).
4. The transmission model
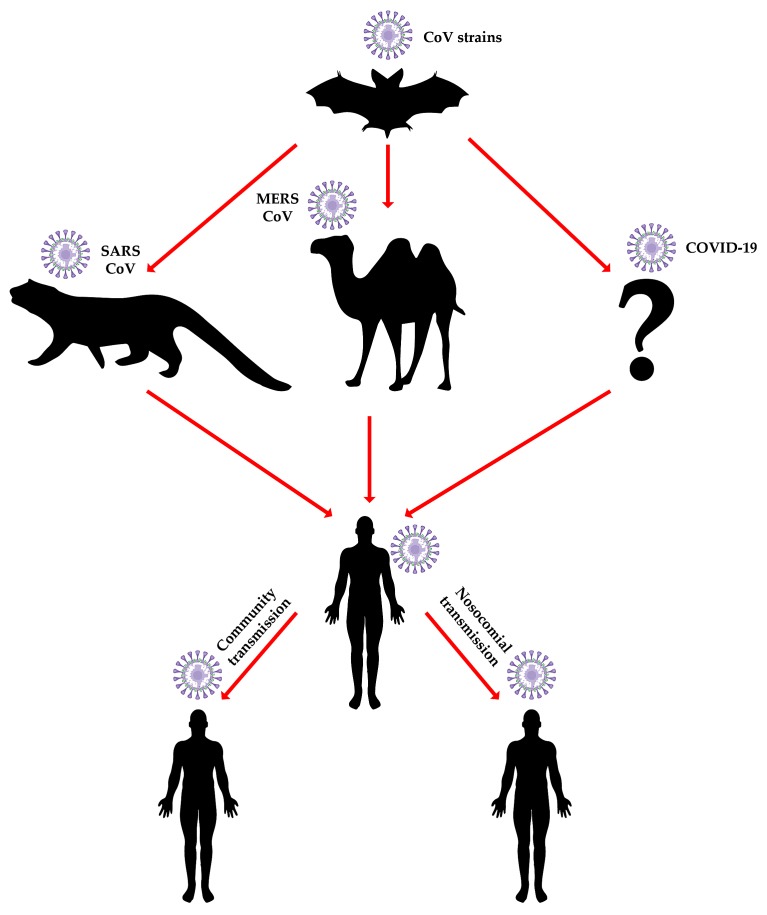
A major question regarding coronavirus epidemiology is 'Why does the bat of all animals play such a central role in coronavirus epidemiology?'. Studies in bats have identified viruses originating in this species, as the potential vector to human infections. Additionally, as bats live in colonies, they present a high risk of transmitting the viruses horizontally (intra-species) which contributes to the vertical (cross-species) spreading ability. This hypothesis is strongly favored by data on another high socioeconomic impact bat-derived virus, the Ebola virus, which was shown to efficiently infect both bat and human cells (22) and to date remains the favored hypothesis for index case infection in the 2014-16 West African Ebolavirus disease outbreak.
Additional evidence of bat to human indirect transmission comes from the COVID-19's Spike protein and ACE2 receptor interactions (23). Indeed, the two highly pathogenic strains of coronaviruses - SARS-CoV and MERS-CoV were identified both in bat species and in animals involved in transmission to humans (20). It is interesting to note that the first highly pathogenic strain of coronavirus, SARS-CoV-1, has a low genetic similarity with other known coronaviruses (39% with bovine coronavirus and 46% with porcine epidemic diarrhea virus) (24). Recently, three comparison studies were made with coronavirus strains from pangolins. The first (February 18, 2020) compared the sequences of COVID-19 with the coronaviruses in illegally trafficked pangolins to show a sequence similarity between 85.5 and 92.4% (25), with the subsequent papers (February 20, 2020), reporting sequence similarities with pangolin coronaviruses at 90.23% (26) and 91.02% (27), respectively. However, the α-coronavirus strains that cause human disease were also originally identified in bats (28); it is therefore not surprising that COVID-19 shares 96% genetic similarity (whole genome level) with a coronavirus isolated from Chinese bat species (21). Another important question is 'Why China?' China is the third-largest country by surface area worldwide but the most populated country. Its varied land characteristics and diverse climate range supports huge biodiversity, which contributes to enabling transmission of viruses between animal populations. Principally, however, co-habitation of animals and humans in close proximity, and gastronomic customs involving the consumption of a variety of exotic animals, including wild fauna such as bats, increases the chance of vertical transmission throughout the food supply chain. Officially, live-bird markets have been closed; however, black-market vendors run illegal slaughterhouses, that are crowded places with poor ventilation, where a number of species are hoarded together: These create ideal conditions for the spreading of the virus through airborne droplets of blood and other secretions, or shared cages, trade tools, and utensils, allowing for an 'amplification' of vertical transmission risk. The prevalence of such culinary and wild animal handling practices in Southern China have been causally linked to both the 2002 SARS-CoV-1 outbreak starting from a market in Shenzen (Guangdong, China) (29) and the entry of SARS-CoV-2 into the human population. Of the original 41 cases presenting with pneumonia of unknown origin, two thirds had links to Huanan Seafood Wholesale Market that also sold live animals (30).
Such a species barrier bridge has been also documented with filovirus disease outbreaks in Africa, wherein high risk infectious agents are routinely detected in bushmeat markets and the associated human population both by PCR and immunoprecipitation methods (31). As with China, human and animal close contact and often co-habitation remains common in rural African areas with practically no barriers to wild environments (e.g., tropical forests). Such exposure data have fed debates on the role of so-called 'herd immunity' through natural exposure to emerging tropical, or perhaps rural, zoonoses. It is noteworthy that, comparable risks have been documented with viruses not typically associated with highly biodiverse geographies or the consumption of wild meat. For example, the 2012 outbreak of MERS-CoV in the Middle East and the Arab peninsula specifically, was shown to involve both local and international city dwellers with no previous exposure to the natural camelid host that continues to be in close contact with rural populations (Fig. 2) (32,33). The direct impact on individual health of zoonoses, and indirect impact on health-care systems as evidenced through the COVID-19 outbreak, underscore how increasingly urban population organisation even among developing nations amplifies the risk of onward human transmission, despite past exposure and indeed immunity in settings with blurred wild-human habitat barriers.
Figure 2.
The transmission model of coronaviruses.
5. Pathogenesis of coronavirus infection
The genome of coronaviruses contains genes for the 4 structural proteins: Envelope (E), membrane (M), nucleocapsid (N) and spike (S). Coronavirus virions are lipid bilayer-enveloped particles variable in size (80-160 nm), characterized by multiple 20-nm spike-like extensions on the surface in the form of 'corolla' or 'flower petals'. At the virion core, a nucleocapsid with icosahedral symmetry contains an electron-dense layer with a center that is clear. Its genomic nucleic acid consists of is single-stranded positive RNA which requires a negative RNA replication cycle intermediate that generates subgenomic protein coding RNAs as well as genomic RNA for virion assembly. The core also features accessory proteins that differ considerably between various types of coronaviruses.
The lipidic envelope features the trimerically-organised Type I transmembrane spike glycoproteins which consist of the ectodomain subunits S1 and S2 protruding externally, a transmembrane anchor, and a tail extending towards the viral core (34). Cell attachment involves the S1 subunit interacting with host cell surface receptors driving endocytosis and, after membrane fusion with the involvement of subunit S2 (35), release of the virion core into the cytosolic milieu. The glycoproteins of coronaviruses mediate attachment, fusion, and entry in the host cells, but different parts of those glycoproteins are involved in each of these processes. These class I viral membrane-fusion proteins undergo structural rearrangements that produce fusion between the viral and cellular membranes. Glycoprotein conformational changes and cathepsin L proteolysis within endosomes are also involved in the pathogenesis of coronavirus (Fig. 3). Thus, whilst cleavage of S protein is required to expose the hydrophobic fusion peptide, it seems that receptor interaction is required for the cleavage to occur (36). Historically, immunotherapies and many exploratory small molecule treatments have targeted such host-pathogen interactions to disrupt infection. However, experience across multiple virus strains indicates such monotherapy to be inefficient due to mutational escape.
Figure 3.
Pathogenesis of coronavirus infections.
Accessory proteins play a definitive role in infectivity, however, their functions are not yet fully understood. It is speculated that some proteins play important roles in countering the immune response; thus, viruses that lack these have a lower infectivity. For example, SARS-CoV-1 has two accessory proteins, 3a and 3b, that play potential roles in the virulence of this strain (37). On the other hand, sometimes a complete infectious particle can be assembled without spike proteins, indicating that accessory proteins can substitute these (38). Therefore, there is a potential risk that a vaccine targeting structural proteins alone, such as the spike protein, might be inefficient, driving evolutionary escape of the virus, either through target protein mutation, or by favouring virions utilizing other accessory proteins for attachment. A some-what more viral escape-proof approach involves targeting of the receptor-binding domain (RBD) which is a conserved domain of S protein (39). Notably, other respiratory viruses that efficiently cross species barriers (e.g., influenza) require close monitoring and annual adjustment of epitope targeting to minimize spread and impact.
The primary receptor used by coronaviruses to enter into target cells is the angiotensin-converting-enzyme II (ACE2) receptor (21,40), although some strains also use other alternative receptors, such as CD209L, for which they have a lower affinity (41). Whilst there is no evidence in pre- or post-publication literature currently that SARS-CoV-2 also utilizes CD209L for cell entry this potential attachment mechanism cannot be excluded until experimental data thereto are produced. Likewise, other, as yet unidentified receptors, facilitate coronavirus cellular entry in the absence of ACE2 in hepatocytes and some enterocytes (42). SARS-CoV-2 in particular, also appears to use the SARS-CoV receptor ACE2 to enter cells. Although the spike protein between SARS-CoV-1 and -2 vary in sequence, Xu et al (43) suggested that the Spike protein binding affinity for ACE2 is conserved as the 3D structure of the receptor binding domain is identical with that of SARS-CoV-1, which would translates to equal infectivity. The analysis of protein-protein interaction using bioinformatics showed that SARS nucleocapsidal proteins bind to human cyclophilin A (hCypA) and this binding was demonstrated by surface plasmon resonance (SPR) technology. The 3D modelling detected the probable binding sites and allowed deduction of important interaction between residue pairs (44). Wan and colleagues observed that several amino acids in the receptor-binding motif of SARS-CoV-2 allow binding to human ACE2, even though with suboptimal strength (45). A recent study demonstrated that a mutation in the Spike protein (N501T) of SARS-CoV-2 enhanced the affinity for ACE2 (45).
ACE2 is highly expressed in the respiratory tract, particularly in epithelial cells of the bronchi, alveoli (both type I and II cells), trachea and bronchial serous glands, as well as in macrophages and alveolar monocytes. Notably, the expression in the lung cells is much higher than in trachea (46). In line with the expression profile of ACE2, viral genome load has been consistently reported to be both more elevated in the lower than in the upper respiratory tract, with lower respiratory specimens being additionally less prone to false negative results (47,48). ACE2 is also diffusely located on other cells, such as mucosal cells of the intestines, endothelial cells of veins and arteries including heart cells, epithelial cells of the renal tubules, epithelial cells of the kidneys, immune cells and cerebral neuronal cells, which may also be susceptible to coronavirus infections. The observation of COVID-19 patient demise on account of severe heart failure brought about by SARS-CoV-2 infection and the higher risk among patients with previous cardiovascular and hypertensive disease has driven multiple hypotheses regarding potential direct mechanisms of viral action on the circulatory system. Thus, ACE2 expression can be increased as a result of using drugs such as ACE inhibitors and angiotensin II type-I receptor blockers (ARBs). Indeed, it was shown that expression of ACE2 is increased in diabetes patients (another high risk COVID-19 group) treated with ACE inhibitors and hypertension patients, treated with ARBs. ACE2 expression can also be increased by thiazolidinediones and ibuprofen. Therefore, in these categories of patients, the risk of infection with COVID-19 is proposed to be higher (49). Yet, whilst the NL63 coronavirus strain binds to the same ACE2 receptor as SARS-CoV-2, it produces only upper respiratory tract disease. This indicates that there are other unknown factors, apart from the presence of receptors that influence the susceptibility of cells to coronavirus infection. Insights into the differentiation factors influencing coronavirus strain-specific outcomes come from recent data on host co-factors mediating SARS-CoV-2 fusion. Thus, SARS-CoV-2 uses the serine protease TMPRSS2 for S protein priming; camostat mesylate, an inhibitor of TMPRSS2, blocked COVID-19 infection of lung cells, but inhibition was more substantial when the endosomal cysteine protease cathepsin B/L (CatB/L) was also inhibited e.g., with E-64d (40). These results point to a complex interplay of host factors in the endosome with the spike protein in mediating virion fusion. It is thus speculated that in infected cells furin-mediated precleavage at the S1/S2 site can promote TMPRSS2-dependent entry, as in the case of MERS-CoV (40). MERS-CoV however is an exception to the SARS coronavirus set, as it uses at hedipeptidyl peptidase 4 (DPP-4) surface antigen as a receptor, and not ACE2. The relative expression of these endosomal factors and similarly active proteins across cell types, as well as alternative receptors that mediate cellular attachment require urgent scrutiny to understand cell type tropism, and, more crucially, extra-pulmonary reservoirs and sites of replication. Some of these extrapulmonary reservoirs have been suggested by CT scans showing shadows and interstitial changes in tissues separate to the lungs (50), which require molecular and cellular studies to confirm direct involvement in virus replication as opposed to pathological findings secondary to SARS-CoV-2 infection.
After coronaviruses infect primary cells, the mature virions can be released and infect other target cells (51). Infective viral particles can be found in sweat, stool, urine and respiratory secretions from patients with other coronaviruses, with viable SARS-CoV-2 so far documented in respiratory droplets, saliva, mechanically generated aerosols, and faeces. After excretion environmental contamination can be substantial (fomites) presenting what is currently believed to be the primary mode of human-to-human transmission for up to 7 days after surface deposition of the virus. The development of atypical pneumonia with rapid respiratory deterioration and failure determined by coronavirus infection is associated with increased levels of activated pro-inflammatory chemokines and cytokines (52). Thus, it has been proposed that Vitamin D plays a role in the modulation of the immune response to infectious agents, based on laboratory findings and observational studies. However, randomized clinical trials have returned inconclusive, often controversial results. Therefore, it has been suggested that cholecalciferol at elevated doses might be useful for the prevention and therapy of infection with COVID-19 (53,54).
In the pathogeny of coronavirus infection, an important role is played by the amplitude of host immunity; for example, canonical interferon levels terminate protein synthesis or even induce cell death. However, the intensity of the immune response can vary, depending on other comorbidities of the patient, explaining the role of these in the evolution of the disease. Indeed, the majority of deaths have occurred among individuals with comorbidities (55). In a nationwide study from China, 2.1% of patients with COVID-19 had cancer, however no patient had liver transplantation or inflammatory bowel disease, 7.4% of the patients had diabetes and 15% hypertension (56). The death rate is expected to be higher in immunocompromised patients, however this is not established to date. On the other hand, it seems that the exacerbated immune reaction in COVID-19 infection is the one that leads to the greatest pulmonary and systemic damage.
MERS-CoV has a lower infectivity compared to SARS-CoV-2; thus, an intense and prolonged exposure is necessary in order for the virus to enter the lungs. MERS-CoV uses DPP-4 as a cell receptor (57). DPP-4 is expressed in a wide variety of epithelial cells with localization in the alveoli, kidneys, liver, small intestine, prostate and activated leukocytes that determine the higher tropism of MERS-CoV compared to other coronaviruses (58).
The genome of SARS-CoV-2 was sequenced during the early stages of the outbreak, enabling the ultrafast development of point-of-care tests based on RT-PCR for the new COVID-19 infection within 2 weeks of its discovery (45), in line with past predictions of rapid response (59) (https://doi.org/10.1039/C7SC03281A). To date, however, these are available only in kit format for benchtop real time PCR systems requiring substantial manual processing (5-8 h minimal data turnaround). Emergency use authorization (EUA) for the use of these/similar assays in a handful of the numerous regulator-approved point-of-care integrated extraction-amplification systems (e.g., BioFire, GeneXpert, etc.) was obtained only on March 22, 2020, an unacceptable 3 months into the outbreak. Consequently, despite the tremendous need for mass screening and isolation of infected cases, sample processing bottlenecks have forced most health authorities and governments worldwide to restrict testing only to clinically complex, hospitalized cases. Conversely, experience from countries with early outbreak experience such as Korea, Singapore, Hong Kong, Taiwan, Italy, and indeed China shows that mass testing, case isolation and contact tracing can reduce human transmission even to zero. Indeed, presently the risk to these countries appears restricted to new case introduction from abroad, justifying measures seeking to halt international travel.
Diagnostic process notwithstanding, a major drawback lies with the diagnostic sensitivity of preferred as opposed to available samples. Thus, data from the Chinese Centres for Disease Control and Prevention, as well as clinical centres at the epicenter of the outbreak, have reinforced World Health Organisation technical diagnostic guidelines (60) that require a lower respiratory sample as well as an upper respiratory sample to rule out SARS-CoV-19 as the causal agent of viral pneumonia and COVID-19-like disease. The simplest upper respiratory sample is presented in the form of an oral and/or nasal swab (or combination of both, given the handful of reported nasal swab negatives in the presence of oral swab positives). Lower respiratory specimens are difficult to obtain as they require either intubated patients (tracheal aspirates), or anesthesia (bronchoalveolar lavage, bronchial brushings). Notwithstanding risk of injury to the patient, these invasive procedures create infectious aerosol generation risks and are contraindicated. The only suitable surrogate sample is lung sputum, but only if this is naturally available, to minimize aerosol generation risk. Unfortunately, the Chinese experience has been that <30% of COVID-19 suspected cases generate sputum, thereby restricting testing to oral or nasal swabs. These, however, have a reported false negative rate of between 10-37% (47,48), with oral swab false negative rates on account of low analytical sensitivity reaching up to 68%. These statistics point underscore the impact of sampling process variability beyond anatomical differences - one healthcare professionals' swabbing technique may differ vastly to another- and emphasize the need for standardizing/automating the sampling process to increase diagnostic test reliability, even for respiratory samples. A significant additional complicating factor is the limited understanding of sample viral load across the exposure-symptomatic-convalescence continuum, onset of shedding, and disparity between genome copy detected by RT-PCR vs infectious virion copy number, which collectively amplify the well-established issues around operator error in sampling methods. Of interest, early data suggest viral shed-ding may indeed start as early as one week ahead of symptoms and transmission may peak up to 3 days before symptoms (61). The data, if accepted and validated in separate studies, will compound the impact of transmission risk attributed to asymptomatic or subclinical individuals and will emphasize the need for more aggressive contact tracing. Testing even onto random mass population screening will be needed to arrest transmission, as anecdotal evidence suggests from a single town in Italy where the combination of lockdown, contact tracing and testing eliminated new cases whilst transmission remained rampant elsewhere in the country (62).
On the other hand, there is particular importance attributed by a number of Western governments to serological assays that monitor the production of antibody responses to pathogens. At first glance these tests are exceedingly appealing due to their lower cost (50-100×) and time-to-results (30-48×) as compared to RT-qPCR in a diagnostic lab, or indeed when using automated point of care RT-qPCR systems (20× lower cost and 6-12× faster). In addition, antibody tests can offer a clear answer as to the spread of exposure that may involve asymptomatic or low-grade respiratory disease, supporting so called 'herd immunity'. However, evidence from multiple Asian countries and Italy show transmission containment was achieved and can be achieved using RT-qPCR which documents infectious cases: antibody responses do not initiate until at least a week after the onset of symptoms, meaning that the deployment of antibody tests for mass screening will have an enhanced risk of false negatives as compared to oronasal swab RT-qPCR by missing pre-symptomatic, asymptomatic, and newly symptomatic cases. Whilst the research and academic value in understanding asymptomatic spread is undisputed, the magnitude of the socioeconomic damage by COVID-19 in redirecting resources away from confirmation of active infection in mildly symptomatic cases and asymptomatic contacts onto exposure numbers, is likely to extend transmission long beyond the period of a few weeks documented in China and Korea into months, extending the impact onto healthcare systems. Furthermore, such an extension of spread risks the development of cycles of infection and socioeconomic damage with the re-introduction of new index cases within country regions and into countries free of the disease. The increasing risk of virus evolution, and its establishment as a seasonal, endemic respiratory disease agent, crucially, does not take into account how immunity to other coronaviruses appears to be short-lived: we simply do not know yet if COVID-19 convalescence will protect from re-infection, and if it does, for how long.
6. Clinical manifestations of coronavirus-associated diseases
The predominant symptoms of SARS-CoV-1 are respiratory in nature, associated with fever and diarrhea. Clinical manifestations among patients are diverse, from mild-moderate to severe-life threatening symptoms. The virus has a mean incubation period of 4.6 days (63), however in the majority of cases, symptoms appear after 10 days (64). In severe cases, the median time from the appearance of symptoms until artificial ventilation is 11 days, and 23.7 days until the patient's death (65). The clinical manifestations are similar to flu-like symptoms, such as fever, a dry cough, myalgia, dyspnea, headache, sore throat, sputum production, rhinorrhea (66,67). In 40-70% of cases, watery diarrhea appears within 1 week after the first manifestations (42). In the elderly, the symptoms can also be associated with confusion, a poor appetite and a decrease in general well-being. In is noteworthy that the symptomatology in children below 12 years is mild; no mortality has been registered among children and teenagers. Asymptomatic cases are reported to be rare (63,68). Increased levels of C-reactive protein, lymphopenia, thrombocytopenia, and increased levels of lactate dehydrogenase have been observed in laboratory tests. The infection manifests in two different stages, with the first-week characterized by flu-like symptoms that improve even if the viral loads persist, and a recurrent period during the second week in which respiratory failure can appear and more than 20% of patients may require mechanic ventilation (69). The respiratory system is the main affected area in the human body where the infection determines the appearance of diffuse alveolar damage, squamous metaplasia, giant-cell infiltrates and increased macrophage levels in the interstitium and the alveoli (46).
Unlike SARS-CoV-1, Middle East Respiratory Syndrome coronavirus (MERS-CoV) has a wide variety of clinical manifestations from asymptomatic to severe respiratory symptoms. The mean incubation period is 5-6 days (70-73). In severe cases, death can occur within 11-13 days after the onset of the disease (70,73). The clinical manifestations are similar to flu-like symptoms, such as general myalgia, fever, chills, malaise associated with confusion, pulmonary symptoms, such as cough, shortness of breath, dyspnea and pneumonia. Extra-pulmonary clinical manifestations include abdominal disorders, nausea, diarrhea, vomiting, acute renal failure and neurological complications (74). The severity of the disease is associated with an increasing age, and with individuals with comorbidities, such as diabetes mellitus, renal and pulmonary diseases. Death can occur after the development of acute respiratory distress syndrome (ARDS), multiorgan failure or septic shock (7). The neurological complications of the infection with MERS-CoV are associated with the in vitro ability of the virus to invade the central nervous system (75), although it was not detected in the CSF. Among the neurological manifestations, confusion has been reported in 25.7% and seizures in 8.6% of cases (76). Severe neurological complications are encephalitis, stroke, polyneuropathy, acute disseminated encephalomyelitis, Guillain Barré syndrome and Bickerstaff's encephalitis. Usually, the neurological complications appear at a delayed time point from the respiratory symptoms, namely 2-3 weeks from the onset of the clinical manifestation of the disease and can be underdiagnosed (74,77-79). Children are rarely affected (74). In laboratory tests, thrombocytopenia, lymphopenia, neutrophilia, leucocytosis, reduced renal functions and increased inflammatory markers have been observed. Similar to SARS-CoV-1, the pathogenesis has not yet been fully elucidated. Viral-mediated lung damage is characterized by diffuse alveolar damage. The virus has been determined in multinucleated epithelial cells, pneumocytes and bronchial submucosal glands. Apart from the respiratory system, the virus has also been identified in the epithelial cells of the proximal renal tubules (58). Acute tubular sclerosis and tubulointerstitial nephritis have been observed in renal biopsies collected from persons infected with MERS-CoV (80).
The latest high clinical burden coronavirus, SARS-CoV-2, presents a wide variety of clinical manifestations which are not yet fully characterized (81,82). The virus has a mean incubation period of 3-7 days, and no more than 14 days (30) - although anecdotal reports likely related to poor contact tracing have suggested potentially up to 24 days of incubation. Outside Hubei province, the median incubation period has been estimated to be 5.1 days (95% CI: 4.5 to 5.8), with 97.% of infected subjects developing symptoms within 11.5 days of infection (83). In a retrospective study on 99 patients that have confirmed COVID-19 pneumonia treated in an infec-tious diseases hospital in Wuhan, China, it was shown that the main clinical manifestations were flu-like symptoms, such as fever and cough (83 and 81% of the cases), shortness of breath (31% of cases), muscle ache (11% of cases), and less frequently confusion (9%), headache (8%), sore throat, rhinorrhea, and chest pain. In less than 2% of patients, extra-pulmonary symptoms and diarrhea, nausea and vomiting were also observed. As complications, ARDS was reported in 17% of cases followed by acute respiratory injury (8%), septic shock (4%), acute renal injury (3%), and ventilator-associated pneumonia (1%). Death occurred in 11% of participants in the study determined by multiple organ failure. The main finding in laboratory tests was lymphopenia. The severity of the disease was associated with co-infections, smoking history, hypertension and age (13). Another retrospective study on 137 patients infected with COVID-19 revealed the same pattern of clinical manifestations with fever, cough and muscle pain, and less frequently diarrhea, headache and even heart palpitations in some cases (50). Lymphopenia was observed in 72.3% of cases associated with normal or decreased white blood cell counts (50). The lung is the main organ affected. In the imaging examination by computer tomography or X-ray, lesions were identified in multiple lung lobes presented as patchy/punctate ground-glass opacities (GGO), patchy consolidation, irregular solid nodules and fibrous stripes (50,84-86). Studies that have investigated the pulmonary pattern of COVID-19 pneumonia have demonstrated that the disease progresses rapidly, and CT re-examination after 3-14 days has revealed significant changes in lung structure. The single GGO observed in the early stages can increase significantly in short-term re-examination. The same has also occurred with fibrous stripes observed in the early stages that consolidate in short-term re-examination. In some cases, the irregular solid nodules observed in the early stages increased and merged in short-term re-examination. Short-term repeated imaging scans should be carried out in patients with COVID-19 pneumonia for monitoring the evolution and for specific management of the patient as the disease can rapidly progress or indeed resolve (86,87). The peak of lung abnormalities was reached on day 10 following the onset of symptoms, followed by a decrease in symptomatology (87).
It was documented that in one third of the severely ill patients, death occurs because the COVID-19 coronavirus produce acute myocardial injury and damage to the cardiovas-cular system in general, that further degrade the state of these already very ill patients (88).
7. Treatment of coronavirus-associated diseases
The treatment for all types of coronaviruses is mainly supportive, as no specific treatment has been discovered to date. For the treatment of SARS-CoV-1, apart from supportive measures, several therapeutic schemes have been implemented, with varying success rates such as antiviral therapy with riba-virin (89), protease inhibitors - lopinavir and ritonavir, alone or associated with ribavirin (67,90), interferon-alfacon-1 (91), systemic corticosteroids (89,92), and convalescent plasma for passive immunotherapy (93). Apart from convalescent plasma, none of these treatments have exhibited significant benefits compared to the side-effects produced.
For MERS-CoV infections, taking as an example the early SARS-CoV-1 epidemic, supportive care was primarily used, and in some instances broad-spectrum antibacterials, antivirals such as ribavirin alone or in combination with interferon-2α2b (94,95) and antifungals have been used to prevent the co-infection with other opportunistic pathogens (96,97). Mycophenolic acid is another drug that exhibited efficacy in monotherapy on a small number of patients; however further studies are required to confirm these effects (98,99).
In the case of the new COVID-19-associated pneumonia, treatment also focuses on supportive care measures and symptomatic treatment. Following the example of SARS-CoV-1 treatment, the administration of systemic corticosteroids was attempted, although this did not exhibit any benefits. In the case of patients with severe evolution, immunoglobulin G was administered (50). The application of early respiratory support improved the prognosis and the recovery of the patients (50). In a 54-year-old Korean man infected with SARS-CoV-2, the administration of the combination lopinavir/ritonavir from day 10 of illness determined the decrease in viral load till no detectable levels (100). The question of whether the decrease in viral load was associated with the natural course of the healing process or with antiviral treatment or both, is being currently investigated in order to confirm the efficacy of antiviral treatment for COVID-19. The efficacy of remdesivir (Gilead Sciences), a nucleotide analog, in inhibiting in vitro and in vivo SARS-CoV-1, MERS-CoV and bat CoV strains that are capable to replicate in human airway epithelial cells render it a good choice for testing on newly affected COVID-19 patients (85,101-103). Taking into consideration that COVID-19 binds to human ACE2 in order to penetrate human cells and to induce severe pneumonia, the renin-angiotensin system, ACEI (angiotensin-converting enzyme inhibitors) and AT1R (angiotensin II type-I receptor) inhibitors have been suggested to modify individual susceptibility to COVID-19 by influencing SARS-Cov-2 virulence. However, further studies are needed to confirm the mechanisms involved (including the role of high ACE2 expression) and to consider possible changes in administration and dosage of antihypertensive drugs (46,49,104). Thus far, the Professional Association for Hypertension Therapy, USA, has warned against changes in hypertensive drugs following concerns about a possible increased susceptibility to COVID-19 (https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/). Interest has arisen also on the 'old', off-patent antimalarial drug chloroquine and its less toxic hydroxychloroquine analog, both of which are quinine derivatives (cinchoca tree bark, South America). Preliminary support from observations of potential inhibitory activity against SARS-CoV-2 (105,106), clearly needs to be confirmed through randomized controlled trials. There are also ongoing trials using monoclonal antibodies. One of them is tocilizumab, an anti-IL6 antibody, currently in phase III clinical trials, with commercial name Actemra produced by Roche (107). Sarilumab, a drug used in rheumatoid arthritis is about to enter clinical trials for COVID-19 infection, according to Regeneron Pharmaceuticals and Sanofi, respectively (108).
In order to facilitate COVID-19 virus replication, certain in vivo conditions, such as the pH value, the temperature, humidity, and the sufficiency of oxygen supply should be ensured. Consequently, in order to hinder the reproduction procedures of the virus, it would be expedient to disturb the environment of the virus, by interfering with the temperature or the pH values or both. SARS-CoV-2 has been speculated to be a seasonal virus, in which case a hindrance of the rapid spread that characterizes it at ambient temperatures over 25°-30° Celsius would be observed. Unfortunately, the sup-tropical/arid environments favored by SARS-CoV-1 and MERS-CoV, as well as the comparatively higher temperatures in Israel, Egypt, the Arab peninsula and now sub-Saharan Africa compared to Hubei province indicate that temperature probably does not affect COVID-19 replication. Likewise, the ambient humidity levels thought to impact aerosol and droplet suspension time/travel distance, and by extension virus transmission, do not seem to affect onward transmission chains across geographical regions. Alteration of the pH value inside the human body within tolerable levels however is achievable; such an intervention would not only suppress replication of the virus, but also may have - although possibly weak - virucidal effect. A pH value change with no negative impact on the health of the patient could be achieved by administering a drug that would lead to urine acidosis and endocellular alkalosis. This drug could be a simple small molecule with hydrophobic weak base properties, which would accumulate in lysosomes after having crossed both plasma and lysosomal membranes by diffusion. Perhaps such a medicine may express both a therapeutic and a prophylactic effect by increasing the environmental barriers to effective virus attachment in early endosomal pathways, and preventing spike proteolysis required for capsid release and cell membrane fusion within lysosomes, in line with cathepsin protease-targeted therapies. Given the success of combination therapy strategies in other antiviral diseases centered on virus fusion and/or replication, promoting endocellular alkalosis during antiviral drug therapy (e.g., replication inhibitors) could amplify antiviral effect and thereby contribute to the reduction of the treatment period (109-113).
8. Prevention strategies
Although since 2004, the World Health Organization issued guidelines for the prevention of re-emergence of new coronavirus virulent strains (114), implementation has evidently been inadequate. Key recommendations include: the availability of the early detection of infected individuals to prevent the spread of emergent and re-emergent infection, particularly to prevent international spread; the development of contingency plans by each country for the management and detection of coronavirus, individualized by the risks that exist locally; preparing global and assisting national risk assessments; updating new discoveries; assisting countries in their efforts to improve protection against infections; assessing the availability of all necessary resources in the case of an outbreak; and, supporting the international scientific collaboration for SARS study. Unfortunately, all of these strategies could not prevent the current outbreak, and after 17 years, the medical, scientific and governmental structures have demonstrated that they are not adequately prepared to effectively contain highly pathogenic viruses. The main measures that should be taken for preventing the dissemination of pathogens in the general population are associated with infection control measures both in healthcare facilities and in the community. Contact tracing and quarantine or the isolation of those suspected of infection along with the education of the population has proven essential in preventing community transmission of the viruses both in developing nations (West African and Democratic Republic of Congo Ebola virus outbreaks), developed nations (e.g., Hong Kong, South Korea SARS and MERS outbreaks), and now practically all G20 nations. The lack of local implementation of what are demonstrably effective measures, as learned since the SARS-CoV-1 outbreak due to scientific chauvinism over healthcare standards between East and West, population anthropology between Africa and Europe in terms of communication strategy and behaviour, or indeed local vs international experience between e.g., the UK and Italy with emerging threats is lamentable. Going forward, the identification of natural reservoirs and intermediate hosts for existing and future risks as identifiable through random sampling and monitoring (21) will become essential in preventing future zoonotic virus outbreaks. Unfortunately, these alarms were raised repeatedly over the past two decades with minimal heed: perhaps the damage brought by COVID-19 onto global economy and healthcare systems will transform the field of tropical emergent disease.
A good example of positive action are the actions of currently more than 40 teams developing vaccines against SARS-CoV-2 infection (115); interest is largely spurred by the unprecedented success and rapid development of Ebolavirus vaccines during the West African outbreak. COVID-19 vaccines can be based on the viral RNA or derivatives: Examples include Innovio and Moderna in USA, as well as CureVac (Germany), and another team at Imperial College in London. RNA and DNA vaccines have the advantage of being quick to develop and high likelihood of safety, though no RNA vaccine or therapeutic currently enjoys regulatory approval. The Institute Pasteur in France is working on a traditional COVID-19 vaccine based on a SARS vaccine. CanSino Biologics developed a recombinant coronavirus vaccine incorporating adenovirus type 5 vector (Ad5). To date clinical trials have been initiated by Moderna Therapeutics and Cansino Biologics (115) with further trials shortly anticipated in the UK and other European countries.
A sobering note, however, is that despite the ample amounts of research performed on coronaviruses over the past 20 years, particularly following the SARS-CoV-1 outbreak, no vaccine is unfortunately yet available for either SARS-CoV-1 or MERS, although some potential vaccines have reached as far as phase I clinical trials (116-118). Some of the strategies used for vaccines are eliciting neutralizing-antibody for S proteins and T-cell responses (119) which seem to be jointly required for convalescence, rather than simply neutralizing antibody titers. The quest for protective immunity to coronavirus is but nascent, and virology offers a plethora of high socioeconomic impact, high prevalence RNA viruses for whom decades of vaccine research have yet to deliver success.
9. Conclusions
The Guangdong region of China was considered a high-risk area for a coronavirus outbreak after the SARS-CoV-1 epidemic in 2002. Nevertheless, although a large amount of research and efforts have been made worldwide to prevent further outbreaks, a coronavirus outbreak occurred again in 2019. Unfortunately, healthcare systems locally, nationally, and now internationally have again been overcome, as with the other two major coronavirus outbreaks. The evidence from COVID-19 is clear that highly aggressive measures are necessary to break the transmission chain in the regions at risk - practically most countries globally at the time of authoring. Unfortunately, there is no specific prevention strategy against the new coronavirus, and its pandemic status restrict the global community to two options: effective measures for the immediate prevention of respiratory infections with predictable and measurable consequences, or ambitions of 'herd' or vaccine-derived immunity at a non-specific time in the future. We can only hope that after this third significant outbreak, sufficient experience has been gained; a joint effort of the worldwide health authorities will be needed in extinguishing the current epidemic. It can only be trusted that this experience will re-emphasize the need for prevention and readiness and for further studies to limit the transmission of animal viruses to humans and mitigation should it arise again in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization, AOD, DC, DT, MV, MA, JMD, MG, VAT, GGO and DAS; validation, research, resources, data reviewing, and writing AOD, DA, OZ, OC, DT, ND, SAM, MG, VAT, GGO; review and editing, AOD, AT, DAS, MV, SAM, JMD, MA, ND and DC. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Groot RJ, Baker SC, Baric R, et al. Family - Coronaviridae. In: Lefkowitz EJ, Adams MJ, Carstens EB, editors. Virus Taxonomy Ninth Report of the International Committee on Taxonomy of Viruses King AMQ. Elsevier; 2011. pp. 806–828. [Google Scholar]
- 2.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 Mar 17; doi: 10.1056/NEJMc2004973. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geller C, Varbanov M, Duval RE. Human coronaviruses: Insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony SJ, Johnson CK, Greig DJ, Kramer S, Che X, Wells H, Hicks AL, Joly DO, Wolfe ND, Daszak P, et al. PREDICT Consortium: Global patterns in coronavirus diversity. Virus Evol. 2017;3:vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goumenou M, Spandidos DA, Tsatsakis A. [Editorial] Possibility of transmission through dogs being a contributing factor to the extreme Covid 19 outbreak in North Italy. Mol Med Rep. doi: 10.3892/mmr.2020.11037. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/. Accesed July 24, 2015.
- 7.World Health Organization Middle East respiratory syndrome coronavirus (MERS-CoV) MERS Monthly Summary. 2013 https://www.who.int/emergencies/mers-cov/en/. Accessed July 9, 2013.
- 8.World Health Organization Coronavir us disease (COVID-2019) situation reports. https://www.who.int/emer-gencies/diseases/novel-coronavirus-2019/situation-reports.
- 9.Movert E, Wu Y, Lambeau G, Kahn F, Touqui L, Areschoug T. Using Patient Pathways to Accelerate the Drive to Ending Tuberculosis. J Infect Dis. 2013;208:2025–2035. doi: 10.1093/infdis/jit359. [DOI] [PubMed] [Google Scholar]
- 10.Chan-Yeung M, Xu RH. SARS: Epidemiology. Respirology. 2003;8(Suppl):S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu RH, He JF, Evans MR, Peng GW, Field HE, Yu DW, Lee CK, Luo HM, Lin WS, Lin P, et al. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mobaraki K, Ahmadzadeh J. Current epidemiological status of Middle East respiratory syndrome coronavirus in the world from 1.1.2017 to 17.1.2018: A cross-sectional study. BMC Infect Dis. 2019;19:351. doi: 10.1186/s12879-019-3987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020 Mar 17; doi: 10.1001/jama.2020.4344. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Novel coronavirus (COVID-19) situation. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd.
- 16.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020 Mar 13; doi: 10.1016/S0140-6736(20)30627-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv L, Li G, Chen J, Liang X, Li Y. Comparative genomic analysis revealed specific mutation pattern between human coronavirus SARS-CoV-2 and Bat-SARSr-CoV RaTG13. bioRxiv. doi: 10.3389/fmicb.2020.584717. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradhan P, Pandey AK, Mishra A, et al. Uncanny similarity of unique inserts in the 2019-nCoV spike protein to HIV-1 gp120 and Gag. bioRxiv. In Press. [Google Scholar]
- 19.Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coro-navirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020 Feb 11; doi: 10.1097/CM9.0000000000000722. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Zhao K, Shi ZL, Zhou P. Bat Coronaviruses in China. Viruses. 2019;11:210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroy EM, Rouquet P, Formenty P, et al. Multiple Ebola Virus Transmission Events and Rapid Decline of Central African Wildlife. Science. 2004;303:387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Wong SK, Li F, Kuhn JH, Huang IC, Choe H, Farzan M. Animal origins of the severe acute respiratory syndrome coronavirus: Insight from ACE2-S-protein interactions. J Virol. 2006;80:4211–4219. doi: 10.1128/JVI.80.9.4211-4219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 25.Lam TTY, Shum MHH, Zhu HC, et al. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. bioRxiv. In Press. [Google Scholar]
- 26.Liu P, Jiang JZ, Hua Y, et al. Are pangolins the intermediate host of the 2019 novel coronavirus (2019-nCoV)? bioRxiv. In Press. [Google Scholar]
- 27.Zhang T, Wu Q, Zhang Z. Pangolin homology associated with 2019-nCoV. bioRxiv. In Press. [Google Scholar]
- 28.Wang LF, Cowled C, editors. Bats and Viruses: A New Frontier of Emerging Infectious Diseases. John Wiley & Sons Inc; 2015. [DOI] [Google Scholar]
- 29.Guan Y, Zheng BJ, He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smiley Evans T, Tutaryebwa L, Gilardi KV, Barry PA, Marzi A, Eberhardt M, Ssebide B, Cranfield MR, Mugisha O, Mugisha E, et al. Suspected Exposure to Filoviruses Among People Contacting Wildlife in Southwestern Uganda. J Infect Dis. 2018;218(Suppl 5):S277–S286. doi: 10.1093/infdis/jiy251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 33.Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV - a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J Virol. 2012;86:2856–2858. doi: 10.1128/JVI.06882-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayanan K, Huang C, Makino S. SARS coronavirus accessory proteins. Virus Res. 2008;133:113–121. doi: 10.1016/j.virusres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoeman D, Fielding BC. Coronavirus envelope protein: Current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Zhou Q, Li Y, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor Article SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffers SA, Tusell SM, Gillim-Ross L, et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung WK, To KF, Chan PK, et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo C, Luo H, Zheng S, Gui C, Yue L, Yu C, Sun T, He P, Chen J, Shen J, et al. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem Biophys Res Commun. 2004;321:557–565. doi: 10.1016/j.bbrc.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fred Hutchinson Cancer Research Center INTERIM guidelines for COVID-19 management in hematopoietic cell transplant and cellular therapy patients, Version 1, 2020. https://www.fredhutch.org/content/dam/www/coronavirus/COVID-19_Interim_Patient_Guidelines_3_9_20.pdf. Accessed March 8 2020.
- 48.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 Mar 11; doi: 10.1001/jama.2020.3786. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang L, Karakiulakis G, Roth M. Are patients with hyper-tension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;2600:30116. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kui L, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 Feb 7; doi: 10.1097/CM9.0000000000000744. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qinfen Z, Jinming C, Xiaojun H, et al. The life cycle of SARS coronavirus in Vero E6 cells. J Med Virol. 2004;73:332-7–332-7. doi: 10.1002/jmv.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 54.Gruber-Bzura BM. Vitamin D and Influenza-Prevention or Therapy? Int J Mol Sci. 2018;19:2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau EH, Hsiung CA, Cowling BJ, Chen CH, Ho LM, Tsang T, Chang CW, Donnelly CA, Leung GM. A comparative epidemiologic analysis of SARS in Hong Kong, Beijing and Taiwan. BMC Infect Dis. 2010;10:50. doi: 10.1186/1471-2334-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Feb 28; doi: 10.1056/NEJMoa2002032. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng DL, Al Hosani F, Keating MK, et al. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates. Am J Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsaad KO, Hajeer AH, Al Balwi M, et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah K, Bentley E, Tyler A, Richards KSR, Wright E, Easterbrook L, Lee D, Cleaver C, Usher L, Burton JE, et al. Field-deployable, quantitative, rapid identification of active Ebola virus infection in unprocessed blood. Chem Sci (Camb) 2017;8:7780–7797. doi: 10.1039/C7SC03281A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization Country & Technical Guidance - Coronavirus disease (COVID-19) https://www.who.int/emer-gencies/diseases/novel-coronavirus-2019/technical-guidance.
- 61.Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA. Serial Interval of COVID-19 Among Publicly Reported Confirmed Cases. Emerg Infect Dis. 2020 Mar 19; doi: 10.3201/eid2606.200357. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crisanti A, Cassone A. In one Italian town, we showed mass testing could eradicate the coronavirus Opinion. The Guardian; 2020. https://www.theguardian.com/comment-isfree/2020/mar/20/eradicated-coronavirus-mass-testing-covid-19-italy-vo. Accessed March 20 2020. [Google Scholar]
- 63.Chiu WK, Cheung PC, Ng KL, et al. Severe acute respiratory syndrome in children: experience in a regional hospital in Hong Kong. Pediatr Crit Care Med. 2003;4:279–283. doi: 10.1097/01.PCC.0000077079.42302.81. [DOI] [PubMed] [Google Scholar]
- 64.Donnelly CA, Ghani AC, Leung GM, et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung GM, Hedley AJ, Ho LM, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141:662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 66.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 67.Chan JW, Ng CK, Chan YH, et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hon KL, Leung CW, Cheng WT, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hui DS, Sung JJ. Severe acute respiratory syndrome. Chest. 2003;124:12–15. doi: 10.1378/chest.124.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Assiri A, McGeer A, Perl TM, et al. KSA MERS-CoV Investigation Team, 2013b. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 73.Ki M. 2015 MERS Outbreak in Korea: Hospital-To-Hospital Transmission. Epidemiol Heal. 2015;37:e2015033. doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arabi YM, Balkhy HH, Hayden FG, et al. Middle East Respiratory Syndrome. N Engl J Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Hameed FM. Spontaneous intracranial hemorrhage in a patient with Middle East respiratory syndrome corona virus. Saudi Med J. 2017;38:196–200. doi: 10.15537/smj.2017.2.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Algahtani H, Subahi A, Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: A report of two cases and review of the literature. Case Rep Neurol Med. 2016;2016:3502683. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JE, Heo JH, Kim HO, et al. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cha RH, Yang SH, Moon KC, et al. A Case Report of a Middle East Respiratory Syndrome Survivor with Kidney Biopsy Results. J Korean Med Sci. 2016;31:635–640. doi: 10.3346/jkms.2016.31.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hui DS, Azhar I, Madani E, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020 Mar 10; doi: 10.7326/M20-0504. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin X, Gong Z, Xiao Z, Xiong J, Fan B, Liu J. Novel Coronavirus Pneumonia Outbreak in 2019: Computed Tomographic Findings in Two Cases. Korean J Radiol. 2020;21:365–368. doi: 10.3348/kjr.2020.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warren TK, Wells J, Panchal RG, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020 Feb 13; doi: 10.1007/s00330-020-06731-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology. 2020 Feb 13; doi: 10.1148/radiol.2020200370. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 Mar 5; doi: 10.1038/s41569-020-0360-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sung JJ, Wu A, Joynt GM, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 92.Tsui PT, Kwok ML, Yuen H, Lai ST. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Widagdo W, Okba NMA, Stalin Raj V, Haagmans BL. MERS-coronavirus: From discovery to intervention. One Heal. 2016;3:11–16. doi: 10.1016/j.onehlt.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calina D, Rosu L, Rosu AF, et al. Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleuresy. Farmacia. 2016;64:946-52–946-52. [Google Scholar]
- 97.Cowling BJ, Lam TTY, Yen HL, Poon LLM, Peiris M. Evidence-Based Options for Controlling Respiratory Virus Transmission. Emerg Infect Dis. 2017;23:171231. doi: 10.3201/eid2311.171231. [DOI] [Google Scholar]
- 98.Zhao Z, Zhang F, Xu M, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- 99.Al-Qahtani AA, Lyroni K, Aznaourova M, et al. Middle east respiratory syndrome corona virus spike glycoprotein suppresses macrophage responses via DPP4-mediated induction of IRAK-M and PPARγ. Oncotarget. 2017;8:9053–9066. doi: 10.18632/oncotarget.14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ. Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: The Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9:e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho A, Saunders OL, Butler T, et al. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg Med Chem Lett. 2012;22:2705-7–2705-7. doi: 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun ML, Yang JM, Sun YP, Su GH. Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:14. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. [DOI] [PubMed] [Google Scholar]
- 105.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 Mar 4; doi: 10.1016/j.ijantimicag.2020.105932. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 asso-ciated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 107.Clinical Trials Arena ROCHE to start Phase III trial of Actemra in Covid-19 patients. https://www.clinicaltrialsarena.com/news/roche-actemra-covid-19-trial/. Accessed March 19 2020.
- 108.Healio Sarilumab enters clinical trial for COVID-19, spotlighting 'key role' for IL-6. https://www.healio.com/rheu-matology/rheumatoid-arthritis/news/online/%7B1957db6e-f7a2-4e5d-939e-d4b5964b2dd3%7D/sarilumab-enters-clinical-trial-for-covid-19-spotlighting-key-role-for-il-6. Accessed March 19, 2020.
- 109.Zhitomirsky B, Assaraf YG. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat. 2016;24:23–33. doi: 10.1016/j.drup.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Kazmi F, Hensley T, Pope C, Funk RS, Loewen GJ, Buckley DB, Parkinson A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells) Drug Metab Dispos. 2013;41:897–905. doi: 10.1124/dmd.112.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adar Y, Stark M, Bram EE, Nowak-Sliwinska P, van den Bergh H, Szewczyk G, Sarna T, Skladanowski A, Griffioen AW, Assaraf YG. Imidazoacridinone-dependent lysosomal photodestruction: A pharmacological Trojan horse approach to eradicate multidrug-resistant cancers. Cell Death Dis. 2012;3:e293–e293. doi: 10.1038/cddis.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ashfaq UA, Javed T, Rehman S, Nawaz Z, Riazuddin S. Lysosomotropic agents as HCV entry inhibitors. Virol J. 2011;8:163. doi: 10.1186/1743-422X-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: Analysis and therapeutic implications. J Pharm Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 114.WHO SARS risk assessment and preparedness framework. 2004 https://www.who.int/csr/resources/publications/CDS_CSR_ARO_2004_2.pdf.
- 115.Craven J. COVID-19 Vaccine Tracker. Regulatory Affairs Professionals Society (RAPS); 2020. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker. Accessed March 21, 2020. [Google Scholar]
- 116.Martin JE, Louder MK, Holman LA, et al. VRC 301 Study Team. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin JT, Zhang JS, Su N, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12:1107–1113. [PubMed] [Google Scholar]
- 118.Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis. 2018;18:410–418. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Janice Oh HL, Ken-En Gan S, Bertoletti A, Tan YJ. Understanding the T cell immune response in SARS coronavirus infection. Emerg Microbes Infect. 2012;1:e23. doi: 10.1038/emi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.