Summary
Objective To investigate the relationship between avascular osteonecrosis (AVN) and corticosteroid treatment given to patients with severe acute respiratory syndrome (SARS).
Methods Longitudinal study of 71 former SARS patients (mainly health care workers) who had been treated with corticosteroids, with an observation time of 36 months. Magnetic resonance images (MRI) and X‐rays of hips, knees, shoulders, ankles and wrists were taken as part of the post‐SARS follow‐up assessments.
Results Thirty‐nine per cent developed AVN of the hips within 3–4 months after starting treatment. Two more cases of hip necrosis were seen after 1 year and another 11 cases of AVN were diagnosed after 3 years, one with hip necrosis and 10 with necrosis in other joints. In total, 58% of the cohort had developed AVN after 3 years of observation. The sole factor explaining AVN in the hip was the total dose of corticosteroids received.
Conclusion The use of corticosteroids in SARS has been debated; opinions conflict about whether the immediate benefits in terms of saving lives compensate for the adverse effects, including AVN.
Keywords: SARS, avascular osteonecrosis, corticosteroids, cohort study
Introduction
During the SARS epidemic, many patients received high‐dose corticosteroid therapy to counter the often very severe symptoms of acute respiratory distress. After the epidemic, there have been several reports in both Chinese and international literature on the occurrence of avascular osteonecrosis (AVN) (2004, 2006; Hong & Du 2004; Griffith et al. 2005; Li et al. 2005). AVN is due to impaired circulation of blood to an area of bone, causing subsequent necrosis (Assouline‐Dayan et al. 2002). The condition may be idiopathic or associated with various conditions such as trauma, corticosteroid usage, alcoholism, infections, blood disorders and autoimmune diseases.
High doses of corticosteroids given over a short period of time to patients who are not predisposed to AVN generally do not confer a high risk of AVN (Wing et al. 1998). But high doses of corticosteroids given over a longer period of time in patients who are predisposed to AVN (such as patients with systemic lupus erythematosus) are strongly correlated with AVN (Felson & Anderson 1987). Questions arose about risk factors for AVN observed in SARS patients. Was it solely associated with the amount and duration of corticosteroid treatment, or were there other predisposing factors?
In this paper, we describe a cohort of patients from Beijing with a follow‐up time of 3 years and investigate the relationship between osteonecrosis incidence, IgG antibody titre at enrolment, and treatment dose of corticosteroids.
Patients and methods
Study population
Patients were diagnosed at the CPAF hospital in Beijing from March 2003 to April 2003 with clinical presentations fulfilling the WHO and National Department of Health definition of probable SARS (WHO 2003; CDC 2004). The diagnosis of SARS was subsequently confirmed serologically by enzyme‐linked immunosorbent assay (ELISA) and neutralization assay for specific antibody against the SARS coronavirus (SARS‐CoV) (Liu et al. 2006). All patients were employees of the hospital and infected by two known sources: inpatients in the hospital at the time or (colleague) medical personnel. Because of the size of the outbreak, many of the infected employees were referred to three other hospitals in the city for treatment and returned to the CPAF hospital around the end of June 2003 for further recovery. At this time, the first case of AVN was found and it was decided to examine the whole group of patients on the presence of AVN, 71 individuals in total. All patients were followed for 3 years up to June 2006. Follow‐up visits were every 6 months.
Treatment of SARS
All SARS patients were given treatment according to the protocols in the various hospitals at the time (Anonymous 2003). Indication for administering corticosteroids included one or more of the following criteria: (1) severe toxic symptoms, persistent high fever >39 °C after the use of analgesics for 3 days and other problems including heat loss; (2) illness progressing within 48 h, lesions expanding by more than 50% and exceeding ¼ of the total lung area on P‐A position chest radiographs; and (3) diagnostic criteria of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). The recommended dose of methylprednisolone for adults was 80–320 mg/day intravenously, but modifications were made according to the needs of individual patients.
Radiological investigation
All SARS patients underwent magnetic resonance imaging (MRI) and X‐ray at the CPAF hospital in Beijing as part of the post‐SARS follow‐up assessments, using instruments from GE (USA). Images were taken of hips, knees, shoulders, ankles and wrists. Two musculoskeletal radiologists (one with more than 20 years experience and one with 5 years experience) interpreted the MRI examinations and findings were reached by consensus. Osteonecrosis was defined as a subchondral or intramedullary area demarcated by a distinct T1 hypo‐intense marginal rim, and encompassing medullary fat in its centre. The ARCO Classification of osteonecrosis was used to describe the condition of the hip (Gardeniers 1993). Digits in the classification refer to ARCO stage (nature of radiographic findings) and the letters refer to the subclass expressing the location of the lesion and the extension of the lesion under weight bearing dome of the acetabulum on the femoral head (A: medial; B: central; C: lateral); no quantification of abnormality is given. ARCO stages 1A, 1B, 2A and 2B are indicated as moderate, and stages 1C and 2C as severe AVN. Osteonecrosis identified in shoulder, knee, ankle and/or wrist was only classified as positive (osteonecrosis present) and negative (osteonecrosis not present).
Treatment of avascular necrosis
All patients with AVN were referred for specialist treatment and received appropriate therapy, including physiotherapy and/or hip replacement, according to standard guidelines. Treatment with corticosteroids was discontinued after diagnosis of AVN.
Data collection and statistical analysis
The bio‐data collected of each patient was age, sex and profession (health worker or non‐health worker). For each patient the following information about corticosteroid treatment was available: total dose of corticosteroids (cumulative prednisolone‐equivalent) given (in mg), maximal dose per day and duration of treatment. Information on the condition of joints was recorded as described above. Finally, the IgG antibody titre against SARS‐CoV during illness was recorded. Other conditions or diseases leading to AVN were ruled out, such as history of bone diseases such as Paget’s disease, fractures and osteoporosis, or diseases that affect the bone metabolism such as end‐stage renal failure, thyrotoxicosis and hyperparathyroidism. No patients were known with alcohol abuse or intravenous drug use, nor with pre‐existing joint pain before SARS.
Univariate logistic regression analysis was performed to identify the association between AVN and the above risk factors. Continuous variables were tested both categorically and continuously. In addition, we tested adding squared terms and used log‐transformed values, but these did not result in significantly better fits of the data. All variables of the univariate analysis with significance P < 0.10 were entered into a multivariate logistic regression model, and the best model was obtained by using the standard backward stepwise (likelihood ratio) method. The software package used for the analysis was SPSS, version 11.0.
Results
A total of 71 former SARS patients were followed for the duration of 36 months. The duration between the start of corticosteroid treatment and the first assessment was 3–4 months. At the time of the first assessment (month 0), 28 patients (39.4%) were diagnosed with AVN (Table 1). All but three patients had bilateral hip involvement and nine of these had bone abnormalities in other joints as well. Severe AVN (ARCO stages 1C and 2C) was present in 11 cases. Eight patients required hip replacement surgery. At month 12, another two patients had developed bilateral hip AVN (both with ARCO stage 1A), bringing the percentage with AVN to 42.3%. Between month 12 and month 36 no new instances of AVN were observed. At month 36 however, a total of 10 new cases were found with bone abnormalities in other joints than the hip, and 1 new case with bilateral AVN of the hip (ARCO stage 1A). In addition, four cases with previous hip involvement had developed bone abnormalities in other joints. This brought the total number of patients with AVN in any joint at 36 months follow‐up to 41 (57.7%). Figures 1a,b give examples of MRI images of a hip and knee joint of patients diagnosed with AVN.
Table 1.
Avascular necrosis (AVN) occurrence during the 3‐year follow‐up of 71 former SARS patients
| Time (months) | ARCO stage of hip joint* | AVN in hip | AVN in hip (1C and 2C) | AVN in other joints but not in hip | AVN in other joints as well as hip | AVN in any joint | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A† | 1B | 1C | 2A | 2B | 2C | |||||||
| n | % (N = 71) | |||||||||||
| 0 | 7 | 6 | 7 | 2 | 2 | 4 | 28 | 11 | 0 | 9 | 28 | 39.4 |
| 6 | 7 | 6 | 7 | 2 | 2 | 4 | 28 | 11 | 0 | 9 | 28 | 39.4 |
| 12 | 9 | 6 | 7 | 2 | 2 | 4 | 30 | 11 | 0 | 9 | 30 | 42.3 |
| 18 | 9 | 6 | 7 | 2 | 2 | 4 | 30 | 11 | 0 | 9 | 30 | 42.3 |
| 24 | 9 | 6 | 7 | 2 | 2 | 4 | 30 | 11 | 0 | 9 | 30 | 42.3 |
| 30 | 9 | 6 | 7 | 2 | 2 | 4 | 30 | 11 | 0 | 9 | 30 | 42.3 |
| 36 | 10 | 6 | 7 | 2 | 2 | 4 | 31 | 11 | 10‡ | 13 | 41 | 57.7 |
*In all but three cases both hip joints were involved, the hip with the highest stage is registered in this table.
†Digit refers to ARCO stage; letter refers to the subclass expressing the location of the lesion and the extension of the lesion under weight bearing dome of the acetabulum on the femoral head (A: medial; B: central; C: lateral); no quantification of abnormality is given.
‡Shoulder: 8; knee: 1; ankle: 1.
Figure 1.
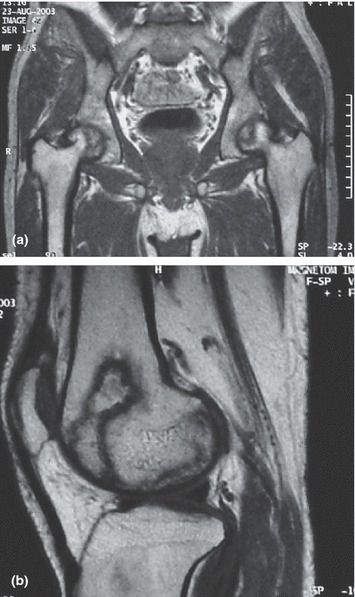
(a) MRI image of hip joints with bilateral AVN in a former SARS patient treated with corticosteroids. (b) MRI image of knee joint with AVN in a former SARS patient treated with corticosteroids.
Table 2 shows the distribution of moderate (n = 20) and severe (n = 11) AVN in the hip, and AVN not in hip (n = 10), according to age, sex, corticosteroid treatment and initial antibody titre. Most patients (41/71) are in the lowest age category (20–29 years), followed by the ages 30–39 years. Very few patients (8/71) are above 40 years. In addition, a slight majority of patients (58%) is female. Age and sex distribution is a reflection of the fact that most patients were healthcare workers. About half of the patients (35/71) received a maximal dose of corticosteroids of 320 or 480 mg. Twenty‐nine patients received a maximal dose of 160 mg or less, but four patients received extreme high maximal doses of 560 mg or more. The average duration of treatment was 34 days, but varied widely between 1 and 63 days. The average total dose was 6000 mg, again with a wide range between 1100 and 25 000 mg. In eight cases, it was not possible to obtain the initial antibody titre against the SARS‐CoV. In the remaining 63 cases, the titres were positive, ranging from 1:20 to more than 1:640. The 10 patients found with AVN in other joints than the hip at the end of the observation period had generally lower treatment indicators (maximal dose, duration of treatment and total dose) than the others. This is not surprising as only individuals with relatively low doses remained to develop AVN in other joints at a later stage.
Table 2.
AVN distribution according to age, sex, corticosteroid treatment and initial antibody titre
| N | AVN other, but not in hip | AVN hip | Total (%) | |||
|---|---|---|---|---|---|---|
| Moderate* | Severe† | |||||
| Total cohort | 71 | 10 | 20 | 11 | 41 | 57.7 |
| Age in years | ||||||
| 20–29 | 41 | 7 | 11 | 5 | 23 | 56.1 |
| 30–39 | 22 | 2 | 6 | 4 | 12 | 54.5 |
| 40–49 | 4 | 0 | 2 | 1 | 3 | 75.0 |
| 50–59 | 4 | 1 | 1 | 1 | 3 | 75.0 |
| Sex | ||||||
| Male | 30 | 1 | 7 | 6 | 14 | 46.7 |
| Female | 41 | 9 | 13 | 5 | 27 | 65.9 |
| Prednisolone‐equivalent dose | ||||||
| Max on any day | ||||||
| ≤160 mg | 29 | 6 | 5 | 1 | 12 | 41.4 |
| 240 mg | 3 | 0 | 3 | 0 | 3 | 100.0 |
| 320 mg | 21 | 4 | 3 | 3 | 10 | 47.6 |
| 480 mg | 14 | 0 | 6 | 6 | 12 | 85.7 |
| 560 mg | 1 | 0 | 1 | 0 | 1 | 100.0 |
| 640 mg | 1 | 0 | 1 | 0 | 1 | 100.0 |
| <840 mg | 2 | 0 | 1 | 1 | 2 | 100.0 |
| Duration in days | ||||||
| <20 days | 5 | 2 | 2 | 0 | 4 | 80.0 |
| 20–29 days | 26 | 3 | 5 | 3 | 11 | 42.3 |
| 30–39 days | 16 | 3 | 2 | 2 | 7 | 43.8 |
| 40–49 days | 11 | 2 | 5 | 2 | 9 | 81.8 |
| 50–59 days | 11 | 0 | 5 | 3 | 8 | 72.7 |
| 60–69 days | 2 | 0 | 1 | 1 | 2 | 100.0 |
| Total dose in mg | ||||||
| 1000–4999 mg | 44 | 9 | 9 | 3 | 21 | 47.7 |
| 5000–9999 mg | 16 | 1 | 5 | 3 | 9 | 56.3 |
| 10 000–14 999 mg | 4 | 0 | 1 | 3 | 4 | 100.0 |
| 15 000–19 999 mg | 2 | 0 | 1 | 1 | 2 | 100.0 |
| 20 000–25 000 mg | 5 | 0 | 4 | 1 | 5 | 100.0 |
| Antibody titre‡ | ||||||
| 1:20–1:40 | 15 | 2 | 5 | 2 | 7 | 46.7 |
| 1:80–1:160 | 23 | 4 | 8 | 6 | 14 | 60.9 |
| 1:280–1:640 | 14 | 3 | 9 | 2 | 11 | 78.6 |
| >1:640 | 11 | 0 | 5 | 1 | 6 | 54.5 |
*Moderate: ARCO stages 1A, 1B, 2A, 2B.
†Severe: ARCO stages 1C, 2C.
‡Missing values: 8.
Table 3 shows risk factors for developing AVN in the hip within 3 years after treatment of SARS with corticosteroids. Univariate logistic regression analysis was performed on 31 cases that developed moderate and severe AVN in the hip among the cohort of 71 surviving SARS patients. There is no statistically significant (P > 0.05) association between AVN in the hip and age and sex of the patients. The association with log antibody titre is nearly statistically significant (P = 0.06). All factors, however, related to treatment with corticosteroids show a strong association (P < 0.01). Multivariate regression analysis including all three treatment factors, log antibody titre and hospital results in a model with only the total dose (cumulative prednisolone‐equivalent dose) remaining statistically significant. In fact, a model only including maximum dose and duration of treatment gives a worse fit of the data (not shown). Repeating the same analysis for those having severe AVN of the hip (n = 11) gives similar results, but with larger confidence intervals. In multivariate analysis, however, maximal dose instead of total dose remains as the statistically significant factor (OR = 1.59; 95% CI: 1.09–2.31; P = 0.02). Testing the risk of severe AVN of the hip (n = 11) out of those developing AVN of the hip (n = 31) did not result in a significant factor (not shown), which suggests that the degree of severity of AVN in the hip is random or because of an as yet unknown factor.
Table 3.
Risk factors for AVN in a cohort of surviving SARS patients
| Risk factor | Odds ratio | Confidence interval (95%) | P‐value |
|---|---|---|---|
| Age (10 years) | 1.40 | 0.84–2.35 | 0.20 |
| Sex (female) | 1.02 | 0.40–2.65 | 0.96 |
| Prednisolone‐equivalent total dose (1000 mg)* | 1.42 | 1.14–1.77 | <0.01 |
| Maximal daily dose (100 mg) | 2.22 | 1.44–3.41 | <0.01 |
| Duration of treatment (weeks) | 1.59 | 1.16–2.18 | <0.01 |
| Lg10 antibody titre† | 2.51 | 0.96–6.60 | 0.06 |
| Hospital* | |||
| Hospital 1 (n = 5) | 0.58 | 0.06–5.81 | 0.64 |
| Hospital 2 (n = 17) | 3.29 | 0.97–11.11 | 0.06 |
| Hospital 3 (n = 16) | 3.83 | 1.09–13.45 | 0.04 |
| Hospital 4 (n = 33) | 1.0 | (Reference category) | |
*In a multivariate analysis of all factors with P < 0.1, only ‘prednisolone‐equivalent total dose’ remained statistically significant. In a model with ‘prednisolone‐equivalent total dose’ and ‘hospital’, the overall significance of ‘hospital’ becomes P = 0.70.
†The antibody titre was not measured for eight persons.
Discussion
In this paper, we describe a cohort of 71 SARS patients who were treated with high doses of corticosteroids during the acute phase of their disease. Out of this group, 28 (39%) developed avascular necrosis of the hips within 3–4 months after starting treatment. Two more cases of hip necrosis were seen after 1 year and another 11 cases of AVN were diagnosed after 3 years, one with hip necrosis and 10 with necrosis in other joints. In total, 58% of the cohort had developed avascular necrosis after 3 years of observation. The sole factor explaining avascular necrosis in the hip was the total dose of corticosteroids received.
The occurrence of AVN of bones in SARS patients was reported soon after the outbreak of SARS in China (Hong & Du 2004; 2004, 2005; Shen et al. 2004). In‐depth analyses of this complication followed subsequently (Griffith et al. 2005; Chan et al. 2006). There are no reports, however, of long‐term follow‐up of a cohort of former SARS patients who were treated with corticosteroids. The present paper has now filled this gap. A limitation to this study is the absence of control patients, which is inherent to the fact that the data was from an outbreak situation, which did not allow randomisation of patients receiving treatment or not. In addition, it is not possible to determine to which extent the disease SARS itself has possibly contributed to AVN in the patients.
The previous reports underline the short duration between onset of SARS and the diagnosis of AVN. Hong and Du (2004) report a mean time of 119 days and Li et al. indicate that all cases with AVN were detected within 6 months from the start of corticosteroid treatment. Chan et al. (2006) describe a median time from admission to MRI examination of the cohort of SARS patients of 6.7 months, with a range of 3.3–9.7 months. In our study we were not able to calculate accurately the time between onset of SARS and the diagnosis of AVN because of missing data, but 28 of the 41 patients with AVN were diagnosed between 3 and 4 months after starting corticosteroid treatment. It is known that in general the interval between corticosteroid administration and the onset of symptoms is rarely less than 6 months and may be more than 3 years (Risteli & Risteli 1993), and a median duration of 5.3 months has been reported (Koo et al. 2002).
The reported prevalence of AVN in SARS patients treated with corticosteroids varies remarkably. In Hong Kong prevalences of 5% (Griffith et al. 2005) and 10% (Chan et al. 2004) were reported, while one hospital in Beijing reported a prevalence of 33% (Li et al. 2005). Our study, which is also from a hospital in Beijing, reports a prevalence of 39% shortly after the end of the SARS epidemic. In all studies the diagnosis of AVN was based on MRI. The prevalence reported in literature of AVN after corticosteroid treatment is approximately 10% (Mankin 1992; Mulliken et al. 1994). The prevalence reported in our study goes far beyond this figure, certainly when taking into account the new cases of AVN found during the whole follow‐up period of 3 years. It is an intriguing question, certainly in the absence of more comprehensive follow‐up studies of former SARS patients treated with corticosteroids, how many of these patients still suffer from AVN and the resulting disabilities. Given the prevalence figures from Beijing, this number should be substantial in the whole of China. Unfortunately, we do not know if this is the case and whether the situation in Beijing was special in terms of patient selection or doses given.
Our study shows an interesting bi‐modal distribution of occurrence of AVN over time. Most of the cases are found within 6 months after starting corticosteroid treatment. These cases all have – often serious – hip involvement. A second group appearing after 30 months are less serious and have primarily other joints involved than the hip. This second group is distinguished from the first group by lower treatment indicators (lower maximal dose, shorter duration of treatment and lower total dose). In the group of 31 cases with AVN of the hip, who were all but one found in the first 12 months of observation, the AVN could be explained completely by the total (cumulative) dose of corticosteroids received. Griffith et al. found the same in Hong Kong (Griffith et al. 2005). In the other study from Hong Kong, high‐dose corticosteroids and an extended duration of treatment were shown to be a significant risk factor for AVN (Chan et al. 2006). In the previous study from a Beijing hospital, however, maximum daily dose was shown to be the most significant risk factor. In our study, we found the same when including only the 11 cases with serious AVN of the hip in the multivariate regression analysis. This could indicate that occurrence per se of AVN is primarily dependent on total dose, while severity of AVN is more related to maximum daily dose.
This study once again underlines the risk of high‐dose corticosteroid treatment for the occurrence of AVN of the bone. In the case of SARS, however, a unique disease with which there was no treatment experience at all, it was felt that corticosteroids were necessary to counter the acute respiratory distress syndrome in critically ill patients to save their lives. Experience was gained in the course of the epidemic and treatment regimens adjusted accordingly. The use of corticosteroids has been debated, with conflicting opinions about steroids being the key component in the treatment of SARS (Gomersall 2004). It has remained uncertain how the aggressive use of corticosteroids during the SARS epidemic has tipped the balance. Has it saved more lives by applying corticosteroids in high doses? And, do immediate benefits in terms of saving lives weigh against the adverse effects, including AVN? Perhaps the low SARS case fatality in mainland China (about 6%), relative to that in other affected countries and areas (10–17%), is to some extent the result of these high‐dose treatments (Feng et al., 2009). It has been suggested that randomised controlled trials are performed to solve the issue of effectiveness of corticosteroids in circumstances of an acute respiratory disease. Such research protocols should be pre‐developed to be initiated promptly, should SARS or a comparable disease return (Gomersall et al. 2004).
Conflicts of interest
The authors have declared that they have no conflicts of interest.
Acknowledgements
The present study was supported by the Commission of the European Community under the Sixth Framework Program Specific Targeted Research Project, SARS Control ‘Effective and Acceptable Strategies for the Control of SARS and new emerging infections in China and Europe’ (Contract No. SP22‐CT‐2004‐003824).
References
- Anonymous (2003) Consensus for the management of severe acute respiratory syndrome. Chinese Medical Journal [in Chinese] 116, 1603–1635. [PubMed] [Google Scholar]
- Assouline‐Dayan Y, Chang C, Greenspan A, Shoenfeld Y & Gershwin ME (2002) Pathogenesis and natural history of osteonecrosis. Seminars in Arthritis and Rheumatism 32, 94–124. [PubMed] [Google Scholar]
- CDC (2004) Revised Diagnoses Criterion for Severe Acute Respiratory Syndrome (SARS). May 29 2004. CDC, China [in Chinese]. Available from: http://www.moh.gov.cn/newshtml/130.htm (Accessed: 15 October 2007). [Google Scholar]
- Chan CW, Chiu WK, Chan CC, Chow EY, Cheung HM & Ip PL (2004) Osteonecrosis in children with severe acute respiratory syndrome. The Pediatric Infectious Disease Journal 23, 888–890. [DOI] [PubMed] [Google Scholar]
- Chan MH, Chan PK, Griffith JF et al. (2006) Steroid‐induced osteonecrosis in severe acute respiratory syndrome: a retrospective analysis of biochemical markers of bone metabolism and corticosteroid therapy. Pathology 38, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT & Anderson JJ (1987) Across‐study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet 1, 902–906. [DOI] [PubMed] [Google Scholar]
- Feng D, De Vlas SJ, Fang LQ et al. (2009) The SARS Epidemic in mainland China: bringing together all epidemiological data. Tropical Medicine & International Health 14 (Suppl. 1), 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeniers JWM (1993) Report of the Committee of Staging and Nomenclature. ARCO News Letter 5, 79–82. [Google Scholar]
- Gomersall CD (2004) Pro/con clinical debate: steroids are a key component in the treatment of SARS. Pro: yes, steroids are a key component of the treatment regimen for SARS. Critical Care 8, 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomersall CD, Joynt GM, Lam P et al. (2004) Short‐term outcome of critically ill patients with severe acute respiratory syndrome. Intensive Care Medicine 30, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JF, Antonio GE, Kumta SM et al. (2005) Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology 235, 168–175. [DOI] [PubMed] [Google Scholar]
- Hong N & Du XK (2004) Avascular necrosis of bone in severe acute respiratory syndrome. Clinical Radiology 59, 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo KH, Kim R, Kim YS et al. (2002) Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clinical Rheumatology 21, 299–303. [DOI] [PubMed] [Google Scholar]
- Li YM, Wang SX, Gao HS et al. (2004) Factors of avascular necrosis of femoral head and osteoporosis in SARS patients’ convalescence. Zhonghua Yi Xue Za Zhi 84, 1348–1353. [PubMed] [Google Scholar]
- Li ZR, Sun W, Qu H et al. (2005) Clinical research of correlation between osteonecrosis and steroid. Zhonghua Wai Ke Za Zhi 43, 1048–1053. [PubMed] [Google Scholar]
- Liu W, Fontanet A, Zhang PH et al. (2006) Two‐year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. The Journal of Infectious Diseases 193, 792–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin HJ (1992) Nontraumatic necrosis of bone (osteonecrosis). The New England Journal of Medicine 326, 1473–1479. [DOI] [PubMed] [Google Scholar]
- Mulliken BD, Renfrew DL, Brand RA & Whitten CG (1994) Prevalence of previously undetected osteonecrosis of the femoral head in renal transplant recipients. Radiology 192, 831–834. [DOI] [PubMed] [Google Scholar]
- Risteli L & Risteli J (1993) Biochemical markers of bone metabolism. Annals of Medicine 25, 385–393. [DOI] [PubMed] [Google Scholar]
- Shen J, Liang BL, Zeng QS et al. (2004) Report on the investigation of lower extremity osteonecrosis with magnetic resonance imaging in recovered severe acute respiratory syndrome in Guangzhou. Zhonghua Yi Xue Za Zhi 84, 1814–1817. [PubMed] [Google Scholar]
- WHO (2003) WHO: Case Definitions for Surveillance of Severe Acute Respiratory Syndrome (SARS). WHO, Geneva. Available from: http://www.who.int/csr/sars/casedefinition/en/ (Accessed: 15 October 2007). [Google Scholar]
- Wing PC, Nance P, Connell DG & Gagnon F (1998) Risk of avascular necrosis following short term megadose methylprednisolone treatment. Spinal Cord 36, 633–636. [DOI] [PubMed] [Google Scholar]


