Abstract
Abstract: To characterize epidemiology and risk factors for respiratory viral infections (RVI) in pediatric lung transplant recipients within the first post‐transplant year, a retrospective multicenter study of pediatric lung transplant recipients from 1988 to 2005 was conducted at 14 centers in the United States and Europe. Data were recorded for 1 year post transplant. Associations between RVI and continuous and categorical risk factors were assessed using Wilcoxon's rank‐sum and χ2 tests, respectively. Associations between time to RVI and risk factors or survival were assessed by multivariable Cox proportional hazards models. Of 576 subjects, 79 subjects (14%) had 101 RVI in the first year post transplant. Subjects with RVI were younger than those without RVI (median ages 9.7, 13; P<0.01). Viruses detected included adenovirus (n=25), influenza (n=9), respiratory syncytial virus (n=21), parainfluenza virus (n=19), enterovirus (n=4), and rhinovirus (n=22). In a multivariable model for time to first RVI, etiology other than cystic fibrosis (CF), younger age, and no induction therapy were independently associated with risk of RVI. Cytomegalovirus serostatus and acute rejection were not associated with RVI. RVI was independently associated with decreased 12‐month survival (hazard ratio 2.6, 95% confidence interval 1.6–4.4). RVI commonly occurs after pediatric lung transplantation with risk factors including younger age and non‐CF diagnosis. RVI is associated with decreased 1‐year survival.
Keywords: lung transplantation, respiratory virus infection, respiratory syncytial virus, adenovirus, pediatric
Lung transplantation is an accepted method of treatment for end‐stage pulmonary diseases. In the pediatric population, the most common indications for lung transplantation are cystic fibrosis (CF) and pulmonary hypertension. Unfortunately, despite the therapeutic potential of lung transplantation, the 1‐year and 5‐year post‐lung transplantation survival rates remain poor at only 80% and 50%, respectively (1). Infection, which occurs in approximately 60–90% of recipients (2, 3, 4), has been reported to account for nearly 40% of all deaths (1). Respiratory viral infections (RVIs) constitute a significant proportion of these infectious episodes with 9–66% of evaluated subjects developing an identifiable RVI in the reported literature (5, 6, 7, 8, 9, 10).
Aside from the direct effects of RVIs, recent studies have reported an association between infection and chronic graft rejection in both the adult and pediatric lung transplant populations. Many groups have reported that viruses such as influenza, parainfluenza, adenovirus, and respiratory syncytial virus (RSV) are associated with the development of bronchiolitis obliterans (BO) and bronchiolitis obliterans syndrome (BOS) (7, 8, 9, 11, 12, 13, 14, 15, 16). In animal models, introduction of respiratory viruses increased allograft rejection (17, 18). Although the underlying mechanisms remain to be elucidated, it is hypothesized that RVIs injure the graft, which activates a cascade of events to culminate in chronic graft rejection.
To date, comprehensive evaluation of RVI in pediatric lung transplant recipients has not been performed. This multicenter retrospective study reports the incidence of RVI, associated pre‐transplant risk factors, and associated post‐transplant outcomes within 1 year after pediatric lung transplantation. We hypothesize that an episode of RVI is associated with increased BOS or mortality in primary pediatric lung transplant recipients within the first year following transplantation.
Materials and methods
Study design
Members of the International Pediatric Lung Transplant Collaborative were invited to participate in this multicenter retrospective cohort study. Fourteen invited centers contributed data. Before study participation, each center obtained approval from its respective Institutional Review Board (United States centers) or Ethics Committee (European centers).
The principal investigator performed a comprehensive retrospective chart review on each patient who underwent primary lung transplant between January 1988 and the time of data collection (range, August 2004–January 2007). The medical chart review included all clinic records, discharge summaries, visit notes, correspondence letters, biopsy reports, microbiology testing results, pulmonary function test measurements, and diagnostic imaging up to 1 year post transplant or until death or retransplantation, if either event occurred before 1 year post transplant. Information gathered included demographic information, induction and initial immunosuppressive therapeutic regimens, prophylaxis regimens, post‐transplant lymphoproliferative disorder (PTLD), acute rejection episodes, BO/BOS, viral and fungal infections, and if applicable, date and cause of death. Patient identifiers were removed from the data at the time of medical chart review.
Therapy regimen
Pre‐transplant evaluation of patients was performed according to the standard protocol of each participating institution. Use of induction immunosuppressive therapy varied over the study period and across participating centers, ranging from no therapy to receipt of lympholytic agents or interleukin‐2 receptor antagonists. After transplantation, most patients received triple‐drug immunosuppression with a calcineurin inhibitor (CNI), prednisone, and either azathioprine or mycophenolate mofetil. Patients had gradual reduction of immunosuppressive therapy as time from transplant increased, if there was no evidence of significant acute or chronic graft rejection on radiographs, transbronchial biopsies, and lung function test results. Target CNI trough serum concentrations varied by center and were not recorded for this study. Additionally, prophylaxis for cytomegalovirus (CMV) and routine transbronchial biopsies to assess for rejection were not standardized across centers and changed over time within centers. Prophylaxis for RVI was routinely prescribed at only 1 institution that administered amantadine during the duration of the study, although individual subjects at other institutions may have received preventative therapies. Vaccination against influenza was routinely recommended at participating sites, although not all subjects may have received vaccination in the first year after transplantation due to the timing of transplantation.
Diagnostic definitions
RVI
Diagnosis was based upon positive viral identification in bronchoalveolar lavage (BAL), bronchial wash, tracheal aspirate, sputum, or nasopharyngeal swab specimens in addition to clinical evidence of infection. Clinical evidence of viral infection was indicated by rhinorrhea, cough, and fever, and supported by suggestive radiographic and/or transbronchial biopsy findings. The site of RVI specimen was recorded when available in the medical chart, although no distinction was made between upper and lower RVI due to the limitations of the data available. Direct fluorescence antibody testing, viral cultures, and polymerase chain reaction (PCR) were the principal diagnostic methods used. The application and specific methodology of each assay varied between and within institutions. Episodes with clinical evidence of viral infection but without identification of specific viral etiologies were excluded from this study. As such, only episodes of infection based upon laboratory documentation were used.
Acute rejection
Diagnosis and grading was performed at each individual center without review by a central pathology core. Grading was based upon the identification of perivascular lymphocytic infiltrates in transbronchial biopsy specimens using a defined classification scheme for pulmonary allograft rejection (19, 20). In addition, clinical suspicion of acute rejection that resulted in enhanced immunosuppression with intravenous methylprednisone or an anti‐lymphocyte agent without definitive pathological evidence was noted.
BO and BOS
Diagnosis and grading of BO was based on histologic identification of fibrous scarring of small conducting airways with partial or complete obliteration of the airway lumen in transbronchial biopsy specimens, as defined by the International Society for Heart and Lung Transplantation (19, 21). Diagnosis of BOS was based on changes in pulmonary function test results as were available in the clinical charts, particularly FEV1 measurements, using criteria that reflect current standards from the International Society of Heart and Lung Transplantation in 1993 and 2002 (22, 23).
Statistical analysis
Patient information was compiled and entered into an ORACLE database. Data analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina, USA), and graphics were produced using R version 2.0.1 software (The R Foundation for Statistical Computing, Vienna, Austria). Comparisons of demographic data and outcomes between the infected and uninfected cohorts were performed using Wilcoxon's rank‐sum tests for continuous variables and χ2 tests or Fisher's exact tests for categorical variables as appropriate. Associations between demographic and clinical factors, post‐transplant morbidities (including first and second episodes of acute rejection, chronic rejection, episodes of PTLD, first CMV infection and pulmonary fungal infection), and time to RVI, and between RVI and times to post‐transplant morbidities and mortality were assessed using Cox's proportional hazards models with follow‐up censored at death, retransplant, or 1 year post transplant. When used as risk factors in these models, RVI and post‐transplant morbidities were modeled as time‐dependent covariates. All tests were 2‐tailed and performed at a significance level of 0.05.
Results
RVI occur frequently within the first year after pediatric lung transplantation
Of the 576 subjects studied, 79 subjects (13.8%) developed 101 episodes of RVI within the first year after transplant. A single documented episode of RVI was most common (75%); however, 25% developed >1 RVI, with no subject having >3 episodes. The mean number of RVI per infected subject was 1.3. Specimens were retrieved from nasopharyngeal swab (n=27, 27%), trachea (n=2, 2%), and BAL (n=54, 53%). The specimen source was not documented in 16/101 (16%). Methodology for viral discovery was recorded in 48 of 101 episodes. Viral culture predominated (n=38, 38%) with additional positive results obtained with direct fluorescent antibody testing (n=4, 4%) and PCR (n=3, 3%).
Early RVI associated with death
RVI occurred early in the post‐transplant course. The mean time to first episode of RVI was 3.4 months (range, 0–11.4) with a median time to first RVI episode of 1.5 months. In the 405 subjects for whom duration of initial post‐transplant hospitalization was available, 67 subjects had 88 episodes of RVI. For these subjects, 28 subjects developed 33 episodes of RVI during the initial hospitalization for a nosocomial rate of 7% in this subset. Subjects with RVI during their initial hospitalization were hospitalized for a median of 43 days (P25, P75: 18, 64) compared with 24 days (P25, P75: 16, 38) days for those with and without RVI after their first hospitalization, respectively. In addition, for subjects with RVI during the first hospitalization, 46% died or were retransplanted within 1 year, compared with 20% without any RVI and 5% with RVI after the first hospitalization. Subjects with RVI during the first hospitalization had increased risk of death or retransplantation within 1 year compared with all subjects as well as those subjects who had RVI after initial hospitalization (hazard ratio [HR] 3.5, 95% confidence interval [CI] 2.0–6.3 P<0.001 and HR 12.5, 95% CI 2.8–55, P<0.001, respectively).
Frequently encountered viruses and seasonal distribution
Of the 101 RVI episodes, the most frequently encountered viral pathogens were adenovirus (24.8%), rhinovirus (21.8%), RSV (20.8%), and parainfluenza viruses (18.8%). The remaining pathogens included herpes simplex virus (HSV), enteroviruses, and influenza A/B (Table 1). The distribution of viral infections varied depending on the age of the subject. Of the 93 subjects <3 years of age, 25 (27%) developed 1 or more RVI episodes. Organisms recovered included adenovirus (14 episodes), parainfluenza (8), RSV (7), rhinovirus (7), enterovirus (2), and influenza (1). In the 481 subjects older than 3 years, only 54 subjects (11%) recorded at least 1 RVI episode. In this group, rhinovirus (15 episodes) and RSV (14) occurred more frequently than adenovirus (11), parainfluenza (11), influenza (8), and enterovirus (2). A seasonal distribution of RVI was observed for some viruses (Fig. 1). The viruses most commonly recovered during the winter months were influenza and RSV. Adenovirus occurred more frequently in the spring months while parainfluenza and rhinovirus peaked in the fall and spring. Herpesvirus infection occurred rarely. HSV presented as pneumonitis in 4 of the 5 documented episodes. The single subject with varicella died secondary to disseminated varicella zoster virus infection with associated pneumonitis.
Table 1.
Viruses recovered in the first year after transplantation
| Virus | N=101 |
|---|---|
| Adenovirus | 25 |
| Rhinovirus | 22 |
| Respiratory syncytial virus | 21 |
| Parainfluenza | 19 |
| Influenza A/B | 9 |
| Herpes simplex virus | 5 |
| Enteroviruses | 4 |
| Varicella | 1 |
Figure 1.
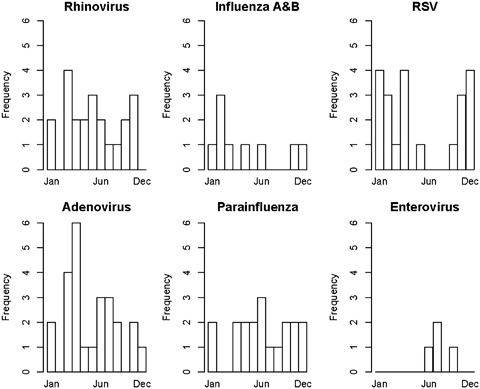
Events by virus detected and month detected. RSV, respiratory syncytial virus.
Univariable risks for RVI
Several features distinguished subjects with RVI within the first year post transplant from their uninfected cohorts (Table 2). Specifically, subjects with RVI were more likely to be younger in age (RVI: 9.7 years [25–75%: 1.7, 13.2]; without RVI: 13.1 years [9.0, 16.1]; P<0.001) and have an underlying diagnosis other than CF (P<0.001). Subjects who received living donor or bilateral cadaveric transplants had the highest proportions of RVI compared with single lung and heart–lung transplant recipients. A significant difference between subjects with and without RVI was observed in the distribution of transplant type (P<0.028); however, small sample size in some groups limited the ability to perform pair‐wise comparisons.
Table 2.
Demographic of subjects with and without respiratory viral infections (RVI)
| RVI | No RVI | P‐value 1 | |||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Age at transplant (years) | 79 | 9.7 (1.7, 13.2) 2 | 495 | 13.1 (9.0, 16.1) 2 | <0.001 3 |
| Female | 49 | 62.0 | 281 | 56.5 | 0.36 |
| Caucasian | 66 | 83.5 | 448 | 90.6 | 0.052 |
| Cystic fibrosis | 22 | 27.8 | 284 | 57.3 | <0.001 |
| Transplant type | 0.028 4 | ||||
| Single left or right | 1 | 1.3 | 13 | 2.6 | |
| Bilateral cadaveric | 65 | 82.3 | 335 | 67.5 | |
| Heart and lung transplant | 9 | 11.4 | 125 | 25.2 | |
| Living donor | 4 | 5.0 | 23 | 4.6 | |
| No induction therapy | 52 | 65.8 | 250 | 54.6 | 0.012 |
| Home‐going regimen: cyclosporine | 64 | 81.0 | 349 | 70.2 | 0.048 |
χ2 test.
2 Median (25, 75).
3 Wilcoxon's rank‐sum test.
4 Fisher's exact test.
In addition to age at transplant, factors univariably associated with time to RVI included induction therapy and home‐going regimen (Table 3). Younger age at transplant was associated with increased risk of RVI, with the youngest transplant recipients (0–5.4 years of age) having the greatest risk (HR 4.7, 95% CI 2.1, 10.8). The increased risk of RVI diminished as age at transplantation reached 15–16 years. Subjects who did not receive induction therapy, had an underlying etiology other than CF, or were treated with cyclosporine (versus tacrolimus) had increased risk of RVI (P=0.012, P<0.001, P=0.049, respectively). Significantly increased risk of RVI was not seen with prior episodes of CMV, acute rejection (≥grade A2 or multiple episodes of treated rejection), or BOS.
Table 3.
Univariable risks for respiratory viral infection (RVI)
| Univariable hazard ratios for time to RVI | |||
|---|---|---|---|
| Risk factor | Number in analysis | Hazard ratio (95% CI) | P‐value |
| Age at transplant in years (quintiles) | |||
| 16.4–21 | 115 | Reference | <0.01 |
| 14–16.4 | 113 | 0.85 (0.29, 2.5) | |
| 11.4–14 | 116 | 2.5 (1.02, 6.0) | |
| 5.5–11.4 | 113 | 3.2 (1.3, 7.5) | |
| 0–5.4 | 117 | 4.7 (2.1, 10.8) | |
| Year of transplant (quintiles) | |||
| 2002–2005 | 122 | Reference | 0.063 |
| 1985–1992 | 88 | 1.1 (0.41, 2.8) | |
| 1993–1996 | 142 | 1.9 (0.91, 4.1) | |
| 1997–1998 | 92 | 2.4 (1.07, 5.2) | |
| 1999–2001 | 132 | 2.5 (1.2, 5.2) | |
| No induction therapy | 532 | 1.9 (1.2, 3.1) | 0.012 |
| Home‐going regimen: cyclosporine | 576 | 1.8 (1.00, 3.1) | 0.049 |
| Diagnosis other than cystic fibrosis | 575 | 3.2 (2.0, 5.3) | <0.001 |
| Transplant type | |||
| Heart/lung | 134 | Reference | 0.073 |
| Single | 14 | 1.1 (0.14, 8.7) | |
| Living donor | 27 | 2.5 (0.76, 8.0) | |
| Bilateral cadaveric | 400 | 2.5 (1.2, 4.9) | |
| CMV before RVI | 576 | 1.2 (0.60, 2.3) | 0.66 |
| Any rejection before RVI | 576 | 0.78 (0.47, 1.3) | 0.34 |
| Two rejections before RVI | 576 | 0.67 (0.38, 1.2) | 0.17 |
| A2 rejection before RVI | 576 | 1.06 (0.63, 1.8) | 0.83 |
| Two A2 rejections before RVI | 576 | 0.90 (0.44, 1.9) | 0.78 |
| Two treated rejections before RVI | 576 | 0.77 (0.40, 1.5) | 0.43 |
| BOS before RVI | 576 | 1.7 (0.50, 5.5) | 0.69 |
CI, confidence interval; CMV, cytomegalovirus; A2, grade of rejection; BOS, bronchiolitis obliterans syndrome.
Independent predictors for RVI
In a multivariable Cox proportional hazards model (Table 4), age <15 years at the time of transplant and an underlying etiology other than CF remained significant risk factors for the development of RVI (age <15, HR 3.4, 95% CI [1.2, 9.4], P=0.018; and non‐CF, HR 3.0, 95% CI [1.7, 5.2], P<0.001). While subjects with CF were more likely to be older, the interaction between these factors was not statistically significant. In a second multivariable model, the receipt of induction therapy was found to be protective against RVI (HR 0.58, 95% CI 0.35, 0.96, P=0.03) while age younger than 15 years and etiology other than CF remained significant risks. Prior episodes of CMV, episodes of acute rejection, and home‐going immunosuppression including the use of cyclosporine were not significantly associated with subsequent RVI in the multivariable models.
Table 4.
Multivariable risks for respiratory virus infection (RVI)
| Multivariable models for time to RVI | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Variable | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value |
| No cystic fibrosis | 3.0 (1.7, 5.2) | <0.001 | 2.8 (1.7, 4.8) | <0.001 |
| Age at transplant <15 years | 3.4 (1.2, 9.4) | 0.018 | 2.6 (1.3, 5.3) | 0.009 |
| No induction treatment | 1.7 (1.0, 2.8) | 0.034 | ||
CI, confidence interval.
RVI not a risk for subsequent morbidity within 1 year after transplantation
An episode of preceding RVI was not significantly associated with the development of subsequent post‐transplant morbidity within the first post‐transplant year, including acute rejection episodes (any, treated, or ≥grade A2), CMV infection or disease, and development of BOS (P>0.1 for all morbidities; data not shown).
RVI is a risk factor for death
RVI is a risk factor for death or retransplantation within the first year after pediatric lung transplantation. RVI remained a predictor of death or retransplantation within the first year after transplantation by univariable analysis (HR 2.5, 95% CI 1.5, 4.0; P<0.001) and remained predictive when previously reported risks for death in this population were added to the model, including pulmonary fungal infections and BOS within 1 year after transplantation(Table 5). The site of viral recovery was not associated with mortality or retransplantation. Virus was recovered from BAL in 76% (13/17) who died or received a second transplant compared with 71% (30/42) who survived to 1 year post transplant.
Table 5.
Multivariable survival model
| Multivariable survival model with time‐dependent events | ||
|---|---|---|
| Variable | Hazard ratio (95% CI) | P‐value |
| RVI | 2.6 (1.6, 4.4) | <0.001 |
| A2 rejection | 0.46 (0.29, 0.74) | <0.001 |
| Lung fungal | 2.4 (1.5, 3.9) | <0.001 |
| BOS | 7.4 (4.0, 13.4) | <0.001 |
| Tx type: single versus bilateral/other | 1.03 (0.32, 3.3) | 0.96 |
| Tx type: heart/lung versus bilateral/other | 1.7 (1.1, 2.6) | 0.014 |
| Tx type: living donor versus bilateral/other | 3.6 (1.8, 7.3) | <0.001 |
CI, confidence interval; RVI, respiratory viral infection; A2, grade of rejection; BOS, bronchiolitis obliterans syndrome; Tx, transplant.
Discussion
In the pediatric population, lung transplantation is an accepted treatment for a variety of end‐stage pulmonary diseases. However, despite advances in surgical care and management, the pediatric mortality rate post lung transplant has remained relatively unchanged and is among the highest rate of all pediatric solid organ transplant procedures. RVI represents a potential obstacle to achieving improved outcomes. Previous studies have demonstrated that RVIs occur in many adult lung transplant recipients and have been associated with post‐transplant morbidity including BOS. In a small cohort of pediatric subjects, adenovirus infection was associated with graft failure and death (7). However, the current scientific literature lacks a comprehensive examination of the risk factors for and the impact of RVI in pediatric lung transplant. This retrospective study evaluated the incidence and impact of RVI in pediatric lung transplant recipients from multiple centers throughout the United States and Europe.
Compared with previous reports of RVI in adult lung transplant recipients ranging from 9% to 66% (7, 8, 9, 24), RVI occurred in 13.8% of the pediatric subjects in this cohort. The seasonal variation of the viruses encountered reflects that seen in the general pediatric population. Clinicians should be aware of circulating community acquired viral infections to assist not only with the preparation of the patient for hospital discharge but also with evaluation of patients who return with acute illness. In addition, the viruses recovered are similar to those reported in previous studies (8, 9). Rhinovirus, which until recently was recovered with conventional viral culture, comprised a significant proportion of the RVI (20%), similar to that reported in adults (9). This reflects the importance of testing method, as newly available molecular testing becomes more widely available for respiratory specimens to detect not only rhinovirus but also other emerging viruses such as coronavirus and human metapneumovirus (9, 24, 25).
Risk factors for RVI have not been previously evaluated after lung transplantation. In this cohort, younger age at the time of transplant, absence of induction therapy, etiology other than CF, and a cyclosporine‐based immunosuppressive regimen were identified as independent risk factors for the development of RVI within the first year following lung transplantation. Younger age and etiology of transplant are both independently significant risk factors without evidence of an interaction. Younger children may be at increased risk for RVI for several reasons including immunologic naivety and increased exposure to infectious peers as seen in the general population. In addition, the proportion of subjects with adenovirus <3 years of age was significantly higher than the older cohort. Although this study did not distinguish primary adenoviral infection from reactivation of latent virus, younger children have been shown to harbor virus at higher levels in their tonsils that reactivated after in vitro stimulation (26). This provides another potential mechanism for increased risk of RVI in young transplant recipients. The observation that young age is associated with RVI development contrasts with what has been observed with pulmonary fungal infections in pediatric lung transplant recipients, where an older age is an independent risk factor. This suggests that patients may be at risk for distinctly different infections based on the age at transplantation.
The lack of induction therapy as an independent risk factor for RVI appears to be counterintuitive, as these subjects may be expected to be less immunosuppressed in the early post‐transplant period. Lack of induction therapy was not associated with increased episodes of acute rejection, including enhanced immunosuppression with intravenous steroids, which could have increased relative immunosuppressive state in this group, theoretically increasing risk for RVI. Interestingly, acute rejection episodes were not associated with increased risk of RVI. Further studies are required to elucidate the mechanism through which induction therapy could reduce an individual's susceptibility to RVI.
Although preceding RVI was not significantly associated with post‐transplant morbidity such as BOS in our study, this may be a product of an inherent limitation of only assessing outcomes up to 1 year after transplant. BOS developed in only 20% of subjects within the first year after transplantation; extended follow‐up and evaluation of the association between RVI and BOS should be performed in future populations to assess this relationship that has been reported in adult populations (8, 9, 11, 12, 13, 14, 15). However, RVI, especially RVI within the hospitalization for transplantation, was found to be a risk factor for post‐transplant mortality or retransplantation within the first year following pediatric lung transplantation.
As with any retrospective study, there are limitations related to the study design, availability of data, and selection bias. In addition, the participating institutions utilized independent protocols for pre‐transplant evaluation, induction, and immunosuppressive therapies, and surveillance and diagnosis of rejection and infection. The use of induction immunosuppressive therapy also varied over the study period. To minimize the effects of these temporal and inter‐center differences, data collection were performed by the principal investigator at all participating sites with strict adherence to specific predetermined definitions.
A second limitation of this study involves RVI diagnosis and diagnostic testing. Most participating centers did not routinely evaluate for RVI with each episode of cold symptoms, potentially underestimating the number of episodes of RVI. In addition, as mentioned previously, testing methods varied both across and within centers during the study period, which did not include the most recent era when molecular diagnostics have allowed for increased sensitivity in the identification of RVI from respiratory viral specimens, including previously unidentified viruses like human metapneumovirus. Prospective studies for intercurrent cold symptoms and which use molecular diagnostics to detect the vast number of respiratory viruses, including newly discovered human metapneumovirus, coronaviruses, and bocaviruses, will improve the understanding of RVIs and their potential impact after pediatric lung transplantation.
Further, the lack of differentiation between upper and lower RVI is another limitation. The types of infecting agents, and most importantly impact, are significantly different between the 2 classes of RVI; RVI of the lower tract have been reported to carry more serious risks for subsequent mortality and rejection post transplant (8). Unfortunately, the locations of the RVI could not be accounted for in this study owing to limitations in the availability of information, specifically the site of specimen collection for viral culture and diagnosis. In addition, the exclusion of clinical episodes along with the lack of routine screening protocols for RVI during the period for which data were collected likely underestimates the actual incidence of RVI in this population. Underestimation of RVI, especially if more severe episodes were detected, may overestimate the impact of RVI in this population. A prospectively collected cohort, including molecular evaluation for all symptomatic episodes, is in process. The duration of viral shedding was not addressed in the study; however, reports in the literature indicate that viral shedding for both rhinovirus and RSV may be prolonged in transplant recipients (27, 28, 29). Clinicians should consider this possibility with continuation or recurrence of symptoms after an RVI. Although nosocomial and community‐acquired RVI were not specifically delineated in the data collection, a significant number of early nosocomial infections were detected with the analysis of a large subset of subjects for which duration of transplant hospitalization was known. Increased awareness and enhanced infection control measures could decrease the risk of early RVI in this at‐risk population.
The limited examination of outcomes to 1 year after transplant also requires comment. Although infection is the most serious complication beyond 1 month but <1 year post transplant, it remains a potentially serious complication regardless of the time since transplant. Limiting analysis of outcomes to 1 year post transplant may have censored the true occurrence of RVI in the patient population. Furthermore, it is possible that the relationships reported between RVI and pretransplant risk factors and negative outcomes, such as chronic graft rejection and/or retransplantation, may not manifest within the first year post transplant, limiting the ability to identify significant associations. In this case, the HRs of the univariable and multivariable Cox proportional models may be artificially low. Moreover, truly significant risk factors and events may have been excluded altogether. In addition, the impact of newer RVI therapeutics such as cidofovir (for adenovirus) and ribavirin (for RSV and human metapneumovirus) may impact the outcome related to treated RVI (30, 31, 32). Future studies are planned to examine associations between RVI and post‐transplant morbidities such as acute and chronic graft rejection beyond 1 year post transplant.
This study provides the first evaluation of RVI in the pediatric lung transplant population across a wide range of pediatric transplant programs in the United States and Europe. Despite the inherent limitations of the study design, the data indicate that younger age and etiology for transplant other than CF are independent risk factors for the development of RVI within the first year after pediatric lung transplantation. In addition, a single episode of RVI is associated with death or retransplantation at 1 year post transplant even when controlling for other known mortality risks. Future studies must prospectively evaluate the incidence of RVI in this population, differentiating upper tract and lower tract RVI and following for associations between RVI and later outcomes beyond 1 year post transplant.
Acknowledgements:
This manuscript represents a collaborative effort among multiple pediatric lung transplant programs. In addition to the authors listed, we wish to acknowledge the contributions of the following individuals: Christian Benden, Craig Cole, Allison Drabble, Jaime Fox, Kelli Harker, Kathy Iurlano, Peter Michelson, Carsten Mueller, Pauline Whitmore, and Barbara Williams.
Funding: Supported by Thrasher Research Fund, National Institutes of Health/K23 RR022956 (LDI).
Presented at the International Society of Heart and Lung Transplantation Annual Meeting, April 2008.
References
- 1. Waltz DA, Boucek MM, Edwards LB, et al Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric lung and heart–lung transplantation report–2006. J Heart Lung Transplant 2006; 25 (8): 904–911. [DOI] [PubMed] [Google Scholar]
- 2. Kanj SS, Tapson V, Davis RD, Madden J, Browning I. Infections in patients with cystic fibrosis following lung transplantation. Chest 1997; 112 (4): 924–930. [DOI] [PubMed] [Google Scholar]
- 3. Kramer MR, Marshall SE, Starnes VA, Gamberg P, Amitai Z, Theodore J. Infectious complications in heart–lung transplantation. Analysis of 200 episodes. Arch Intern Med 1993; 153 (17): 2010–2016. [PubMed] [Google Scholar]
- 4. Maurer JR, Tullis DE, Grossman RF, Vellend H, Winton TL, Patterson GA. Infectious complications following isolated lung transplantation. Chest 1992; 101 (4): 1056–1059. [DOI] [PubMed] [Google Scholar]
- 5. Milstone AP, Brumble LM, Barnes J, et al A single‐season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J 2006; 28 (1): 131–137. [DOI] [PubMed] [Google Scholar]
- 6. Hopkins P, McNeil K, Kermeen F, et al Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med 2008; 178 (8): 876–881. [DOI] [PubMed] [Google Scholar]
- 7. Bridges ND, Spray TL, Collins MH, Bowles NE, Towbin JA. Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg 1998; 116 (4): 617–623. [DOI] [PubMed] [Google Scholar]
- 8. Khalifah AP, Hachem RR, Chakinala MM, et al Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med 2004; 170 (2): 181–187. [DOI] [PubMed] [Google Scholar]
- 9. Kumar D, Erdman D, Keshavjee S, et al Clinical impact of community‐acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant 2005; 5 (8): 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerna G, Vitulo P, Rovida F, et al Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol 2006; 78 (3): 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vilchez RA, Dauber J, Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection – myth or reality? Am J Transplant 2003; 3 (3): 245–249. [DOI] [PubMed] [Google Scholar]
- 12. Vilchez RA, McCurry K, Dauber J, et al Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2 (3): 287–291. [DOI] [PubMed] [Google Scholar]
- 13. Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest 2001; 119 (4): 1277–1280. [DOI] [PubMed] [Google Scholar]
- 14. Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNA aemia dynamics after lung transplantation. Transplantation 2003; 75 (12): 2064–2068. [DOI] [PubMed] [Google Scholar]
- 15. Vilchez R, McCurry K, Dauber J, et al Influenza and parainfluenza respiratory viral infection requiring admission in adult lung transplant recipients. Transplantation 2002; 73 (7): 1075–1078. [DOI] [PubMed] [Google Scholar]
- 16. Bando K, Paradis IL, Komatsu K, et al Analysis of time‐dependent risks for infection, rejection, and death after pulmonary transplantation. J Thorac Cardiovasc Surg 1995; 109 (1): 49–57; discussion 57–49. [DOI] [PubMed] [Google Scholar]
- 17. Winter JB, Gouw AS, Groen M, Wildevuur C, Prop J. Respiratory viral infections aggravate airway damage caused by chronic rejection in rat lung allografts. Transplantation 1994; 57 (3): 418–422. [DOI] [PubMed] [Google Scholar]
- 18. Kuo E, Bharat A, Goers T, et al Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg 2006; 82 (3): 1043–1050. [DOI] [PubMed] [Google Scholar]
- 19. Yousem SA, Berry GJ, Cagle PT, et al Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996; 15 (1 Part 1): 1–15. [PubMed] [Google Scholar]
- 20. Berry GJ, Brunt EM, Chamberlain D, et al A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. The international society for heart transplantation. J Heart Transplant 1990; 9 (6): 593–601. [PubMed] [Google Scholar]
- 21. Trulock EP, Christie JD, Edwards LB, et al Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart–lung transplantation report. J Heart Lung Transplant 2007; 26 (8): 782–795. [DOI] [PubMed] [Google Scholar]
- 22. Cooper JD, Billingham M, Egan T, et al A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 1993; 12 (5): 713–716. [PubMed] [Google Scholar]
- 23. Estenne M, Maurer JR, Boehler A, et al Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002; 21 (3): 297–310. [DOI] [PubMed] [Google Scholar]
- 24. Larcher C, Geltner C, Fischer H, Nachbaur D, Muller LC, Huemer HP. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant 2005; 24 (11): 1891–1901. [DOI] [PubMed] [Google Scholar]
- 25. Dare R, Sanghavi S, Bullotta A, et al Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol 2007; 45 (2): 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garnett CT, Talekar G, Mahr JA, et al Latent species C adenoviruses in human tonsil tissues. J Virol 2009; 83 (6): 2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaiser L, Aubert JD, Pache JC, et al Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med 2006; 174 (12): 1392–1399. [DOI] [PubMed] [Google Scholar]
- 28. Blanchard SS, Gerrek M, Siegel C, Czinn SJ. Significant morbidity associated with RSV infection in immunosuppressed children following liver transplantation: case report and discussion regarding need of routine prophylaxis. Pediatr Transplant 2006; 10 (7): 826–829. [DOI] [PubMed] [Google Scholar]
- 29. Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 2008; 27 (3): 282–288. [DOI] [PubMed] [Google Scholar]
- 30. Doan ML, Mallory GB, Kaplan SL, et al Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant 2007; 26 (9): 883–889. [DOI] [PubMed] [Google Scholar]
- 31. Glanville AR, Scott AI, Morton JM, et al Intravenous ribavirin is a safe and cost‐effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant 2005; 24 (12): 2114–2119. [DOI] [PubMed] [Google Scholar]
- 32. Raza K, Ismailjee SB, Crespo M, et al Successful outcome of human metapneumovirus (hMPV) pneumonia in a lung transplant recipient treated with intravenous ribavirin. J Heart Lung Transplant 2007; 26 (8): 862–864. [DOI] [PMC free article] [PubMed] [Google Scholar]


