Summary
Swine enteric coronaviruses, including porcine epidemic diarrhoea virus (PEDV) and porcine deltacoronavirus (PDCoV), have emerged and spread throughout the North American swine industry over the last four years. These diseases cause significant losses within the pork industry and within the first year after PEDV introduction, approximately 10% of the US herd died due to the disease. Similar to other enteric coronaviruses, such as transmissible gastroenteritis virus (TGEV), these emerging swine enteric coronavirus diseases (SECD) are age‐dependent, with high morbidity and mortality in neonatal pigs. Since the introduction of SECD, research has focused on investigating viral pathogenesis through experimental inoculation, increasing maternal antibody for neonatal protection, understanding transmission risks through feed and transportation, and outlining the importance of biosecurity in preventing SECD introduction and spread. A survey of swine professionals conducted for this review revealed that the majority of respondents (75%) believe SECD can be eradicated and that most herds have been successful at long‐term elimination of SECD after exposure (80%). However, unique properties of SECD, such as ineffective immunity through parenteral vaccination and a low oral infectious dose, play a major role in management of SECD. This review serves to describe the current knowledge of SECD and the characteristics of these viruses which provide both opportunities and challenges for long‐term disease control and potential eradication from the US swine population.
Keywords: coronavirus, porcine deltacoronavirus, porcine epidemic diarrhoea virus, review, swine, transmission
1. INTRODUCTION
In April 2013, porcine epidemic diarrhoea (PED) emerged as a new swine enteric disease in US swine (Stevenson et al., 2013). Within 1 year after introduction, the virus had spread to most swine‐producing areas and caused the loss of approximately 7 million pigs, affecting primarily neonates within the first few weeks of life. The economic losses due to the introduction of PED are substantial, with estimates of $300,000 lost per year for a single 700‐sow farrow‐to‐finishing herd (Weng, Weersink, Poljak, de Lange, & von Massow, 2016). The causative agent, PED virus (PEDV), is an enveloped, positive‐sense RNA virus in the family Coronaviridae and genus Alphacoronavirus with a 28 kb genome (Kocherhans, Bridgen, Ackermann, & Tobler, 2001). The virus was first described in Europe almost 40 years ago (Pensaert & de Bouck, 1978; Wood, 1977) and had been primarily maintained as an endemic pathogen in European and Asian swine populations until its introduction into North American in 2013. Since the 1990s, significant outbreaks of PED have occurred in The Czech Republic, Belgium, Hungary, Korea, China, Italy and Thailand (Song & Park, 2012) with a re‐emergence of PED as a major swine disease in China around 2010 (Sun et al., 2012; Wang et al., 2013). Sequence analysis of US strains showed greater than 99% nucleotide identity to strains circulating in China between 2011 and 2012, indicating a probable Chinese origin of the US strain (Chen et al., 2014; Huang et al., 2013). Since 2013, PED has been a significant disease in the US swine industry, with 39 states and >3,750 premises being confirmed as positive for the virus as of December 2017 (USDA, 2017).
In January 2014, porcine deltacoronavirus (PDCoV) emerged as another new swine enteric disease in US swine (Marthaler, Jiang, Collins, & Rossow, 2014; Wang, Byrum, & Zhang, 2014a). The virus is an enveloped, positive‐sense RNA virus in the family Coronaviridae and genus Deltacoronavirus with a 25.4 kb genome (Wang et al., 2014a). Prior to its US introduction, PDCoV had previously only been described in China in 2012 (Woo et al., 2012). Sequence analysis of US strains showed approximately 99% nucleotide identity to the PDCoV strains previously detected in Hong Kong in 2012 (Wang, Byrum, & Zhang, 2014b). Since its introduction in 2014, PDCoV has spread throughout most swine‐producing states, albeit at a lower prevalence rate than PEDV, with 21 states and >540 premises being confirmed as positive for the virus as of December 2017 (USDA, 2017). Subsequent to US introduction, PDCoV has since been detected in Thailand and Korea (Janetanakit et al., 2016; Lee & Lee, 2014).
Clinical disease and lesions caused by these two viruses are clinically indistinguishable from transmissible gastroenteritis virus (TGEV), a closely related virus in the family Coronaviridae and genus Alphacoronavirus associated with diarrhoea in young pigs. However, all three viruses are antigenically distinct and cross‐protection does not occur between PEDV, PDCoV and TGEV (Hofmann & Wyler, 1989; Jung, Hu, & Saif, 2016; Ma et al., 2015; Pensaert & de Bouck, 1978; Pensaert, Debouck, & Reynolds, 1981; Zhang, 2016). Since PEDV and PDCoV emerged into the US swine population, much research has been performed to understand the factors around pathogenesis, immunity, introduction, transmission and management of these diseases; however, SECD continue to be introduced into new herds each week and gaps in knowledge remain. Tissue tropism, diagnosis, viral structure and proteins, physiochemical properties and pathophysiology of PEDV and PDCoV have been reviewed elsewhere (Jung & Saif, 2015; Jung et al., 2016; Lee, 2015; Zhang, 2016). The current review describes the characteristics of these viruses, such as a low oral infectious dose, survival in feed and prolonged viral shedding, which create both opportunities and challenges for future management and potential eradication.
2. THE US EXPERIENCE
The first case of PEDV confirmed by a veterinary diagnostic laboratory was in Ohio during the week of 15 April 2013 (AASV, 2013). Soon thereafter, PEDV spread rapidly and within weeks had been detected in most swine‐producing states in the United States (Figure 1). By 3 June 2013, a mere 8 weeks after the initial case, PEDV was present in 12 states and Iowa had 85 total positive cases reported. And by approximately 14 months after the introduction of PEDV, it was estimated that 50% of the breeding herds in the United States had been infected (Goede & Morrison, 2016; Goede et al., 2015). Stevenson et al. (2013) described one of the first outbreaks on four different swine farms in Iowa, reporting high mortality in suckling pigs with diarrhoea and vomiting in pigs of varying ages. PEDV was sequenced and compared between the four unassociated farms; strains were close to 100% identical and suggested that the virus introduced on all four farms had originated via the same source (Stevenson et al., 2013). Retrospective analysis of serum collected from feral swine (n = 368) in six states between 2011 and 2013 was all negative for PEDV on ELISA, suggesting a lack of viral circulation in wild pigs prior to introduction in commercial swine (Scott et al., 2016).
Figure 1.
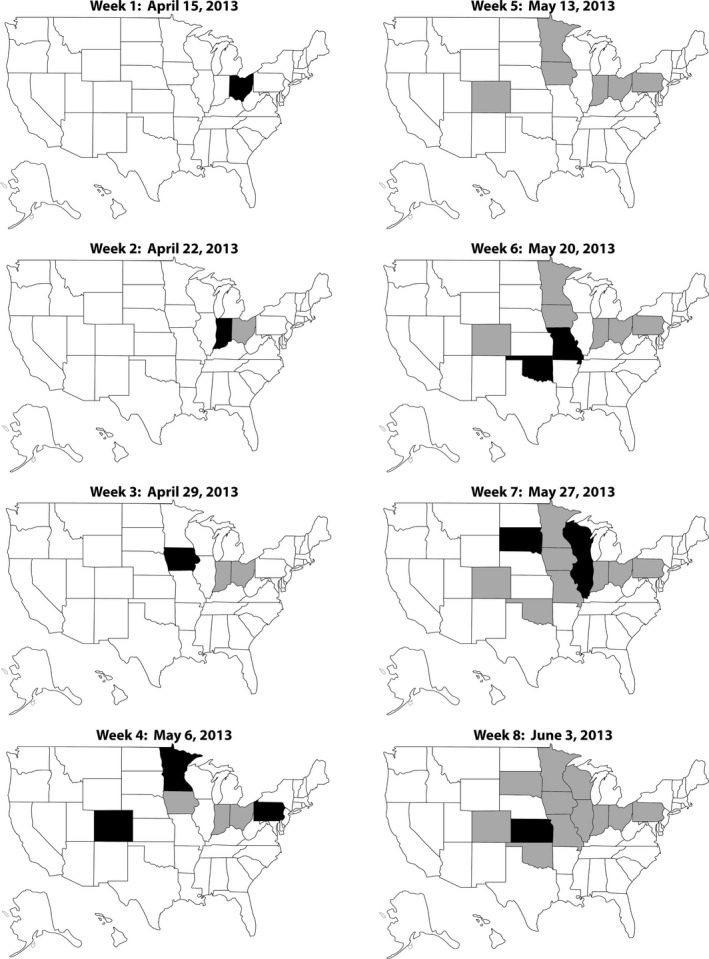
Rapid dissemination of porcine epidemic diarrhoea virus (PEDV) throughout the United States in the first 8 weeks after introduction. States shown in black represent new positives during each week; states shown in grey represent those identified as positive during previous weeks. Adapted from data compiled by the Iowa State University Veterinary Diagnostic Laboratory. https://www.aasv.org/aasv%20website/Resources/Diseases/PED/LABSUMTOT_WK_STATE.pdf
The first case of PDCoV confirmed by a veterinary diagnostic laboratory was in Ohio during January to February 2014 (Wang et al., 2014a). Soon thereafter, PDCoV spread to several swine‐producing states and by the week of 30 March 2014, approximately 10 weeks after its introduction, PDCoV had spread to six additional states, including Iowa, Illinois, Indiana, Michigan, Minnesota and Montana (USDA, 2014). Wang et al. (2014a) described the initial outbreak in Ohio, where both sows and young pigs were reported to have watery diarrhoea. However, death loss in neonatal pigs described in this outbreak was significantly less (30%–40% mortality) than what had been reported for PEDV (approaching 100%) (Wang et al., 2014a). Retrospective PCR analysis of samples submitted to the Iowa State University Diagnostic Laboratory for enteric disease revealed the presence of PDCoV as early as August 2013 (Sinha, Gauger, Zhang, Yoon, & Harmon, 2015). Antibody testing suggested an even earlier exposure of US swine to PDCoV, with samples from 2010 being positive for anti‐PDCoV IgG (Thachil, Gerber, Xiao, Huang, & Opriessnig, 2015).
Disease control measures highlighted in the United States include demarcating a line of separation between outside sources and the farm, cleaning and disinfecting barns and equipment, ensuring replacement animals were negative, and enhancing biosecurity procedures for personnel as well as delivery and transport vehicles. A comprehensive informational guide has been compiled by the National Pork Board on PEDV and can be found online (https://library.pork.org/media/?mediaId=41A7CFB3-8856-4DE7-82216ADAF811B745).
On 5 June 2014, PEDV and PDCoV became reportable diseases in the United States by USDA, and a Situation Report is published weekly at http://www.aphis.usda.gov. New positive accessions continue to be detected in the United States every week; two new confirmed PEDV‐positive premises were reported during the week of 19–25 November 2017 (USDA, 2017). Although over 4,000 premises in the United States have been confirmed positive for PEDV and/or PDCoV since reporting was initiated, approximately 14% of these locations have successfully eradicated the virus and have been confirmed as negative for SECD (USDA, 2017). However, this number likely underestimates the true percentage of successful eradications as inherently fewer tests are used to confirm a negative result in those herds lacking clinical disease. Moreover, this underscores an important gap in knowledge for true prevalence throughout the United States due to inconsistent reporting as well as the percentage and protocols of herds with success in eradicating these pathogens after introduction.
3. THE CANADIAN EXPERIENCE
The Canadian PED experience has been considerably different than that in the United States. PED broke in Canada several months after its US introduction, with the first confirmed case in Ontario reported in January 2014 (Kochhar, 2014). Ojkic et al. (2015) described the initial PEDV outbreak in Canada as a rapidly spreading diarrhoeal disease non‐responsive to antimicrobial therapy with vomiting and 100% mortality in pigs less than 1 week of age in a farrow‐to‐finish swine herd (Ojkic et al., 2015). The Canadian PEDV isolate was >99% identical to the United States isolate (Pasick et al., 2014). With the benefit of the experience of PED in the United States, the Canadians (including swine veterinarians, producers and regulatory authorities) were better prepared and more able to contain the spread of the virus throughout the country. The source of the virus was linked to feed (Pasick et al., 2014; Pasma, Furness, Alves, & Aubry, 2016); the company recalled the feed, and the outbreak stopped spreading. Infected sites were voluntarily shut down, and regional spread was contained. There have been sporadic PED outbreaks primarily in Quebec and Ontario associated with trucks which have been quickly contained; the losses due to PED in Canada have been significantly less than those which have occurred in the United States. Most recently, there has been a PED outbreak in Manitoba that has spread through the local region, including 90 sites as of 7 November 2017 (Manitoba, 2017). The outbreak is expected to remain localized to this general area and thus far, 22 of the 90 outbreak sites are now presumed negative for PED.
Disease control measures highlighted in Canada include restricting swine movement to non‐shedding pigs, creating buffer zones around positive sites and enhancing biosecurity of transportation, slaughter facilities and personnel. A 1‐page biosecurity guideline list for barns, personnel and sites has been compiled by ManitobaPork and can be found online (http://www.manitobapork.com/images/PEDv_Biosecurity_Cheat_Sheet.pdf).
4. CLINICAL DISEASE AND VIRAL SHEDDING
Disease caused by PEDV peaks in the late fall and early winter months in cold temperatures, with the number of confirmed US cases peaking between January and February over the last 3 years (USDA, 2017). Production losses may be doubled for those herds infected in colder periods of the year compared to those initially exposed during warmer months (Goede & Morrison, 2016). Clinical disease and positive cases typically decline in late spring, summer and early fall. Infection of neonatal pigs usually results in significant mortality rates (approaching 100%), caused by malabsorptive diarrhoea and dehydration as a result of small intestinal enterocyte necrosis and villous atrophy (Figure 2). Infection of grow‐finish animals results in moderate morbidity but typically little to no mortality, with vomiting, lethargy and mild to moderate diarrhoea. Infections in adult swine may be inapparent due to minimal to no gastrointestinal signs; however, some sows and gilts may have mild diarrhoea, reduced activity and inappetance upon naïve exposure.
Figure 2.
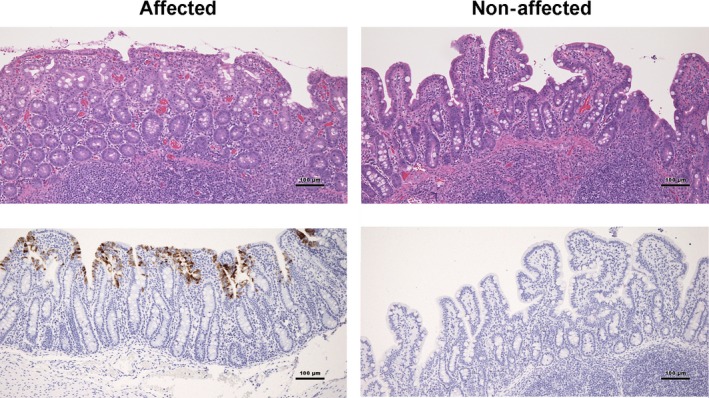
Small intestinal villous atrophy associated with porcine epidemic diarrhoea virus (PEDV) infection. Images are shown of 4‐week‐old pigs 7 days of post‐infection with PEDV. The affected pig has villous atrophy in the small intestine (H&E stain, top left panel) with positive immunohistochemical staining of villous enterocytes (brown stain, bottom left panel). No significant microscopic lesions are noted in the non‐affected pig (H&E stain, top right panel) with enterocytes negative for PEDV staining on immunohistochemistry (bottom right panel). Images kindly provided by Dr. Jerome Nietfeld [Colour figure can be viewed at http://wileyonlinelibrary.com]
The increased severity of diarrhoea, followed by extensive dehydration and death losses, in neonatal suckling pigs is thought to be in part due to a reduced rate of enterocyte replacement on intestinal villi after cell death caused by viral infection and underdeveloped innate defence in the small intestine (Annamalai, Saif, Lu, & Jung, 2015; Jung & Saif, 2015; Moon, 1971; Moon, Norman, & Lambert, 1973). This inverse relationship between severity of disease and age of pigs has been experimentally investigated for PEDV by Shibata et al. (2000). Specifically, 100% morbidity and 100% mortality were documented in 2‐ and 7‐day‐old pigs, 60%–100% morbidity and 0% mortality were documented in 2‐ and 4‐week‐old pigs, and 0% morbidity and 0% mortality were documented in 8‐ and 12‐week‐old pigs (Shibata et al., 2000).
Several experimental infection studies using US strains since 2013 and 2014 have also provided information on the age‐dependency, transmission, morbidity and mortality of PEDV and PDCoV (Table 1). In 1‐day‐old caesarean‐derived colostrum‐deprived (CD/CD) pigs, microscopic lesions and clinical signs appeared within 12‐18 hours post‐infection (hpi) with PEDV and progressed to severe dehydration and diarrhoea within 36–72 hpi (Madson et al., 2015). In 2‐ to 4‐day‐old pigs inoculated with PDCoV, emesis and diarrhoea were both noted by 2 days of post‐infection (dpi) coupled with moderate to severe dehydration and lethargy. Even a single day seemed to impact response to PDCoV in this study, where pigs inoculated at 2 days of age had a mortality rate of 50.1% compared to 21.5% mortality of pigs inoculated at 3 days of age (Vitosh‐Sillman et al., 2016). In 3‐ and 4‐week‐old pigs, emesis, although inconsistent among individual pigs, was the first clinical sign of PED between 2 and 3 dpi and may be considered as a potential first sign of exposure (Madson et al., 2014; Niederwerder et al., 2016). Soon thereafter, diarrhoea, lethargy, dehydration and reduced appetite are common clinical signs lasting approximately 5–8 days in weaned pigs.
Table 1.
Outcome following experimental infection with North American swine enteric coronavirus isolatesa
| Age | Primary clinical outcome | Duration of clinical signs | Mortality | Duration of sheddingb | References | |
|---|---|---|---|---|---|---|
| PEDV | 1 dc | 100% severe diarrhoea | 18 hpid | ND | 12 hpid | Madson et al., (2015) |
| 10–35 dc | 100% severe watery diarrhoea | 25 hpid | ND | 24 hpid | Jung et al., (2014) | |
| 3 w | 70% watery diarrhoea, reduced ADG, emesis | 2–10 dpi | 0% | 1–24 dpi | Madson et al., (2014) | |
| 3 w | Depression, inappetence, diarrhoea, emesis | 1–11 dpi | 0% | 1–18 dpi | Pasick et al., (2014) | |
| 4 w | 30% watery diarrhoea, ±emesis, dehydration | 3–8 dpi | 0% | 2–28 dpi | Niederwerder et al., (2016) | |
| 4 we | 100% mild‐moderate diarrhoea | 2–9 dpi | 0% | 1–42 dpi | Crawford et al., (2015) | |
| 5 w | Reduced ADG | 0–7 dpi | 0% | 1–5 dpi | Curry et al., (2017) | |
| Gilts | Reduced ADG | 0–7 dpi | 0% | ND | Schweer, Schwartz et al., (2016) | |
| PDCoV | 2–4 d | 100% diarrhoea, ±emesis, dehydration, lethargy | 2–12 dpi | 27% | 2–21 dpi | Vitosh‐Sillman et al., (2016) |
| 5 d | 100% profuse watery diarrhoea | 2–7 dpi | 0% | 2–7 dpi | Chen et al., (2015) | |
| 10 d | 100% severe watery diarrhoea | 1–10 dpi | 0% | 1–21 dpi | Ma et al., (2015) | |
| 10 dc | Moderate diarrhoea, ±emesis | 3 dpid | ND | 1 dpid | Ma et al., (2015) | |
| 19 dc | 100% severe watery diarrhoea, emesis | 1 dpid | ND | 1 dpid | Ma et al., (2015) | |
| 5 w | No morbidity | None | 0% | 2–5 dpi | Curry et al., (2017) | |
| Dams | 100% diarrhoea, inappetence | 3–9 dpi | 0% | 2–35 dpi | Vitosh‐Sillman et al., (2016)) |
Key: PEDV, porcine epidemic diarrhoea virus; PDCoV, porcine deltacoronavirus; d, days; w, weeks; m, months; ADG, average daily gain; dpi, day post‐infection; hpi, hour post‐infection; ND, not determined.
Length of shedding reported as the first and last positive faecal sample or swab in the group.
Caesarean‐derived colostrum‐deprived or gnotobiotic pigs used for inoculation.
Timing is first detection of clinical disease or faecal shedding; pigs were euthanized and not followed to assess duration.
Pigs were exposed through direct contact with an inoculated pig.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Perhaps the most interesting finding from the experimental studies was the discrepancy between clinical disease duration and the length of time virus was detected in faeces or the intestine. In almost all experimental infection trials (Table 1), virus was detected prior to the initiation of clinical disease and well past resolution of clinical signs. For example, in weaned pigs at 3 and 4 weeks of age, PEDV shedding in faeces could be detected approximately 1 day prior to the development of clinical signs and 2–3 weeks after clinical disease abates (Madson et al., 2014; Niederwerder et al., 2016). Specifically, although 4‐week‐old pigs inoculated with PEDV had mostly resolved clinical disease by 8 dpi, viral shedding was detected until 28 dpi and the majority of pigs maintained PEDV in the small intestine until 42 dpi (Niederwerder et al., 2016). Similarly, 2‐ to 4‐day‐old pigs infected with PDCoV had resolved clinical disease by 12 dpi, but maintained faecal shedding until 21 dpi and still had virus detected in the small intestine until 42 dpi. Additionally, dams in this study maintained faecal shedding and PDCoV in the small intestine until 35 dpi, 26 days after resolution of clinical disease (Vitosh‐Sillman et al., 2016). In another study, 10‐day‐old piglets challenged with PDCoV had resolved clinical disease by 10 dpi and histopathologic lesions by 21 dpi; however, PDCoV RNA was still detectable in faeces, intestine and blood at 21 dpi when the study concluded (Ma et al., 2015). Prolonged PDCoV shedding has also been reported in 14‐day‐old gnotobiotic pigs after experimental infection (Hu, Jung, Vlasova, & Saif, 2016). Taken together, these studies provide evidence that pigs can shed low levels of virus for several weeks post‐infection and may harbour virus in the intestine for over 1 month after clinical disease subsides. Additionally, it is worth noting that virus was often detected at the conclusion of these studies post‐euthanasia and that the exact time period of viral persistence in tissues remains unknown. Moreover, diagnosing a true negative on faecal swabs is challenging due to a lack of viral shedding in some pigs harbouring virus in the intestine.
In an effort to investigate the potential for chronic PEDV infection and transmission, Crawford et al. (2015) designed a study where naïve sentinel pigs were sequentially exposed to PEDV‐infected pigs at weekly intervals. Although PEDV‐infected animals had intermittent viral shedding through 42 dpi, naïve sentinel pigs only became infected when introduced at 7 and 14 dpi, but not at later times (Crawford et al., 2015). This suggests that the presence of virus in the faeces and intestine several weeks after inoculation may not pose a risk for productive transmission. However, long‐term persistence of PEDV has been reported in the field (Park & Lee, 2009; Pijpers, van Nieuwstadt, Terpstra, & Verheijden, 1993), and healthy pigs should be considered as potential SECD carriers post‐exposure. For example, a recent interview from Manitoba Pork in Canada stated that long‐term shedding up to 70 dpi had been a challenge to current disease management in the field (Hamblin, 2017).
Diagnosis of SECD is typically performed through PCR detection of nucleic acid in faeces, blood or oral fluids. Faeces or faecal swabs are considered the sample of choice during acute disease as they often contain the highest titres of PDCoV and PEDV. Viral detection in serum is more variable, with most suckling or nursery pigs developing a low‐level viremia during acute infection (Chen et al., 2015; Jung et al., 2014; Niederwerder et al., 2016). Dams infected with PDCoV or PEDV may fail to develop a detectable viremia. For example, suckling pigs infected with PDCoV had detectable viremia between 2 and 5 dpi, whereas infected dams lacked a detectable viremia (Vitosh‐Sillman et al., 2016). Oral fluids have also been shown to be a valuable tool for PCR surveillance of PEDV and PDCoV, maintaining positive results for approximately 4 weeks post‐infection (Niederwerder et al., 2016; Vitosh‐Sillman et al., 2016). Antibody detection may be used for herd surveillance but is less useful in acute diagnoses as antibody production is typically delayed, with the majority of pigs not seroconverting until at least two weeks post‐exposure (Niederwerder et al., 2016; Pasick et al., 2014). However, due to intermittent faecal shedding in more chronic or endemic infections, antibody testing may be more reliable to detect ongoing exposures within a herd.
5. LONG‐TERM IMPACT OF SECD ON PIG GROWTH AND PRODUCTION
Although mortality approaches 100% in neonatal pigs and death losses are relatively easy to quantify during an acute outbreak, less is known about the impact of SECD on growth and production during an endemic infection or during recovery after an acute outbreak. Goede and Morrison (2016) quantified the production impacts of PED through data collected from 429 exposed herds between April 2013 and July 2014 by the Swine Health Monitoring Project (SHMP). Based on this data, the mean time required to produce consistently negative piglets on PCR (as defined by four consecutive samples representing 30 or more litres) was 29.5 weeks after the outbreak. The median time to return to 100% of baseline production, as measured by the number of pigs weaned per week, was 21 weeks. Additionally, these authors noted that 6% of herds participating in the SHMP failed to return to 100% of baseline production approximately 1 year after exposure. These data provide evidence that production losses occur for several months after an acute outbreak and that for a small number of herds, decreased production may be long‐term, continuing for >1 year after the initial exposure (Goede & Morrison, 2016).
Other experimental trials have monitored weight gain in infected pigs to estimate the long‐term impacts of SECD. For example, in 3‐week‐old pigs experimentally infected with PEDV, average daily gain (ADG) was significantly lower than control pigs during the first week post‐infection. Although subsequent weekly ADG was similar between the control and infected pigs for the remainder of the 5‐week study, infected pigs maintained lower overall weights (Madson et al., 2014). In another study (Curry et al., 2017), similar findings were reported in 5‐week‐old pigs infected with PEDV; ADG in the first seven days after PEDV infection was significantly less than the ADG of non‐infected controls (p < .001). Even though ADG was not significantly different between the two groups during any other time of the 6‐week study, body weight for the PEDV‐infected pigs was on average 6.5 kg less than the control pigs at 42 dpi (Curry et al., 2017). In growing pigs approximately 7 weeks of age, Schweer, Schwartz et al. (2016) compared the growth performance characteristics of pigs infected with PEDV alone, pigs infected with a combination of PEDV and porcine reproductive and respiratory syndrome virus (PRRSV), and uninfected controls. Infection with PEDV reduced the ADG of gilts compared to the controls by almost half (0.66 kg compared to 0.35 kg) over the 7 days of post‐infection. Interestingly, infection of pigs with PRRSV and PEDV in combination resulted in a greater reduction in ADG than with either singular infection, indicating the importance of co‐infections in the pathogenesis of both viruses (Schweer, Schwartz et al., 2016).
Field studies on the impact of PEDV through the evaluation of production records have reported similar effects. Alvarez, Sarradell, Morrison, and Perez (2015) compared the records of either nursery or wean to finish pigs (3–13 weeks of age) before and after the introduction of PEDV in a large Midwestern swine system. Prior to PEDV exposure, mean monthly mortality rates were 4.3%–4.8% in growing pigs. After PEDV exposure, mortality rates increased on average by 12.5%. With regard to average daily gain, a mean reduction in 0.16 lb was documented in the PEDV‐exposed groups. Interestingly, PEDV did not result in a significant decrease in average daily feed intake. This study underscores production losses in the field through mortality and reduced feed conversion as well as highlights a research need for understanding how to feed pigs with SECD (Alvarez et al., 2015).
Although fewer studies have been completed with PDCoV, results are inconsistent with PEDV. In the same Curry et al. (2017) study described above, researchers evaluated the effects of PDCoV infection on growth performance in 5‐week‐old pigs and found no significant differences with non‐infected controls. However, it should be noted that PDCoV shedding was relatively low in concentration and short‐lived when compared to PEDV‐infected pigs from the same study (Curry et al., 2017). Chen et al. (2015) found that 5‐day‐old pigs inoculated with PDCoV had lower ADG in the first few days after infection compared to control pigs; however, ADG differences were not statistically significant, and no significant reduction in body weight was seen at either 4 or 7 dpi (Chen et al., 2015). Although this evidence suggests that PEDV has a greater production impact on growth, additional research is needed to compare the growth impact of PDCoV and PEDV in side‐by‐side controlled experimental studies.
The long‐term impact of PEDV and potentially PDCoV on performance may be due to the systemic inflammatory effects on feed intake and utilization, localized effects on enterocyte health and function, or a combination of the two. For example, infection with PEDV results in apoptosis of epithelial cells, increased gastrointestinal permeability, disruption of tight and adherens junctions of enterocytes, and reduced goblet cells present in the small intestine (Jung, Eyerly, Annamalai, Lu, & Saif, 2015; Jung & Saif, 2017; Kim & Lee, 2014; Schweer, Pearce et al., 2016). These disruptions to normal gastrointestinal anatomy and physiology likely impact digestive capacity and function. Studies evaluating how SECD impacts growth performance long‐term through the finishing period to market are lacking; additional research is necessary to determine the lifelong effects of SECD on weight as well as determine the nutrient requirements for pigs with enterocyte compromise post‐infection. However, data thus far suggest that PEDV‐infected pigs may require an extended period in the grow/finish phase to reach market weight and that reduced weight gain early on post‐infection may be a largely unrecognized long‐term economic loss due to SECD.
6. TRANSMISSION
Faecal–oral direct or indirect fomite exposure is the primary routes of transmission for SECD, with aerosol transmission also being considered as a possible route for viral spread. Alonso et al. (2014) evaluated the detection and infectivity of PEDV collected from air samples in both experimentally infected as well as naturally infected field premises. Although PEDV was detected in air samples from the experimental setting and at distances of up to 10 miles in the field setting, only the PEDV collected in air samples from experimentally inoculated pigs was shown to be infectious to other pigs through bioassay. The authors attributed this difference to a lower PEDV titre in field samples as well as environmental conditions affecting the infectivity of the airborne virus (Alonso et al., 2014). When compared to porcine reproductive and respiratory syndrome virus (PRRSV) and highly pathogenic avian influenza (HPAIV), PEDV was detected at the highest concentration in air samples collected from naturally infected herds (Alonso et al., 2017).
When studies have included contact controls to assess transmission from cohoused infected pigs, faecal–oral transmission is typically rapid and occurs within 24 hr of contact (Crawford et al., 2015; Niederwerder et al., 2016; Vitosh‐Sillman et al., 2016). Although faecal–oral transmission occurs quickly after experimental inoculation, productive transmission depends on both the oral dose and the age of the pigs. For example, Thomas et al. (2015) reported that an oral dose as low as 10 ml of 0.00562 TCID50/ml was capable of causing infection in a 6‐day‐old pig whereas a dose of 10 ml of 5.62 TCID50/ml was required for productive oral transmission in 24‐day‐old pigs. Interestingly, the minimum infectious dose for 6‐day‐old pigs was below the limit of detection on PCR. These data underscore the age‐dependent susceptibility to SECD and the extremely small amount of virus necessary to cause infection in neonatal pigs through oral exposure (Thomas et al., 2015).
Experimental aerosol transmission studies have had mixed results with regard to SECD. For example, 4‐week‐old aerosol control pigs housed in the same room as PEDV‐infected pigs in a BLS‐3 facility failed to develop productive infections, despite PEDV nucleic acid being detected at low levels in nasal swabs of control animals (Niederwerder et al., 2016). In contrast, Vitosh‐Sillman et al. (2016) investigated aerosol transmission of PDCoV in a similar manner to the above study, albeit in a BSL‐2 facility, and found that aerosol control dams and piglets developed diarrhoea due to productive transmission approximately 2 days after the infected pigs were inoculated (Vitosh‐Sillman et al., 2016). Rate of air exchange and disinfectant protocols in a BSL‐2 versus a BSL‐3 facility likely contributed to the differences in these two studies.
It would appear that productive aerosol transmission can occur with SECD in experimental conditions. In Niederwerder et al. (2016) and Vitosh‐Sillman et al. (2016), extensive examination of the respiratory tract revealed no evidence of infection in respiratory‐associated tissues. Thus, the mechanism by which aerosol transmission occurs is likely due to ingestion of aerosolized virus via the faecal–oral route. This is in stark contrast to the tissue tropism of transmissible gastroenteritis virus (TGEV), where infection of the respiratory tract is a known characteristic of pathogenesis (Furuuchi & Shimizu, 1976; Kemeny, Wiltsey, & Riley, 1975; O'Toole, Brown, Bridges, & Cartwright, 1989).
7. RISK FACTORS FOR INTRODUCTION
Although several possibilities have been considered for how PEDV and PDCoV were introduced and rapidly disseminated throughout pig farms in the United States and North America (Figure 3), the exact mechanism by which these two viruses entered and spread are not completely understood. Aerosols, neighbouring farms, breaches in biosecurity, contaminated transport vehicles, feed and feed totes have all been implicated as potential contributors (Alonso et al., 2014; Bowman, Krogwold, Price, Davis, & Moeller, 2015; Dee et al., 2014; Lowe et al., 2014; Pasick et al., 2014; Scott et al., 2016). Alvarez, Goede, Morrison, and Perez (2016) investigated the spatial and temporal epidemiology of PEDV outbreaks in both the southeastern and midwestern regions of the United States to understand the role of neighbouring farms in local spread. Farms infected with PEDV had significant spatial clustering, with negative herds located <2 km from an acutely infected farm having a significant risk of subsequent exposure (Alvarez et al., 2016). Lowe et al. (2014) investigated truck trailers for evidence of PEDV contamination after transporting pigs to harvest facilities in June 2013. Samples collected from 575 trailer floors showed that 6.6% were positive prior to unloading pigs and another 5.2% became PEDV‐positive sometime, while pigs were being unloaded (Lowe et al., 2014). These data suggest that transport vehicles may (i) serve as fomites for SECD spread and (ii) become contaminated with SECD and potentially other pathogens at locations where pigs congregate from multiple sources, such as abattoirs.
Figure 3.
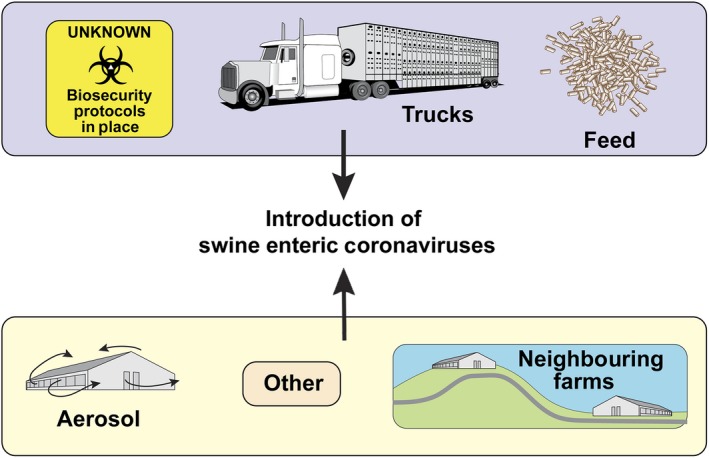
Routes considered important for swine enteric coronavirus diseases (SECD) introduction and transmission. Trucks, personnel, feed, aerosols, equipment and other fomites may be transferred between farms, resulting in introduction and transmission of SECD [Colour figure can be viewed at http://wileyonlinelibrary.com]
After PEDV was introduced into the United States, several investigations revealed an epidemiological link to contaminated feed as a vehicle for introduction and transmission. A root cause investigation performed by USDA revealed that flexible intermediate bulk containers or feed totes may have served as fomites in the spread of PEDV. A study performed by this group showed that PEDV remained viable on tote material for 35 days at room temperature (Scott et al., 2016). Further investigations into the epidemiologic link between PEDV and contaminated feed have demonstrated that PCR‐positive feed is not always infectious in a bioassay. For example, Bowman, Krogwold et al. (2015) investigated pelleted feed as a potential common source for PEDV introduction affecting multiple sites and various age groups within an Ohio swine operation. Although PEDV nucleic acid was detected in an unopened bag of feed used on the operation, the feed did not result in productive PEDV infection when fed to naïve pigs for bioassay testing (Bowman, Krogwold et al., 2015). Similar results were described in Pasick et al. (2014) during an investigation into the route of PEDV introduction into Canada. Spray‐dried porcine plasma (SDPP) imported from the United States and the associated complete feed were found to contain low levels of PEDV nucleic acid on qPCR. However, only the PEDV‐positive SDPP was confirmed as infectious to 3‐week‐old pigs when orally administered for bioassay testing; inconclusive results were obtained for the contaminated complete feed. Furthermore, infection caused by the PEDV‐positive SDPP was transmissible to contact controls (Pasick et al., 2014). In contrast to these results, Opriessnig, Xiao, Gerber, Zhang, and Halbur (2014) found that PEDV‐contaminated SDPP did not result in productive transmission to 3‐week‐old pigs (Opriessnig et al., 2014). These trials serve as a reminder of the important distinction between being positive for nucleic acid, being positive for infectious virus and being capable of causing clinical disease.
In further experimental trials investigating the link between SECD and feed, it was demonstrated that the minimum oral infectious dose of PEDV in conventional 10‐day‐old piglets is extremely low in contaminated feed (5.6 × 101 TCID50/g), indicating that only a small volume of faecal material from a shedding piglet is needed to create a significant amount of infectious feed (Schumacher et al., 2016). Other research has shown that infection with PEDV can easily result from the consumption of contaminated feed via natural feeding behaviour (Dee et al., 2014). Furthermore, PEDV is capable of surviving for several weeks in contaminated feed ingredients under simulated shipping conditions with varying temperature and humidity in a transboundary model from China. Specifically, soybean meal, vitamin D, lysine and choline were all shown to support PEDV survival during the 37‐day transboundary model (Dee et al., 2016). In another study evaluating the survival of PEDV, PDCoV and TGEV at room temperature over a 56‐day trial, soybean meal was also found to promote coronavirus survival when compared to complete feed and other feed ingredients (Trudeau et al., 2017).
Porcine epidemic diarrhoea virus has been identified as a biological hazard for feed mills, and biosecurity protocols have been outlined for this risk, such as procedures to reduce the likelihood of PEDV entering the facility through ingredients, people and trucks as well as preventing cross‐contamination across manufacturing equipment (Cochrane, Dritz, Woodworth, Stark et al., 2016). Once PEDV is introduced into a swine feed manufacturing facility, there is significant dissemination of viral RNA throughout surfaces and equipment (Huss et al., 2017), making decontamination and viral elimination a challenge. Several mitigation tools have been investigated for this risk. For example, abrasive ingredients, such as rice hulls, may be used to flush equipment and reduce viral contamination of subsequent feed (Gebhardt et al., 2016). In addition, feed additives, such as medium chain fatty acids (MCFA) and formaldehyde, are effective at reducing PEDV contamination in complete feed and feed ingredients (Cochrane, Dritz, Woodworth, Huss et al., 2016; Cochrane, Saensukjaroenphon et al., 2016; Dee et al., 2015, 2016). Ionizing radiation and the application of heat, such as during the pelleting or spray‐drying process, will also reduce viral load and infectivity of PEDV‐contaminated feed and feed ingredients (Cochrane et al., 2017; Gerber et al., 2014; Trudeau et al., 2016).
The lesson learned from PED has made it necessary to quantitate the risk that feed may play in the potential introduction and transmission of other endemic and foreign animal diseases. Consistent with understanding and mitigating this risk, recent research studies funded by the Swine Health Information Center (SHIC) have focused on investigating the survival of viruses important to the US swine industry through imported feed ingredients (Swine Health Information Center, 2016, 2017). Viruses such as Seneca virus A (surrogate for foot and mouth disease virus), bovine herpesvirus‐1 (surrogate for pseudorabies virus), feline calicivirus (surrogate for vesicular exanthema virus), and porcine reproductive and respiratory syndrome virus (PRRSV) have all been shown to be capable of maintaining infectivity for weeks under simulated shipping conditions from China (Swine Health Information Center, S, 2017).
8. HERD AND FARM MANAGEMENT
Several management and elimination strategies have been utilized for control of swine enteric coronaviruses. Figure 4 diagrams practises which may be employed for management or eradication upon SECD exposure, including potential outcomes. To manage SECD, initiating and maintaining herd immunity is typically performed through feedback and potentially vaccination. Controlled exposure through feedback of infectious SECD in the form of faeces, ingesta or homogenized intestines is commonly used to simultaneously stimulate immunity in sows and replacement gilts through programs designed to “load, close and expose” the herd. Stimulating gastrointestinal immunity in sows and gilts through controlled exposure increases the beneficial lactogenic immunity through IgA in colostrum or milk consumed by neonatal pigs. Lactogenic immunity is critical for protecting piglets from the high morbidity and mortality of SECD and mitigating the significant losses that can occur within naïve herds (Langel, Paim, Lager, Vlasova, & Saif, 2016). For example, Goede et al. (2015) compared the PEDV challenge response of 3‐day‐old piglets from sows previously exposed to a mild PEDV isolate through whole‐herd feedback to 3‐day‐old piglets from sows naïve to the virus. Piglets born to sows previously exposed through feedback had a 33% increase in survival, a 57% reduction in diarrhoea and reduced viral loads (estimated 200‐fold to 400‐fold lower) in the intestines compared to 3‐day‐old piglets born to naïve dams. In this study, lactogenic immunity, detected by ELISA as anti‐PEDV IgA in colostrum or milk, was still present 7 months after sows had received the feedback and provided partial cross‐protection against a different PEDV strain (Goede et al., 2015).
Figure 4.
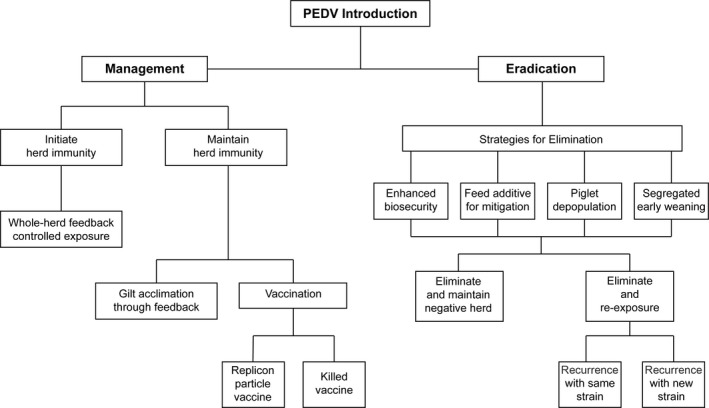
Disease control strategies and potential outcomes following swine enteric coronavirus exposure. Once porcine epidemic diarrhoea virus (PEDV) is introduced into a swine herd, strategies for disease control are utilized to stimulate immunity for management or eliminate the virus for eradication
Although administering PEDV feedback to reproducing females has clear benefits, such as stimulating immunity and providing benefits to offspring, feedback is generally considered to have several unknowns and potential risks. Importantly, there is no widely accepted standard feedback protocol shown to have the highest efficacy in controlled experimental conditions. This leads to several factors that vary considerably between herds administering feedback, such as (i) virus concentration and volume of material administered, (ii) physical material administered (i.e., faeces, homogenized intestines, ingesta or a mix), (iii) frequency and timing of administration, and (iv) procedures to ensure material is free of other infectious agents (i.e., multiplex PCR, metagenomic sequencing, bacterial culture and herd history). As a result, feedback procedures utilized in the field result in inconsistent outcomes, such as the robustness and duration of the sow or gilt immune response.
Several potential vaccine products have been developed or are in development for SECD and have been reviewed elsewhere (Crawford, Lager, Kulshreshtha, Miller, & Faaberg, 2016; Gerdts & Zakhartchouk, 2017). Even so, the currently available vaccines are generally considered to provide incomplete protection in naïve animals. Subsequent to feedback administration or natural exposure, killed vaccines may effectively booster immunity and could provide an alternative for repeated virulent SECD exposure through the oral route. In Gillespie, Song, Inskeep, Stone, and Murtaugh (2017), PEDV‐neutralizing antibodies in milk and colostrum were significantly increased (approximately fivefold) in vaccinated sows with pre‐existing immunity compared to non‐vaccinated sows with pre‐existing immunity. However, this same response to an inactivated vaccine was not seen in naïve sows (Gillespie et al., 2017). Overall, use of feedback and vaccines in the field vary considerably in practice and a standardized procedure for stimulating and maintaining uniform SECD immunity throughout the herd is a much‐needed resource for the industry.
Numerous strategies can be employed for SECD elimination post‐exposure, such as enhanced biosecurity, virus mitigation through a feed additive, piglet depopulation and segregated early weaning (Figure 4). Both PEDV and PDCoV are enveloped viruses and fairly susceptible to a wide range of disinfectants. The Quebec swine health team (EQSP) created a list of over 20 potential disinfectants which may be used to inactivate SECD, including recommended dilution rates, contact times and application rates. For example, Virkon® (potassium peroxymonosulfate; DuPont, Wilmington, DE) is recommended at a 1.0% dilution rate for 10 min of contact time, and Synergize™ (quaternary ammonium chloride and glutaraldehyde; Preserve International, Reno, NV) is recommended at a 0.4% dilution rate for 5–12 min of contact time (EQSP, 2015).
Additionally, experimental studies have been performed to assess the efficacy of disinfection or cleaning procedures on eliminating the risk of SECD spread through fomites or facilities. In Huss et al. (2017), a quaternary ammonium compound, a 5% sodium hypochlorite solution, and heat (60°C) were investigated in succession for their ability to disinfect a feed manufacturing facility contaminated with PEDV. Significant reductions in viral contamination resulted after each step in the disinfection protocol; however, PEDV RNA was still detectable in a small number of samples even after all three steps (Huss et al., 2017). It is unknown whether the detection of RNA in this study indicated the presence of infectious virus. In Bowman, Krogwold et al. (2015); Bowman, Nolting et al. (2015), several disinfectants, including a phenol, quaternary ammonium compound, sodium hypochlorite, oxidizing agent and quaternary ammonium/glutaraldehyde combination, were investigated for their ability to inactivate PEDV in the presence or absence of faeces. Although PEDV RNA was often detected at low levels on PCR after application of the disinfectant, the virus was found to be non‐infectious on virus isolation and/or bioassay (Bowman, Nolting et al., 2015). Holtkamp et al. (2017) investigated accelerated hydrogen peroxide (AHP) as a potential disinfectant on metal contaminated with faecal material to mimic trailer conditions in the field. Dilutions of 1:16 and 1:32 at 30 min of contact time were found to effectively eliminate infectivity of PEDV as shown through pig bioassay. Similar to other studies, it is important to note that most samples maintained positive or suspect Ct values on PCR after treatment with AHP (Holtkamp et al., 2017). Taken together, these studies demonstrate that disinfectants may effectively inactivate PEDV but may not eliminate genetic material, highlighting another important gap in knowledge for distinguishing between PCR‐positive/infectious and PCR‐positive/non‐infectious.
An emerging area of study is the role of direct‐fed microbials and probiotics as potential therapeutics to improve the response of pigs to SECD. For example, Canning et al. (2017) recently reported that feeding the bacterial species Bacillus subtilis to 2‐week‐old pigs infected with PEDV reduced intestinal pathology as shown by decreased atrophic enteritis scores; however, no differences were detected in diarrhoea or average daily gain when compared to non‐treated infected controls (Canning et al., 2017). Interestingly, the presence of normal flora seemed to enhance disease after challenge with a cell culture‐adapted PDCoV in Ma et al. (2015), as conventional 10‐day‐old pigs had more severe disease than 10‐day‐old gnotobiotic pigs post‐challenge with the same virus and inoculation dose. However, small numbers of pigs were used for these studies (3–4 piglets/group), and further investigation is needed to delineate the beneficial or detrimental role of normal flora in SECD pathogenesis (Ma et al., 2015). Overall, the microbiome is a relatively new area of study and additional research to determine the potential application of microbiome therapeutics in the prevention or treatment of SECD would benefit the industry.
9. SURVEY OF SWINE PROFESSIONALS
During the months of May and June 2017, an online swine enteric coronavirus survey was distributed to swine veterinarians and managers through several mechanisms, including the National Pork Board Swine Health Committee, the American Association of Swine Veterinarians list‐serve and the National Pork Board list‐serve. The purpose of the survey was to compile information and opinions from stakeholders on the current SECD situation in the field. The survey allowed individuals to provide SECD information on up to five unique swine herds, including information on size, production type, location, SECD status and exposure, source of SECD introduction and methods for SECD control. Individuals were also asked to give their opinion on whether or not PEDV and PDCoV could be eradicated from the US swine population. Over a 4‐week period, 40 swine veterinarians and managers provided information on 83 swine herds. Summarized results are presented in Table 2.
Table 2.
Survey results on swine enteric coronavirus prevalence and management in the fielda
| Herd Size | 0‐99: 2 (2) | 100‐999: 4 (5) | 1,000‐4,999: 56 (68) | 5,000‐9,999: 15 (18) | 10,000+: 6 (7) | |||||
| Production Type | Farrow to Finish: 16 (19) | Farrow to Nursery: 0 (0) | Farrow to Wean: 60 (72) | |||||||
| Nursery: 1 (1) | Wean to Finish: 2 (2) | Finishing: 4 (5) | ||||||||
| Location | Swine‐Dense: 54 (65) | Not Swine‐Dense: 29 (35) | ||||||||
| SECD Status | Never Been Exposed: 24 (29) | Exposed and Eliminated (No Reintroduction): 47 (57) | ||||||||
| Endemic: 3 (4) | Exposed, Eliminated and Recent Reintroduction (Within the Last Year): 9 (11) | |||||||||
| SECD Exposureb | PEDV and PDCoV Simultaneously: 3 (5) | PEDV and PDCoV at Separate Times: 3 (5) | ||||||||
| PEDV Alone: 50 (85) | PDCoV Alone: 3 (5) | |||||||||
| Source of SECD Introductionc | Trucks: 19 (26) | Aerosol: 7 (10) | ||||||||
| Feed: 21 (29) | Neighboring Farms: 6 (8) | |||||||||
| Other: 6 (8) | Unknown (Biosecurity Protocols in Place): 13 (18) | |||||||||
| Methods for SECD Controld | Controlled Exposure (Gilt Acclimation): 23 (30) | Early Segregated Weaning: 22 (29) | ||||||||
| Load‐Close‐Expose (Whole Herd): 59 (77) | Piglet Depopulation: 36 (47) | |||||||||
| Killed Vaccine: 11 (14) | Enhanced Biosecurity: 43 (56) | |||||||||
| Replicon Particle Vaccine: 8 (10) | Feed Additive: 1 (1) | |||||||||
| Can We Eradicate SECD?e | Yes: 30 (75) | No: 10 (25) | ||||||||
Data are shown as the number (per cent) of herds or individuals responding in each category; 83 total herds represented.
Per cent is based on number of herds with SECD exposure (n = 59).
Per cent is based on number of herds with responses (n = 72).
Question allowed multiple answers (select all that apply); per cent is based on number of herds with responses (n = 77).
Opinion question; per cent is based on number of individuals with responses (n = 40).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The vast majority of swine herds represented in the survey contained between 1,000 and 9,999 pigs (71/83; 85.5%) and were either farrow‐to‐finish or farrow‐to‐wean production sites (76/83; 91.5%). Approximately two‐thirds of the herds were located in swine‐dense regions and approximately 71% (59/83) of herds reported exposure to SECD. Perhaps the most interesting result of the survey was the low percentage of herds with active SECD infection (12/83; 14.5%). The remaining herds had either never been exposed to SECD (24/83; 28.9%) or had been exposed and had eliminated the virus without a recent reintroduction (47/83; 56.6%). Of the 59 herds reporting SECD exposure, the vast majority of these herds (50/59; 85%) reported exposure to PEDV alone, with only a minor population reporting PDCoV exposure either singularly (3/59; 5%) or in combination with PEDV (6/59; 10%). Taken together, these survey data suggest that most herds have had long‐term success in maintaining a negative status or eliminating PEDV after exposure. However, it is important to consider that the absence of SECD clinical signs and/or faecal shedding does not always translate to virus eradication. Underlying immunity may suppress clinical disease and to determine a truly negative status, it may be necessary to comingle naïve animals into the herd or re‐test animals after stress, such as transportation.
Questions on SECD introduction and methods for SECD control provided mixed results. With regard to the probable source of virus introduction on farms, respondents provided answers for 72 individual herds. All six possible routes of introduction included in the survey were well represented in these herds; routes including trucks, feed and unknown (biosecurity protocols in place) comprised the majority of herds (53/72; 73.6%). Additionally, aerosols, neighbouring farms and other were possible routes that each garnered approximately 10% of the herd responses. The survey question addressing methods utilized for SECD control were also varied, and most herds utilized several strategies in combination. This question allowed multiple answers (select all that apply), and 77 herds were represented in the responses provided. The most commonly used practice selected was whole‐herd feedback through a load‐close‐expose protocol (59/77; 76.6%). Approximately one‐third of herds continued to use controlled exposure for gilt acclimation (23/77; 29.9%). With regard to vaccination, approximately 25% of herds reported to use either the killed (11/77; 14%) or replicon particle (8/77; 10%) vaccines. Neonatal pig management strategies, including early segregated weaning (22/77; 29%) and piglet depopulation (36/77; 47%), were also reported. The majority of respondents had implemented enhanced biosecurity as a method of control (43/77; 56%), whereas only a single herd reported using a feed additive as a management tool for SECD mitigation.
The final survey inquiry included an opinion question about the feasibility of SECD eradication. Perhaps surprising was the overwhelming response of “yes” by respondents on the ability of the US swine industry to eradicate SECD (30/40; 75%). Overall, survey results were very similar when compared between herd locations being considered swine‐dense and those considered not swine‐dense (Figure 5). Although only 40 individuals and 83 herds were represented in this survey, the data provide valuable insight into field observations and a better understanding of the current opinions of swine industry stakeholders with regard to SECD.
Figure 5.
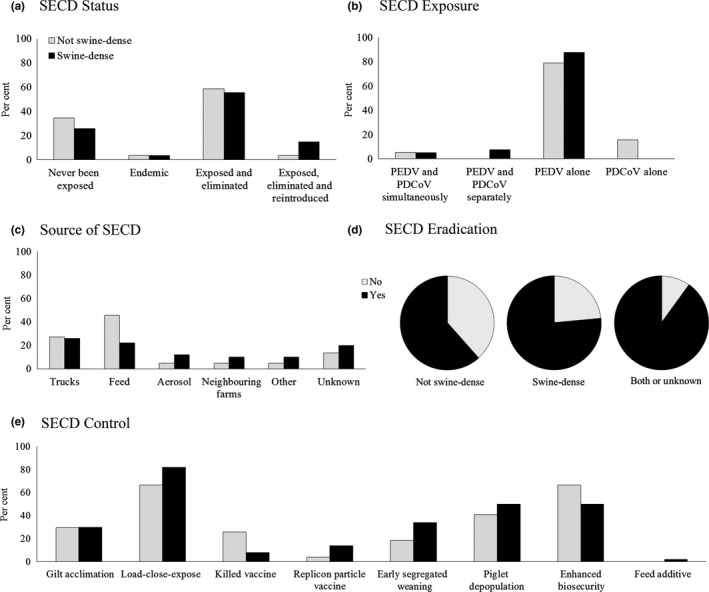
Swine enteric coronavirus survey responses comparing swine‐dense and not swine‐dense herds. Data are shown as the per cent of herds considered swine‐dense (black bars) and not swine‐dense (grey bars) with each response in regard to status (a), exposure (b), source (c) and methods for control (e). In (d), data are shown as the proportion of each group with yes or no responses to the possibility of eradicating porcine epidemic diarrhoea virus (PEDV) and PDCoV. The category of both or unknown represents individuals who included information for both swine‐dense and not‐swine‐dense herds or only answered this opinion question
10. CONCLUSION
Since the first appearance of PEDV and PDCoV in the United States and Canada in 2013–2014, significant efforts have been made to understand the risks for introduction, characteristics of pathogenesis and spread, and strategies for disease control. The appearance of these two viruses underscores the risk of other potential diseases being introduced into US and Canadian swine herds. Since SECD introduction, the United States has primarily focused on disease management with the number of positive cases and premises gradually declining each winter. In Canada, disease elimination has been the primary focus, and SECD has now been largely contained to a single province in the country. Nevertheless, several challenges have been encountered by both countries, such as continued virus introduction on new farms and long‐term shedding of exposed pigs. As demonstrated on the survey and by the Canadian experience, the success of eliminating these viruses in many herds post‐introduction provides hope for a potential future eradication from one or both countries; however, the cost of virus introduction into naïve herds and the continued detection of new positive cases highlights the significant challenges that would be faced in a SECD elimination program.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Funding was provided by the National Pork Checkoff #16‐266. The authors would like to thank the swine professionals who participated in the survey and Mal Hoover for her assistance with the illustrations.
Niederwerder MC, Hesse RA. Swine enteric coronavirus disease: A review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transbound Emerg Dis. 2018;65:660–675. 10.1111/tbed.12823
REFERENCES
- AASV (2013). PEDV Positive Cases ascertained from the VDL's (ISU, KSU, OADDL, SDSU, & UMN) (https://www.aasv.org/aasv%20website/Resources/Diseases/PED/LABSUMTOT_WK_STATE.pdf).
- Alonso, C. , Goede, D. P. , Morrison, R. B. , Davies, P. R. , Rovira, A. , Marthaler, D. G. , & Torremorell, M. (2014). Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Veterinary research, 45, 73 10.1186/s13567-014-0073-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, C. , Raynor, P. C. , Goyal, S. , Olson, B. A. , Alba, A. , Davies, P. R. , & Torremorell, M. (2017). Assessment of air sampling methods and size distribution of virus‐laden aerosols in outbreaks in swine and poultry farms. Journal of veterinary diagnostic investigation, 29, 298–304. 10.1177/1040638717700221 [DOI] [PubMed] [Google Scholar]
- Alvarez, J. , Goede, D. , Morrison, R. , & Perez, A. (2016). Spatial and temporal epidemiology of porcine epidemic diarrhea (PED) in the Midwest and Southeast regions of the United States. Preventive Veterinary Medicine, 123, 155–160. 10.1016/j.prevetmed.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Alvarez, J. , Sarradell, J. , Morrison, R. , & Perez, A. (2015). Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS ONE, 10, e0120532 10.1371/journal.pone.0120532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai, T. , Saif, L. J. , Lu, Z. , & Jung, K. (2015). Age‐dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Veterinary immunology and immunopathology, 168, 193–202. 10.1016/j.vetimm.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, A. S. , Krogwold, R. A. , Price, T. , Davis, M. , & Moeller, S. J. (2015). Investigating the introduction of porcine epidemic diarrhea virus into an Ohio swine operation. BMC veterinary research, 11, 38 10.1186/s12917-015-0348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, A. S. , Nolting, J. M. , Nelson, S. W. , Bliss, N. , Stull, J. W. , Wang, Q. , & Premanandan, C. (2015). Effects of disinfection on the molecular detection of porcine epidemic diarrhea virus. Veterinary microbiology, 179, 213–218. 10.1016/j.vetmic.2015.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning, P. , Ruston, C. , Madson, D. , Bates, J. , Skoland, K. , Davenport, J. , … Karriker, L. (2017). Effect of direct‐fed microbial Bacillus subtilis C‐3102 on enteric health in nursery pigs after challenge with porcine epidemic diarrhea virus. Journal of Swine Health and Production, 25, 129–137. [Google Scholar]
- Chen, Q. , Gauger, P. , Stafne, M. , Thomas, J. , Arruda, P. , Burrough, E. , … Zhang, J. (2015). Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5‐day‐old neonatal piglets. Virology, 482, 51–59. 10.1016/j.virol.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Li, G. , Stasko, J. , Thomas, J. T. , Stensland, W. R. , Pillatzki, A. E. , … Zhang, J. (2014). Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. Journal of clinical microbiology, 52, 234–243. 10.1128/JCM.02820-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane, R.A. , Dritz, S.S. , Woodworth, J.C. , Huss, A.R. , Stark, C.R. , Saensukjaroenphon, M. , … Jones, C.K . (2016). Assessing the Effects of Medium Chain Fatty Acids and Fat Sources on Porcine Epidemic Diarrhea Virus Viral RNA Stability and Infectivity. In Kansas Agricultural Experiment Station Research Reports.
- Cochrane, R. A. , Dritz, S. S. , Woodworth, J. C. , Stark, C. R. , Huss, A. R. , Cano, J. P. , … Jones, C. K. (2016). Feed mill biosecurity plans: a systematic approach to prevent biological pathogens in swine feed. Journal of Swine Health and Production, 24, 154–164. [Google Scholar]
- Cochrane, R. A. , Saensukjaroenphon, M. , Dritz, S. S. , Woodworth, J. C. , Huss, A. R. , Stark, C. R. , … Jones, C. K. (2016). Evaluating the inclusion level of medium chain fatty acids to reduce the risk of PEDV in feed and spray‐dried animal plasma. Journal of animal science, 94(Suppl. 2), 50 Abstract. 10.2527/msasas2016-107 [DOI] [Google Scholar]
- Cochrane, R. A. , Schumacher, L. L. , Dritz, S. S. , Woodworth, J. C. , Huss, A. R. , Stark, C. R. , … Jones, C. K. (2017). Effect of pelleting on survival of porcine epidemic diarrhea virus‐contaminated feed. Journal of animal science, 95, 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, K. , Lager, K. M. , Kulshreshtha, V. , Miller, L. C. , & Faaberg, K. S. (2016). Status of vaccines for porcine epidemic diarrhea virus in the United States and Canada. Virus research, 226, 108–116. 10.1016/j.virusres.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Crawford, K. , Lager, K. , Miller, L. , Opriessnig, T. , Gerber, P. , & Hesse, R. (2015). Evaluation of porcine epidemic diarrhea virus transmission and the immune response in growing pigs. Veterinary research, 46, 49 10.1186/s13567-015-0180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry, S. M. , Gibson, K. A. , Burrough, E. R. , Schwartz, K. J. , Yoon, K. J. , & Gabler, N. K. (2017). Nursery pig growth performance and tissue accretion modulation due to porcine epidemic diarrhea virus or porcine deltacoronavirus challenge. Journal of animal science, 95, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee, S. , Clement, T. , Schelkopf, A. , Nerem, J. , Knudsen, D. , Christopher‐Hennings, J. , & Nelson, E. (2014). An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naive pigs following consumption via natural feeding behavior: proof of concept. BMC veterinary research, 10, 176 10.1186/s12917-014-0176-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee, S. , Neill, C. , Clement, T. , Singrey, A. , Christopher‐Hennings, J. , & Nelson, E. (2015). An evaluation of porcine epidemic diarrhea virus survival in individual feed ingredients in the presence or absence of a liquid antimicrobial. Porcine Health Management, 1, 9 10.1186/s40813-015-0003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee, S. , Neill, C. , Singrey, A. , Clement, T. , Cochrane, R. , Jones, C. , … Nelson, E. (2016). Modeling the transboundary risk of feed ingredients contaminated with porcine epidemic diarrhea virus. BMC veterinary research, 12, 51 10.1186/s12917-016-0674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- EQSP (2015). Useful Information on Disinfectants after Contamination with Novel Swine Enteric Coronavirus Diseases (SECD) (http://www.manitobapork.com/images/producers/pdfs/may-2017/Disinfectants-Proven-Effective-against-Coronaviruses-EQSP-150424.pdf, Quebec swine health team).
- Furuuchi, S. , & Shimizu, Y. (1976). Effect of ambient temperatures on multiplication of attenuated transmissible gastroenteritis virus in the bodies of newborn piglets. Infection and immunity, 13, 990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt, J.T. , Woodworth, J.C. , Jones, C.K. , Gauger, P.C. , Tokach, M.D. , DeRouchey, J.M. , … Dritz, S.S . (2016). Evaluation of the Effects of Flushing Feed Manufacturing Equipment with Chemically‐Treated Rice Hulls on Porcine Epidemic Diarrhea Virus Cross Contamination During Feed Manufacturing. In Kansas Agricultural Experiment Station Research Reports. [DOI] [PMC free article] [PubMed]
- Gerber, P. F. , Xiao, C. T. , Chen, Q. , Zhang, J. , Halbur, P. G. , & Opriessnig, T. (2014). The spray‐drying process is sufficient to inactivate infectious porcine epidemic diarrhea virus in plasma. Veterinary microbiology, 174, 86–92. 10.1016/j.vetmic.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts, V. , & Zakhartchouk, A. (2017). Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Veterinary microbiology, 206, 45–51. 10.1016/j.vetmic.2016.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, T. , Song, Q. , Inskeep, M. , Stone, S. , & Murtaugh, M. P. (2017). Effect of booster vaccination with inactivated porcine epidemic diarrhea virus on neutralizing antibody response in mammary secretions. Viral immunology, 31, 62–68. [DOI] [PubMed] [Google Scholar]
- Goede, D. , & Morrison, R. B. (2016). Production impact & time to stability in sow herds infected with porcine epidemic diarrhea virus (PEDV). Preventive Veterinary Medicine, 123, 202–207. 10.1016/j.prevetmed.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Goede, D. , Murtaugh, M. P. , Nerem, J. , Yeske, P. , Rossow, K. , & Morrison, R. (2015). Previous infection of sows with a “mild” strain of porcine epidemic diarrhea virus confers protection against infection with a “severe” strain. Veterinary microbiology, 176, 161–164. 10.1016/j.vetmic.2014.12.019 [DOI] [PubMed] [Google Scholar]
- Hamblin, J . (2017). Previously Infected Pigs Spread PED Longer Than Previously Thought (https://www.aasv.org/news/story.php?id=10384).
- Hofmann, M. , & Wyler, R. (1989). Quantitation, biological and physicochemical properties of cell culture‐adapted porcine epidemic diarrhea coronavirus (PEDV). Veterinary microbiology, 20, 131–142. 10.1016/0378-1135(89)90036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp, D. J. , Myers, J. , Thomas, P. R. , Karriker, L. A. , Ramirez, A. , Zhang, J. , & Wang, C. (2017). Efficacy of an accelerated hydrogen peroxide disinfectant to inactivate porcine epidemic diarrhea virus in swine feces on metal surfaces. Canadian Journal of Veterinary Research, 81, 100–107. [PMC free article] [PubMed] [Google Scholar]
- Hu, H. , Jung, K. , Vlasova, A. N. , & Saif, L. J. (2016). Experimental infection of gnotobiotic pigs with the cell‐culture‐adapted porcine deltacoronavirus strain OH‐FD22. Archives of virology, 161, 3421–3434. 10.1007/s00705-016-3056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.W. , Dickerman, A.W. , Pineyro, P. , Li, L. , Fang, L. , Kiehne, R. , … Meng, X.J. (2013). Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio, 4, e00737‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss, A. R. , Schumacher, L. L. , Cochrane, R. A. , Poulsen, E. , Bai, J. , Woodworth, J. C. , … Jones, C. K. (2017). Elimination of porcine epidemic diarrhea virus in an animal feed manufacturing facility. PLoS ONE, 12, e0169612 10.1371/journal.pone.0169612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetanakit, T. , Lumyai, M. , Bunpapong, N. , Boonyapisitsopa, S. , Chaiyawong, S. , Nonthabenjawan, N. , … Amonsin, A. (2016). Porcine Deltacoronavirus, Thailand, 2015. Emerging infectious diseases, 22, 757–759. 10.3201/eid2204.151852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Eyerly, B. , Annamalai, T. , Lu, Z. , & Saif, L. J. (2015). Structural alteration of tight and adherens junctions in villous and crypt epithelium of the small and large intestine of conventional nursing piglets infected with porcine epidemic diarrhea virus. Veterinary microbiology, 177, 373–378. 10.1016/j.vetmic.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Hu, H. , & Saif, L. J. (2016). Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus research, 226, 50–59. 10.1016/j.virusres.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , & Saif, L. J. (2015). Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Veterinary Journal, 204, 134–143. 10.1016/j.tvjl.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , & Saif, L. J. (2017). Goblet cell depletion in small intestinal villous and crypt epithelium of conventional nursing and weaned pigs infected with porcine epidemic diarrhea virus. Research in veterinary science, 110, 12–15. 10.1016/j.rvsc.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Jung, K. , Wang, Q. , Scheuer, K. A. , Lu, Z. , Zhang, Y. , & Saif, L. J. (2014). Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerging infectious diseases, 20, 662–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny, L. J. , Wiltsey, V. L. , & Riley, J. L. (1975). Upper respiratory infection of lactating sows with transmissible gastroenteritis virus following contact exposure to infected piglets. The Cornell Veterinarian, 65, 352–362. [PubMed] [Google Scholar]
- Kim, Y. , & Lee, C. (2014). Porcine epidemic diarrhea virus induces caspase‐independent apoptosis through activation of mitochondrial apoptosis‐inducing factor. Virology, 460–461, 180–193. 10.1016/j.virol.2014.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans, R. , Bridgen, A. , Ackermann, M. , & Tobler, K. (2001). Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes, 23, 137–144. 10.1023/A:1011831902219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar, H. S. (2014). Canada: Porcine epidemic diarrhea in Canada: an emerging disease case study. The Canadian Veterinary Journal, 55, 1048–1049. [PMC free article] [PubMed] [Google Scholar]
- Langel, S. N. , Paim, F. C. , Lager, K. M. , Vlasova, A. N. , & Saif, L. J. (2016). Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus research, 226, 93–107. 10.1016/j.virusres.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. (2015). Porcine epidemic diarrhea virus: An emerging and re‐emerging epizootic swine virus. Virology journal, 12, 193 10.1186/s12985-015-0421-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , & Lee, C. (2014). Complete genome characterization of Korean porcine deltacoronavirus strain KOR/KNU14‐04/2014. Genome Announcements, 2, e01191–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, J. , Gauger, P. , Harmon, K. , Zhang, J. , Connor, J. , Yeske, P. , … Main, R. (2014). Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerging infectious diseases, 20, 872–874. 10.3201/eid2005.131628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Zhang, Y. , Liang, X. , Lou, F. , Oglesbee, M. , Krakowka, S. , & Li, J. (2015). Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio, 6, e00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson, D. M. , Arruda, P. H. , Magstadt, D. R. , Burrough, E. R. , Hoang, H. , Sun, D. , … Yoon, K. J. (2015). Characterization of porcine epidemic diarrhea virus isolate US/Iowa/18984/2013 infection in 1‐day‐old Cesarean‐derived colostrum‐deprived piglets. Veterinary pathology, 53, 44–52. [DOI] [PubMed] [Google Scholar]
- Madson, D. M. , Magstadt, D. R. , Arruda, P. H. , Hoang, H. , Sun, D. , Bower, L. P. , … Yoon, K. J. (2014). Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3‐week‐old weaned pigs. Veterinary microbiology, 174, 60–68. 10.1016/j.vetmic.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Manitoba (2017). Porcine Epidemic Diarrhea (PED) Virus (http://www.gov.mb.ca/agriculture/animals/animal-health/porcine-epidemic-diarrhea.html, Manitoba Government).
- Marthaler, D. , Jiang, Y. , Collins, J. , & Rossow, K. (2014). Complete Genome Sequence of Strain SDCV/USA/Illinois121/2014, a Porcine Deltacoronavirus from the United States. Genome Announcements, 2, e00218–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, H.W. (1971). Epithelial cell migration in the alimentary mucosa of the suckling pig. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine 137, 151–154. 10.3181/00379727-137-35533 [DOI] [PubMed] [Google Scholar]
- Moon, H. W. , Norman, J. O. , & Lambert, G. (1973). Age dependent resistance to transmissible gastroenteritis of swine (TGE). I. Clinical signs and some mucosal dimensions in small intestine. Canadian Journal of Comparative Medicine, 37, 157–166. [PMC free article] [PubMed] [Google Scholar]
- Niederwerder, M. C. , Nietfeld, J. C. , Bai, J. , Peddireddi, L. , Breazeale, B. , Anderson, J. , … Hesse, R. A. (2016). Tissue localization, shedding, virus carriage, antibody response, and aerosol transmission of Porcine epidemic diarrhea virus following inoculation of 4‐week‐old feeder pigs. Journal of veterinary diagnostic investigation, 28, 671–678. 10.1177/1040638716663251 [DOI] [PubMed] [Google Scholar]
- Ojkic, D. , Hazlett, M. , Fairles, J. , Marom, A. , Slavic, D. , Maxie, G. , … Burlatschenko, S. (2015). The first case of porcine epidemic diarrhea in Canada. The Canadian Veterinary Journal, 56, 149–152. [PMC free article] [PubMed] [Google Scholar]
- Opriessnig, T. , Xiao, C. T. , Gerber, P. F. , Zhang, J. , & Halbur, P. G. (2014). Porcine epidemic diarrhea virus RNA present in commercial spray‐dried porcine plasma is not infectious to naive pigs. PLoS ONE, 9, e104766 10.1371/journal.pone.0104766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, D. , Brown, I. , Bridges, A. , & Cartwright, S. F. (1989). Pathogenicity of experimental infection with ‘pneumotropic’ porcine coronavirus. Research in veterinary science, 47, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. , & Lee, C. (2009). Clinical examination and control measures in a commercial pig farm persistently infected with porcine epidemic diarrhea(PED) virus. Journal of Veterinary Clinics, 26, 463–466. [Google Scholar]
- Pasick, J. , Berhane, Y. , Ojkic, D. , Maxie, G. , Embury‐Hyatt, C. , Swekla, K. , … Alexandersen, S. (2014). Investigation into the role of potentially contaminated feed as a source of the first‐detected outbreaks of porcine epidemic diarrhea in Canada. Transboundary and Emerging Diseases, 61, 397–410. 10.1111/tbed.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasma, T. , Furness, M. C. , Alves, D. , & Aubry, P. (2016). Outbreak investigation of porcine epidemic diarrhea in swine in Ontario. The Canadian Veterinary Journal, 57, 84–89. [PMC free article] [PubMed] [Google Scholar]
- Pensaert, M. B. , & de Bouck, P. (1978). A new coronavirus‐like particle associated with diarrhea in swine. Archives of virology, 58, 243–247. 10.1007/BF01317606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert, M. B. , Debouck, P. , & Reynolds, D. J. (1981). An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus‐like agent, CV 777, and several coronaviruses. Archives of virology, 68, 45–52. 10.1007/BF01315166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers, A. , van Nieuwstadt, A. P. , Terpstra, C. , & Verheijden, J. H. (1993). Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. The Veterinary record, 132, 129–131. 10.1136/vr.132.6.129 [DOI] [PubMed] [Google Scholar]
- Schumacher, L. L. , Woodworth, J. C. , Jones, C. K. , Chen, Q. , Zhang, J. , Gauger, P. C. , … Dritz, S. S. (2016). Evaluation of the minimum infectious dose of porcine epidemic diarrhea virus in virus‐inoculated feed. American journal of veterinary research, 77, 1108–1113. 10.2460/ajvr.77.10.1108 [DOI] [PubMed] [Google Scholar]
- Schweer, W. P. , Pearce, S. C. , Burrough, E. R. , Schwartz, K. , Yoon, K. J. , Sparks, J. C. , & Gabler, N. K. (2016). The effect of porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus challenge on growing pigs II: Intestinal integrity and function. Journal of animal science, 94, 523–532. 10.2527/jas.2015-9836 [DOI] [PubMed] [Google Scholar]
- Schweer, W. P. , Schwartz, K. , Burrough, E. R. , Yoon, K. J. , Sparks, J. C. , & Gabler, N. K. (2016). The effect of porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus challenge on growing pigs I: Growth performance and digestibility. Journal of animal science, 94, 514–522. 10.2527/jas.2015-9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, A. , McCluskey, B. , Brown‐Reid, M. , Grear, D. , Pitcher, P. , Ramos, G. , … Singrey, A. (2016). Porcine epidemic diarrhea virus introduction into the United States: Root cause investigation. Preventive Veterinary Medicine, 123, 192–201. 10.1016/j.prevetmed.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, I. , Tsuda, T. , Mori, M. , Ono, M. , Sueyoshi, M. , & Uruno, K. (2000). Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Veterinary microbiology, 72, 173–182. 10.1016/S0378-1135(99)00199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, A. , Gauger, P. , Zhang, J. , Yoon, K. J. , & Harmon, K. (2015). PCR‐based retrospective evaluation of diagnostic samples for emergence of porcine deltacoronavirus in US swine. Veterinary microbiology, 179, 296–298. 10.1016/j.vetmic.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, D. , & Park, B. (2012). Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes, 44, 167–175. 10.1007/s11262-012-0713-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, G. W. , Hoang, H. , Schwartz, K. J. , Burrough, E. R. , Sun, D. , Madson, D. , … Yoon, K. J. (2013). Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. Journal of veterinary diagnostic investigation, 25, 649–654. 10.1177/1040638713501675 [DOI] [PubMed] [Google Scholar]
- Sun, R. Q. , Cai, R. J. , Chen, Y. Q. , Liang, P. S. , Chen, D. K. , & Song, C. X. (2012). Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerging infectious diseases, 18, 161–163. 10.3201/eid1801.111259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swine Health Information Center, S . (2016). SHIC Funded Research Identifies Feed Biosecurity as Critical to Global Animal Health (http://www.swinehealth.org/shic-funded-research-identifies-feed-biosecurity-as-critical-to-global-animal-health/).
- Swine Health Information Center, S . (2017). Modeling the transboundary survival of foreign animal disease pathogens in contaminated feed ingredients, Sundberg, P., ed. (http://www.swinehealth.org/pathogen-transmission-white-paper/).
- Thachil, A. , Gerber, P. F. , Xiao, C. T. , Huang, Y. W. , & Opriessnig, T. (2015). Development and application of an ELISA for the detection of porcine deltacoronavirus IgG antibodies. PLoS ONE, 10, e0124363 10.1371/journal.pone.0124363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. T. , Chen, Q. , Gauger, P. C. , Gimenez‐Lirola, L. G. , Sinha, A. , Harmon, K. M. , … Zhang, J. (2015). Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naive conventional neonatal and weaned pigs. PLoS ONE, 10, e0139266 10.1371/journal.pone.0139266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau, M. P. , Verma, H. , Sampedro, F. , Urriola, P. E. , Shurson, G. C. , & Goyal, S. M. (2017). Environmental persistence of porcine coronaviruses in feed and feed ingredients. PLoS ONE, 12, e0178094 10.1371/journal.pone.0178094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau, M. P. , Verma, H. , Sampedro, F. , Urriola, P. E. , Shurson, G. C. , McKelvey, J. , … Goyal, S. M. (2016). Comparison of thermal and non‐thermal processing of swine feed and the use of selected feed additives on inactivation of porcine epidemic diarrhea virus (PEDV). PLoS ONE, 11, e0158128 10.1371/journal.pone.0158128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA (2014). Swine Enteric Coronavirus Disease Testing Summary Report (https://www.aphis.usda.gov/animal_health/animal_dis_spec/swine/downloads/secd_sit_rep_11_14_14.pdf, Animal and Plant Health Inspection Service).
- USDA (2017). Swine Enteric Coronavirus Disease (SECD) Situation Report (https://www.aphis.usda.gov/animal_health/animal_dis_spec/swine/downloads/secd_sit_rep_11_30_17.pdf, Animal and Plant Health Inspection Service).
- Vitosh‐Sillman, S. , Loy, J. D. , Brodersen, B. , Kelling, C. , Doster, A. , Topliff, C. , … Hesse, R. (2016). Experimental infection of conventional nursing pigs and their dams with Porcine deltacoronavirus. Journal of veterinary diagnostic investigation, 28, 486–497. 10.1177/1040638716654200 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Byrum, B. , & Zhang, Y. (2014a). Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerging infectious diseases, 20, 1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Byrum, B. , & Zhang, Y. (2014b). Porcine coronavirus HKU15 detected in 9 US states, 2014. Emerging infectious diseases, 20, 1594–1595. 10.3201/eid2009.140756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. M. , Niu, B. B. , Yan, H. , Gao, D. S. , Yang, X. , Chen, L. , … Wang, C. Q. (2013). Genetic properties of endemic Chinese porcine epidemic diarrhea virus strains isolated since 2010. Archives of virology, 158, 2487–2494. 10.1007/s00705-013-1767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, L. , Weersink, A. , Poljak, Z. , de Lange, K. , & von Massow, M. (2016). An economic evaluation of intervention strategies for Porcine Epidemic Diarrhea (PED). Preventive Veterinary Medicine, 134, 58–68. 10.1016/j.prevetmed.2016.09.018 [DOI] [PubMed] [Google Scholar]
- Woo, P. C. , Lau, S. K. , Lam, C. S. , Lau, C. C. , Tsang, A. K. , Lau, J. H. , … Yuen, K. Y. (2012). Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. Journal of virology, 86, 3995–4008. 10.1128/JVI.06540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, E. N. (1977). An apparently new syndrome of porcine epidemic diarrhoea. The Veterinary record, 100, 243–244. 10.1136/vr.100.12.243 [DOI] [PubMed] [Google Scholar]
- Zhang, J. (2016). Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus research, 226, 71–84. 10.1016/j.virusres.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]


