Abstract
Vibrio vulnificus is a Gram‐negative, curved, obligate halophilic marine bacterium that exclusively exists in coastal seawaters. Previous studies revealed that V. vulnificus is one of the most dangerous foodborne zoonotic pathogens for human beings. However, it remains unknown whether marine mammals can be infected by V. vulnificus. In May 2016, a captive spotted seal (Phoca largha) died due to septicemia induced by V. vulnificus. Upon post‐mortem examination, V. vulnificus was isolated, identified, and named as BJ‐PH01. Further analysis showed that BJ‐PH01 belongs to biotype 1 and the Clinical genotype. Furthermore, we performed an epidemiological investigation of V. vulnificus in six aquariums in northern China. As a result, V. vulnificus was successfully isolated from all investigated aquariums. The positive rates ranged from 20% to 100% in each investigated aquarium. During the investigation, 12 strains of V. vulnificus were isolated, and all 12 isolates were classified into biotype 1. Eleven of the 12 isolates belonged to the Clinical genotype, and one isolate belonged to the Environmental genotype. All 12 isolated V. vulnificus strains showed limited antibiotic resistance. Overall, our work demonstrated that V. vulnificus is frequently distributed in aquariums, thus constituting a threat to captive marine mammals and to public health.
Keywords: epidemiology, Phoca largha, Septicemia, Vibrio vulnificus
1. INTRODUCTION
Vibrio vulnificus (V. vulnificus) is a Gram‐negative bacterium naturally existing in estuarial and coastal environments throughout the world. V. vulnificus infection is a highly lethal disease, which is responsible for 95% of all seafood‐related deaths in the United States, with a fatality rate of approximately 50%. Due to its capacity to cause rapid death among high‐risk populations, V. vulnificus is considered one of the most dangerous foodborne pathogens for humans (Baker‐Austin & Oliver, 2016; Oliver, 2005).
Vibrio vulnificus causes human infection through oral ingestion and wound infection (Kashimoto et al., 2015; Madiyal et al., 2016). Oral ingestion mainly leads to primary septicemia when people consume contaminated raw or uncooked seafood (Kim, Bae, Ma, & Kim, 2015). Symptoms of primary septicemia include fever, chills, nausea, and hypotension. Wound infection is generally acquired through the exposure of a preexisting wound to contaminated seawater or shellfish, resulting in fulminant necrotizing skin and soft tissue infections (Huang et al., 2016; Kotton, Soboh, & Bisharat, 2015). Previous reports indicated that V. vulnificus can only affect humans and other primates. Although V. vulnificus accumulates in oysters due to their filter feeding of particles from seawater, V. vulnificus infection causes no clinical signs in shellfish. Therefore, it is difficult to identify V. vulnificus‐containing oysters without laboratory detection.
The incidence of V. vulnificus infection in humans is associated with multiple risk factors, including (a) seawater temperature and salinity, (b) season, (c) gender and age, (d) preexisting chronic diseases, and (e) bacterium virulence. As reported, the majority of human infections occur in the subtropical regions from April to November. People over the age of 40 are predominantly infected. Moreover, males are more susceptible than females (Ito et al., 2012), perhaps due to the role of estrogen in protecting against the bacterium's endotoxins (Miyamoto et al., 1999; Soucy, Boivin, Labrie, & Rivest, 2005). As mentioned previously, patients with underlying chronic diseases, including alcoholism, diabetes, cancer, or renal diseases, are more susceptible to V. vulnificus (Bross, Soch, Morales, & Mitchell, 2007a). Several other risk factors contribute to the high pathogenicity of V. vulnificus in humans, such as capsule, iron, and the vcg gene (Jones & Oliver, 2009).
Marine mammals are important inhabitants of tropical and temperate regions, especially offshore areas of the sea (Schipper et al., 2008). There is an overlap in the distributions of V. vulnificus and marine mammals. No evidence has shown that marine mammals can be infected by V. vulnificus; however, the concerns cannot be excluded. Here, we provide evidence that marine mammals can also be infected by V. vulnificus. Further investigation showed that V. vulnificus is ubiquitous in aquariums, thus revealing a novel threat to captive marine animals and human beings in aquariums.
2. MATERIALS AND METHODS
2.1. Ethics statement
Animal studies were performed in strict accordance with the Guidelines for the Care and Use of Animals in Research, which is issued by the Institute of Zoology, Chinese Academy of Sciences. This study was evaluated and approved by the Animal Ethics Committee of Institute of Zoology, Chinese Academy of Sciences. All experiments were conducted in a Biosafety Level 2 (BSL‐2) facility.
2.2. Tissue sample collection and handling
2.2.1. Sample collection
An autopsy was performed after the spotted seal died. Pathological lesions were observed and recorded. Tissue samples (i.e., lung, liver, stomach, spleen, intestine, kidney, and heart) were sterilely collected. A festered sample was collected from the trauma injury. All samples were transported on ice and analyzed immediately.
2.2.2. Sample handling
Tissue samples were cultured on blood agar (Oxoid, UK), MacConkey agar (Oxoid, UK), chocolate agar (Oxoid, UK), and thiosulphate‐citrate‐bile‐salt‐sucrose (TCBS) agar (Oxoid, UK). All plates were placed in aerobic or anaerobic conditions at 37°C for 18–24 hr. From each plate, at least 24 single colonies were selected for identification, and all the selected colonies were preliminarily identified by 16S rDNA sequencing. The results were further confirmed by the BD Phoenix automated Microbiology System (BD Diagnostic Systems, Sparks, MD.) (Stefaniuk, Baraniak, Gniadkowski, & Hryniewicz, 2003) and species‐specific PCR amplification. Several known viral pathogens that have been reported to infect marine mammals were also detected by virus‐specific PCR, including type A influenza virus (IAV), phocine distemper virus (PDV), coronavirus, and rotavirus. The primers used in this study are listed in Table 1 (Chatzidaki‐Livanis, Jones, & Wright, 2006; Gómara, Wong, Blome, Desselberger, & Gray, 2002; Hill et al., 1991; Lau et al., 2005; Miller et al., 2011; Reynaud, Pitchford, De Decker, Wikfors, & Brown, 2013; Sea, 2015; Warner & Oliver, 2008).
Table 1.
Primers used in this study
| Gene | Sequences | Target (bp) |
|---|---|---|
| vvhA | CGCCACCCACTTTCGGGCC | 519 |
| CCGCGGTACAGGTTGGCGC | ||
| NanA | GCGGTGATCGATCAAATTGCTG | 618 |
| CCCTTGGTTGAACGCCTCAAT | ||
| mtlABC | GCCCAACATCGGGGCATTTA | 569 |
| GGCCAGCTTCTGAAGCCTG | ||
| Ary | CCAGACCCGAGCGGATATGC | 638 |
| GCGTGTGCGGGCCCCAGA | ||
| SerE | TGTTGTTCTTGCCCACTCTC | 665 |
| CGCGCTTAGATTTGTCTCACC | ||
| Bt2 | AGAGATGGAAGAAACAGGCG | 344 |
| GGACAGATATAAGGGCAAATGG | ||
| vcgC | AGCTGCCGATAGCGATCT | 277 |
| R:CGCTTAGGATGATCGGTG | ||
| vcgE | CTCAATTGACAATGATCT | 277 |
| CGCTTAGGATGATCGGTG | ||
| CPS1 | TCGCGTTATCTGATCAACCA | 294 |
| CGATGGAATCGTGTGATCAGT | ||
| CPS2 | GAACCTTCTGCGATGTTTGATGG | 381 |
| CGATGGAATCGTGTGATCAGT | ||
| IAV | GACCAATCCTGTCACCTCTGA | 251 |
| GTATATGAGGCCCATRCAACT | ||
| CDV | GTGACTGCTCCTGATACTGC | 477 |
| ACCAACTCCCATAGCATAAC | ||
| Rotavirus | GACGGVGCRACTACATGGT | 382 |
| GTCCAATTCATNCCTGGTG | ||
| Coronavirus | GGTTGGGACTATCCTAAGTGTGA | 440 |
| CCATCATCAGATAGAATCATCATA |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.3. Epidemiological investigation of V. vulnificus
2.3.1. Sample collection
Samples (including water samples and animal body surface swabs) were collected from six aquariums in northern China. In detail, 100‐ml water samples were collected from the pools in which the marine animals (i.e., spotted seal, turtle, dolphin, shark, whale, and saltwater fish) were kept (Table 2). Animal (i.e., turtle, spotted seal) body surface swabs were collected using medical degreasing cotton. Freshwater samples and freshwater fish body surface swabs were also collected as negative controls. In total, 54 samples were collected. All the samples were stored on ice, transported to the Institute of Zoology, Chinese Academy of Sciences, and analyzed immediately.
Table 2.
Vibrio Vulnificus can be detected from all the six investigated aquarium
| Aquariums | No. of collected samplesa | No. of positive samples | Positive rate (%) |
|---|---|---|---|
| BJ‐BZ | 12 | 4 | 33.30 |
| BJ‐PH | 10 | 10 | 100 |
| BJ‐SAR | 4 | 4 | 100 |
| BJ‐FG | 2 | 1 | 50 |
| SJZ | 10 | 2 | 20 |
| XA | 16 | 4 | 25 |
Note. aSamples collected from fresh water were not included in this table.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.3.2. Sample handling
All samples were examined using procedures in the Bacteriological Analytical Manual of The Food and Drug Administration (FDA) (Kaysner & DePaola, 2004). In detail, water samples were serially diluted and cultured by 1% NaCl alkaline peptone water (APW) at 37°C for 18–24 hr. Body surface swabs were diluted in a 5‐ml volume of sterile phosphate‐buffered saline (PBS) followed by vortexing for 45 s. The supernatant was cultured in 1% NaCl alkaline peptone water (APW) at 37 °C for 18–24 hr. The resulting products were diluted and cultured on blood agar and then detected by V. vulnificus vvhA gene‐specific PCR (Warner & Oliver, 2008), 16S rDNA sequencing, and the BD Phoenix Automated Microbiology System (BD).
2.4. Biotyping of V. vulnificus
Biotyping of isolated V. vulnificus was performed as previously reported (Bisharat, Agmon, Finkelstein, Raz, Ben‐Dror, Lerner, Soboh, Colodner, Cameron, & Wykstra, 1999). Briefly, ONPG testing, indole production, 1% NaCl ornithine decarboxylase testing, D‐sorbitol fermentation, D‐mannitol fermentation, and lactose fermentation analysis were performed to identify the biotypes of the isolated V. vulnificus.
2.5. Genotyping of V. vulnificus
Genotyping was conducted to identify the virulence genes of the isolated V. vulnificus. In our study, vcg (virulence‐correlated gene), serE (serovar E) gene, cps (capsular polysaccharide) gene, bt2 (biotype 2) gene, ary (arylsulfatase) gene, mtlABC (mannitol/fructose‐specific phosphotransferase system IIA protein) gene, and nanA (N‐acetylneuraminate lyase) gene were detected using virulence gene‐specific PCR as listed in Table 1.
2.6. Animal experiment
The V. vulnificus isolated from the seal (BJ‐PH01) was cultured in 1% APW and quantified by a colony forming unit (CFU) assay on blood agar. The inoculum was serially diluted to 108, 107, 106, 105, 104, and 103 CFU/ml 6‐week‐old female BALB/c mice were intraperitoneally (for each group, n = 4) (Strom & Paranjpye, 2000) or intramuscularly (for each group, n = 4) (Hor, Chang, Chang, Lei, & Ou, 2000; Lee & Chuang, 2010) injected with 0.1 ml of inoculum. The mice were observed each day after inoculation, and dead mice were examined. Pathological information was observed and recorded. Tissue samples of the dead mice were also collected.
Susceptibility tests were conducted by the Kirby‐Bauer method according to the recommendations of the National Committee for Clinical Laboratory Standards (M45) (Clinical & institute, 2015). In detail, V. vulnificus was cultured in Mueller‐Hinton broth for 14–16 hr. The direct colony suspension method was adopted to produce the inoculum suspension. The inocula were cultured on Mueller‐Hinton Agar for disk diffusion. The plates were placed at 35°C for 16–20 hr. The selected antibiotics included amikacin, levofloxacin, chloramphenicol, gentamicin, piperacillin/tazobactam, trimethoprim‐sulfamethoxazole, cefepime, ceftazidime, ciprofloxacin, piperacillin, cefotaxime, and tetracycline.
3. RESULTS
3.1. A spotted seal died due to V. vulnificus infection
Since March 2016, a captive spotted seal (Phoca largha) had suffered from a traumatic injury on the tail, which had not healed over a 2‐month treatment. The mental condition and health status were not clearly affected during the course of treatment. In May 2016, the seal began vomiting, became depressed, and died within 24 hr of the onset of clinical manifestations. Upon necropsy, severe pneumonia (Figure 1a), extensive intestinal hemorrhage (Figure 1b), and liver necrosis (Figure 1c) were observed. No lesions were noted on the heart, kidney, stomach, or spleen. We preliminarily speculated that the seal had died due to sepsis induced by trauma fester or other lethal infections.
Figure 1.

Autopsy of dead spotted seals. (a) Swelling and hemorrhage of lung tissues; (b) extensive hemorrhage was observed in the intestine; (c) severe necrosis was observed in the liver [Colour figure can be viewed at http://wileyonlinelibrary.com]
To explore the cause of death, we conducted bacteriological analysis (Figure 2a) and virological testing. Consequently, we failed to detect any known viral pathogens from the dead seal. In addition, V. vulnificus was successfully isolated from the fester, blood, and several tissue samples (i.e., lung, liver, intestine, and spleen) (Figure 2b). The result was confirmed by 16S rDNA sequencing, V. vulnificus vvhA (cytolysin/hemolysin) gene‐specific PCR amplification (Figure 2c), the BD Phoenix Automated Microbiology System, and electron scanning microscope (SEM) analysis (Figure 2d). The isolated V. vulnificus was named BJ‐PH01. These results suggested that the seal died due to septicemia induced by V. vulnificus.
Figure 2.

Isolation and identification of Vibrio vulnificus. (a) Scheme of bacteria isolation and identification from the dead seal; (b) V. vulnificus forms opaque colonies on blood agar; (c) V. vulnificus‐specific gene detection by PCR. The result showed that BJ‐PH01 was positive for the vvhA gene. Further analysis showed that BJ‐PH01 belongs to the vcgE genotype. Lane M: DL2000 marker; lanes 1‐7: vvhA, vcgC, vcgE, serE, cps0, cps1, and bt2, respectively. (d) Observation of BJ‐PH01 using electron scanning microscopy [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.2. Virulence of the isolated V. vulnificus
Several virulence factors of V. vulnificus have been reported previously, such as toxin, LPS, and capsule (Jones & Oliver, 2009). Possession of an antiphagocytic capsule is one of the absolute requirements for virulence. Encapsulated cells produce opaque colonies, and only opaque cells are able to utilize transferrin‐bound iron. As shown in Figure 2b, the V. vulnificus isolate BJ‐PH01 grew opaque colonies on blood agar. This result prompted us to consider that BJ‐PH01 is virulent in animal models (Simpson, White, Zane, & Oliver, 1987). Several animal models have been constructed to explore the increased susceptibility to V. vulnificus infections after the injection of iron‐containing compounds. Here, we conducted an animal experiment using intraperitoneal (i.p.) injections or intramuscular (i.m.) injections and concurrent i.p. injections with PBS. Six‐ to eight‐week‐old female BALB/c mice were included in the experiment. The results showed that, when infected by 1.4 × 106 PFU V. vulnificus, the mice died within 12 hr post‐infection. The median lethal dose of the isolated V. vulnificus BJ‐PH01 was 3.16 × 105 CFU. Severe hemorrhage was observed at the inoculation site and tails (Figure 3a). Furthermore, extensive edema and hemorrhage were noted in the lungs (Figure 3b) and intestines of the dead mice (Figure 3c), and V. vulnificus could be re‐isolated from the dead mice.
Figure 3.
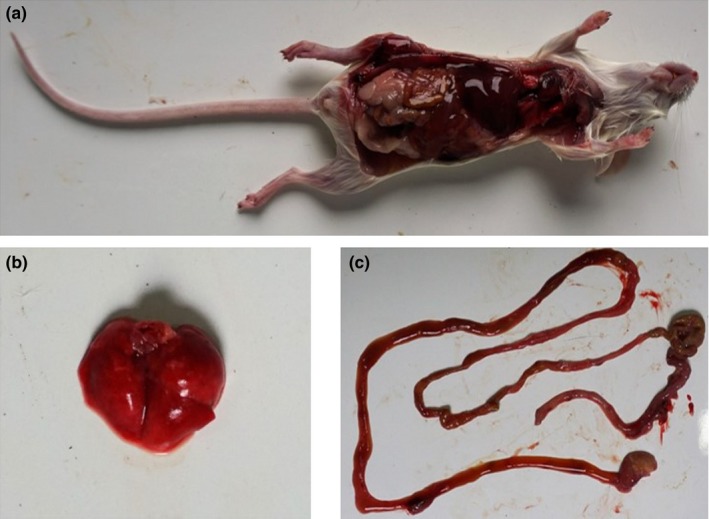
Experimental infection of mice using the isolated Vibrio vulnificus BJ‐PH01. (a) The mouse died within 12 hr post‐injection; upon necropsy, swelling and hemorrhage of the lung (b) and intestine (c) were observed [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.3. Epidemiological investigation of V. vulnificus in aquariums in northern China
Having demonstrated that V. vulnificus can be detected in aquariums, we next asked whether this lethal bacterium is widespread in aquariums. To explore the prevalence of V. vulnificus in aquariums, we performed an epidemiological investigation of V. vulnificus. In this analysis, we successfully collected 54 samples from six aquariums in northern China (Table 2). The detection of V. vulnificus was performed as described above. V. vulnificus was isolated from all six of the investigated aquariums. The average positive rate was 44.4%, ranging from 20% to 100% (Table 2). Finally, 12 strains of V. vulnificus were successfully isolated and identified. In addition to V. vulnificus, other bacteria of the genus Vibro were also frequently isolated, such as Vibrio parahaemolyticus, Vibrio alginolyticus, and Vibrio fluvialis.
The result indicated that V. vulnificus is widely distributed in coastal environments as well as aquariums, which are artificial salt‐water environments. Together with other species of Vibrio, V. vulnificus in aquariums poses a novel threat to captive marine animals and humans.
3.4. Biotyping and genotyping of isolated V. vulnificus
Vibrio vulnificus can be classified into three biotypes based on biochemical characteristics. To explore the biotype of the isolated V. vulnificus, we performed an assay of biochemical identification as previously reported (Bisharat, Agmon, Finkelstein, Raz, Ben‐Dror, Lerner, Soboh, Colodner, Cameron, Wykstra, et al., 1999). All the tested V. vulnificus samples were positive for the indole test, ONPG test, and ornithine decarboxylase test (Table 3). The results indicated that all 12 isolated strains belonged to biotype 1. In addition, biotype 1 V. vulnificus was classified into Clinical (C) or Environmental (E) genotypes based on its virulence‐correlated gene (vcg) (Warner & Oliver, 2008); our analysis showed that 11 of the 12 isolated V. vulnificus strains were classified into the C genotype, and only one of the 12 belonged to the E genotype (Table 4).
Table 3.
Biotype of isolated Vibrio Vulnificus
| Test | Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BJ‐PH01 | BJ‐PH02 | BJ‐PH03 | BJ‐PH04 | BJ‐PH05 | BJ‐PH06 | BJ‐SAR01 | BJ‐BZ01 | SJZ01 | XA01 | XA02 | XA03 | |
| ONPG test | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ |
| Ornithine decarboxylase | − | − | − | − | − | − | − | − | − | − | − | − |
| Indole production | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ |
| D‐sorbitol fermentation | − | − | − | − | − | − | − | − | − | ﹢ | − | − |
| D‐mannitol fermentation | − | − | − | − | − | − | − | − | − | − | − | − |
| Lactose fermentation | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ | ﹢ |
| Citrate | − | − | − | − | − | − | − | − | − | − | − | − |
| Biotype | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 4.
Genotype of isolated Vibrio vulnificus
| Strain | Source | Virulence genes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| vvhA | vcg | serE | CPS type | bt2 | ary | mtlABC | nanA | ||
| BJ‐PH01 | Spotted seal | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐PH02 | Turtle limbs | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐PH03 | Turtle limbs | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐PH04 | Turtle limbs | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐PH05 | Turtle limbs | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐PH06 | Turtle shell | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐SAR01 | Turtle shell | ﹢ | C | − | 0 | − | ﹢ | ﹢ | ﹢ |
| BJ‐BZ01 | Water | ﹢ | C | − | 1 | − | ﹢ | ﹢ | ﹢ |
| SJZ01 | Water | ﹢ | E | − | 0 | − | ﹢ | ﹢ | ﹢ |
| XA01 | Water | ﹢ | C | − | 1 | − | ﹢ | ﹢ | ﹢ |
| XA02 | Water | ﹢ | C | − | 1 | − | ﹢ | ﹢ | ﹢ |
| XA03 | Water | ﹢ | C | − | 1 | − | ﹢ | ﹢ | ﹢ |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
A previous study demonstrated that genotypic markers cannot unequivocally predict virulence, although several genes were putatively reported to be linked to virulence. Here, we investigated the genes ary (arylsulfatase), mtlABC (mannitol/fructose‐specific phosphotransferase system IIA protein), and nanA (N‐acetylneuraminate lyase) by PCR in our study (Table 4). The ary gene has been demonstrated to be associated with virulence of clinical strains by providing a pathogen with sulfur within the host, thus providing an immune evasion approach (Morrison et al., 2012). Positive Ary PCRs were obtained in all of the isolated strains. Although mtlABC appears to be linked to pathogen virulence, the precise role of mtlABC is not yet understood (Reynaud et al., 2013). In our experiments, we obtained positive mtlABC results for all of the isolated strains. The nanA gene has been demonstrated to be involved in sialic acid metabolism and is essential for V. vulnificus virulence (Kim, Hwang, Kim, & Choi, 2011). Positive nanA gene PCR results were obtained for all of the isolated strains. These results indicate that the 12 isolated V. vulnificus strains display a potential threat to mammals, including marine animals and humans.
3.5. Antibiotic analysis of V. vulnificus
The disease progression of V. vulnificus infection is often acute, and it is therefore important to provide timely treatment with proper antibiotics. We conducted a further analysis to understand its sensitivity to major antibiotics. The result showed that all isolated V. vulnificus strains were sensitive to antibiotics. In detail, V. vulnificus was sensitive to levofloxacin, tetracycline, piperacillin, and gentamicin (Table 5).
Table 5.
Antibiotic susceptibility tests of the BJ‐PH01 using the agar disk disffusion method
| Test | Zone diameter (mm) | Resulta |
|---|---|---|
| Amikacin | ≥16 | I |
| Levofloxacin | ≥20 | S |
| Chloramphenicol | ≥17 | I |
| Gentamycin | ≥21 | S |
| Piperacillin/Tazobactam | ≥21 | S |
| Sulfamethoxazole trimethoprim | ≥21 | S |
| Cefepime | ≥21 | S |
| Ceftazidime | ≥22 | S |
| Ciprofloxacin | ≥23 | S |
| Piperacillin | ≥21 | S |
| Cefotaxime | ≥20 | I |
| Tetracycline | ≥26 | S |
Note. aS: susceptible; I: intermediate; R: resistant.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
Vibrio vulnificus is a foodborne pathogen of humans. Here, we provide evidence that marine mammals can also be infected by V. vulnificus. Epidemiological investigations of V. vulnificus showed that this lethal bacterium can frequently be detected in aquariums, thus constituting a novel threat to marine animals, workers, and tourists in relevant aquariums. Antibiotic drug resistance is an increasing concern due to the overuse of antibiotics. Fortunately, all of the isolated V. vulnificus isolates in our study were sensitive to levofloxacin, tetracycline, piperacillin, and gentamicin. No drug‐resistant V. vulnificus isolates were isolated.
The minimum dose capable of causing human infection is currently unknown (Strom & Paranjpye, 2000). Previous studies have suggested that the dose may be fewer than 1,000 organisms. Animal experiments have been helpful in researching disease syndromes produced by V. vulnificus. However, they have no instructive value for determining the infectious dose 50 (ID50) for human infections (Strom & Paranjpye, 2000). In our research, we found that the median lethal dose of isolated V. vulnificus BJ‐PH01 was 3.16 × 105 CFU. These data have limited value in determining virulence for humans at present, but they may contribute to future research in studying the correlation between infectious dose for animal models and humans.
Marine mammals are a diverse group of species that includes cetaceans, pinnipeds, sirenians, sea otters, and polar bears. Protection for marine mammals from multiple threats has increased since the enactment of the Marine Mammal Protection Act in 1972. However, the lack of scientific data, confusion about permitting requirements, failure to adopt appropriate management, and inappropriate human activities have impaired our protection efforts (Schipper et al., 2008). Infectious disease is one of the most important risks to wildlife animals. Scientific research on marine mammal infectious diseases has been relatively insufficient. Previously demonstrated pathogens of marine mammals include viruses (e.g., adenovirus, calicivirus, herpesvirus, morbillivirus, poxvirus, influenza virus, retrovirus) (Goldstein et al., 2009; Maness et al., 2011; Ohishi et al., 2010; Ramis, van Riel, van de Bildt, Osterhaus, & Kuiken, 2012; Rivera, Nollens, Venn‐Watson, Gulland, & Wellehan, 2010), bacteria (e.g., Actinomycetes, Brucella, Clostridia, Erysipelothrix rhusiopathiae, Pasteurella multocida, and Mycoplasma) (Waltzek, Cortés‐Hinojosa, Wellehan, & Gray, 2012), and parasites (e.g., Acanthocephalans, Toxoplasma gondii, Sarcocystis, Coccidia, and Parafilaroides decorus) (Hernandez‐Orts et al., 2015; Jensen, Aars, Lydersen, Kovacs, & Åsbakk, 2010). Here, we demonstrated that spotted seals can be infected by V. vulnificus. As no host tropism of V. vulnificus has been reported, one can speculate that other marine mammals might also be infected with V. vulnificus. Therefore, we should pay more attention to abnormal deaths of wild marine mammals to accumulate scientific data.
In addition to V. vulnificus, other opportunistic pathogens of the Vibrio genus were also detected in this investigation, such as Vibrio parahaemolyticus, Vibrio alginolyticus, and Vibrio fluvialis. Vibrio parahaemolyticus is a marine organism native to estuarine waters around the world. In Asia, Vibrio parahaemolyticus is a common cause of foodborne disease (Sakata, Yonekita, & Kawatsu, 2018), while Vibrio alginolyticus is implicated in wound infections and otitis. Vibrio alginolyticus was recently recognized as a human pathogen after excessive exposure to seawater (Gao et al., 2017). Vibrio fluvialis has been considered an emerging pathogen that induces foodborne diarrhea (Ramamurthy, Chowdhury, Pazhani, & Shinoda, 2014). These results suggest that V. vulnificus is not the only threat to marine animals and public health in aquariums.
The major limitation of our research is that we failed to perform Koch's postulate test because it was impossible for us to confirm the infection using a healthy seal. Due to limited background information, the typical clinical manifestations of V. vulnificus infected seals are poorly understood. We provided a diagnosis mainly based on the detection and isolation of V. vulnificus from the visceral tissues and blood, which indicated that V. vulnificus induced septicemia (Bross, Soch, Morales, & Mitchell, 2007b).
Previous studies have demonstrated that V. vulnificus can survive under nutrient starvation in seawater for years without losing its pathogenic potential for animals and humans (Marco‐Noales, Biosca, & Amaro, 1999). Thus, it is difficult to eliminate V. vulnificus without scientific measures, and it is necessary to take measures to control the water quality in aquariums.
We demonstrated that V. vulnificus can infect and kill marine mammals. However, it was difficult to evaluate the risk of infection of marine mammals by V. vulnificus: (a) V. vulnificus is an opportunistic pathogen, and one or more predisposing factors are required to initiate disease; (b) the minimum dose for V. vulnificus to cause an infection is poorly understood, even in humans; (c) the interface between V. vulnificus and marine mammals in the wild has never been studied. Nevertheless, the prevalence of V. vulnificus is a persistent threat to marine mammals.
CONFLICT OF INTEREST
No conflict of interest exists in the submission, and all authors approved the publication.
ACKNOWLEDGEMENTS
This work was funded by grants from the State Forestry Administration of China, Chinese Academy of Sciences (CZBZX‐1).
Li M, Zhao L, Ma J, et al. Vibrio vulnificus in aquariums is a novel threat to marine mammals and public health. Transbound Emerg Dis. 2018;65:1863–1871. 10.1111/tbed.12967
REFERENCES
- Baker‐Austin, C. , & Oliver, J. D. (2016). Rapidly developing and fatal Vibrio vulnificus wound infection. IDCases, 6, 13 10.1016/j.idcr.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisharat, N. , Agmon, V. , Finkelstein, R. , Raz, R. , Ben‐Dror, G. , Lerner, L. , & Wykstra, D. L. (1999). Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. The Lancet, 354(9188), 1421–1424. 10.1016/S0140-6736(99)02471-X [DOI] [PubMed] [Google Scholar]
- Bross, M. H. , Soch, K. , Morales, M. R. , & Mitchell, R. B. (2007a). Vibrio vulnificus Infection: Diagnosis and Treatment. Alcoholism, 65, 32. [PubMed] [Google Scholar]
- Bross, M. H. , Soch, K. , Morales, R. , & Mitchell, R. B. (2007b). Vibrio vulnificus infection: diagnosis and treatment. Diabetes, 35, 20. [PubMed] [Google Scholar]
- Chatzidaki‐Livanis, M. , Jones, M. K. , & Wright, A. C. (2006). Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon. Journal of Bacteriology, 188(5), 1987–1998. 10.1128/JB.188.5.1987-1998.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2015). M45 Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Wayne, PA: Clinical and Laboratory Standards Institute. [DOI] [PubMed] [Google Scholar]
- Gao, X. , Liu, Y. , Liu, H. , Yang, Z. , Liu, Q. , Zhang, Y. , & Wang, Q. (2017). Identification of the regulon of AphB and its essential roles in LuxR and exotoxin Asp expression in the pathogen vibrio alginolyticus. Journal of Bacteriology, 199(20), 10.1128/jb.00252-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, T. , Mazet, J. A. K. , Gill, V. A. , Doroff, A. M. , Burek, K. A. , & Hammond, J. A. (2009). Phocine distemper virus in northern sea otters in the Pacific Ocean, Alaska, USA. Emerging Infectious Diseases, 15(6), 925–927. 10.3201/eid1506.090056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómara, M. I. , Wong, C. , Blome, S. , Desselberger, U. , & Gray, J. (2002). Molecular characterization of VP6 genes of human rotavirus isolates: Correlation of genogroups with subgroups and evidence of independent segregation. Journal of Virology, 76(13), 6596–6601. 10.1128/JVI.76.13.6596-6601.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Orts, J. S. , Viola, M. N. , Garcia, N. A. , Crespo, E. A. , Gonzalez, R. , Garcia‐Varela, M. , & Kuchta, R. (2015). A checklist of the helminth parasites of marine mammals from Argentina. Zootaxa, 3936(3), 301–334. 10.11646/zootaxa.3936.3.1 [DOI] [PubMed] [Google Scholar]
- Hill, W. E. , Keasler, S. , Trucksess, M. , Feng, P. , Kaysner, C. , & Lampel, K. (1991). Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Applied and Environmental Microbiology, 57(3), 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor, L. I. , Chang, Y. K. , Chang, C. C. , Lei, H. Y. , & Ou, J. T. (2000). Mechanism of high susceptibility of iron‐overloaded mouse to Vibrio vulnificus infection. Microbiology and Immunology, 44(11), 871–878. 10.1111/j.1348-0421.2000.tb02577.x [DOI] [PubMed] [Google Scholar]
- Huang, K. C. , Weng, H. H. , Yang, T. Y. , Chang, T. S. , Huang, T. W. , & Lee, M. S. (2016). Distribution of fatal Vibrio vulnificus necrotizing skin and soft‐tissue infections: a systematic review and meta‐analysis. Medicine (Baltimore), 95(5), e2627 10.1097/MD.0000000000002627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H. , Shibayama, A. , Abe, M. , Antoku, S. , Nawata, H. , Isonishi, M. , Kato, S. (2012). Vibrio vulnificus septicemia and necrotizing fasciitis in the patients with liver cirrhosis and diabetes mellitus. Journal of Diabetes Mellitus, 2, 122–125. 10.4236/jdm.2012.21020 [DOI] [Google Scholar]
- Jensen, S. K. , Aars, J. , Lydersen, C. , Kovacs, K. M. , & Åsbakk, K. (2010). The prevalence of Toxoplasma gondii in polar bears and their marine mammal prey: Evidence for a marine transmission pathway? Polar Biology, 33(5), 599–606. 10.1007/s00300-009-0735-x [DOI] [Google Scholar]
- Jones, M. K. , & Oliver, J. D. (2009). Vibrio vulnificus: Disease and pathogenesis. Infection and Immunity, 77(5), 1723–1733. 10.1128/IAI.01046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimoto, T. , Iwasaki, C. , Gojo, M. , Sugiyama, H. , Yoshioka, K. , Yamamoto, Y. , & Ueno, S. (2015). Vibrio vulnificus detected in the spleen leads to fatal outcome in a mouse oral infection model. FEMS Microbiology Letters, 362(7), 10.1093/femsle/fnv005 [DOI] [PubMed] [Google Scholar]
- Kaysner, C. A. , & DePaola, A. Jr. (2004). Bacteriological analytical manual. Chapter 9: Vibrio. Washington, DC: US Food and Drug Administration; Retrieved from https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070830.htm [Google Scholar]
- Kim, C. S. , Bae, E. H. , Ma, S. K. , & Kim, S. W. (2015). Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: A case report. BMC Infectious Diseases, 15, 422 10.1186/s12879-015-1163-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. S. , Hwang, J. , Kim, M. H. , & Choi, S. H. (2011). Cooperative regulation of the Vibrio vulnificus nan gene cluster by NanR protein, cAMP receptor protein, and N‐Acetylmannosamine 6‐Phosphate. Journal of Biological Chemistry, 286(47), 40889–40899. 10.1074/jbc.M111.300988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton, Y. , Soboh, S. , & Bisharat, N. (2015). Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infectious Disease Reports, 7(3), 5901 10.4081/idr.2015.5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, S. K. , Woo, P. C. , Li, K. S. , Huang, Y. , Tsoi, H.‐W. , Wong, B. H. , & Yuen, K.‐Y. (2005). Severe acute respiratory syndrome coronavirus‐like virus in Chinese horseshoe bats. Proceedings of the National Academy of Sciences of the United States of America,102(39), 14040–14045. 10.1073/pnas.0506735102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. J. , & Chuang, Y. C. (2010). Ibuprofen augments pro‐inflammatory cytokine release in a mouse model of Vibrio vulnificus infection. Microbiology and Immunology, 54(9), 542–550. 10.1111/j.1348-0421.2010.00249.x [DOI] [PubMed] [Google Scholar]
- Madiyal, M. , Eshwara, V. K. , Halim, I. , Stanley, W. , Prabhu, M. , & Mukhopadhyay, C. (2016). A rare glimpse into the morbid world of necrotising fasciitis: Flesh‐eating bacteria Vibrio vulnificus . Indian Journal of Medical Microbiology, 34(3), 384–386. 10.4103/0255-0857.188361 [DOI] [PubMed] [Google Scholar]
- Maness, H. T. D. , Nollens, H. H. , Jensen, E. D. , Goldstein, T. , LaMere, S. , Childress, A. , & Wellehan, J. F. X. (2011). Phylogenetic analysis of marine mammal herpesviruses. Veterinary Microbiology, 149(1), 23–29. 10.1016/j.vetmic.2010.09.035 [DOI] [PubMed] [Google Scholar]
- Marco‐Noales, E. , Biosca, E. G. , & Amaro, C. (1999). Effects of salinity and temperature on long‐term survival of the eel pathogen Vibrio vulnificus biotype 2 (serovar E). Applied and Environment Microbiology, 65(3), 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D. S. , Finnie, J. , Bowden, T. R. , Scholz, A. C. , Oh, S. , Kok, T. , & Li, P. (2011). Preclinical efficacy studies of influenza A haemagglutinin precursor cleavage loop peptides as a potential vaccine. Journal of General Virology, 92(5), 1152–1161. 10.1099/vir.0.028985-0 [DOI] [PubMed] [Google Scholar]
- Miyamoto, N. , Mandai, M. , Suzuma, I. , Suzuma, K. , Kobayashi, K. , & Honda, Y. (1999). Estrogen protects against cellular infiltration by reducing the expressions of E‐Selectin and IL‐6 in endotoxin‐induced uveitis. The Journal of Immunology, 163(1), 374–379. [PubMed] [Google Scholar]
- Morrison, S. S. , Williams, T. , Cain, A. , Froelich, B. , Taylor, C. , Baker‐Austin, C. , & Gibas, C. J. (2012). Pyrosequencing‐based comparative genome analysis of Vibrio vulnificus environmental isolates. PLoS ONE, 7(5), e37553 10.1371/journal.pone.0037553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi, K. , Ando, A. , Suzuki, R. , Takishita, K. , Kawato, M. , Katsumata, E. , & Maruyama, T. (2010). Host–virus specificity of morbilliviruses predicted by structural modeling of the marine mammal SLAM, a receptor. Comparative Immunology, Microbiology and Infectious Diseases, 33(3), 227–241. 10.1016/j.cimid.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Oliver, J. (2005). Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiology and Infection, 133(03), 383–391. 10.1017/S0950268805003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy, T. , Chowdhury, G. , Pazhani, G. P. , & Shinoda, S. (2014). Vibrio fluvialis: An emerging human pathogen. Frontiers in Microbiology, 5, 91 10.3389/fmicb.2014.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis, A. J. , van Riel, D. , van de Bildt, M. W. G. , Osterhaus, A. , & Kuiken, T. (2012). Influenza A and B virus attachment to respiratory tract in marine mammals. Emerging Infectious Diseases, 18(5), 817–820. 10.3201/eid1805.111828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud, Y. , Pitchford, S. , De Decker, S. , Wikfors, G. H. , & Brown, C. L. (2013). Molecular typing of environmental and clinical strains of Vibrio vulnificus isolated in the northeastern USA. PLoS ONE, 8(12), e83357 10.1371/journal.pone.0083357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera, R. , Nollens, H. H. , Venn‐Watson, S. , Gulland, F. M. D. , & Wellehan, J. F. X. (2010). Characterization of phylogenetically diverse astroviruses of marine mammals. Journal of General Virology, 91(1), 166–173. 10.1099/vir.0.015222-0 [DOI] [PubMed] [Google Scholar]
- Sakata, J. , Yonekita, T. , & Kawatsu, K. (2018). Development of a rapid immunochromatographic assay to detect contamination of raw oysters with enteropathogenic Vibrio parahaemolyticus . International Journal of Food Microbiology, 264, 16–24. 10.1016/j.ijfoodmicro.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Schipper, J. , Chanson, J. S. , Chiozza, F. , Cox, N. A. , Hoffmann, M. , Katariya, V. , & Young, B. E. (2008). The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science, 322(5899), 225–230. 10.1126/science.1165115 [DOI] [PubMed] [Google Scholar]
- Sea, N. (2015). Virulence profiles of Vibrio vulnificus in german coastal waters, a comparison of north sea and baltic sea isolates. International Journal of Environmental Research and Public Health, 12, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, L. , White, V. , Zane, S. , & Oliver, J. (1987). Correlation between virulence and colony morphology in Vibrio vulnificus . Infection and Immunity, 55(1), 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy, G. , Boivin, G. , Labrie, F. , & Rivest, S. (2005). Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. The Journal of Immunology, 174(10), 6391–6398. 10.4049/jimmunol.174.10.6391 [DOI] [PubMed] [Google Scholar]
- Stefaniuk, E. , Baraniak, A. , Gniadkowski, M. , & Hryniewicz, W. (2003). Evaluation of the BD Phoenix automated identification and susceptibility testing system in clinical microbiology laboratory practice. European Journal of Clinical Microbiology and Infectious Diseases, 22(8), 479–485. 10.1007/s10096-003-0962-y [DOI] [PubMed] [Google Scholar]
- Strom, M. S. , & Paranjpye, R. N. (2000). Epidemiology and pathogenesis of Vibrio vulnificus . Microbes and Infection, 2(2), 177–188. 10.1016/S1286-4579(00)00270-7 [DOI] [PubMed] [Google Scholar]
- Waltzek, T. B. , Cortés‐Hinojosa, G. , Wellehan, J. F. X. Jr , & Gray, G. C. (2012). Marine mammal zoonoses: A review of disease manifestations. Zoonoses and Public Health, 59(8), 521–535. 10.1111/j.1863-2378.2012.01492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, E. B. , & Oliver, J. D. (2008). Multiplex PCR assay for detection and simultaneous differentiation of genotypes of Vibrio vulnificus biotype 1. Foodborne Pathogens and Disease, 5(5), 691–693. 10.1089/fpd.2008.0120 [DOI] [PubMed] [Google Scholar]


