Abstract
Feline kobuvirus (FeKoV) is a newly discovered organism, classified under the species Aichivirus A of the genus Kobuvirus. Since it was first reported in 2013, molecular evidence for FeKoV in the feline population has been restricted to two countries: Korea and Italy. In this study, we collected faecal samples from cats in southern China and detected the FeKoV RNA in these samples. A prevalence rate of 9.9% (8/81) was identified by RT‐PCR, and all positive samples were obtained from diarrhoeic animals. In addition, FeKoV was shown positive associated with diarrhoea in cats, with a correlation coefficient of 0.25. Next, we designed three primer pairs with degenerate bases, which targeted the conservative overlapping region of the entire published FeKoV genome, and sequenced the near‐complete genome of the first Chinese field FeKoV strain, WHJ‐1, using long‐fragment PCR. Finally, we analysed WHJ‐1's homology and phylogeny using the polyprotein gene. The results indicated that FeKoV has rapidly mutated since it was first discovered. This study will help to better understand FeKoV's epidemiology, evolutionary pattern and genetic diversity.
Keywords: diarrhoeic cat, FeKoV China, feline kobuvirus, homology analysis, phylogenetic analysis
1. INTRODUCTION
According to the International Committee on Taxonomy of Viruses (ICTV), the Kobuvirus genus belongs to the family Picornaviridae of the order Picornavirales. The genus Kobuvirus was first established in 1999 and currently contains six officially recognized species, Aichivirus A (formerly Aichi virus), Aichivirus B (formerly Bovine kobuvirus), Aichivirus C (porcine kobuvirus), Aichivirus D (kagovirus 1), Aichivirus E (rabbit picornavirus) and Aichivirus F (bat kobuvirus) (Adams, King, & Carstens, 2013; Adams et al., 2017; Pringle, 1999). Other members of the Kobuvirus genus have been detected in sheep, goats, rodents and wild boar (Lee et al., 2012; Phan et al., 2011; Reuter, Boros, Pankovics, & Egyed, 2010; Reuter et al., 2013). The species Aichivirus A includes six types: Aichi virus, canine kobuvirus, murine kobuvirus, Kathmandu sewage kobuvirus, roller kobuvirus and feline kobuvirus (FeKoV) (Adams et al., 2017).
Kobuviruses are small, nonenveloped, icosahedral particles with linear, positive‐sense ssRNA genomes of 8.2–8.4 kb (Lee et al., 2012; Reuter, Boldizsar, & Pankovics, 2009; Yamashita et al., 1998; Yu et al., 2011). Their genome is composed of a 5′ untranslated region (UTR), a large open reading frame (ORF), and a 3′ UTR. The ORF encodes a single polyprotein precursor, which is cleaved into three structural viral proteins (VP0, VP1 and VP3) and eight nonstructural proteins (L, 2A, 2B, 2C, 3A, 3B, 3C and 3D). Kobuviruses have similar genome organizations to those described in other picornaviruses, 5′‐L‐VP0‐VP3‐VP1‐2A‐2B‐2C‐3A‐3B‐3C‐3D‐3′ (Choi, Lee, Lee, & Oem, 2015; Kapoor et al., 2011; Pankovics et al., 2016; Reuter et al., 2009).
The first serological evidence supporting kobuviral infections in cats was reported in the United Kingdom by N. Carmona‐Vicente et al. in 2013 (Carmona‐Vicente et al., 2013). Using the Aichi virus as an antigen, IgG antibody to this organism was detected in 67 of 97 cat serum samples, indicating an Aichi virus crossreactive kobuviral infection is common in cats. The first FeKoV molecular evidence was reported in Korea by Joon‐Yee Chung et al. in 2013 (Chung et al., 2013). Using reverse transcription polymerase chain reaction (RT‐PCR), six of 39 collected faecal samples from Korean cats were confirmed positive for kobuvirus RNA. Phylogenetic analysis revealed that FeKoV (12D240, FK‐13) is most closely related to the canine kobuvirus (Choi et al., 2015). In 2015, Barbara Di Martino et al. screened faecal samples obtained from asymptomatic and diarrhoeic cats for FeKoV RNA in Italy (Di Martino, Profio, Melegari, Marsilio, & Martella, 2015). FeKoV RNA was found in five of 37 diarrhoeic cats but was undetected in asymptomatic cats. The full‐length genome sequence of one Italian FeKoV strain (FeKoV/TE/52/IT/13) was sequenced, and it displayed a high nucleotide identity (96.0%) to the Korean strain, FK‐13.
Until now, molecular reports of the kobuvirus in cats have been restricted to Korea and Italy, and only three FeKoV strain genomes have been sequenced (Cho et al., 2014; Choi et al., 2015; Di Martino et al., 2015). Therefore, it is important to investigate and genetically characterize FeKoV infections in other countries to assess the global epidemiology of this emerging virus. Our study determined that FeKoV is present in China. We evaluated its molecular prevalence in cats in China, sequenced the near‐complete genome of one Chinese FeKoV strain and analysed its homology and phylogeny based on the sequence.
2. MATERIALS AND METHODS
2.1. Sample collection
From December 2016 to November 2017, 81 feline faecal samples were collected from Shenzhen City and Guangdong City from 29 asymptomatic cats and 52 diarrhoeic cats. Samples were collected under the animal ethics guidelines and approved by the Animal Care and Use Committee of South China Agricultural University. Samples were stored at −80°C immediately after collection.
2.2. Viral DNA/RNA extraction and cDNA synthesis
Faecal samples were suspended in phosphate‐buffered saline (PBS) at 10% wt/vol. The mixture was centrifuged at 4,000 g at 4°C for 20 min, and the supernatant was filtered using a 0.22‐μm filter (Millipore, USA). Viral RNA from the resulting 200‐μl filtrates was extracted using a TIANamp Virus DNA/RNA Kit (TianGen, Beijing, China) per the manufacturer's protocol. Seven microlitres of extracted RNA was used for random‐primed reverse transcription (RT) using the HiScript®II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China).
2.3. Sample screening and genome sequencing
The FeKoV screening RT‐polymerase chain reaction (RT‐PCR) was performed using one published primer pair with broad reactivity with various kobuvirus species (Reuter et al., 2009). The primer pair, UNIV‐kobu‐F and UNIV‐kobu‐R, was designed targeting a 216‐bp fragment of the 3D RdRp region of the three prototype kobuviruses (Aichi virus, bovine kobuvirus and porcine kobuvirus). FeKoV RNA was detected by PCR using PrimeSTAR®HS (premix) (Takara, Dalian, China). The PCR conditions were 35 cycles at 98°C for 10 s, 50°C for 15 s and 72°C for 30 s, followed by 1 cycle at 72°C for 5 min. The PCR product was electrophoresed in a 1% ethidium‐bromide‐stained agarose gel, and the faecal samples yielding PCR products of ≈220 bp were considered FeKoV RNA‐positive. The nucleic acids contained in the agarose gel were then purified and cloned into the PLB vector using the Lethal‐Based Fast Cloning Kit (TianGen, Beijing, China). After bacterial transformation, positive clones were sequenced using the Sanger method (BGI, Guangzhou, China). Two common feline enteric pathogens, the feline parvovirus and feline enteric coronavirus, were also detected in the FeKoV‐positive faecal samples.
To acquire genome information on the FeKoV strain in the FeKoV‐positive samples, three overlapping primer pairs were designed to target the nearly complete FeKoV genome (Figure 1). PCR was performed using Q5® high‐fidelity DNA polymerase (NEB, Ipswich, UK). The amplified products were then purified and sequenced. The extreme 5′ sequence was determined by a 5′‐RACE Kit (TaKaRa, Dalian, China) per the manufacturer's protocol. Nucleotide sequences were edited using the Seqman module of the DNAStar package (version 7.1.0). The FeKoV genome sequence identified in this study was submitted to GenBank and assigned the number, MF598159.
Figure 1.
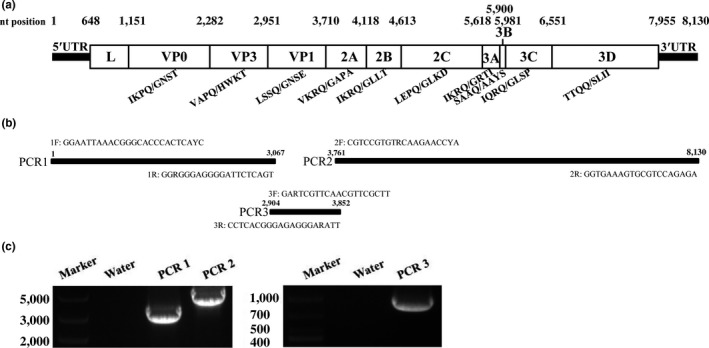
PCR strategy for the Chinese FeKoV WHJ‐1 genome. (a) Schematic genome organization of the Chinese FeKoV strain WHJ‐1: Each polyprotein gene is indicated in a box. The nucleotide (nt) position of each gene is shown above the gene‐boxes. The predicted cleavage sites are shown on each gene border and below the gene‐boxes. (b) Sequence‐specific primers were designed based on the conserved regions of three published FeKoV strains. Primer names and target regions are indicated. F = forward and R = reverse. (c) PCR results for the WHJ‐1 genome: Water indicates the control PCR reaction using water as the PCR template
2.4. Homology and phylogenetic analysis
Thirty‐eight kobuvirus polyprotein ORF sequences were retrieved from the NCBI database (https://www.ncbi.nlm.nih.gov/). Their strain names and accession numbers are shown in Figure 2. The WHJ‐1 polyprotein ORF sequence was then aligned with the kobuvirus polyprotein gene sequences using Bioedit (version 7.0.9.0). The nucleotide and amino acid homologies between these sequences were calculated by the Megalign module of the DNAStar package (version 7.1.0). Finally, a neighbour‐joining tree based on maximum composite likelihood was constructed using a 1,000‐bootstrap value in Mega (version 5.05).
Figure 2.
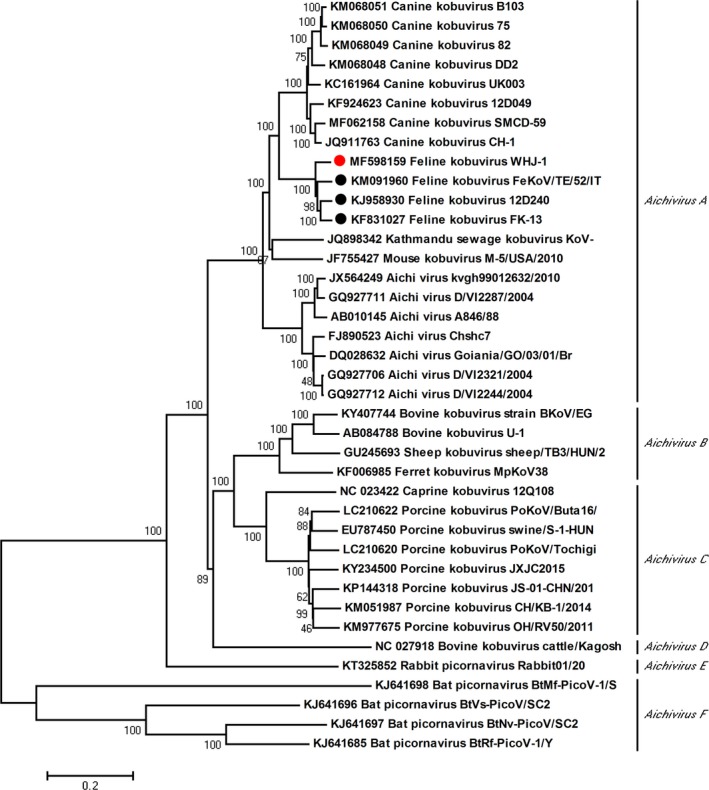
Phylogenetic analysis of the FeKoV strain in China. The neighbour‐joining tree was established using Mega (version 5.05) based on the Chinese FeKoV WHJ‐1 polyprotein gene and 38 kobuvirus strains using maximum composite likelihood and the 1,000‐bootstrap value. The Chinese FeKoV strain is indicated by a red circle, and the Korean and Italian FeKoV strains are shown as black circles
2.5. Virus isolation
Faecal samples that were only positive for FeKoV were processed for viral isolation, which was performed by inoculating 0.5 ml of the filtrate onto confluent cell layers grown in 25‐cm2 flasks at 37°C. Six cell types (CRFK, MDCK, A549, PK‐15, DF‐1, Vero) were used and grown in Dulbecco's Modified Eagle's Medium (Gibco, Shanghai, China) supplemented with 100 μg/ml of streptomycin, 100 units/ml of penicillin and 10% foetal calf serum. After inoculating for 1 hr, the inocula were removed and fresh medium was added. Negative noninoculated controls were also cultivated. Cultures were inspected daily by inverted microscopy for cytopathic effects (CPE) until 4 days postinoculation. The cultures were frozen and used for further passage. Culture lysates and supernatant were also collected for FeKoV RT‐PCR.
3. RESULTS
3.1. FeKoV prevalence in China
To detect the presence of FeKoV, RNA was extracted from the collected faecal samples and reverse transcribed into cDNA. After PCR with the primer pairs, UNIV‐kobu‐F and UNIV‐kobu‐R, and agarose gel electrophoresis, eight PCR products presented bright bands of ≈220 bp. The subsequent sequencing and BLAST hits indicated that all eight faecal samples were FeKoV‐positive, with a prevalence of 9.9% (8/81). All positive samples were obtained from diarrhoeic cats. No FeKoV RNA was detected in samples from asymptomatic cats. Among the FeKoV‐positive faecal samples, three samples and two samples were positive for feline parvovirus and feline enteric coronavirus, respectively, while three samples were positive only for FeKoV. In addition, the sequenced 216‐bp fragment of the eight field FeKoV strains had nucleotide and amino acid sequence identities of 98.1%–100% and 98.6%–100% with each other and the highest nucleotide sequence identity of 93.5% with the Korean FeKoV strain, 12D240, when compared with that of the reported FeKoV strains. One field FeKoV strain demonstrated here was named WHJ‐1.
To elucidate the possibility of a correlation between FeKoV and feline diarrhoea, the data were processed for calculating the Phi coefficient of association. The obtained correlation coefficient was 0.25. In addition, the p value of one‐tailed and one‐tailed Fisher exact probability test was 0.023 (p < 0.05) and 0.046 (p < 0.05), respectively. The result suggested a positive association between FeKoV and feline diarrhoea.
3.2. Sequencing the FeKoV viral genome in China
To acquire the WHJ‐1 genome, all FeKoV genome sequences available in the NCBI database were retrieved and aligned, and three primer pairs were designed based on the FeKoV genome conservative region (Figure 1a,b). After agarose gel electrophoresis, the PCR products amplified by the three primer pairs had bands of ≈3,000, 950, and 4,400 bp, respectively (Figure 1c). After sequencing and assembly, the near‐complete genome of WHJ‐1 was obtained, including a 647‐nucleotide 5′ UTR, a 7,311‐nucleotide complete polyprotein ORF gene and a 172‐nucleotide partial 3′ UTR. WHJ‐1 had a 5′ UTR of 647 nucleotides, with one nucleotide (nt position: 371) longer than that of the Korean Feline kobuvirus strain FK‐13. A nucleotide comparison between their 5′ UTR indicated 27 other base substitutions. The alignment of the nucleotide comparison between their 5′ UTR was shown in Figure S1. As with FeKoV strains from other countries, WHJ‐1 encodes a putative polyprotein precursor of 2,437 amino acids. Thirteen unique amino acid mutation sites were observed in the WHJ‐1 polyprotein when compared with those of other FeKoV strains, including A671T, Y684C, S1024P, S1027P, V1054I, S1800N, P1841S, A1869S, H1870F, P2097S, V2101L, Q2114R and D2139E. The cleavage site positions in the FeKoV polyprotein were predicted by alignment using the Korean representative FeKoV strain, 12D240. WHJ‐1 encodes the 11 putative cleaved viral proteins of L, VP0, VP3, VP1, 2A, 2B, 2C, 3A, 3B, 3C, 3D, with 168, 377, 223, 253, 136, 165, 335, 94, 27, 190 and 468 amino acid lengths, respectively (Figure 1a). The polyprotein's predicted cleavage sites were Q/G, Q/H, Q/A and Q/S. Several conserved picornavirus α‐motifs were predicted in the putative viral proteins, such as the potential myristoylation motifs, G216GASS, G239SRYS and G269KVAS, at the N‐terminus of the VP0 hypothetical protein, the conserved amino acid motifs, H1048WAI and NC1110THFV, in 2A, the conserved nucleotide‐binding domain of helicase G1453PPGTGKS in 2C, and the active‐site, G1919LCG, of the viral cysteine protease 3C.
3.3. Homology and phylogenetic analysis of FeKoV from China
The nucleotide and amino acid sequences of the WHJ‐1 polyprotein and cleaved viral proteins were obtained and compared with those of three FeKoV strains and four representative kobuviruses (Table 1). WHJ‐1 had a higher homology with FeKoV than with kobuviruses of other origins when analysing each gene at both the nucleotide and the amino acid levels. In addition, except the 3A gene homology between WHJ‐1 and the canine and mouse kobuviruses, higher nucleotide and amino acid identities were found in each gene between WHJ‐1 and the canine kobuvirus compared with mouse, human and bovine kobuviruses. Among the polyprotein genes of the three FeKoV strains, WHJ‐1 had a higher nucleotide identity with the Italian strain, FeKoV/TE/52/IT/13 (93.2%), and a higher amino acid homology with the Korean strain, FK‐13 (98.6%). The highest nucleotide identity was found between the FK‐13 3B gene and the 12D240 2A gene, with a value of 95.1%, and a highest amino acid identity was found between the FeKoV/TE/52/IT/13 2B protein, the FK‐13 3A and 3B proteins, and the 12D240 VP0 and 2B proteins, with a value of 100%. The highest nucleotide divergence was found in the FeKoV/TE/52/IT/13 3B gene, with a value of 88.9%, and the highest amino acid divergence was found in the 12D240 L protein, with a value of 83.9%.
Table 1.
Nucleotide/amino acid identities (%) between the Chinese FeKoV strain, WHJ‐1 and other kobuvirus representatives
| Gene | Feline | Canine | Mouse | Human | Bovine | ||
|---|---|---|---|---|---|---|---|
| FeKoV/TE/52/IT/13 | FK‐13 | 12D240 | US‐PC0082 | M5/USA/2010 | A846/88 | U‐1 | |
| L | 93.5/98.8 | 92.2/97.6 | 92.5/83.9 | 70.4/67.7 | 63.9/57.5 | 63.5/55.8 | 46.4/29.2 |
| VP0 | 93.0/99.2 | 92.7/99.2 | 93.0/100.0 | 79.7/85.4 | 76.1/82.6 | 75.0/73.5 | 61.7/59.1 |
| VP3 | 94.7/98.2 | 94.4/99.1 | 94.5/98.6 | 83.4/90.4 | 79.5/87.7 | 78.2/86.8 | 63.8/58.9 |
| VP1 | 92.0/95.7 | 92.0/98.4 | 91.7/96.4 | 74.6/74.2 | 70.0/71.7 | 68.6/65.6 | 49.9/33.2 |
| 2A | 94.9/95.6 | 94.9/97.1 | 95.1/97.1 | 76.5/73.5 | 71.3/69.9 | 67.0/64.0 | 58.9/56.4 |
| 2B | 91.5/100.0 | 91.1/99.4 | 91.7/100.0 | 84.4/92.1 | 77.2/81.2 | 78.6/81.2 | 57.2/54.5 |
| 2C | 93.5/98.2 | 93.6/99.7 | 93.0/99.7 | 85.4/92.2 | 82.7/91.9 | 78.8/87.8 | 64.9/67.7 |
| 3A | 94.0/96.8 | 93.6/100.0 | 91.1/97.9 | 77.3/83.0 | 68.4/75.5 | 65.6/67.0 | 55.8/47.8 |
| 3B | 88.9/92.6 | 95.1/100.0 | 93.8/96.3 | 81.5/85.2 | 81.5/92.6 | 75.3/74.1 | 63.0/66.7 |
| 3C | 93.3/96.8 | 90.4/97.4 | 92.1/96.8 | 84.6/91.1 | 81.1/85.3 | 79.8/83.2 | 60.3/49.5 |
| 3D | 93.2/97.9 | 93.7/97.9 | 93.8/98.1 | 84.2/92.3 | 82.2/89.5 | 80.8/87.6 | 69.9/75.0 |
| Polyprotein | 93.2/97.8 | 93.0/98.6 | 93.0/97.5 | 81.1/85.8 | 77.0/82.0 | 75.6/78.0 | 60.9/56.8 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Phylogenetic analysis of the kobuvirus polyprotein ORF gene indicated the Chinese FeKoV strain, WHJ‐1, was clustered with the Italian and Korean FeKoV strains (Figure 2). In addition, the phylogenetic tree showed that FeKoV was most closely related to the canine kobuvirus, as indicated by the nucleotide and amino acid homology analyses. Both FeKoV and the canine kobuvirus were grouped in Aichivirus A, the species that comprise the genus Kobuvirus together with Aichivirus B‐F.
3.4. Viral cultures of FeKoV from China
After serial passages, no CPE were observed in the cultured cells, and no kobuvirus RNA was detected in the culture supernatant or cells by RT‐PCR.
4. DISCUSSION
In this study, we provided the first evidence to support FeKoV circulation in the Chinese feline population, demonstrating that FeKoV is not geographically restricted to Korea and Italy (Cho et al., 2014, 2015; Choi et al., 2015; Chung et al., 2013; Di Martino et al., 2015). All FeKoV‐positive faecal samples were collected from diarrhoeic cats, with a prevalence rate of 8/52, similar to those identified in Korea (6/39) and Italy (5/37) (Chung et al., 2013; Di Martino et al., 2015). No FeKoV was found in asymptomatic cats in this study, nor in another study in Italy. However, a study conducted by Yoon‐Young Cho et al. detected FeKoV RNA in healthy cats (two of 19) in Korea in 2015, although a higher prevalence rate of 15/52 was found in diarrhoeic cats (Cho et al., 2015). FeKoV prevalence studies in China, Korea and Italy indicate FeKoV is commonly detected in diarrhoeic cats (Di Martino et al., 2015). In addition, our field study in China indicated FeKoV was positively associated with feline diarrhoea. However, the relationship between FeKoV infection and diarrhoea in cats requires further epidemiological and experimental studies.
Although the genomes of four FeKoV strains have been sequenced by separate research groups, the detailed primer sequences and PCR strategies remain uncharacterized. To obtain the FeKoV genome in China, PCR primers with degenerate bases targeting the consensus region of three FeKoV genome sequences were designed using high‐fidelity DNA polymerase. Based on these, two long PCR fragments of ≈3,000 and 4,400 bp and a short PCR fragment of ≈900 bp were acquired to assemble the near‐complete genome of the first Chinese FeKoV strain, WHJ‐1. Our viral sequencing strategy may help to determine the genomic sequences of other FeKoV strains. Moreover, considering the highly genetically variable kobuvirus characteristics, the WHJ‐1 genomic information may also contribute to the primer design applied to the FeKoV viral detection method.
Bayesian evolutionary analysis based on the FeKoV VP1 gene showed that the time to the most recent common ancestor was 5.3 years, and the substitution rate was 1.29 × 10−2 substitutions/site/year, demonstrating that FeKoV emerged recently in the feline population and mutated rapidly (Cho et al., 2015). The polyprotein gene for the field FeKoV strain, WHJ‐1, has a nucleotide identity of 93.0%–93.2% with that of the Italian and Korean strains, indicating FeKoV's genetic variation during 2013 and 2017. In addition, analysing the WHJ‐1 polyprotein showed 13 unique amino acid variant sites. Cats are important companion animals who maintain close contact with humans. Previous studies have demonstrated the zoonotic potential of carnivore enteric viruses (Di Martino et al., 2015). The FeKoV's highly genetically variable characteristic emphasizes the importance of continued worldwide surveillance.
To isolate FeKoV, we used six cell types originating from cats, dogs, humans, pigs, chickens and monkeys but detected no FeKoV replication in any of these cell lines after serial passages. To date, kobuvirus culturing remains an important problem to be solved. To our knowledge, except for the Aichi virus strain, A846/88, and the Bovine kobuvirus strain, U‐1, other attempts to isolate kobuviruses have failed (Reuter, Kecskemeti, & Pankovics, 2010; Yamashita et al., 1991, 2003). The lack of pure viral stocks after culturing makes it difficult to study kobuviral pathogenicity.
In conclusion, in this study, we first determined FeKoV presence in faecal samples from diarrhoeic cats in southern China. We also sequenced the near‐complete genome of one field FeKoV strain using long‐fragment PCR. Homology analysis indicated FeKoV's genetic variation. Our study indicated FeKoV was associated with feline diarrhoea. Further study is required to isolate this virus and study its pathogenicity in cats.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by The National Natural Science Foundation of China (31672563), The Special Fund for Agro‐scientific Research in the Public Interest (201303042), The National Key Research and Development Program of China (2016YFD0501004, 2016YFD0501010), The Promote Scientific and Technological Development Program (2013B040200032), The Guangdong Provincial Key Laboratory of Prevention and Control for Severe Clinical Animal Diseases (2017B030314142).
Lu G, Zhang X, Luo J, et al. First report and genetic characterization of feline kobuvirus in diarrhoeic cats in China. Transbound Emerg Dis. 2018;65:1357–1363. 10.1111/tbed.12916
REFERENCES
- Adams, M. J. , King, A. M. , & Carstens, E. B. (2013). Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Archives of Virology, 158, 2023–2030. 10.1007/s00705-013-1688-5 [DOI] [PubMed] [Google Scholar]
- Adams, M. J. , Lefkowitz, E. J. , King, A. M. Q. , Harrach, B. , Harrison, R. L. , Knowles, N. J. , … Davison, A. J. (2017). Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Archives of Virology, 162, 2505–2538. 10.1007/s00705-017-3358-5 [DOI] [PubMed] [Google Scholar]
- Carmona‐Vicente, N. , Buesa, J. , Brown, P. A. , Merga, J. Y. , Darby, A. C. , Stavisky, J. , … Radford, A. D. (2013). Phylogeny and prevalence of kobuviruses in dogs and cats in the UK. Veterinary Microbiology, 164, 246–252. 10.1016/j.vetmic.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. Y. , Lim, S. I. , Kim, Y. K. , Song, J. Y. , Lee, J. B. , & An, D. J. (2014). Molecular characterization of the full kobuvirus genome in a cat. Genome Announcements, 2, 10.1128/genomeA.00420-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. Y. , Lim, S. I. , Kim, Y. K. , Song, J. Y. , Lee, J. B. , & An, D. J. (2015). Molecular evolution of kobuviruses in cats. Archives of Virology, 160, 537–541. 10.1007/s00705-014-2259-0 [DOI] [PubMed] [Google Scholar]
- Choi, J. W. , Lee, M. H. , Lee, K. K. , & Oem, J. K. (2015). Genetic characteristics of the complete feline kobuvirus genome. Virus Genes, 50, 52–57. 10.1007/s11262-014-1144-y [DOI] [PubMed] [Google Scholar]
- Chung, J. Y. , Kim, S. H. , Kim, Y. H. , Lee, M. H. , Lee, K. K. , & Oem, J. K. (2013). Detection and genetic characterization of feline kobuviruses. Virus Genes, 47, 559–562. 10.1007/s11262-013-0953-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, B. , Profio, F. Di. , Melegari, I. , Marsilio, F. , & Martella, V. (2015). Detection of feline kobuviruses in diarrhoeic cats, Italy. Veterinary Microbiology, 176, 186–189. 10.1016/j.vetmic.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, A. , Simmonds, P. , Dubovi, E. J. , Qaisar, N. , Henriquez, J. A. , Medina, J. , … Lipkin, W. I. (2011). Characterization of a canine homolog of human Aichivirus. Journal of Virology, 85, 11520–11525. 10.1128/JVI.05317-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. H. , Jeoung, H. Y. , Lim, J. A. , Song, J. Y. , Song, D. S. , & An, D. J. (2012). Kobuvirus in South Korean black goats. Virus Genes, 45, 186–189. 10.1007/s11262-012-0745-6 [DOI] [PubMed] [Google Scholar]
- Pankovics, P. , Boros, A. , Biro, H. , Horvath, K. B. , Phan, T. G. , Delwart, E. , & Reuter, G. (2016). Novel picornavirus in domestic rabbits (Oryctolagus cuniculus var. domestica). Infection, Genetics and Evolution : Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 37, 117–122. 10.1016/j.meegid.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, T. G. , Kapusinszky, B. , Wang, C. , Rose, R. K. , Lipton, H. L. , & Delwart, E. L. (2011). The fecal viral flora of wild rodents. PLoS Pathogens, 7, e1002218 10.1371/journal.ppat.1002218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, C. R. (1999). Virus taxonomy–1999. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1998. Archives of Virology, 144, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, G. , Boldizsar, A. , & Pankovics, P. (2009). Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae. Archives of Virology, 154, 101–108. 10.1007/s00705-008-0288-2 [DOI] [PubMed] [Google Scholar]
- Reuter, G. , Boros, A. , Pankovics, P. , & Egyed, L. (2010). Kobuvirus in domestic sheep, Hungary. Emerging Infectious Diseases, 16, 869–870. 10.3201/eid1605.091934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, G. , Kecskemeti, S. , & Pankovics, P. (2010). Evolution of porcine kobuvirus infection, Hungary. Emerging Infectious Diseases, 16, 696–698. 10.3201/eid1604.090937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, G. , Nemes, C. , Boros, A. , Kapusinszky, B. , Delwart, E. , & Pankovics, P. (2013). Porcine kobuvirus in wild boars (Sus scrofa). Archives of Virology, 158, 281–282. 10.1007/s00705-012-1456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, T. , Ito, M. , Kabashima, Y. , Tsuzuki, H. , Fujiura, A. , & Sakae, K. (2003). Isolation and characterization of a new species of kobuvirus associated with cattle. The Journal of General Virology, 84, 3069–3077. 10.1099/vir.0.19266-0 [DOI] [PubMed] [Google Scholar]
- Yamashita, T. , Kobayashi, S. , Sakae, K. , Nakata, S. , Chiba, S. , Ishihara, Y. , & Isomura, S. (1991). Isolation of cytopathic small round viruses with BS‐C‐1 cells from patients with gastroenteritis. The Journal of Infectious Diseases, 164, 954–957. [DOI] [PubMed] [Google Scholar]
- Yamashita, T. , Sakae, K. , Tsuzuki, H. , Suzuki, Y. , Ishikawa, N. , Takeda, N. , … Yamazaki, S. (1998). Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. Journal of Virology, 72, 8408–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. M. , Xu, Z. Q. , Li, B. W. , Zhang, Q. , Cui, S. X. , Jin, M. , & Duan, Z. J. (2011). Analysis and characterization of the complete genome of a member of a new species of kobuvirus associated with swine. Archives of Virology, 156, 747–751. 10.1007/s00705-010-0907-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


