Abstract
The ability of cells to form and release multiple classes of extracellular vesicles (EVs) is an increasingly well-recognized phenomenon. EVs are best known as mediators of intercellular communication. However, in a recent issue of Nature, Keller et al. show that they function as decoys to mitigate bacterial toxins.
The ability of cells to form and release multiple classes of extracellular vesicles (EVs) is an increasingly well-recognized phenomenon. EVs are best known as mediators of intercellular communication. However, in a recent issue of Nature, Keller et al. show that they function as decoys to mitigate bacterial toxins.
Main Text
The bacterium Staphylococcus aureus (S. aureus) was originally described in 1880 by Sir Alexander Ogston (Ogston, 1882). Since then, strains of S. aureus that are resistant to different antibiotics, first penicillin and then methicillin, began to emerge. Methicillin-resistant S. aureus (MRSA) is now endemic in many hospitals and is one of the leading causes of infections contracted in the healthcare setting (Klein et al., 2007). Thus, there is a clear need to better understand the process of bacterial infection and especially how host cells respond to these potentially deadly threats. Mammalian organisms combat bacterial infections by rapidly inducing innate immune responses. In a recent issue of Nature, Keller et al. (2020) describe a previously unknown mechanism of innate immunity that involves the ability of cells to produce exosomes, a specific class of extracellular vesicles (EVs), that function as a protective barrier and prevent bacterial toxins from reaching their target cells.
Exosomes are small EVs, ~30–120 nm in diameter, that originate in the endo-lysosomal trafficking pathway as intraluminal vesicles within multivesicular bodies. They are released from cells when multivesicular bodies fuse with the plasma membrane. Microvesicles (MVs), which represent the second major class of EVs, are larger than exosomes (0.2–2.0 μm in diameter) and are derived as a result of their outward budding and release from the plasma membrane. Both exosomes and MVs engage and transfer their associated protein and nucleic acid cargo to other cells, thus altering the cells’ behavior. This form of intercellular communication has been studied extensively in the context of cancer, where it has been shown that EVs shed by cancer cells interact with neighboring cancer cells as well as with non-cancerous cells that reside either locally within the tumor microenvironment or at distant sites. These interactions promote aggressive phenotypes including accelerated cell growth, therapy resistance, invasiveness, and metastasis (Latifkar et al., 2018). Recently, exosomes released by melanoma cells have also been shown to engage and inactivate immune cells, thus enabling tumor cells to evade an immune response (Chen et al., 2018).
The work described in Keller et al. (2020) now sheds new light on how EVs, and in particular exosomes, are produced by cells as a mechanism to protect against pathogenic agents by acting as cellular decoys. This discovery stemmed from earlier findings, which showed that mice and cells depleted of the autophagy-related protein 16-1 (ATG16L1) were far more susceptible to MRSA strains that release the pore-forming alpha-toxin compared to wild-type mice and cells (Maurer et al., 2015). Keller et al. (2020) then determined the mechanistic basis by which ATG16L1 mediated this effect, showing that it was dependent on changes in the expression level of the metalloprotease ADAM10. Alpha-toxin is known to bind ADAM10 when it is expressed on the surfaces of cells and to mediate the formation of pores. Interestingly, in cells lacking ATG16L1, the levels of ADAM10 detected on their plasma membranes was increased, which led to a corresponding increase in alpha-toxin-mediated pore formation and cell death. Since autophagy-related machinery has been demonstrated to regulate exosome biogenesis (Guo et al., 2017), the authors suspected that ATG16L1 decreases the sensitivity of cells to MRSA by influencing the release of exosomes containing ADAM10. Indeed, cells expressing sufficient amounts of ATG16L1 were found to produce large quantities of exosomes containing the metalloprotease, while cells depleted of ATG16L1 released far fewer of these exosomes, leading to the idea that the shedding of greater numbers of exosomes containing ADAM10 creates a protective barrier surrounding the cells. Thus, alpha-toxins released by MRSA strains in the vicinity of a cell would more likely bind to an exosome, causing the toxins to undergo oligomerization and to become inactivated, thereby highlighting a form of innate immunity that was not previously appreciated.
The authors next examined whether the formation and release of exosomes containing ADAM10 might be increased when cells were treated with a variety of different bacteria (Keller et al., 2020). The results from these experiments showed that S. aureus, as well as other pathogens, including Streptococcus pneumoniae, Citrobacter rodentium, and Salmonella enterica, were similarly capable of enhancing the production of exosomes, suggesting that the release of these EVs represents a general mechanism used by cells to reduce the infectivity of bacterial pathogens. The authors then carried out two additional in vivo experiments that greatly solidified their findings. In the first of these experiments, proteomic analysis was performed on exosomes that were isolated from plasma collected from MRSA-infected mice. The results elegantly show that most of the exosomes originated in the liver, a hotspot for MRSA infection (Surewaard et al., 2018). In the second experiment, the authors wanted to see whether exosomes were protective in vivo. Exosomes isolated from the blood of donor mice treated with an attenuated form of S. aureus were injected into recipient mice that were being exposed to a lethal dose of the bacteria. While control animals rapidly died from the infection, animals that received exosome treatments lived significantly longer. When taken collectively, the results of this study suggest that injecting exosomes, or potentially even liposomes containing ADAM10 (Duong et al., 2019), could provide a new strategy for combating bacterial infections and might offer an especially useful treatment for individuals with compromised immune systems or when antibiotic resistance develops.
In conclusion, the study by Keller et al. (2020) provides a striking and novel example of how exosomes can impact biological responses in surprising ways; in this case, by serving as decoy agents to protect host cells against bacterial pore-forming toxins (Figure 1 ). These findings now join a growing list of examples in which exosomes, and EVs in general, either act to protect parental cells from which they are derived against incoming signals that otherwise would compromise cell survival or provide a mechanism for intercellular communication that confers cells with a biological advantage. In some cases, the actions of these vesicles have deleterious effects with regard to disease progression and pathological disorders. Included among such examples are the ability of exosomes shed by aggressive cancer cells to block immune surveillance and thus interfere with immune therapy (Chen et al., 2018) and the actions of larger EVs (i.e., MVs) in stimulating VEGF receptors on endothelial cells and activating the initial stages of tumor angiogenesis in a manner that is insensitive to the anti-angiogenesis drug bevacizumab (Feng et al., 2017). However, in other cases, like the study described here, they provide a beneficial effect to the health of the organism. There are likely to be many more instances of how EVs are used to provide a benefit or survival advantage to their parental cells. For example, in light of the striking challenges we are facing due to the COVID-19 outbreak, an intriguing question is whether cells might also use exosomes to mitigate viral infections. Given the ever-increasing appreciation of their biological significance, with both good and bad consequences, it will be critical to address fundamental questions regarding the biogenesis of exosomes and other EVs in specific cellular and biological contexts. It also will be increasingly important to design novel strategies to manipulate their production and shedding, either to increase their numbers when advantageous to good health or to inhibit their generation when it contributes to diseases such as metastatic cancer and neurodegenerative disorders.
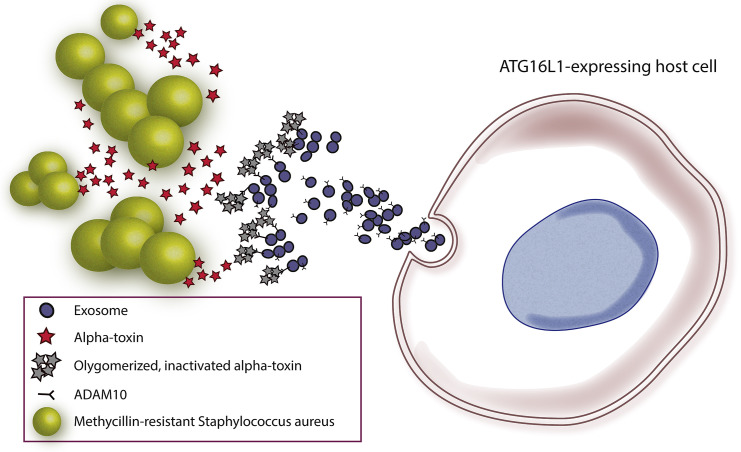
Figure 1.
ATG16L1-Expressing Cells Release Exosomes that Neutralize S. aureus Alpha-Toxin
Upon exposure to S. aureus, ATG16L1-expressing cells release large amounts of exosomes that have the metalloprotease ADAM10 on their surfaces. The alpha-toxin released by S. aureus binds to these exosomes and becomes oligomerized and inactivated, providing a mechanism of innate immunity that effectively diminishes the virulence of pathogenic bacteria (i.e., MRSA).
References
- Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong N., Curley K., Brown A., Campanelli A., Do M.A., Levy D., Tantry A., Marriott G., Lu B. Decoy exosomes as a novel biologic reagent to antagonize inflammation. Int. J. Nanomedicine. 2019;14:3413–3425. doi: 10.2147/IJN.S196975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Zhang C., Lum D., Druso J.E., Blank B., Wilson K.F., Welm A., Antonyak M.A., Cerione R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017;8:14450–14477. doi: 10.1038/ncomms14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Chitiprolu M., Roncevic L., Javalet C., Hemming F.J., Trung M.T., Meng L., Latreille E., Tanese de Souza C., McCulloch D. Atg5 Disassociates the V1V0-ATPase to Promote Exosome Production and Tumor Metastasis Independent of Canonical Macroautophagy. Dev. Cell. 2017;43:716–730.e7. doi: 10.1016/j.devcel.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Keller M.D., Ching K.L., Liang F.-X., Dhabaria A., Tam K., Ueberheide B.M., Unutmaz D., Torres V.J., Cadwell K. Decoy exosomes provide protection against bacterial toxins. Nature. 2020;579:260–264. doi: 10.1038/s41586-020-2066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Smith D.L., Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg. Infect. Dis. 2007;13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifkar A., Cerione R.A., Antonyak M.A. Probing the mechanisms of extracellular vesicle biogenesis and function in cancer. Biochem. Soc. Trans. 2018;46:1137–1146. doi: 10.1042/BST20180523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer K., Reyes-Robles T., Alonzo F., 3rd, Durbin J., Torres V.J., Cadwell K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe. 2015;17:429–440. doi: 10.1016/j.chom.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. Micrococcus Poisoning. J Anat Physiol. 1882;17:24–58. [PMC free article] [PubMed] [Google Scholar]
- Surewaard B.G.J., Thanabalasuriar A., Zeng Z., Tkaczyk C., Cohen T.S., Bardoel B.W., Jorch S.K., Deppermann C., Bubeck Wardenburg J., Davis R.P. α-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe. 2018;24:271–284.e3. doi: 10.1016/j.chom.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]