STRUCTURED SUMMARY
Background:
Celiac disease (CD) is a widespread autoimmune disease triggered by dietary gluten that can lead to severe gastrointestinal symptoms. Because there is no available treatment other than a lifelong gluten-free diet, many patients continue to experience chronic symptoms.
Aim:
In this analysis we report on the efficacy of latiglutenase, an orally administered enzyme treatment, for improving multiple gluten-induced symptoms and consequent quality of life (QOL) due to inadvertent gluten consumption.
Methods:
This analysis is based on data from the CeliAction study of symptomatic patients (ALV003–1221; NCT01917630). Patients were treated with latiglutenase or placebo for 12 weeks and instructed to respond to a symptom diary daily and to multiple QOL questionnaires at weeks 0, 6, and 12 of the treatment periods as secondary endpoints. The results were stratified by serostatus.
Results:
398 patients completed the 12-week CDSD study. In seropositive, but not seronegative, CD patients a statistically significant and dose-dependent improvement was seen in the severity and frequency of abdominal pain, bloating, tiredness, and constipation. In subjects receiving 900 mg latiglutenase, improvements (p-values) in the severity of these symptoms for week 12 were 58% (0.038), 44% (0.023), 21% (0.164), and 104% (0.049) respectively, relative to placebo-dosed subjects. The reduction in symptoms trended higher for more symptomatic patients. Similar results were observed for the QOL outcome measures.
Conclusions:
Although this study was not powered to definitively establish the benefit of latiglutenase in seropositive CD patients, such patients appear to show symptomatic and QOL benefit from using latiglutenase with meals.
Keywords: Celiac disease, latiglutenase, therapy, symptoms
INTRODUCTION
Celiac disease (CD) is a chronic inflammatory disorder of the small intestine triggered by exposure to gluten proteins and affecting about 1% of most populations.1,2,3 The pathological lesion of villous atrophy in the proximal epithelium of the small intestinal is due to an immune response to wheat, rye, or barley. The treatment of CD has been limited to a lifelong gluten-free diet (GFD) which can control but does not cure the disease. While treatment can ameliorate symptoms and damage the diet is not easy or readily achievable by many patients.4,5 Low levels of gluten exposure are common and may cause pain and suffering and ongoing inflammation that can increase the risk of complications including lymphoma, bowel cancer, osteoporosis, anemia, malnutrition, etc.6,7 Patients and families often have a substantial burden to bear to achieve the diet. Further, the cost of care for moderately to severely symptomatic patients, comprising nearly 50% of patients, is more than $10K/year.8
There are several experimental targets for CD in clinical trials,9 however to our knowledge sizable randomized drug trials have only been published for two modes of action – enzyme supplementation therapy10,11 and tight junction modulation in the small intestine.12,13 Latiglutenase (IMGX003, formerly ALV003) is a novel enzyme supplementation therapy comprised of two enzymes that was recently shown to mitigate gluten-induced mucosal injury in CD patients in a gluten-challenge study (ALV003–1021).10 A subsequent “real-world” trial (ALV003–1221), however, did not show evidence of treatment-induced mucosal healing relative to placebo due to what was reported to be due to a trial (Hawthorne) effect, in which the patients changed their behavior during the treatment period by further reducing their gluten intact from their normal GFD.14 In this same study, however, it was shown that statistically and clinically significant reduction in multiple gluten-induced symptoms was observed as a function of latiglutenase dose in a subpopulation of patients who remained seropositive despite being on a GFD for at least one year.15
The pharmacological rationale for latiglutenase therapy in CD is conceptually straightforward. Most immunotoxic gluten peptides are highly resistant to proteolytic activity in the intestine.16 In turn, proteolytic resistance leads to the accumulation of long, metastable gluten-derived intermediates in the small intestinal lumen, which elicit a T-cell dependent response in CD patients. Based on a variety of in vitro, in vivo animal, and ex vivo human studies, it has been suggested that giving exogenous proteases proteases that target the gluten in food could reduce the immunogenic peptides present after gluten exposure and perhaps have a therapeutic role in managing celiac disease.17
In this paper we expand on the preliminary symptom analysis presented earlier15 by presenting additional data and analysis including daily symptom data showing the nature of symptoms manifesting as acute flares that are significantly attenuated by latiglutenase as well as representation of symptom relief in terms of responder analysis relating the percentage of patients who improve by threshold amounts while on treatment relative to placebo. We further provide quality of life (QOL) outcome measures showing commensurate improvement in seropositive patients based on dose of latiglutenase. It is worth reminding that the 3rd Gastroenterology Regulatory Endpoints and Advancement of Therapeutics (GREAT-3) conference sponsored by the U.S. Food and Drug Administration (FDA) in 2015 specifically cited the need to develop treatments that address symptom suffering due to accidental gluten ingestion.18
METHODS
Clinical study design and subjects:
The ALV003–1221 clinical trial (www.clinicaltrials.gov, NCT01917630) was a multi-center, multinational, randomized, double-blind, placebo-controlled, dose-ranging study in symptomatic, established patients with CD. Details of the trial are reported elsewhere.14,15 The symptoms of each subject were recorded for a 4-week baseline period followed by an eligibility and randomization period (2–4 weeks) during which patients underwent serological and endoscopic analysis. The main criterion for randomization in the study was histological evidence for active disease, as judged by a villus height:crypt depth ratio (Vh:Cd) ≤ 2.0. Both seropositive and seronegative CD patients were enrolled and stratified in this study. Qualified subjects entered into a 12-week study period during which either a placebo or a defined dose of latiglutenase (100, 300, 450, 600, 900 mg) was administered orally TID. The patient populations for the data presented here were for seropositive: PBO (n= 54), 600 mg (n=35), 900 mg (n=14) and for seronegative: PBO (n= 68), 600 mg (n=45), 900 mg (n=22). About 20% of these patients were invited to continue for another 12 weeks; however, we don’t use that data in this paper because the population of seropositive patients across the different doses was too small to draw any statistical conclusions. Each participant gave informed consent. All biological samples were coded to maintain blinding, and all investigators performing sample analysis were unaware of the patients’ diagnostic status or the study results.
Celiac Disease Symptom Diary (CDSD©):
The CDSD is a patient reported outcome (PRO) instrument that consists of a daily diary recorded across seven-day periods that assesses common celiac symptoms (abdominal pain, bloating, tiredness, nausea, diarrhea, and constipation). Patients were instructed to complete the CDSD diary each evening recording the presence or absence of individual symptoms occurring over the prior 24-hour period. If a given symptom was present on a given day, follow-up questions were asked to establish the severity of each event. Further detail regarding the CDSD and how it is administered and scored are provided elsewhere.15,19,20 Briefly, for all symptoms except constipation each patient’s daily severity score is normalized from 0 to 10 where 0 represents no symptom. The weekly score therefore ranges from 0 to 70. The frequency value is the number of non-zero events, irrespective of severity. A non-stool composite severity score, consisting of all symptoms besides diarrhea and constipation, was also computed. Constipation requires several days of data and is not amenable to a daily score is not recorded other than to measure the number of complete spontaneous bowel movements (CSBMs) per day. A constipation event is defined when less than three bowel events occur for the week. The severity of constipation is then calculated from the number of bowel movements for the week and ranges from 0 to 70. While we did not formally measure of constipation frequency; this was reported by convention as by the number of bowel movements per week, however, in the following we will refer to this as “constipation frequency” for consistency with other measures.
Impact of Celiac Disease Symptoms Questionnaire (ICDSQ):
The ICDSQ© was used to assess the impact of patients’ celiac symptoms over the previous week at Day 1, Week 6, and Week 12. This was extended to Week 18 and Week 24 for the patients who volunteered to continue, but we don’t include those data due to low statistics. The questionnaire was comprised of 14 items with four domains: Daily Activities (4 items), Social Activities (3 items), Emotional Well-being (5 items) and Physical Functioning (2 items). Each item had 5 response options ranging from ‘not at all’ to ‘completely’. Each domain was individually scored and an overall impact score was also calculated giving equal weight to each domain.
Patient Global Impression-Symptoms (PGI-S):
The PGI-S assessed change over time in the severity of symptoms and impact of symptoms on the same visit schedule as for the ICDSQ. Patients were first asked patients to rate their symptom severity over the previous seven days on a 6-point rating scale from ‘no’ to ‘very severe’ symptoms in the PGI-S. For those patients reporting symptoms, the second PGI-S item asked patients to rate how much their celiac symptoms had a negative impact on their Daily Activities, Social Activities, Emotional Wellbeing and Physical functioning using a 5-point rating scale from ‘not at all’ to ‘completely’.
SF-12 v2® Health Survey:
The SF-12 v2 Health Survey, a shorter version of the SF-36 Health Survey, asked patients to answer 12 questions that measure physical and mental health on the same visit schedule as for the ICDSQ and PGI-S.
Symptom and QOL statistical analysis:
The improvement value at each dose (Idose) for symptom and QOL was quantified using the following equation:
| (1) |
where Bdose is the baseline value (i.e., the score of a particular outcome measure in the week prior to the Day 1 visit), and ΔBdose is the change in baseline value for a particular dose in week 6 or week 12 of drug dosing. The subscript PBO represents the placebo dose population. The (1−(ΔBPBO/BPBO)) term in the denominator accounts for the improvement in a symptom or QOL measure due to latiglutenase activity relative to the placebo effect; as a result, Idose can assume values between 0% (corresponding to the placebo effect) and 100% (full recovery in symptom or QOL outcome). P-values for ΔBdose/Bdose) (including dose = PBO) and (ΔBdose/Bdose) - (ΔBPBO/BPBO) were stratified by serostatus and calculated by analysis of covariance (ANCOVA) and were not adjusted for multiplicity.
Serum testing:
The levels anti-transglutaminase 2 (TG2) IgA and IgA and IgG antibodies to deamidated gliadin peptides (DGP) were measured by enzyme linked immunosorbent assay. The trial results were stratified by serologic status as either negative or positive, with positive defined as above the normal range for any of the three serology tests.
RESULTS
Effect of latiglutenase dose on symptom severity and frequency:
To estimate the extent to which latiglutenase dosing improved the frequency and severity of these symptoms above and beyond the placebo effect, the data collected at the two highest drug doses (600 mg and 900 mg) were analyzed according to equation (1), both in week 6 and week 12 of the study. The results reported in Table 1 underscore both a dose and a duration dependence of the symptomatic benefit due to latiglutenase in seropositive patients. The symptom domains showing the greatest benefit from latiglutenase are abdominal pain, bloating, tiredness, and constipation (as measured by the complete spontaneous bowel movements, CSBM). The severity improvements relative to placebo were 58%, 44%, 21%, and 104%, respectively, for abdominal pain, bloating, tiredness, and constipation for the 900 mg dose level for week 12 (end of main trial). (The >100% RIS for constipation is an artifact of the PBO effect being <0%, Supporting Information Table S1). The p-values for these relative to placebo were 0.038, 0.023, 0.164, and 0.049 respectively. The respective values for the composite of 600 mg and 900 mg were 0.008, 0.007, 0.009, and 0.044 (previously reported).15 Symptom frequency also showed meaningful improvement. Similar trends were observed for week 6, but at approximately 80% the improvement of the week 12 results. It should be noted that these significant dose-dependent results were observed despite a considerable trial (Hawthorne)/placebo effect as described in the Supporting Information (Table S1).
Table 1.
Symptom improvements for seropositive patients treated with 600 mg and 900 mg latiglutenase. Data was analyzed according to equation (1) for weeks 6 and 12 of the study. Values of p ≤ 0.05 are noted in bold.
| Week 6 | Abdominal Pain | Bloating | Tiredness | Constipation (CSBM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | |
| 33.9% | 0.165 | 13.9% | 0.445 | 41.6% | 0.021 | 12.2% | 0.364 | 24.9% | 0.070 | 19.1% | 0.074 | 23.2% | 0.487 | 23.0% | 0.230 | |
| Seronegative | 3.4% | 0.946 | 5.6% | 0.771 | 4.5% | 0.817 | 10.8% | 0.364 | 9.6% | 0.451 | 6.8% | 0.422 | −41.9% | 0.675 | −2.7% | 0.785 |
| Total | 16.9% | 0.314 | 8.8% | 0.478 | 19.8% | 0.094 | 10.1% | 0.267 | 16.1% | 0.075 | 11.6% | 0.072 | −11.8% | 0.928 | 6.8% | 0.599 |
| Week 12 | Abdominal Pain | Bloating | Tiredness | Constipation (CSBM) | ||||||||||||
| Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | |
| 57.5% | 0.038 | 38.9% | 0.034 | 44.1% | 0.023 | 19.0% | 0.189 | 20.6% | 0.164 | 9.1% | 0.391 | 104.4% | 0.049 | 37.7% | 0.036 | |
| Seronegative | −38.7% | 0.075 | −12.2% | 0.263 | 2.7% | 0.929 | 13.7% | 0.402 | 15.4% | 0.264 | 16.5% | 0.092 | −22.1% | 0.824 | 3.5% | 0.779 |
| Total | 5.6% | 0.943 | 7.4% | 0.625 | 18.5% | 0.196 | 14.7% | 0.170 | 17.2% | 0.081 | 13.4% | 0.068 | 36.0% | 0.262 | 17.2% | 0.115 |
| Week 6 | Abdominal Pain | Bloating | Tiredness | Constipation (CSBM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | |
| 24.7% | 0.165 | 17.1% | 0.141 | 29.3% | 0.040 | 10.1% | 0.317 | 23.4% | 0.028 | 16.7% | 0.038 | 46.9% | 0.197 | −12.1% | 0.778 | |
| Seronegative | 4.3% | 0.927 | −2.9% | 0.633 | 2.9% | 0.923 | 4.7% | 0.712 | −13.5% | 0.125 | −14.3% | 0.049 | −76.7% | 0.949 | −5.4% | >0.999 |
| Total | 13.5% | 0.301 | 5.7% | 0.555 | 14.2% | 0.160 | 6.4% | 0.404 | 2.9% | 0.604 | −0.1% | 0.926 | 4.5% | 0.505 | −8.3% | 0.866 |
| Week 12 | Abdominal Pain | Bloating | Tiredness | Constipation (CSBM) | ||||||||||||
| Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | Severity | p-value | Freq. | p-value | |
| 48.0% | 0.026 | 28.3% | 0.039 | 31.1% | 0.040 | 6.3% | 0.521 | 36.9% | 0.002 | 22.0% | 0.012 | 43.0% | 0.252 | −9.9% | 0.790 | |
| Seronegative | −10.0% | 0.532 | −9.7% | 0.303 | 11.4% | 0.552 | 5.2% | 0.769 | −2.6% | 0.742 | −6.8% | 0.403 | −41.8% | 0.726 | 2.1% | 0.534 |
| Total | 17.9% | 0.282 | 6.7% | 0.551 | 19.6% | 0.086 | 5.4% | 0.542 | 14.8% | 0.059 | 6.4% | 0.280 | 8.7% | 0.324 | −3.4% | 0.793 |
Nausea and diarrhea are components of the CDSD PRO tool and inexplicably these domains did not show significant benefit due to latiglutenase. However, within measurement uncertainty, they did not show any worsening either. Unexpectedly, seronegative patients showed insignificant benefit from latiglutenase. This was striking as there were no other properties, such as baseline severity and frequency of symptoms nor magnitude of the trial/placebo effect that differentiated seropositive from seronegative patients. We speculate on possible explanations for this observation in the Discussion section.
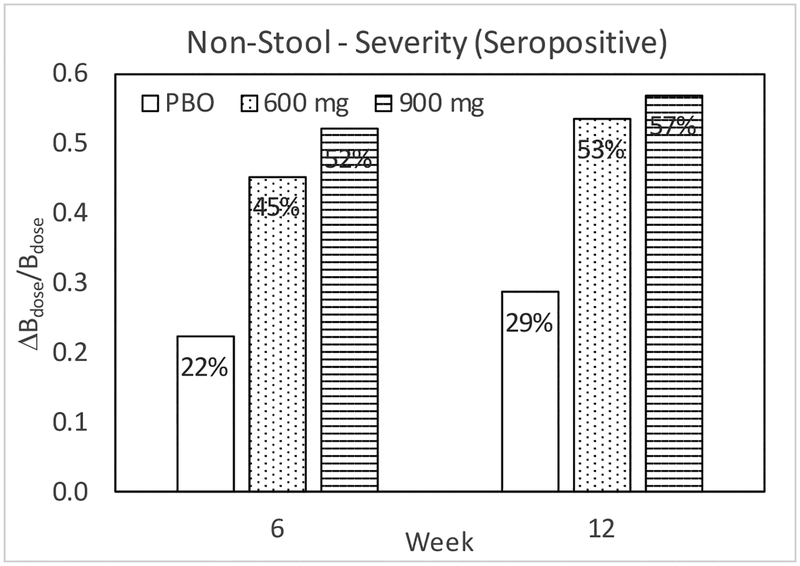
As previously reported, a dose-dependent effect of latiglutenase was observed on the severity and frequency of abdominal pain, bloating, tiredness, and constipation (CSBM) for seropositive patients, but not for seronegative patients in both week 6 and week 12 of the study.15 Although the ALV001–1221 trial was not powered for symptoms, the trend with dose is clearly evident in Table 1. For the Overall Non-Stool GI Specific Severity Score, notable LS-mean differences from PBO in change from Baseline were observed among seropositive patients for 600 – PBO, 900 – PBO, and (600 + 900) – PBO at Week 6 and 12 as tabulated in Table 2. All cases show p < 0.05 values. For the comparable table for seronegative patients (not shown) all cases show p > 0.28 values. Figure 1 shows that these seropositive trends follow a dose dependence.
Table 2.
Analysis of covariance (ANCOVA) for change from baseline for weeks 6 and 12 in CDSD overall non-stool GI specific severity score among seropositive patients
| Week 6 | Week 12 | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | LS-Mean | SE | 95% CI | P value | LS-Mean | SE | 95% CI | P value |
| Placebo (PBO) | −8.47 | 3.09 | (−14.6, −2.38) | −11.35 | 3.11 | (−17.5, −5.20) | ||
| 600 – PBO | −10.98 | 4.97 | (−20.8, −1.17) | 0.029 | −11.08 | 4.93 | (−20.8, −1.34) | 0.026 |
| 900 – PBO | −14.70 | 6.80 | (−28.1, −1.28) | 0.032 | −14.96 | 7.00 | (−28.8, −1.14) | 0.034 |
| (600 + 900) - PBO | −12.84 | 4.73 | (−22.2, −3.49) | 0.007 | −13.02 | 4.81 | (−22.5, −3.53) | 0.007 |
Figure 1.
Change relative to baseline for weeks 6 and 12 in CDSD overall non-stool GI specific severity score among seropositive patients.
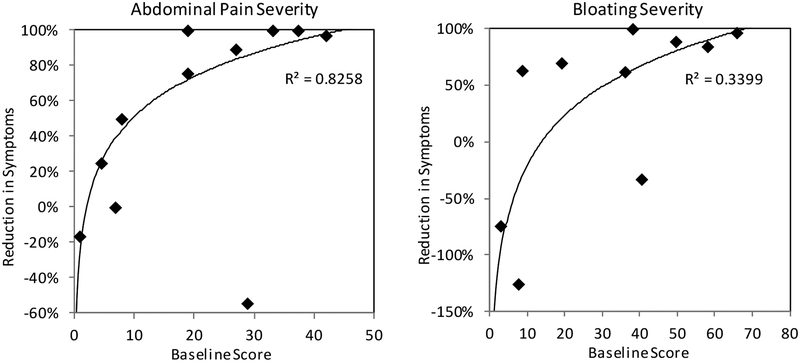
Another noteworthy observation was that the magnitude of RIS for the 600 and 900 mg seropositive latiglutenase arms was greater for those patients experiencing greater (baseline) symptom severity as shown in Figure 2 for abdominal pain and bloating for 900 mg patients. A similar but less distinct trend was observed for tiredness as well as for all symptoms in the 600 mg arm. Finally, segmenting the seropositive populations according to baseline severity did not show any significant efficacy of latiglutenase for nausea or diarrhea nor for any symptom in seronegative patients.
Figure 2.
Dependence of reduction in abdominal pain and bloating severity as a function of baseline symptom scores for seropositive patients on 900 mg treatment. Baseline severity is on a weekly scale of 0 to 70. The trend fit and R2 (logarithmic function) for abdominal pain excludes the outlier point at (x,y) = (28,−58%).
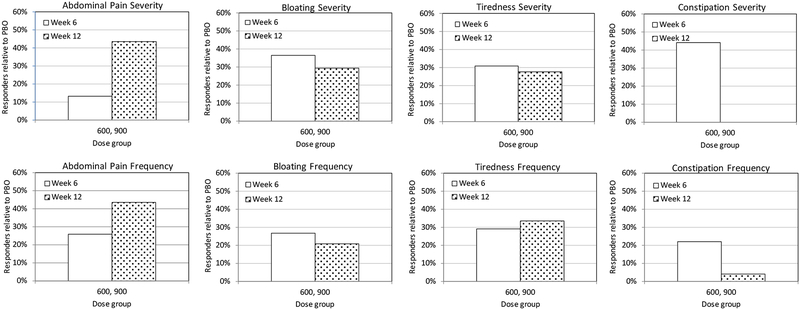
Symptom responder analysis:
We now provide a responder analysis in which we define responders as patients who exceeded a specific RIS threshold in a symptom domain based on the severity and frequency scales. Figure 3 plots the percent of responders relative to PBO, PRBdose, using an equation similar to Eq. 1 and given by
| (2) |
In all cases and for weeks 6 and 12 a positive responder effect is observed relative to PBO (except for a null result for constipation severity). A similar responder analysis using the condition Idose ≥ 50% gave similar results to that for ≥30% (Supporting Information, Figure S2).
Figure 3.
Responder analysis for ALV600+900 relative to PBO for a threshold of ≥30%.
Daily symptom analysis:
We further analyzed the daily CDSD data to determine the frequency of symptom occurrences as a function of severity. This analysis provides additional detail and substantiates the results for dose-dependent symptom improvement and increased improvement for patients with greater baseline severity. These results are presented in the Supporting Information.
QOL dependence on serostatus:
In Figure S5 (Supporting Information) there is a very noticeable trend toward positive benefit for seropositive patients and non-positive benefit for seronegative patients for individual components of the QOL instruments ICDSQ, PGI and SF12v2 on combined 600 mg and 900 mg dose that is consistent for weeks 6 and 12.
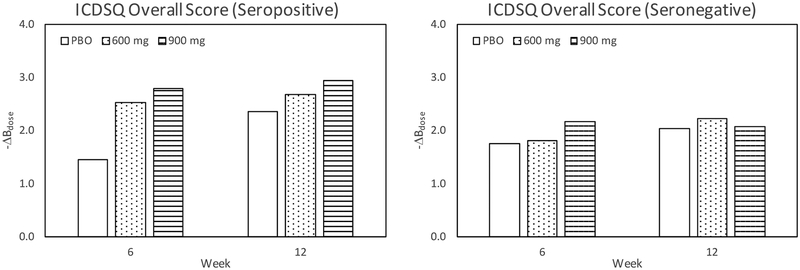
Table 3 and Figure 4 show results for the ICDSQ overall score for seropositive and seronegative patients for weeks 6 and 12. The overall score for the composite of 600 mg and 900 mg treated patients has p = 0.022 for week 6. Figure 4 plots the ΔBdose values for the overall score and shows a distinct dose dependent QOL benefit for seropositive, but not for seronegative patients, although statistical significance is not met for week 12 due to the much greater PBO effect.
Table 3.
Analysis of covariance (ANCOVA) for change from baseline for weeks 6 and 12 in ICDSQ overall score among seropositive patients.
| Week 6 | Week 12 | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | LS-Mean | SE | 95% CI | P value | LS-Mean | SE | 95% CI | P value |
| Placebo (PBO) | −1.45 | 0.35 | (−2.13, −0.76) | −2.36 | 0.35 | (−3.04, −1.67) | ||
| 600 - PBO | −1.08 | 0.56 | (−2.19, 0.02) | 0.055 | −0.32 | 0.56 | (−1.43, 0.78) | 0.565 |
| 900 - PBO | −1.35 | 0.74 | (−2.81, 0.11) | 0.070 | −0.59 | 0.75 | (−2.08, 0.89) | 0.433 |
| (600+900) - PBO | −1.22 | 0.52 | (−2.25, −0.18) | 0.022 | −0.46 | 0.53 | (−1.50, 0.59) | 0.388 |
Figure 4.
Negative change from baseline of ICDSQ overall score for weeks 6 and 12 among seropositive and seronegative patients. The data on the left plot are from Table 3.
Tables 4 and 5 tabulate the change from baseline for the SF-12v2 QOL instrument for weeks 6 and 12 for the physical and mental component scores, respectively. These results also show a statistically significant improvement in these components for seropositive patients in the 600 mg and 900 mg dose. Interestingly both measures decrease in going from week 6 to week 12, a trend also observed by the ICDSQ instrument. This appears to be due less to a decline in QOL improvement and more to an increased placebo effect for week 12.
Table 4.
Analysis of covariance (ANCOVA) for change from baseline for weeks 6 and 12 in SF-12v2: physical component score among seropositive patients (positive values denote improvement).
| Week 6 | Week 12 | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | LS-Mean | SE | 95% CI | P value | LS-Mean | SE | 95% CI | P value |
| Placebo (PBO) | 3.2 | 3.0 | (−2.8, 9.2) | 6.5 | 2.9 | (0.8, 12.3) | ||
| 600 - PBO | 7.5 | 4.9 | (−2.2, 17.1) | 0.129 | 3.8 | 4.7 | (−5.6, 13.1) | 0.424 |
| 900 - PBO | 14.2 | 6.5 | (1.3, 27.1) | 0.031 | −1.0 | 6.4 | (−13.7, 11.7) | 0.876 |
| (600+900) - PBO | 10.8 | 4.6 | (1.7, 19.9) | 0.020 | 1.4 | 4.5 | (−7.5, 10.3) | 0.755 |
Table 5.
Analysis of covariance (ANCOVA) for change from baseline for weeks 6 and 12 in SF-12v2: mental component score among seropositive patients (positive values denote improvement).
| Week 6 | Week 12 | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | LS-Mean | SE | 95% CI | P value | LS-Mean | SE | 95% CI | P value |
| Placebo (PBO) | 0.8 | 2.3 | (−3.8, 5.4) | 8.2 | 2.1 | (3.9, 12.4) | ||
| 600 - PBO | 13.0 | 3.8 | (5.6, 20.4) | <0.001 | 6.8 | 3.5 | (−0.0, 13.6) | 0.051 |
| 900 - PBO | 7.9 | 5.0 | (−2.0, 17.8) | 0.117 | 6.2 | 4.7 | (−3.0, 15.4) | 0.184 |
| (600+900) - PBO | 10.4 | 3.5 | (3.5, 17.4) | 0.004 | 6.5 | 3.3 | (0.1, 13.0) | 0.048 |
DISCUSSION
There is an urgent need for non-dietary therapies for celiac disease. The ALV003–1221 “real-world” trial attempted to show mucosal healing for the treatment arms as a corollary to the successful ALV003–1021 “gluten-challenge” trial that showed protection of the mucosa for the treatment arm. The former trial, however, did not demonstrate significant mucosal healing for the latiglutenase arms relative to the placebo arm; instead all arms improved comparably. It was clear that the patient population improved their GFDs while on the trial accounting for improvements in the villous height to crypt depth ratio (Vh:Cd) as well as a strong improvement in symptom and QOL assessments for the PBO arm. Equally clear was that there was still sufficient unintended gluten ingestion in at least some patients to cause frequent symptom responses that were alleviated by latiglutenase treatment. CD patients even when increasing their diligence of a GFD still cannot avoid eliminating gluten entirely.
Although the ALV003–1221 trial was not powered for symptom improvement nor was it a primary endpoint, the serostatus stratified analysis showed surprisingly strong and statistically significant improvement for most CD-related symptom domains and almost all QOL component measurements for seropositive patients for high doses of latiglutenase. The principle trends were: (i) dose-dependent improvement in symptoms and QOL, (ii) greater reduction in symptoms for more symptomatic patients, (iii) consistent results for week 6 and 12 showing well developed symptom improvement by week 6 and small but continued improvement by week 12 for symptoms, and, (iv) consistency of the severity and frequency reduction in symptoms in a responder analysis. Oddly whereas the symptom benefit maintained and even improved for week 12 vs. week 6, the opposite trend was observed for the QOL components. This may be due to patients self-normalizing from the previous reporting period such that instead of reporting a change from baseline they are biased toward reporting a change from the last reporting period. However, it is also evident in Figure 4 and Tables 3–5 that the placebo effect increased significantly from Week 6 to Week 12, which then diminished the change from baseline relative to placebo for the 600 mg and 900 mg patients.
A perplexing question is why the latiglutenase benefit is statistically significant for seropositive, but not seronegative patients. A potential explanation is that the symptoms observed in seronegative patients may not be predominantly due to gluten exposure or even to CD, but to other gastrointestinal ailments, such as functional GI syndromes that are common in CD patients.21 A potential reason why this group would be enriched in the ALV003–1221 study is that the enrollment criteria set minimum symptom requirements in order to address the population of moderately to severely symptomatic patients. Another less likely explanation is that there may be a population of seronegative patients whose residual biopsy measured villous atrophy is not due to ongoing inadvertent gluten exposure.22
Another question is why latiglutenase reduces the symptoms of abdominal pain, bloating, tiredness, and constipation, but not nausea and diarrhea. We have no plausible explanations at this time; however, we do observe an increasing symptom benefit for all symptoms including nausea and diarrhea with increasing time on a GFD, which we plan to explore further in future studies.
CONCLUSION
The ALV003–1221 trial stratified the patient population with the intention to explore the impact of serostatus on responsiveness to the primary and secondary endpoints. While the study results did not demonstrate conclusive evidence of latiglutenase-induced mucosal healing as the primary endpoint, post analysis of the symptom and QOL data supporting secondary endpoints showed for seropositive patients statistically and clinically significant evidence of latiglutenase-induced reduction of several key symptoms associated with gluten ingestion in CD patients and a correlation to QOL improvement. The principle conclusions of this work include:
A dose-dependent reduction in symptoms was observed for seropositive, but not seronegative patients for abdominal pain, bloating, tiredness, and constipation (CSBM). Nausea and diarrhea were not significantly responsive to latiglutenase.
A trial (Hawthorne)/placebo effect was observed for the major symptoms and QOL assessments for seropositive and seronegative patients (RIS typically 20–30%) further substantiating the trial effect for histology.
Greater reduction in symptom severity and frequency was observed for more symptomatic patients in the week 6 and week 12 data.
Patients experience symptom flares that reduce in frequency and severity under latiglutenase treatment.
The selection of subjects for a trial of a drug therapy that targets gluten will test most effectively if the subjects are likely to be exposed to gluten such as those that are seropositive.
Supplementary Material
Acknowledgments:
We are grateful to Matthew Dickason of ImmunogenX for key insights and critical comments. We also wish to acknowledge Phil Lavin for important comments on the statistics, Kellee Howard for modifying outcome measures and overseeing regulatory issues and Sarah Acaster for helping develop the CDSD PRO and performing statistical calculations.
Grant Support: Clinical trial NCT01917630 was sponsored by Alvine Pharmaceuticals; all data from this trial is presently owned by ImmunogenX. The data analysis reported here was supported in part by a grant from the National Institutes of Health (R01 DK063158 to C.K.).
Footnotes
Conflict of Interest Statement: JAS is a founder of and owns stock in ImmunogenX.
JAM has received grant support from the National Institutes of Health, Alvine Pharmaceuticals, and Alba Therapeutics; receives ongoing support from Oberkotter Foundation and Broad Medical Research Program at CCFA; serves on the advisory board of ImmunogenX; was a consultant to GlaxoSmithKline (GSK), Genentech, and Glenmark Pharmaceuticals Ltd; and is a consultant to ImunnosanT, Institute for Protein Design (PvP Biologics), Takeda Pharmaceutical Company, Ltd., Innovate Biopharmaceuticals, Inc., and Intrexon.
PHRG is an advisor to ImmusanT and ImmunogenX.
CK is a director of Protagonist Pharmaceuticals and an advisor to Sitari Pharmaceuticals, and holds stock in both companies.
JASV is a founder of and owns stock in ImmunogenX.
REFERENCES
- 1.Lohi S, Mustalahti K, Kaukinen K et al. Increasing prevalence of celiac disease over time. Aliment Pharmacol Ther 2007;26:1217–25. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009;137:1912–33. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol 2010;26:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syage JA, Kelly CP, Dickason MA, Cebolla Ramirez A, Leon F, Dominguez R, Sealey-Voyksner JA. Determination of gluten consumption in celiac disease patients on a gluten-free diet. Am J Clin Nutr 2018;107:201–207. [DOI] [PubMed] [Google Scholar]
- 5.Lerner BA, Phan Vo LT, Yates S, et al. Detection of gluten in gluten-free labeled restaurant food: Analysis of crowd-sourced data. Am J Gastroenterol 2019;00:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CP, Bai JC, Liu E, Leffler DA. Celiac disease: clinical spectrum and management. Gastroenterol 2015;148:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green PHR, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol 2015;135:1099–1106. [DOI] [PubMed] [Google Scholar]
- 8.Guandalini S, Tundia N, Thakkar R, Macaulay D, Essenmacher K, Fuldcore M. Direct cost in patients with celiac disease in the U.S.A.: A retrospective claims analysis. Dig Dis Sci 2016;10:2823–2830. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb K, Dawson J, Murray JA. Development of drugs for celiac disease: review of endpoints for Phase 2 and 3 trials. Gastroenterology Report 2015, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahdeaho M-L, Kaukinen K, Laurila K, …Adelman DC, Maki M. Glutenase ALV003 Attenuates Gluten-Induced Mucosal Injury in Patients With Celiac Disease. Gastroenterology 2014;146:1649–1658. [DOI] [PubMed] [Google Scholar]
- 11.Wolf C, Siegel JB, Tinberg C, et al. Engineering of kuma030: A gliadin peptidase that rapidly degrades immunogenic gliadin peptides in gastric conditions. J Am Chem Soc 2015;137:13106–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leffler DA, Kelly CP, Green PHR, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology 2015;148:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daveson AJM, Ee HC, Andrews JM, King T, Goldstein KE, et al. Epitope-Specific Immunotherapy Targeting CD4-Positive T Cells in CeliacDisease: Safety, Pharmacokinetics, and Effects on Intestinal Histology andPlasma Cytokines with Escalating Dose Regimens of Nexvax2 in a Randomized, Double-Blind, Placebo-Controlled Phase 1 Study. EBioMedicine 2017;26:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray JA, Kelly CP, Green PH, Marcantonio A, Wu TT, Mäki M, Adelman DC. No Difference Between Latiglutenase and Placebo in Reducing Villous Atrophy or Improving Symptoms in Patients with Symptomatic Celiac Disease. Gastroenterology 2017;152:787–798. [DOI] [PubMed] [Google Scholar]
- 15.Syage JA, Murray JA, Green PHR, Khosla C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig Dis Sci 2017;62:2428–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, and Khosla C Structural basis for gluten intolerance in Celiac Sprue. Science 2002;297:2275–2279. [DOI] [PubMed] [Google Scholar]
- 17.Gass J, Bethune MT, Siegel M, Spencer A, and Khosla C Combination enzyme therapy for gastric digestion of dietary gluten in celiac sprue patients. Gastroenterology 2007;133:472–480. [DOI] [PubMed] [Google Scholar]
- 18.Leffler D, Kupfer SS, Lebwohl B, Bugin K, Griebel D, et al. , “Development of Celiac Disease Therapeutics: Report of the Third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics Workshop.” Gastroenterology 2016;151:407–411. [DOI] [PubMed] [Google Scholar]
- 19.Leffler DA, Acaster S, Gallop K, Adelman DC. A Novel Patient-Derived Conceptual Model of the Impact of Celiac Disease in Adults: Implications for Patient-Reported Outcome and Health-Related Quality-of-Life Instrument Development. Value in Health 2017;20:637–643. [DOI] [PubMed] [Google Scholar]
- 20.Hindryckx P, Leveseque BG, Holvoet T et al. Disease activity indices in celiac disease: systematic review and recommendations for clinical trials, Gut 2018;67:61–69. [DOI] [PubMed] [Google Scholar]
- 21.Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clinical Gastroenterology and Hepatology 2013;11:359–365. [DOI] [PubMed] [Google Scholar]
- 22.Pallav K Leffler DA, Tariq S, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Alim Pharm & Ther 2011;35:380–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.