Despite recent advances in the management of subpopulations of AML, overall outcomes remain unsatisfactory. Encouraging results were seen with alvocidib (formerly known as flavopiridol), a CDK9 inhibitor with pan-cyclin-dependent kinase (CDK) activity, followed by cytarabine and mitoxantrone (FLAM) in serial phase I-II studies in newly diagnosed AML patients [1–7]. Therefore, we conducted a randomized phase II clinical trial comparing FLAM to cytarabine and daunorubicin (7 + 3) in newly diagnosed AML patients 18–70 years of age with non-favorable risk cytogenetics [8]. We previously published results of this trial revealing no significant differences in overall survival (OS) between FLAM and 7 + 3 despite significantly higher complete remission (CR) rates with FLAM. It is imperative to analyze long-term OS on this study as a secondary endpoint. Furthermore, we hypothesized that the survival trends may change with longer follow-up. We report the final long-term OS results of this trial.
This study randomized 165 patients between FLAM (n = 109) and 7 + 3 (n = 56) with a median age of 59 years (range: 19–70 years) across 10 institutions. Overall, 41% of patients had adverse-risk features based on 2010 European Leukemia Net Guidelines [9]. The primary endpoint of this trial was the rate of CR (including CR with incomplete hematologic recovery: CRi) with FLAM and 7 + 3. FLAM led to significantly increased CR rates when compared with 7 + 3 with 1 or 2 cycles of treatment (70% vs. 47%, p = 0.003, and 70% vs. 57%, p = 0.08, respectively). Despite the improvement in CR rates with FLAM, our earlier published results did not reveal significant differences in overall survival (OS) and event-free survival (EFS) between the arms, with median follow-up 811 days (range: 1–1217 days) by reverse Kaplan-Meier methodology.
The final database lock was on November 21, 2017. The first and last patient enrolled on this study on May 25, 2011 and July 26, 2013, respectively. Median follow-up by reverse Kaplan-Meier methodology is 1644 days (4.5 years: range: 1–2203 days). Overall, 76/109 (70%) patients died on the FLAM arm compared with 39/56 (70%) patients on the 7 + 3 arm (Table 1). Relapses were seen in 31/70 (44%) and 18/32 (56%) CR patients who received FLAM and 7 + 3 (1 or 2 cycles), respectively. Post-remission therapy was not standardized between both arms on this trial. Of the CR patients on the FLAM arm, 46% received a second cycle of FLAM consolidation, 28% received high dose cytarabine (HiDAC) consolidation, 17% underwent an allogeneic stem cell transplant (SCT) without consolidation therapy, 7% did not receive post-remission therapy due to poor performance status, and 3% either re-lapsed or received subsequent salvage chemotherapy for persistent cytogenetic abnormalities while in CR. In contrast, among the CR patients on the 7 + 3 arm, 81% received HiDAC consolidation, 9% underwent a SCT without consolidation therapy, 6% did not receive post-remission due to poor performance status and 3% refused further therapy. An allogeneic stem cell transplant (SCT) was performed in 50% of patients on both arms. Receipt of SCT was not significantly associated with OS in either arm, and there was no effect modification between treatment arm and receipt of SCT on OS using a time-varying covariate analysis approach (interaction p = 0.32). Fig. 1A and B display the Kaplan-Meier estimates of OS and EFS for FLAM and 7 + 3. The EFS analysis censored patients at the time of receipt of SCT or alternative therapy. Median OS was 17.5 months for FLAM and 23.1 months for 7 + 3 (p = 0.5). Five-year OS rates were 28% (95% confidence interval (CI): 21–39%) and 26% (95% CI: 16–43%) for FLAM and 7 + 3, respectively. Median EFS for FLAM vs. 7 + 3 = 9.7 vs. 3.4 months, respectively (p = 0.18). Five-year EFS rates for FLAM vs. 7 + 3 were 19% (95% CI: 10%–35%) and 26% (95% CI: 14%–48%), respectively. If we remove the censoring and follow patients for relapse or death post-SCT, the median EFS for FLAM vs. 7 + 3 = 9.8 vs. 7.7 months, respectively (p = 0.48). Five-year EFS rates for FLAM vs. 7 + 3 were 25% (95% CI: 18%–35%) and 16% (95% CI: 8%–30%), respectively. The majority of long-term survivors (i.e., alive ≥ 5 years after randomization) on both arms received a SCT in CR1. Two long-term survivors on the FLAM arm received only 1 cycle of consolidation in CR1 without a SCT.
Table 1.
Causes of Death.
| Survival Outcomes | FLAM (n = 109) | 7 + 3 (n = 56) |
|---|---|---|
| Causes of Death | 47 (62%) | 31 (79%) |
| Leukemia | 9 (12%) | 1 (3%) |
| Infection | 8 (11%) | 5 (13%) |
| Post-SCT complications | 3 (4%) | 0 |
| Hemorrhage/Coagulopathy | 2 (3%) | 1 (3%) |
| TLS | 1 (1%) | 1 (3%) |
| Multi-organ Failure | 1 (1%) | 0 |
| Stroke | 5 (7%) | 0 |
| Unknown Causes # of Deaths (%) | 76 (70%) | 39 (70%) |
Fig. 1. Kaplan-Meier estimates by treatment arm.
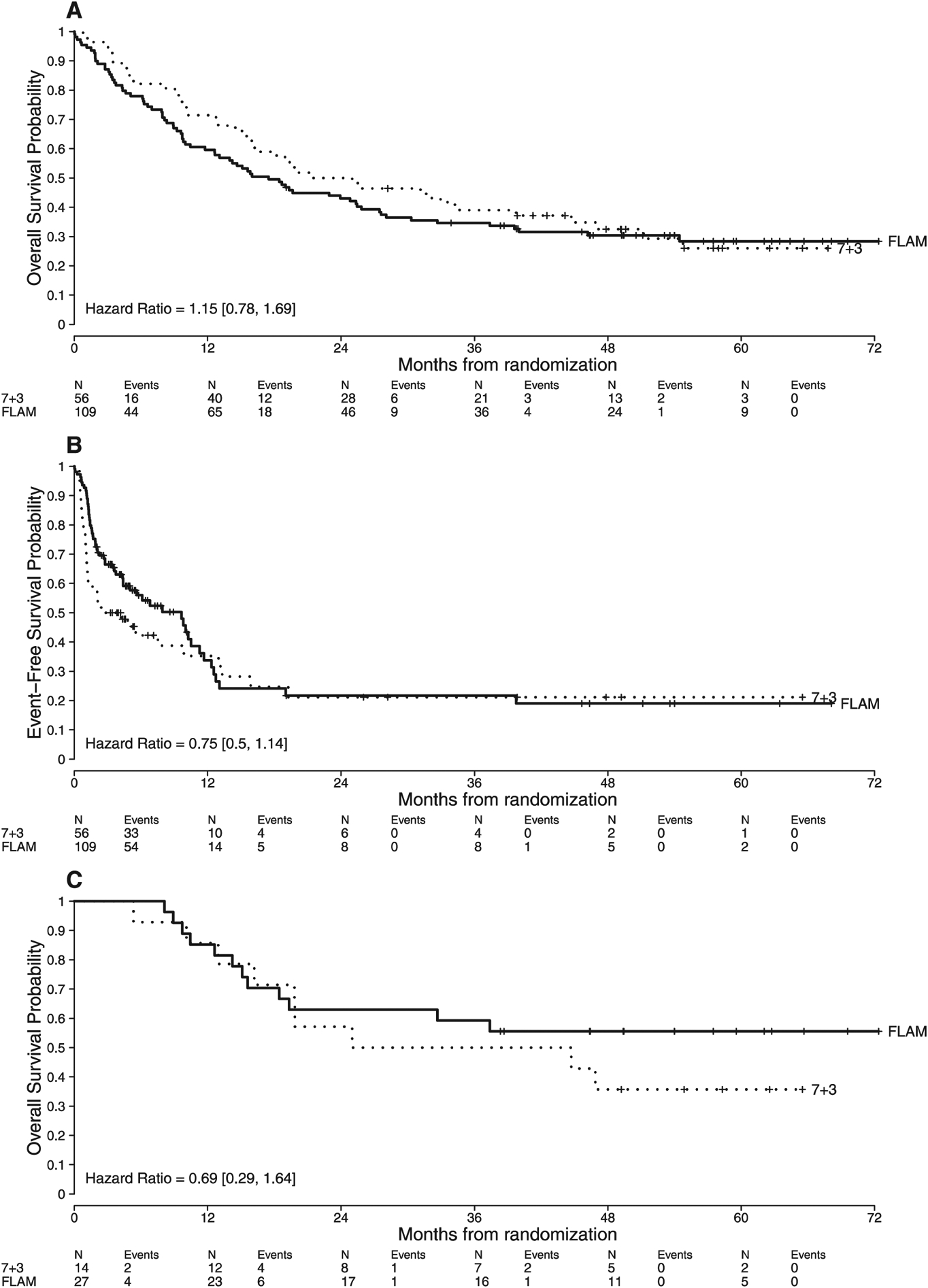
(A): OS for the entire cohort.
(B): EFS for the entire cohort.
(C): OS in patients < 50 years.
Subset analyses on this study revealed that younger patients (< 50 years) had significantly improved CR rates with FLAM versus 1 cycle of 7 + 3 (CR rates = 96% vs. 57%, respectively) when compared with older patients (≥ 50 years: CR rates = 61% vs. 43%, respectively) when testing for heterogeneity of treatment effect (p = 0.04). Therefore, we analyzed long-term OS and EFS of FLAM versus 7 + 3 in those < 50 years and ≥ 50 years. As expected, patients < 50 years had improved OS when compared with those ≥ 50 years on both arms (Median OS = 46.9 months versus 15.9 months, respectively). In patients < 50 years, median OS was not reached with FLAM versus 34.9 months with 7 + 3 (Fig. 1C). Long-term OS appeared to be better in patients < 50 years who were randomized to FLAM with 5-year OS = 56% (95% CI: 40–78%) versus 36% (95% CI: 18–72%) with 7 + 3, compared with those ≥ 50 years (19% versus 22%, respectively), but the interaction was not statistically significant (p = 0.15).
OS analyses need to be interpreted cautiously in this clinical trial given the 2:1 randomization (FLAM: n = 109 versus 7 + 3: n = 56), heterogeneity in the post-remission therapies between both arms, and the lack of power to detect an OS difference between both regimens. The FLAM regimen was initially studied as 1 induction cycle followed by a subsequent consolidation course identical to induction in those who achieve CR. However, those randomized to FLAM had the option of either FLAM or HiDAC consolidation at the time of CR, whereas those randomized to 7 + 3 received varying cycles of HiDAC consolidation, which confounds the OS analyses. Future randomized trials should stipulate the post-remission therapy and number of courses for both arms explicitly. Additionally, when compared to contemporary randomized phase II-III clinical trials, those on the 7 + 3 arm appeared to have better than expected outcomes. In the randomized phase III study of 7 + 3 with high dose (90 mg/m2) compared with standard dose (45 mg/m2) daunorubicin conducted by the Eastern Cooperative Oncology Group (ECOG) in newly diagnosed AML patients, median OS was 23.7 months in patients on the high dose daunorubicin arm [10]. However, 15.6% of patients on the ECOG study had favorable-risk cytogenetics and the upper age limit was 60 years. In contrast, our study excluded favorable-risk cytogenetics and the upper age limit was 70 years. Patients ≥ 60 years have worse outcomes compared with their younger counterparts [11–13]. In the present study, 44% and 55% of patients were ≥ 60 years on the FLAM and 7 + 3 arms, respectively, thus making direct comparisons challenging for similarly designed randomized phase II-III clinical trials in AML in younger or older patient populations.
Despite the lack of improvement in OS when compared with 7 + 3, these findings corroborate alvocidib’s activity in AML and support further development. Retrospective mitochondrial profiling revealed that NOXA, a BH3 pro-apoptotic protein that antagonizes myeloid leukemia cell-1 (MCL-1), priming in the bone marrow was significantly higher in patients achieving CR compared with non-responders (45% versus 5%, respectively) treated with FLAM [14]. A high NOXA priming score reflects dependence of leukemic cells on MCL-1 for survival. Further investigation of alvocidib (FLAM) is being performed in a biomarker-driven phase II clinical trial in newly diagnosed and re-lapsed/refractory AML patients with high NOXA (≥ 40%) priming scores (NCT02520011). Additionally, a phase I study of alvocidib followed by 7 + 3 in newly diagnosed AML with non-favorable cytogenetics is currently enrolling patients (NCT03298984).
Since inception of this trial, Gemtuzumab Ozogamicin (GO) was FDA-approved in combination with 7 + 3 for newly diagnosed CD33+ AML. This approval was based on findings from the ALFA-0701 randomized phase 3 trial of 7 + 3 (daunorubicin 60 mg/m2) with or without GO on days 1, 4 and 7 in newly diagnosed AML patients between 50 and 70 years of age. EFS, but not CR, was improved with the addition of GO. Subset analyses suggest that the benefit of GO was largely seen in the non-adverse risk patient population [15]. 7 + 3 + GO would be an appropriate comparison arm for further studies of alvocidib in AML, though it is noteworthy that ALFA-0701 used lower doses of daunorubicin and did not include patients < 50 years.
In summary, our final analysis reveals that FLAM leads to higher CR rates compared with 1 or 2 cycles of 7 + 3 induction in newly diagnosed AML patients 18–70 years with non-favorable cytogenetics but no significant difference was seen in OS or EFS. Subset analyses suggest that younger patients are more likely to achieve CR on FLAM compared with 7 + 3. Ongoing trials of alvocidib will provide further direction for its role in the current treatment armamentarium for AML.
Acknowledgements
The authors would like to thank the patients, families, research staff, nurses, and investigators from each participating institution.
Funding
This study was supported in part by NCI Cooperative Agreement U01 CA70095 and NCI Cancer Center Support Grant 2P30 CA06973-46.
Footnotes
Conflict of interest
JFZ received research funding and honoraria from advisory boards from Tolero.
JEK-Data Safety and Monitoring Board (DSMB) Member- Tolero
Contributor Information
Joshua F. Zeidner, The Johns Hopkins Hospital, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, United States; University of North Carolina, Lineberger Comprehensive Cancer Center, Chapel Hill, NC, United States.
Matthew C. Foster, University of North Carolina, Lineberger Comprehensive Cancer Center, Chapel Hill, NC, United States
Amanda L. Blackford, The Johns Hopkins Hospital, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, United States
Mark R. Litzow, Mayo Clinic, Rochester, MN, United States
Lawrence E. Morris, The Blood and Marrow Transplant Program at Northside Hospital, Bone Marrow Transplant Group of Georgia, Atlanta, GA, United States
Stephen A. Strickland, Vanderbilt-Ingram Cancer Center, Nashvile, TN, United States
Jeffrey E. Lancet, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States
Prithviraj Bose, Virginia Commonwealth University, Massey Cancer Center, Richmond, VA, United States; University of Texas, MD Anderson Cancer Center, Houston, TX, United States.
M. Yair Levy, Texas Oncology, Baylor Charles A. Simmons Cancer Center, Dallas, TX, United States.
Raoul Tibes, Mayo Clinic, Scottsdale, AZ, United States; New York University School of Medicine, Laura & Isaac Perlmutter Cancer Center, New York, NY, United States.
Ivana Gojo, The Johns Hopkins Hospital, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, United States; University of Maryland Medical Center, Stewart Greenebaum Cancer Center, Baltimore, MD, United States.
References
- [1].Karp JE, Ross DD, Yang W, et al. , Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial, Clin. Cancer Res 9 (2003) 307–315. [PubMed] [Google Scholar]
- [2].Karp JE, Passaniti A, Gojo I, et al. , Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in re-lapsed and refractory adult acute leukemias, Clin. Cancer Res 11 (2005) 8403–8412. [DOI] [PubMed] [Google Scholar]
- [3].Karp JE, Smith BD, Levis MJ, et al. , Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia, Clin. Cancer Res 13 (2007) 4467–4473. [DOI] [PubMed] [Google Scholar]
- [4].Karp JE, Blackford A, Smith BD, et al. , Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia, Leuk. Res 34 (2010) 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Karp JE, Smith BD, Resar LS, et al. , Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias, Blood 117 (2011) 3302–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karp JE, Garrett-Mayer E, Estey EH, et al. , Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia, Haematologica 97 (2012) 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zeidner JF, Karp JE, Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia, Leuk. Res 39 (2015) 1312–1318. [DOI] [PubMed] [Google Scholar]
- [8].Zeidner JF, Foster MC, Blackford AL, et al. , Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia, Haematologica 100 (2015) 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dohner H, Estey EH, Amadori S, et al. , Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet, Blood 115 (2010) 453–474. [DOI] [PubMed] [Google Scholar]
- [10].Fernandez HF, Sun Z, Yao X, et al. , Anthracycline dose intensification in acute myeloid leukemia, N. Engl. J. Med 361 (2009) 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mrozek K, Marcucci G, Nicolet D, et al. , Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia, J. Clin. Oncol 30 (2012) 4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lowenberg B, Ossenkoppele GJ, van Putten W, et al. , High-dose daunorubicin in older patients with acute myeloid leukemia, N. Engl. J. Med 361 (2009) 1235–1248. [DOI] [PubMed] [Google Scholar]
- [13].Lancet JE, Cortes JE, Hogge DE, et al. , Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML, Blood 123 (2014) 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smith BD, Warner S, Whatcott C, et al. , An alvocidib-containing regimen is highly effective in AML patients through a mechanism dependent on MCL-1 expression and function, J. Clin. Oncol 33 (2015) abstract 7062. [Google Scholar]
- [15].Castaigne S, Pautas C, Terre C, et al. , Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study, Lancet 379 (2012) 1508–1516. [DOI] [PubMed] [Google Scholar]


