Abstract
Introduction
Metformin is a first-line drug for treating type 2 diabetes that regulates the differentiation of mesenchymal stem cells. Its effects on human dental pulp cells (DPCs) remain unknown. This study aimed to investigate the effects of metformin on proliferation and differentiation of DPCs.
Methods
Live/dead viability assay kit was used to examine the effects of metdormin on cell viability of DPCs. Cell proliferation was analyzed using a Cell Counting Kit-8 (CCK-8) kit. Levels of phosphorylated and unphosphorylated AMPK were quantified by Western blot analysis in response to metformin and the AMPK signaling inhibitor compound C. The effects of compound C on the metformin-induced odontoblast differentiation of DPCs were determined by alkaline phosphatase activity (ALP) assay, von Kossa staining, and the expression of odontoblastic markers was evaluated by reverse transcription polymerase chain reaction analysis.
Results
DPCs exhibited mesenchymal stem cell characteristics using flow cytometry. Different doses of metformin were shown to be cytocompatible with DPCs, yielding > 90% cell viability. None of the concentrations of metformin up to 50 μM affected cell proliferation. Western blot assay showed that DPCs express functional organic cation transporter-1 (OCT-1), a transmembrane protein that mediates the intracellular uptake of metformin. Metformin significantly activated the AMPK pathway in a dose-dependent manner. In addition, it stimulated alkaline phosphatase activity, enhanced mineralized nodule formation, and increased the expression of odontoblastic markers including DSPP, DMP-1, Runx2, and OCN. Moreover, pretreatment with compound C, a specific AMPK inhibitor, markedly reversed metformin-induced odontoblastic differentiation and cell mineralization.
Conclusions
This study shows that metformin can induce DPC differentiation and mineralization in an AMPK-dependent manner, and that this well-tolerated anti-diabetic drug has potential in regenerative endodontics as well as in other regenerative applications.
Keywords: Metformin, odontoblast, proliferation, AMPK
Introduction
The prevalence of diabetes is increasing globally, and the situation is particularly alarming in Asia. The prevalence of diabetes in China has increased dramatically from around 1% in 1980 to the most recent estimate of 9.7%, according to a nationwide survey (1). Cummulative evidence shows that type 2 diabetes mellitus (T2DM) is associated with aberrant bone formation that can lead to skeletal fractures (2–4). Metformin, a relatively inexpensive and well-tolerated anti-hyperglycemic biguanide, is widely used by millions of diabetic patients as the first-line treatment for T2DM. As a highly hydrophilic cationic compound, metformin relies on polyspecific cell membrane organic cation transporters (OCTs) of the solute carrier 22A (SLC22A) gene family to facilitate its intracellular uptake and action (5). Several studies indicate that metformin has a potential osteogenic effect by promoting the differentiation of mesenchymal stem cells (MSCs) and preosteoblasts. Furthermore, metformin is also able to reverse the deleterious effects of advanced glycation end products on these cells (6–9). Kanazawa and colleagues (10) demonstrated that metformin can induce the differentiation and mineralization of preosteoblasts into osteoblastic cells via activation of the AMPK signaling pathway. Human dental pulp cells (DPCs) share similar gene expression profiles and differentiation capability to that of other MSCs (11, 12). However, it remains unknown whether metformin is able to induce the odontoblastic differentiation of DPCs.
DPCs possess multipotent differentiation potential and the ability to form dentin-pulp-like complex throughout life. When the dental pulp is confronted by trauma, microbes, or chemicals, a host of inflammatory cytokines are released (13). These insults can also stimulate the underlying progenitor pulp cells to differentiate into odontoblasts, which are capable of secreting dentin matrix proteins as part of reparative dentinogenesis (14). Odontoblasts secrete several collagenous and non-collagenous proteins, such as type-1 collagen, osteopontin, dentin matrix protein 1 (DMP-1), and dentin sialophospho protein (DSPP), which are unique biological markers for the odontoblast/osteoblast-like differentiations of DPCs (15, 16). DPCs are potentially superior to other stem cells for regenerative medicine applications including bone tissue engineering. For example, bone marrow MSCs require an invasive procedure to harvest; in contrast, DPCs are easy to harvest from donors including children losing their primary teeth and teenagers having their wisdom teeth removed, which are otherwise discarded as medical waste. Therefore, DPCs are considered to be of great promise for dental repair/regeneration as well as other tissue engineering applications.
Therefore, the present study was designed to examine the effects of metformin on the proliferation and odontoblastic differentiation of DPCs. The role of AMPK signaling in metformin-mediated odontoblastic differentiation was also investigated.
Materials and Methods
Cell Cultures
DPCs were isolated and characterized as described previously (17). Dental pulp tissues were obtained from explants of clinically healthy dental pulp from human adult third molars that were removed from individuals undergoing tooth extraction for orthodontic treatment. The procedure was approved by the Institutional Review Board of the University of Maryland Baltimore. Briefly, pulp tissues were minced and digested in a solution of 3 mg/mL of collagenase type I and 4 mg/mL dispase for 30–60 min at 37°C. Cell suspension was obtained by passing the digested tissue through a 70-μm cell strainer. The cells were pelleted and seeded in culture dishes, and incubated in α-MEM supplemented with 20% FBS, 100 units/mL penicillin G, 100 mg/mL streptomycin, and 50 mg/mL ascorbic acid (Sigma-Aldrich, St. Louis, MO) at 37 °C in 5% CO2. Non-adherent cells were removed 48 h after the initial plating. The medium was replaced every 3 days. When primary culture became subconfluent, after approximately 1–2 weeks, cells were collected by trypsinization and subcultured at 5000 cells per cm2 in growth medium. To analyze the cell surface antigen expressions, the cells from the second passage were used. The cells were washed and resuspended in PBS supplemented with 3% FBS that contained saturating concentrations (1:100 dilution) of the following reagents: FITC-conjugated anti-human monoclonal antibodies, anti-CD73-phycoerythrin (PE) (BD Biosciences, San Jose, CA), anti-CD90-PE (BioLegend, San Jose, CA), anti-CD105-PE (BioLegend), anti-CD34-PE (BD Biosciences), anti-CD45-PE (BD Biosciences), or anti-HLA-DR (PE) (BD Biosciences) for 1 h at room temperature in the dark. As a negative control, PE-conjugated nonspecifc mouse IgG1 (BioLegend) were substituted for the primary antibodies. DPCs from the fourth to fifth passages were used for subsequent experiments.
Before the experiments, DPCs were serum starved in 0.5% fetal calf serum for 18 hours. Different concentrations of metformin were added, and the cells were incubated for the indicated time periods. In the experiments involving the AMPK inhibitor Compound C (EMD Chemicals, San. Diego, CA), Compound C was added 1 hour before the addition of metformin, as described below.
Cell Viability
DPCs were seeded in 24-well plates at a density of 3 × 104 cells/well. At 1 and 7 days, cells were stained by live/dead viability assay kit (Invitrogen Life Technologies, Carlsbad, CA) as we previously described (18). Cells were washed with PBS, followed by incubation with the dye. Live cells were stained green with 2 mM calcein AM and dead cells were marked red with 4 mM ethidium homodimer-1 (EthD-1), and they were examined using epifluorescence microscopy (Eclipse TE2000-S, Nikon, Melville, NY). The percentage of live cells and the live cell density were calculated as previously described (18). Three random sections were analyzed for each sample.
Cell Proliferation Assays
A cell counting kit (CCK-8, Dojindo, Tokyo, Japan) was used to evaluate cell proliferation at 1, 3, 5, and 7 days as described previously (19). Three replicates in each group were used for this assay (n = 3). CCK-8 is based on the WST-8 reaction that produces an orange formazan dye in an amount that is directly related with the number of viable cells. The cell proliferative rate was determined via the absorbance at an optical density of 450 nm (OD450nm) using a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA) according to the manufacturer’s protocol.
Western Blot Analysis
Cells were harvested and lysed in lysis buffer: 20 mmol/L Na2PO4 at pH 7.4, 150 mmol/L NaCl, 1% Triton X-100, 1% aprotinin, 1 mmol/L phenymethysulfonyl fluoride, 10 mg/mL leupeptin, 100 mmol/L NaF, and 2 mmol/L Na3VO4. Lysates were centrifuged at 12,000 rpm for 15 min. The supernatant was collected, and the protein content was determined using the Bio-Rad protein assay. SDS-PAGE sample buffer (10 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.2 M DTT) was added to the lysates. Lysates were heated to 100 °C for 8 min, and 20 μg of the total protein was loaded in each well of a 10% SDS-PAGE gel. Western blot analysis was performed as reported previously (20, 21). The following primary antibodies were used: OCT1 antibody (Sigma-Aldrich, St. Louis, MO), OCT2 antibody (Sigma-Aldrich), OCT3 antibody (Sigma-Aldrich), Phospho-AMPKα (Thr-172) (p-AMPK) (Cell Signaling Technology, Beverly, MA), total AMPK antibody (Cell Signaling Technology), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Santa Cruz Biotech, Santa Cruz, CA).
Alkaline Phosphatase Activity
DPCs were pre-incubated with 10 μM Compound C 1 hour and then exposed to 50 μM metformin; this procedure was repeated at day 3. After the treatment, the cells were scraped into cold PBS and then sonicated in an ice bath and centrifuged at 1500 g for 5 min. Then, the ALP activity was measured in the supernatant using ALP assay mixtures containing 0.1 M diethanolamine, 1 mM MgCl2, and 10 mg/mL p-nitrophenyl phosphate. After incubation at 37 °C for 30 min, the reaction was stopped by the addition of NaOH, and the absorbance was measured at 410 nm using a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA).
Reverse Transcriptase PCR (RT-PCR) and Real Time Quantitative PCR (qPCR)
The expression levels of ALP, DSPP, DMP-1, Runx2, OCN mRNA were determined by SYBR green real-time reverse transcription-PCR (RT-PCR) as we previously described (22, 23). Total RNA were extracted using Trizol reagent. Quantitative determination of RNA levels were performed in triplicate in three independent experiments. Real-time PCR and data collection were performed with an ABI PRISM 7500 sequence detection system. The housekeeping gene GAPDH was used as an internal control to normalize the expression levels of different genes. For each primer set, the melting curves were performed to ensure that a single peak was produced, The data for gene expression were analyzed with the △△Ct method. The primers used for the amplification of the indicated genes are listed in Table 1.
TABLE 1.
List of Reverse Transcriptase Polymerase Chain Reaction Primers
| Gene | Forward | Reverse |
|---|---|---|
| ALP | CTATCCTGGCTCCGTGCTC | GCTGGCAGTGGTCAGATGTT |
| DSPP | TGGAGCCACAAACAGAAGCAA | TCCAGCTACTTGAGGTCCATC |
| DMP-1 | GTGAGTGAGTCCAGGGGAGATAA | TTTTGAGTGGGAGAGTGTGTGC |
| Runx2 | GACTGTGGTTACCGTCATGGC | ACTTGGTTTTTCATAACAGCGGA |
| OCN | CTCACACTCCTCGCCCTATT | TTGGACACAAAGGCTGCAC |
| GAPDH | TCAACGACCCCTTCATTGAC | ATGCAGGGATGATGTTCTGG |
Von Kossa Staining
Specific calcifications were detected by von Kossa staining (24). Briefly, DPCs were plated in six-well plates at a density of 1 × 105 cells per well and cultured in DMEM supplemented with 10% FBS, 50 mg/mL ascorbic acid, 10 mmol/L sodium β-glycerophosphate and 10 nmol/L dexamethasone. DPCs were then pre-treated with 10 μM compound C for 1 hour prior to the addition of 50 μM metformin. This treatment was repeated every 3 d. After 14 d of treatment, the cells were treated with a 5% silver nitrate solution and exposed to ultraviolet light for 30 min. This solution was then neutralized with 5% sodium thiosulfate for 2 min, and the cells were rinsed with distilled water for 5 min. Finally, the cells were stained with nuclear fast red (Sigma-Aldrich) for 1 min. To quantify the mineralization, the calcium content was measured by a quantitative colorimetric method using a calcium assay kit following the manufacturer’s instructions. The calcium content of the cell layer was determined at day 14 of odontogenic culture. The absorbance of the solutions was read at 570 nm using a UV-visible light spectrophotometer.
Statistical Analysis
All experiments were repeated at least three times. Data are expressed as mean ± standard deviation (SD). Results of at least three independent experiments (always performed with cells isolated from different donors) were compared by OneWay ANOVA. Differences between groups were evaluated with Tukey’s post-test. A P value < .05 was considered significant.
Results
Identification of Stem Cell Phenotypic Markers in Primary DPCs
The surface markers of DPCs were analyzed by flow cytometry. Consistent with other mesenchymal stem cell populations (Fig. 1), the majority of DPCs exhibited intense expression of mesenchymal surface molecular markers (CD73–99.9%, CD90–99.8%, and CD105–99.4%). On the other hand, DPCs exhibited weak expression of surface markers for hematopoietic system-derived cells (CD34–0.1% and CD45–1.3%). Embryonic stem cells pluripotency marker HLA-DR (Class II histocompatibility Antigen) was 0.2%. Weak staining of PE-conjugated nonspecific mouse IgG1 control antibody confirmed the specificity of primary antibody binding. These results indicate that the DPCs possessed typical MSC characteristics.
Figure 1.
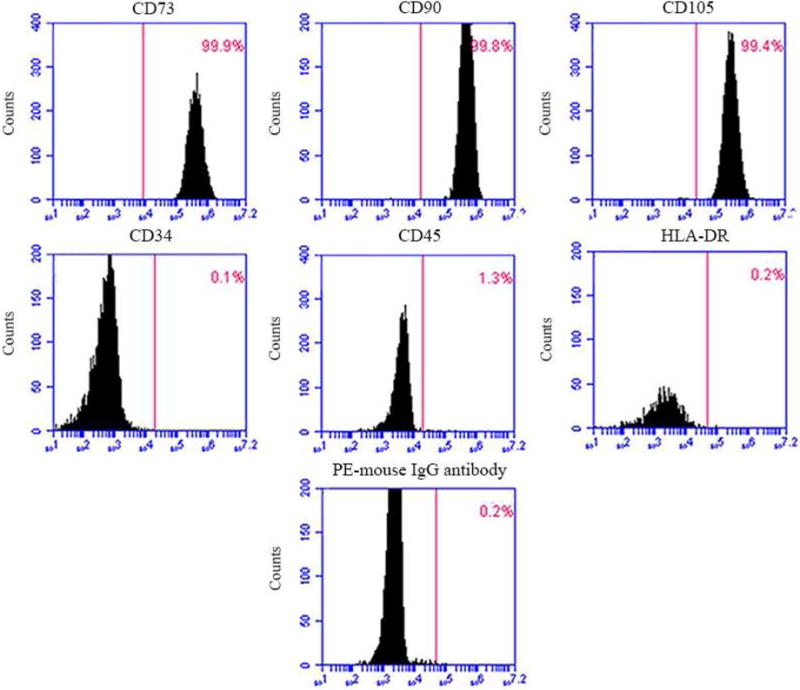
DPCs phenotype by flow cytometry. The expression of a series of cell surface markers associated with the mesenchymal stem cell (MSC) phenotype was investigated using flow cytometry. Analysis of molecular surface antigen markers in DPCs by flow cytometry indicated that cells were negative for CD34 and CD45, whereas they were positive for CD73, CD90, CD105; PE-conjugated non-specific mouse IgG1 served as negative control.
DPC Viability and Cell Proliferation
In order to evaluate the effects of metformin treatment on DPCs’ cytocompatibility, cellular viability was assessed using the live/dead staining in culture. Representative live/dead staining images at 1 day and 7 days are shown in Figure 2A. There were numerous live cells (stained green) and a few dead cells (stained red). Cell number significantly increased from day 1 to day 7 most likely due to cell proliferation. In Figure 2B, the percentages of live cells in all four groups were approximately 90% and were not significantly different among the three doses of metformin (P > .05). As shown in Figure 2C, the live cell density increased with time due to proliferation, with no significant difference among the four groups (P > .05). None of the concentrations of metformin up to 50 μM affected cell proliferation as determined by CCK-8 assay (Fig. 2D). Overall, these results demonstrate the non-cytotoxic nature of 50 μM metformin, a dose used in other experiments of this study.
Figure 2.
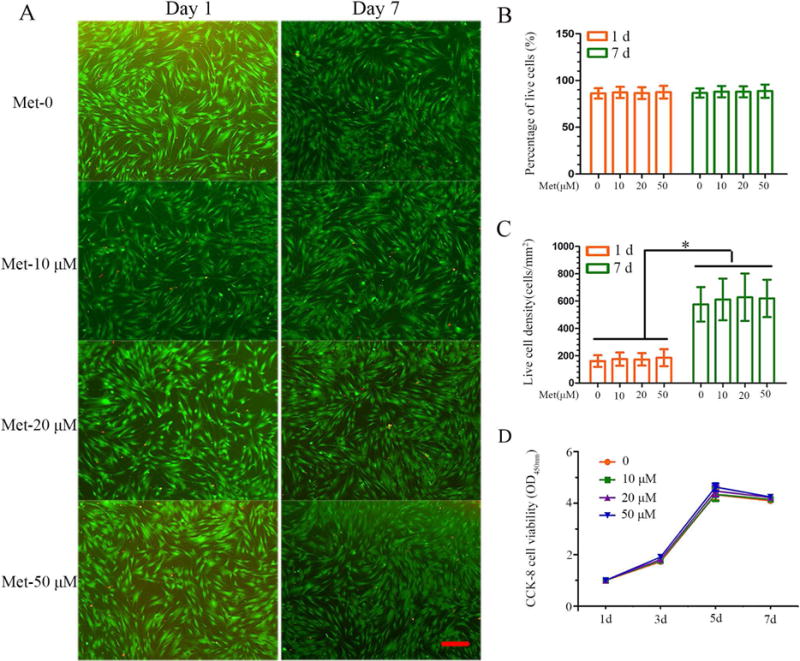
Effect of metformin on cell viability and proliferation of DPCs. (A) Representative live/dead images of metformin-treated DPCs at days 1 and 7 of culture, with live cells stained green and dead cells shown in red. In all four groups, live cells were abundant, and dead cells were few (scale bar = 100 μm). (B) Percentage of live cells of DPCs was around 90%. Data represent mean ± SD of 3 experiments with triplicates. (C) All groups exhibited increasing live cell density. Data represent mean ± SD of 3 experiments with triplicates. *P < .05. (D) Metformin has no effect on the cell proliferation. Data represent mean ± SD of 3 experiments with triplicates.
AMPK activation in Response to Metformin in DPCs
Protein expression levels of OCT1, OCT2 and OCT3, encoded by the SLC22A gene family, facilitate metformin transport across cellular membranes (5). Western blotting was performed to determine OCT expression levels in DPCs as previously demonstrated (20). Among the members of the SLC22A family of transporters frequently associated with metformin uptake, only OCT-1 was expressed in these cells (Fig. 3A). To determine whether AMPK phosphorylation, a surrogate marker of metformin action, also occurs in DPCs following metformin treatment we treated DPCs with metformin for 24 h. The expression levels of phospho-AMPK (relative to AMPK) in metformin-treated cells were markedly increased. Phospho-AMPK expression levels were notably up-regulated in metformin-treated cells with increasing doses, compared with the control group, as shown in Figure 3B. The small molecule compound C has been widely used as a selective AMPK inhibitor (25). We used compound C to prevent AMPK phosphorylation in response to metformin. As depicted in Figure 3C, metformin-induced phospho-AMPK was downregulated following pretreatment with 10 μM compound C. These results confirmed that in DPCs metformin triggers activation of the AMPK pathway as confirmed by the inhibitory action of compound C.
Figure 3.
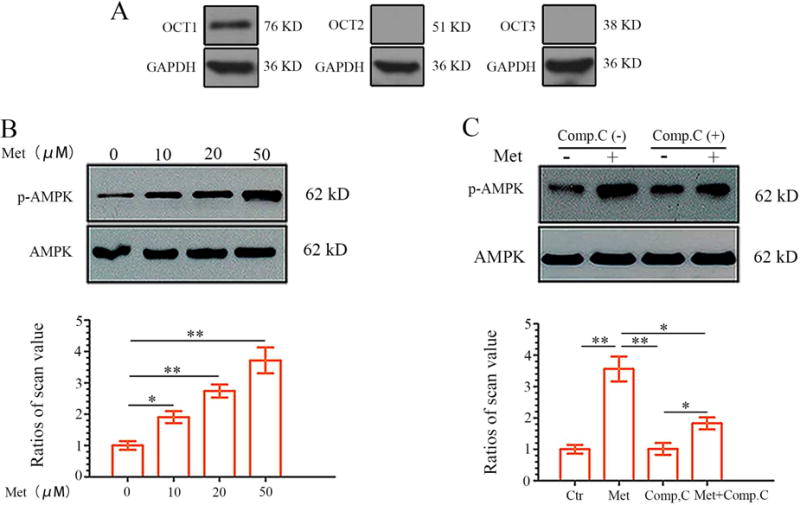
Metformin up-regulates AMPK phosphorylation in DPCs in a dose-dependent manner. (A) Whole cell extracts were analyzed by western blotting to determine expression levels of OCTs in DPCs. As shown, only OCT-1 expression was detected in DPCs. (B) DPCs were treated with different concentrations of metformin for 24 hours. Lower panels show the ratios of band densities of phosphor-AMPK to AMPK. Data represent mean ± SD of 3 experiments with triplicates. *P < .05. **P < .001. (C) Confluent DPCs cells were preincubated with the AMPK inhibitor Compound C (10 μM) for 1 hour before treatment with metformin. Compound C decreases metformin-induced AMPK phosphorylation. Data represent mean ± SD of 3 experiments with triplicates. *P < .05. **P < .001.
Metformin-induced AMPK Activation Increases Alkaline Phosphatase Activity, Alkaline Phosphatase mRNA Expression and Mineralization
To understand whether metformin affects the odontoblastic differentiation of DPCs, an ALP activity assay was performed. To this end, ALP activity significantly increased in the metformin-treated group until day 14 when it was more than 2-fold higher than the control group (Fig. 4A). The increased ALP activity was significantly reduced following pretreatment with Compound C. The results of the ALP mRNA expression were consistent with ALP assay (Fig. 4B). Next, we investigated the mineralized nodule formation, an index of terminal odontoblastic differentiation, in DPCs after 14 days of incubation with metformin in the abscence or presence of Compound C. Treatment of DPCs with metformin increased mineralized nodule formation and calcium content. Conversely, a decrease in calcified nodule formation (Fig. 4C) and calcium content (Fig. 4D) was observed in cells treated with metformin in the presence of Compound C. These results suggest that the downstream effects of metformin on the odontoblastic maturation of DPCs is mediated through the activation of the AMPK signaling pathway.
Figure 4.
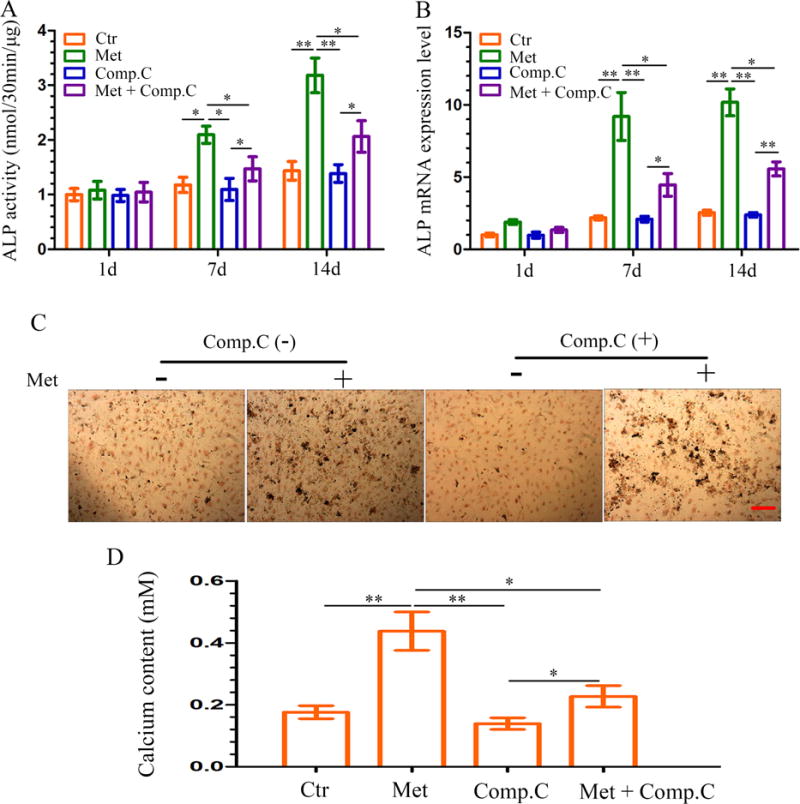
Effect of metformin-induced ALP activity and mineralized nodule formation in DPCs. (A–B) DPCs were treated with metformin (50 μM) in the absence or presence of Compound C (10 μM, pretreatment for 1 h); cells were retreated every 3 days. ALP activity (A) and ALP mRNA expression (B) were measured at each time point. Data represent mean ± SD of 3 experiments with triplicates. *P < .05. **P < .001. (C) DPCs were cultured in osteogenic induction medium for 14 days, mineralized nodule formation was assessed by von Kossa staining (scale bar = 100 μm). (D) On the 14th day, the calcium content was determined. Data represent mean ± SD of 3 experiments with triplicates. *P < .05. **P < .001.
Metformin Triggers DPC Odontoblastic Differentiation in an AMPK-dependent Manner
To further investigate the effects of metformin on odontoblastic differentiation of DPCs, cells were exposed to metformin for 1, 7 and 14 days. Metformin markedly upregulated the mRNA expression of critical odontoblastic genes including DSPP, DMP-1, Runx2 and OCN mRNA. In contrast, expression of these genes was significantly inhibited by the addition of compound C prior to metformin treatment (Fig. 5). These results further indicate that AMPK is a key mediating factor controlling metformin-induced odontoblastic differentiation of DPCs.
Figure 5.
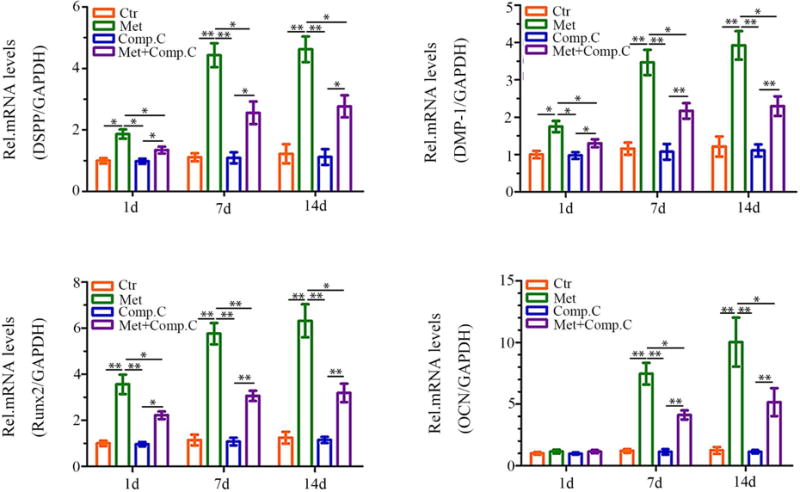
Effects of metformin treatment on the odontoblastic differentiation of DPCs. DPCs cells were preincubated with Compound C (10 μM) for 1 hour before treatment with metformin. The mRNA expression of DSPP, DMP-1, Runx2, and OCN was analyzed using real-time RT-PCR. Data represent mean ± SD of 3 experiments with triplicates. *P < .05. **P < .001.
Discussion
Stem cell-based therapies are rapidly emerging as a potential strategy for tissue regeneration in many diseases and conditions. The goal of cellular therapy in regenerative medicine and dentistry seeks to replace or restore the biological functions of damaged tissues or organs (26). Dental pulp cells derived from dental pulp tissues represent a population of mesenchymal stem/progenitor cells residing in the pulp chamber. The high proliferative potential of DPCs makes this cell population suitable for stem cell-based regeneration and preferentially useful for dentine repair. In addition, they form dentin- and bone-like tissues in vivo (27). Several studies have indicated that continued dentin-like tissue formation is associated with DPCs (28–30). Therefore, DPCs appear as an ideal cellular source of stem cells with an immense intrinsic differentiating potential to be used in endodontic tissue engineering and regeneration.
Metformin is widely used by millions of diabetic patients as the first-line treatment for T2DM. As an insulin-sensitizing drug, it is safe, non-toxic and well-tolerated. The effect of metformin on cell proliferation has been reported in various types of cells (7, 8, 31). Our observations in the present study indicate that different doses of metformin have no significant effect on DPC proliferation. Furthermore, metformin was found to not alter the viability of DPCs, suggesting that metformin is safe and non-toxic. The effect of metformin on cell proliferation is not consistent with that previously reported for rat marrow mesenchymal stem cells (8). Cortizo et al. reported that treatment of two osteoblast-like cell lines (MC3T3E1 and UMR106) with metformin for 24 h led to a dose-dependent increase in cell proliferation as determined by the crystal violet bioassay (8). These conflicting observations may result from differences in experimental procedures or cell types.
Metformin has been reported to significantly activate AMPK in preosteoblastic MC3T3E1 cells in a dose- and time-dependent manner (10). Interestingly, this study showed that the addition of the antiviral agent adenine 9-β-D-arabinofuranoside, a specific AMPK inhibitor, attenuated the metformin-induced responses. These results suggested that metformin induced the differentiation and mineralization of osteoblast-like cells via the activation of AMPK signaling. To investigate whether AMPK signaling pathway is also involved in metformin-induced DPC odontoblastic differentiation, we assessed the activation of AMPK signaling in metformin-treated DPCs under conditions designed to induce odontoblastic differentiation and its role in odontoblastic differentiation with the use of AMPK inhibitors. Among the concentration gradients in our experiment, we found that in DPCs 50 μM metformin significantly up-regulated phospho-AMPK expression levels. Thus, this concentration was selected for the experiments in the present study. Compound C, a widely used inhibitor of AMPK activation (32), was also used to test the effect of metformin on AMPK phosphorylation. The phosphorylation of AMPK was partially inhibited by Compound C. It is important to mention that the potentially complex mechanisms by which metformin regulates DPCs odontoblastic differentiation have not been explored before, and here we demonstrate that metformin in fact exerts an stimulating effect on DPC-driven odontoblastogenesis.
ALP activity is most often used as an early marker of odontoblastic differentiation (33) and plays an important role in mineralization. In the current study, less ALP activity was seen when AMPK signaling pathway was inhibited by Compound C; consistent with this finding, less mineralized nodules were also observed in DPCs treated with Compound C at the late stage of odontoblastic differentiation. These results indicate that the AMPK signaling pathway is in part controlling the effects of metformin on the odontoblastic differentiation of DPCs.
Morever, the gene expression levels of related odontoblastic markers such as DSPP, DMP-1, Runx2, and OCN were measured to investigate the effects of AMPK-signaling on the differentiation ability of odontoblasts in vitro. Runx2, of the runt domain gene family, is an essential transcription factor that controls bone and tooth development by regulating osteoblast and odontoblastic differentiation (34, 35). DSPP and DMP-1 are members of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family. DSPP was originally considered to be dentine-specific marker. Although several studies have also recently shown its expression in bone (36–38), DSPP remains a major marker of odontoblastic differentiation. DMP-1, also expressed in early osteoblastic differentiation, was related to the matrix mineralization of bone and dentin (39, 40). OCN can be expressed by odontoblasts and is present in the dentin matrix, and it is also considered to be a reparative molecule within the dental pulp (41–43). In the present study, DSPP, DMP-1, Runx2 and OCN mRNA levels were upregulated in metformin-treated DPCs. However, no effect was observed by metformin on the mRNA level of OCN during the early stages of treatment. These results may be attributed to the fact that OCN was expressed at the later mineralization stage (44). Compound C siginificantly inhibited the metformin-induced upregulation of DSPP, DMP-1, Runx2 and OCN mRNAs, thereby providing further evidence that the AMPK signaling pathway plays an important role in metformin-mediated DPC odontoblastic differentiation.
In summary, this study demonstrated that metformin can enhance the odontoblastic differentiation of DPCs through activation of the AMPK signaling pathway. These results suggest that metformin may play an important pharmacological role in triggering odontoblastic differentiation, and may provide new insights into the therapy of pulpal wound. Further in vivo experiments should be performed using an animal model to demonstrate the possibility of future clinical applications and to determine the effects of metformin on the regeneration of the dentin-pulp complex.
Acknowledgments
Wei Qin and Xianling Gao contributed equally to this study.
This study was supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research Grant R01 DE023578 (A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest related to this study.
These results indicate that metformin may promote dental pulp tissue healing and repair through AMPK signaling and provide new insights into the therapy of pulpal wound.
References
- 1.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Krakauer JC, McKenna MJ, Buderer NF, Whitehouse FW, Parfitt AM, et al. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–82. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–99. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 4.Carnevale V, Romagnoli E, D’Erasmo E. Skeletal involvement in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004;20:196–204. doi: 10.1002/dmrr.449. [DOI] [PubMed] [Google Scholar]
- 5.Nies AT, Koepsell H, Damme K, et al. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–67. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 6.Jang WG, Kim EJ, Bae IH, et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48:885–93. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Xue J, Li X, et al. Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J Pharm Pharmacol. 2008;60:1695–700. doi: 10.1211/jpp.60/12.0017. [DOI] [PubMed] [Google Scholar]
- 8.Cortizo AM, Sedlinsky C, McCarthy AD, et al. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol. 2006;536:38–46. doi: 10.1016/j.ejphar.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Molinuevo MS, Schurman L, McCarthy AD, et al. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010;25:211–21. doi: 10.1359/jbmr.090732. [DOI] [PubMed] [Google Scholar]
- 10.Kanazawa I, Yamaguchi T, Yano S, et al. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun. 2008;375:414–19. doi: 10.1016/j.bbrc.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–35. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 13.Chidiac JJ, Al-Asmar B, Rifai K, et al. Inflammatory mediators released following application of irritants on the rat injured incisors. The effect of treatment with anti-inflammatory drugs. Cytokine. 2009;46:194–200. doi: 10.1016/j.cyto.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Paula-Silva FW, Ghosh A, Silva LA, et al. TNF-alpha promotes an odontoblastic phenotype in dental pulp cells. J Dent Res. 2009;88:339–44. doi: 10.1177/0022034509334070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang YC, Hwang IN, Oh WM, et al. Influence of TGF-beta1 on the expression of BSP, DSP, TGF-beta1 receptor I and Smad proteins during reparative dentinogenesis. J Mol Histol. 2008;39:153–60. doi: 10.1007/s10735-007-9148-8. [DOI] [PubMed] [Google Scholar]
- 16.Papagerakis P, Berdal A, Mesbah M, et al. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002;30:377–85. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim JY, Kim DS, Auh QS, et al. Role of Protein Phosphatase 1 in angiogenesis and odontoblastic differentiation of human dental pulp cells. J Endod. 2017;43:417–24. doi: 10.1016/j.joen.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502–10. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Saito T. Nephronectin stimulates the differentiation of MDPC-23 cells into an odontoblast-like phenotype. J Endod. 2017;43:263–71. doi: 10.1016/j.joen.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Patel H, Younis RH, Ord RA, et al. Differential expression of organic cation transporter OCT-3 in oral premalignant and malignant lesions: potential implications in the antineoplastic effects of metformin. J Oral Phathol Med. 2013;42:250–6. doi: 10.1111/j.1600-0714.2012.01196.x. [DOI] [PubMed] [Google Scholar]
- 21.Vitale-Cross L, Molinolo AA, Martin D, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5:562–73. doi: 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P, Liu X, Zhao L, et al. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015;18:236–48. doi: 10.1016/j.actbio.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Qin W, Xing Q, et al. TRIM33 is essential for osteoblast proliferation and differentiation via BMP pathway. J Cell Physiol. 2017;232:3158–69. doi: 10.1002/jcp.25769. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Yi W, Wang E, Wang M. Effects of light-emitting diode irradiation on the osteogenesis of human umbilical cord mesenchymal stem cells in vitro. Sci Rep. 2016;6:37370. doi: 10.1038/srep37370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vucicevic L, Misirkic M, Janjetovic K, et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- 26.Lymperi S, Ligoudistianou C, Taraslia V, et al. Dental Stem Cells and their Applications in Dental Tissue Engineering. Open Dent J. 2013;7:76–81. doi: 10.2174/1874210601307010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caton J, Bostanci N, Remboutsika E, Mitsiadis TA, et al. Future dentistry: cell therapy meets tooth and periodontal repair and regeneration. J Cell Mol Med. 2011;15:1054–65. doi: 10.1111/j.1582-4934.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20:715–22. doi: 10.1016/j.tcb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abou NE, Chrzanowski W, Salih VM, et al. Tissue engineering in dentistry. J Dent. 2014;42:915–28. doi: 10.1016/j.jdent.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Griss T, Vincent EE, Egnatchik R, et al. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 2015;13:e1002309. doi: 10.1371/journal.pbio.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blas-Garcia A, Apostolova N, Ballesteros D, et al. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology. 2010;52:115–25. doi: 10.1002/hep.23647. [DOI] [PubMed] [Google Scholar]
- 33.Min KS, Lee YM, Hong SO, et al. Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod. 2010;36:447–52. doi: 10.1016/j.joen.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Jin B, Choung PH. Recombinant human plasminogen activator inhibitor-1 accelerates odontoblastic differentiation of human stem cells from apical papilla. Tissue Eng Part A. 2016;22:721–32. doi: 10.1089/ten.tea.2015.0273. [DOI] [PubMed] [Google Scholar]
- 35.Posa F, Di Benedetto A, Colaianni G, et al. Vitamin D effects on osteoblastic differentiation of mesenchymal stem cells from dental tissues. Stem Cells Int. 2016;2016:9150819. doi: 10.1155/2016/9150819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba O, Qin C, Brunn JC, et al. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112:163–70. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim JK, Shukla R, Casagrande L, et al. Differentiating dental pulp cells via RGD-dendrimer conjugates. J Dent Res. 2010;89:1433–8. doi: 10.1177/0022034510384870. [DOI] [PubMed] [Google Scholar]
- 38.Kim IS, Song YM, Hwang SJ. Osteogenic responses of human mesenchymal stromal cells to static stretch. J Dent Res. 2010;89:1129–34. doi: 10.1177/0022034510375283. [DOI] [PubMed] [Google Scholar]
- 39.Balic A, Mina M. Identification of secretory odontoblasts using DMP1-GFP transgenic mice. Bone. 2011;48:927–37. doi: 10.1016/j.bone.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher LW. DMP1 and DSPP: evidence for duplication and convergent evolution of two SIBLING proteins. Cells Tissues Organs. 2011;194:113–8. doi: 10.1159/000324254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egbuniwe O, Idowu BD, Funes JM, et al. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle. 2011;10:3912–9. doi: 10.4161/cc.10.22.18093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goto N, Fujimoto K, Fujii S, et al. Role of MSX1 in osteogenic differentiation of human dental pulp stem cells. Stem Cells Int. 2016;2016:8035759. doi: 10.1155/2016/8035759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou H, Wang G, Song F, et al. Investigation of human dental pulp cells on a potential injectable poly(lactic-co-glycolic acid) microsphere scaffold. J Endod. 2017;43:745–50. doi: 10.1016/j.joen.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]


