Abstract
Patients with dry mouth often have an acidic oral environment lacking saliva to provide calcium (Ca) and phosphate (P) ions. There has been no report on tooth remineralization in acidic pH4 and CaP ion-lacking solutions. The objective of this study was to develop a novel method of combining poly(amido amine) (PAMAM) with adhesive containing nanoparticles of amorphous calcium phosphate (NACP) for dentin remineralization in pH4 and CaP-lacking solution for the first time. Demineralized dentin was tested in four groups: (1) dentin control, (2) dentin with PAMAM, (3) dentin with NACP adhesive, (4) dentin with PAMAM+NACP adhesive. Dentin samples were examined by scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS) and hardness testing. Increasing the NACP filler level in adhesive from 0 to 40 wt% did not negatively affect the dentin bond strength (p>0.1). NACP adhesive released CaP ions and neutralized the acid. PAMAM alone failed to achieve dentin remineralization in lactic acid. NACP alone induced slight dentin remineralization in lactic acid (p>0.1). In contrast, the novel PAMAM+NACP group in the pH4 and CaP-lacking solution completely remineralized the pre-demineralized dentin, increasing its hardness which approached that of healthy dentin (p>0.1). In conclusion, dentin remineralization via PAMAM+NACP adhesive in pH4 and CaP-lacking acid was achieved for the first time, when conventional remineralization methods such as PAMAM or NACP did not work. The novel PAMAM+NACP method is promising to increase the longevity of the composite-tooth bond, inhibit caries, remineralize lesions and protect tooth structures, even for patients with dry mouth and an acidic oral environment.
Keywords: Remineralization, poly (amido amine), calcium phosphate nanoparticles, adhesive, lactic acid, dentin hardness
INTRODUCTION
Resin composites are the main material for tooth cavity restorations due to their esthetics, direct-filling capability and enhanced performance.1 Composites are bonded to tooth structure through adhesives.2–5 The bonded interface is considered the weak link in the restoration,4 and secondary (recurrent) caries at the margins is a main reason for restoration failure.6 Therefore, there is a need for the development of bond protection and caries-inhibition strategies.
Dentin bonding involves the infiltration of adhesive monomers into the demineralized collagen fibrils to form a hybrid layer (HL).2–4 In the oral environment, the collagen scaffold may be damaged by oral bacteria, enzymes and fluids, which could result in the degradation of HL.7,8 Since the bond longevity is limited by the stability of HL,7 protecting HL becomes a key approach to improving the stability of restoration-dentin bonds. Remineralization of denuded HL is an effective strategy for strengthening the resin-dentin bond, because minerals can protect external risk factors from attacking HL. On the other hand, remineralization is also an effective repair process for caries lesions.9 Cariogenic bacteria produce acids, leading to tooth decay and secondary caries.10 The net loss or gain in mineral over time ultimately determines whether the tooth decay will advance, stabilize, or regress. Therefore, developing a novel approach for remineralization-promotion and demineralization-prevention would help prolong the longevity of the composite-dentin bond and inhibit secondary caries.
Among dental patients, individuals with a higher caries risk often have more secondary caries, reducing the restoration longevity and increasing the failure rate.11 Saliva reduction and dry mouth contribute to caries.12–14 This is because saliva plays an important role in the inhibition of caries.12–15 In healthy people, the saliva fluid covers the teeth at all times. The constant flow of saliva helps remove food debris and bacteria by swallowing.12–15 Saliva has a strong buffer capacity due to its components of phosphates, bicarbonates, and urea.12–15 With frequent flows, saliva can penetrate into bacteria biofilms and neutralize the acids produced by cariogenic bacteria.12–15 In addition, saliva contains high concentrations of calcium (Ca) and phosphate (P) ions, which help decrease the solubility of hydroxyapatite. The large amounts of Ca and P ions in saliva also favor and promote tooth remineralization.12–15
However, many people suffer from reduced salivary gland functions. About 30% of the population reports dry mouth.13 Many factors can cause hyposalivation or xerostomia, such as nutrition deficiency, excessive intake of alcohol, certain medicine, salivary dysfunction, certain systemic diseases, Sjögren’s syndrome, etc.16 Saliva flow can drop to only 15%-33% of its normal level due to hyposalivation.15,16 This means minimal saliva penetration into biofilm to neutralize acids and supply Ca and P ions.15 Low salivary flow occurs often in seniors.12 Indeed, root caries increases with aging, from 7% among young people, to 56% in seniors ≥ 75 years of age.17 In addition, patients with head and neck cancers taking radiation therapy can have extreme saliva reduction,18–21 causing rampant radiation caries.
Unfortunately, to date, there is no effective method for people with dry mouth to inhibit caries. While saliva substitutes are used to relieve the sensation of dry mouth, they offer little to protect the teeth.22 Various remineralization agents are used for remineralization.23–32 They are somewhat effective for healthy individuals, but are ineffective for patients with severe saliva reduction.12,13,15 For example, casein phosphopeptide amorphous calcium phosphate (CPP-ACP) did not reduce radiation caries.33 Therefore, a new remineralization approach needs to be developed that is effective even in an acidic environment where Ca and P ions are deficient, in order to induce remineralization for individuals with reduced saliva.
Poly (amido amine) (PAMAM) dendrimers possess mono-dispersed molecular weights, a highly-branched topological architecture and abundant reactive functional groups, making them desirable nucleation templates for inducing tooth remineralization.34–41 For example, amine-terminated PAMAM (PAMAM-NH2) successfully induced newly-generated minerals precipitated in demineralized dentin and collagen fibrils.37,39 Polyhydroxy-terminated PAMAM (PAMAM-OH) induced deep dentinal tubule occlusion.35 Carboxylic-terminated PAMAM (PAMAM-COOH) also achieved intrafibrillar remineralization by absorbing Ca and P ions within the collagen matrix.34 In addition, phosphate-terminated PAMAM (PAMAM-PO3H2) remineralized the demineralized dentin in vivo in rats.40
Another way for remineralization is using calcium phosphate (CaP)-releasing resins.42 Nanoparticles of amorphous calcium phosphate (NACP) were mixed into adhesives.43–47 Due to the small particle sizes, NACP readily flowed with adhesive into dentinal tubules to form resin tags.43–46 NACP adhesive released high levels of Ca and P ions,45 and rapidly neutralized acids.48 Indeed, NACP induced dentin remineralization in vitro.48 It would be interesting to combine PAMAM with an NACP adhesive to achieve even more effective remineralization. PAMAM can attract Ca and P ions to form minerals.34–40 However, its remineralization capability relies on the supply of Ca and P ions which may be deficient in cases with dry mouth. Our pilot study used a cyclic artificial saliva/lactic acid regimen, in which remineralization relied on the artificial saliva with a neutral pH containing Ca and P ions.48 To date, there has been no report on combining PAMAM with an NACP adhesive for remineralization by immersing in an acidic solution that contained no Ca and P ions.
Therefore, the objectives of this study were to: (1) develop a novel remineralization method that is effective even in an acidic solution with no initial Ca and P ions, and (2) investigate the effects of combining PAMAM with NACP adhesive on dentin remineralization, acid neutralization, and dentin hardness. It was hypothesized that: (1) PAMAM alone could not remineralize in lactic acid solution; (2) NACP adhesive could release Ca and P ions, neutralize the acid, raise the pH, and promote dentin remineralization in lactic acid; (3) The novel PAMAM+NACP adhesive method would achieve the greatest dentin remineralization, and restore the full hardness for demineralized dentin.
MATERIAS AND METHODS
PAMAM synthesis
The synthesis of PAMAM dendrimers was described previously.49,50 It included a two-step interactive sequence to produce amine-terminated structures. First, iterative sequencing involved alkylation with methyl acrylate (MA) followed by amidation with excess 1, 2-ethylenediamine (EDA). The alkylation step produced ester-terminated intermediates called “half-generations”. The second step involved amidation of ester-terminated intermediates with a large excess of EDA to produce amine terminated intermediates that were called “full-generations”. The third generation PAMAM-NH2 (G3-PAMAM-NH2) was synthesized for further carboxyl modification. 2 g of dry G3-PAMAM-NH2 was dissolved in 25 mL dimethyl sulfoxide (DMSO) in a round-bottom container. Subsequently, 15 mL of the DMSO solution containing 5.15 g succinic anhydride (molar ratio of SAH/–NH2 = 5:1) was added into this solution. The two solutions were reacted for 24 h without oxygen. The DMSO solution was dialyzed against distilled water to remove the excess succinic anhydride and the organic solvent. The first two generations of PAMAM dendrimers are linear molecules, while the third generation is a sphere macromolecule with more functional ending groups, which supplies more nucleation locations to attract Ca and P ions. The present study used the third generation PAMAM-COOH (G3-PAMAM-COOH, Chenyuan Dendrimer Tech., Weihai, China), because previous studies showed that G3-PAMAM-COOH effectively induced minerals in demineralized human teeth.34,50 In this article, the term “PAMAM” refers to G3-PAMAM-COOH. A PAMAM solution was prepared by dissolving 100 mg of PAMAM powder in 10 mL of distilled water to achieve a concentration of 10 mg/mL.34,50 The chemical structure of PAMAM is shown in Appendix Figure 1.
NACP-containing adhesive fabrication
NACP [Ca3(PO4)2] were synthesized via a spray-drying technique.51,52 Briefly, calcium carbonate (CaCO3, Fisher, Fair Lawn, NJ) and dicalcium phosphate (CaHPO4, Baker Chemical, Phillipsburg, NJ) were dissolved into an acetic acid solution to obtain Ca and P ion concentrations of 8 and 5.333 mmol/L, respectively. The solution was sprayed into a heated chamber to evaporate the distilled water and volatile acid. The dried particles were collected by an electrostatic precipitator, yielding NACP with a mean particle size of 116 nm.51,52
The primer contained pyromellitic glycerol dimethacrylate (PMGDM) (Esstech, Essington, PA) and 2-hydroxyethyl methacrylate (HEMA) (Esstech, Essington, PA) at a mass ratio 3.3/1, with 50 wt% acetone solvent (all mass fractions).44,46 The adhesive contained PMGDM and ethoxylated bisphenol A dimethacrylate (EBPADMA) (Sigma-Aldrich, St, Louis, MO) at a 1:1 mass ratio.46 It was rendered light-curable with 1% phenylbis (2,4,6- trimethylbenzoyl) phosphine oxide (Sigma–Aldrich).46 Then 10 wt% of HEMA and 5 wt% of bisphenol A glycidyl dimethacrylate (BisGMA) (Esstech) were added. This adhesive is referred to as “PEHB”. Accordingly, the following five adhesives were made:
0 wt% NACP + 100 wt% PEHB
10 wt% NACP + 90 wt% PEHB;
20 wt% NACP + 80 wt% PEHB;
30 wt% NACP + 70 wt% PEHB;
40 wt% NACP + 60 wt% PEHB.
To test the dentin bond strength, two commercial bonding agents, Prime & Bond NT (Dentsply, Milford, DE) and Scotchbond Multi-Purpose (SBMP, 3 M, St. Paul, MN) were used as controls. NT is a total-etching one-bottle i system and contained <10% methyl methacrylate, 30 wt% typical methacrylates, and 60 wt% acetone. NT was combined with a self-cure activator (SCA) at 1:1 ratio to enable dual-cure. SBMP primer contained 10–20 wt% copolymer of acrylic and itaconic acids, 35–45 wt% HEMA, and 40–50 wt% water. SBMP adhesive contained 30–40 wt% HEMA and 60–70 wt% BisGMA.
Dentin shear bond testing
Extracted caries-free human molars were obtained from the dental school clinics following a protocol approved by the University of Maryland Institutional Review Board. The tips of the crowns were cut off using a diamond saw (Isomet, Buehler, Lake Bluff, IL). The tooth was then ground with 320 grit SiC paper until occlusal enamel was completely removed.45,46 After etching with 37 wt% phosphoric acid gel for 15 s and rinsed with water, a primer was applied and rubbed in for 15 s, and the solvent was removed with air.45 An adhesive was applied and photo-cured for 10 s (Optilux-VCL401, Demetron, Danbury, CT). A stainless-steel iris with a central opening (diameter = 4 mm, thickness = 1.5 mm) was held against the adhesive-treated dentin surface. The central opening was filled with a composite (TPH, Caulk/Dentsply, Milford, DE) and photo-cured for 1 min.46 The bonded samples were immersed in water at 37 °C for 1 day or 14 days. Then the dentin shear bond strength, SD, was measured following previous studies.45,46 Briefly, a chisel was held parallel to the composite-dentin interface and loaded via a Universal Testing Machine (MTS, Eden Prairie, MN) at 0.5 mm/min until the bond failed. SD was calculated as: SD = 4P/(πd2), where P is the load at failure, and d is the diameter of the composite.45,46 Twelve measurements were made for each group.
Preparation of dentin samples
Dentin squares of approximately 4 × 4 × 1 mm were prepared by cutting perpendicular to the long axis of the tooth 4 mm above the cemento-enamel junction. Dentin samples were demineralized with 37 wt% phosphoric acid for 15 s, following a previous study.31 The demineralized dentin samples were ultra-sonicated in distilled water for 10 min to remove any debris, and then stored at 4 °C in phosphate-buffered saline (PBS, pH 7.4).
Remineralization in acidic solution without any initial Ca and P ions
The demineralized dentin samples were tested in four groups as described below.
Control. Each demineralized dentin sample was coated with 100 μL of distilled water, and then air dried to serve as control.34,50
PAMAM. Each demineralized dentin sample was coated with 100 μL of the PAMAM solution, which was maintained on dentin surface for 1 hour to ensure that PAMAM macromolecules were immobilized on dentin. Then the sample was rinsed with water to remove any loose PAMAM molecules.34 PAMAM macromolecules could adhere to demineralized dentin by electrostatic interactions.34
NACP. PEHB adhesive containing 40 wt% NACP was selected for remineralization testing. This was because the dentin bond testing showed that adding 10–40 wt% NACP in PEHB did not negatively affect dentin bond strength, and previous studies showed higher NACP filler level had more Ca and P ion release.45 NACP adhesive paste was placed into a rectangular mold of 2 × 2 × 12 mm following previous studies,45,46 and photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each side. A demineralized dentin was placed in contact with three NACP adhesive bars,53 because when immersed in 1 mL solution, this would yield a sample/solution volume ratio of 0.14/1, the same as that in a previous study.53 Appendix Figure 2 showed how the adhesive bars were placed on top of the dentin.
PAMAM+NACP. Each demineralized dentin sample was first coated with 100 μL of PAMAM solution, and then three NACP adhesive bars were placed as in (3).
Six samples were tested for each group (n = 6). A 1.5 mL conical vial was used to store each sample which was completely immersed in 1 mL of the following solution. A 133 mmol/L sodium chloride (NaCl) solution was buffered to pH 4 using 50 mmol/L lactic acid to simulate a cariogenic condition (referred to as “lactic acid solution”).53 Each day, each specimen of the four groups was immersed in 1 mL of fresh lactic acid solution for 24 hours (h) at 37 °C. This was repeated for 14 days (d). The 24 h lactic acid solution immersion every day simulated the most challenging oral environment of patients with the most severe saliva reduction or saliva deficiency.18–21 The rationale was that if dentin remineralization could be achieved in this most extremely challenging condition, then remineralization would be more readily achieved under more favorable oral conditions.
Ca and P ion concentration measurement
At 1, 3, 5, 7, 10 and 14 d, the Ca and P ion concentrations were measured for the lactic acid solution where the samples were immersed. At each time, the 1 mL solution was completely removed and replaced by fresh lactic acid. The Ca and P ion concentrations in the collected solution was measured via a spectrophotometric method (DMS-80 UV-visible, Varian, Palo Alto, CA) using known standards and calibration curves, following a previous study.51 The ion release from NACP adhesive could be sustained for longer than 70 days in a pH 4 solution as previously measured in vitro, and the release was estimated to be able to last longer than 2 years in the oral environment according to a previous study.45
Acid neutralization
At 1, 3, 5, 7, 10, and 14 d, the pH of the lactic acid solutions of the four groups was measured. Every day, each sample was immersed in the lactic acid solution for 24 h. During the 24 h, the pH was constantly monitored with a combination pH electrode (Orion, Cambridge, MA).
Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS)
At 14 d, each dentin was cut into two halves along the midline using the diamond band saw (Buehler). One half was used to examine the occlusal section (the observed surface was perpendicular to the tubule axis). The other was used to examine the longitudinal section (the observed surface was parallel to the tubule axis). They were immersed in 1 wt% glutaraldehyde in PBS for 4 h at 4 °C, subjected to graded ethanol dehydrations, and rinsed with 100 wt% hexamethyldisilazane.53 Then they were sputter-coated with gold and examined via SEM (JEOL 5300, Peabody, MA). Meanwhile, the chemical components of the dentin samples were examined from the SEM images using EDS (INCA350, Oxford, UK).
Dentin hardness measurement
A hardness tester (Tukon 2100B, Instron, Canton, MA) was used to measure dentin hardness for the four groups with a Vickers diamond indenter at a load of 20 g and a dwell time of 10 s.36,54 In addition, hardness of sound dentin, and dentin after acid-etching but without the lactic acid solution immersion, were also measured as comparative controls. The hardness results showed that the PAMAM+NACP adhesive group increased the demineralized dentin hardness at 14 d, but the value was still lower than that of sound dentin. To observe how long it would take for PAMAM+NACP adhesive to restore the demineralized dentin hardness to the healthy dentin level, the samples were immersed in the lactic acid solution for another 14 d (for a total of 28 d). Six indentations were made in each dentin, and six dentin samples were tested for each group, yielding 36 indentations per group at each time period.
Statistical analysis
Statistical analysis was performed by SPSS 13.0. All data were checked for normal distribution with the Kolmogorov–Smirnov test. One-way and two-way analyses of variance (ANOVA) were applied to detect the significant effects of the variables. Tukey’s multiple comparison tests were used at a p value of 0.05.
RESULTS
The dentin shear bond strength results are plotted in Fig. 1 after immersion for: (A) 1 day, and (B) 14 days (mean ± sd; n = 12). Increasing the NACP filler level from 10 wt% to 40 wt% in PEHB had no significant effect (p > 0.1). Water-aging for 14 days did not affect dentin bond strength compared to that at 1 day (p > 0.1).
Figure 1.
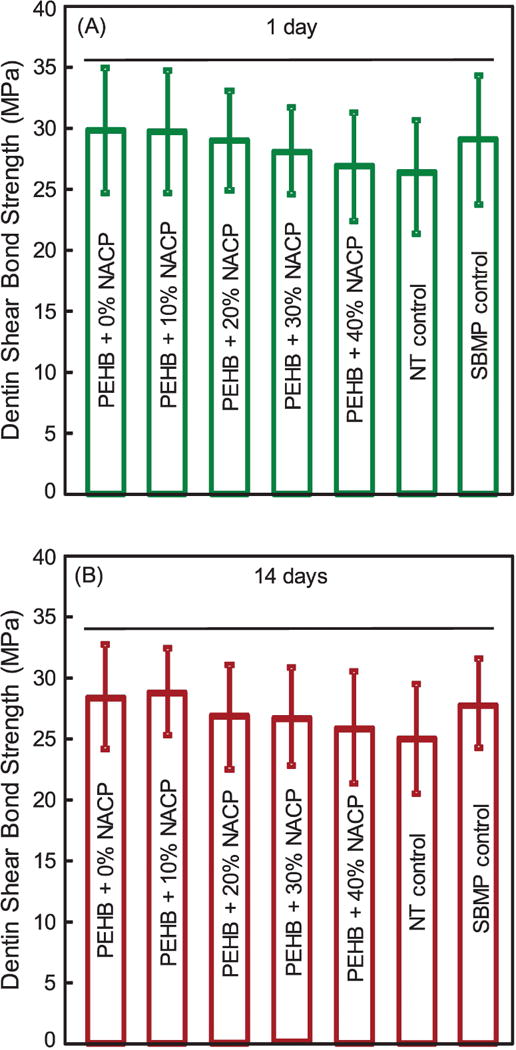
Dentin shear bond strength using extracted human molar, tested after storage in distilled water for (A) 1 day, and (B) 14 days (mean ± SD; n = 12). Horizontal line indicates values that are not significantly different from each other (p > 0.1).
The Ca and P ion concentrations in the lactic acid solution are plotted in Fig. 2 (mean ± sd; n = 6). PAMAM and control groups had Ca and P concentrations near 0. NACP and PAMAM+NACP had ion release from NACP adhesive. The ion concentrations decreased from 1 d to 14 d due to the use of a fresh solution each day. Even changing to a fresh solution for immersion daily, NACP and PAMAM+NACP at 14 d still had much higher ion concentrations than PAMAM and control, indicating continued release from NACP adhesive.
Figure 2.

Ca and P ion concentrations (mean ± SD; n = 6). (A) Ca ion concentration of lactic acid solution, (B) P ion concentration, (C) Ca and P concentrations at 1 and 14 d for NACP adhesive and PAMAM+NACP, (D) Ca and P concentrations at 1 and 14 d for control and PAMAM. NACP and PAMAM+NACP groups had much higher Ca and P concentrations than PAMAM group and control group. The Ca and P concentrations decreased over time due to the use of a fresh lactic acid solution each day.
The different groups affected the pH as shown in Fig. 3 (mean ± sd; n = 6): (A) at 15 min, (B) 60 min, (C) 3 h, and (D) 24 h, after the samples were immersed in a fresh lactic acid solution with an initial pH of 4. This measurement was done from 1 d to 14 d. For PAMAM and control groups, the pH stayed at nearly 4. In contrast, for NACP and PAMAM+NACP groups, at 1 d, the pH was increased to 6.1 at 15 min, 6.5 at 60 min, 6.9 at 3 h, and 6.9 at 24 h. The pH showed a slight decrease over time because a fresh pH 4 solution was used each day to immerse the sample. At 14 d, the pH reached 5.5 at 15 min and 6.5 at 3 h. These pH values were much higher than those without the NACP adhesive (p < 0.05).
Figure 3.

Acid neutralization. The lactic acid pH was measured after the sample was immersed for: (A) 15 minutes, (B) 60 minutes, (C) 3 hours, and (D) 24 hours (mean ± SD; n = 6). This was repeated for 14 days. (E) The time it took to raise the pH from 4 to 5.5. NACP and PAMAM+NACP neutralized the acid and increased the pH. The acid neutralization ability of NACP adhesive decreased over time due to the use of a fresh acid solution each day. PAMAM and control groups showed no acid neutralization, with pH staying near 4.
Fig. 3E plots the time it took to increase the pH to above 5.5 for the lactic acid with an initial pH of 4. For NACP and PAMAM+NACP groups, the NACP adhesive rapidly increased the pH from the cariogenic pH 4 to a safe pH of ≥ 5.5. At 1 d, it took 8 minutes to raise the pH from 4 to 5.5. At 14 d, the pH increased from 4 to 5.5 in 15 minutes. In contrast, PAMAM and control failed to raise the pH, which stayed at nearly 4.
Representative SEM images of dentin perpendicular to dentinal tubules are shown in Fig. 4. All pictures were taken after daily treatment of 24 h immersion in a fresh lactic acid with an initial pH of 4 and without any initial Ca and P ions, and after this was repeated for 14 d. For PAMAM and control, all tubules were open without any minerals (A-D). Exposed collagen fibrils were visible at a higher magnification (C, D). NACP adhesive alone achieved some remineralization, with a small number of minerals in the surface and tubules (E, G). For PAMAM+NACP (F, H), there were much more minerals regenerated in dentin.
Figure 4.

Typical SEM images of demineralized dentin perpendicular to dentinal tubule axis after 14 d lactic acid immersion: (A, C) control, (B, D) PAMAM, (E, G) NACP, and (F, H) PAMAM+NACP. Exposed collagen fibrils were visible in (A-D). Images E-H showed remineralization in demineralized dentin. For PAMAM+NACP in image H, numerous needle-like minerals precipitated in dentin.
Typical SEM images of dentin parallel to dentinal tubule axis are shown in Fig. 5. These images were taken on dentin cross-sections in a subsurface region of 2 to 30 μm beneath the external dentin surface. For PAMAM and control, there were no minerals in the tubules (A-D), and exposed collagen fibrils were visible at a higher magnification (C, D). For NACP group, a small amount of minerals were precipitated in the tubules (E, G). For PAMAM+NACP, numerous mineral crystals were regenerated in the tubules (F, H).
Figure 5.

Representative SEM images of demineralized dentin subsurface cross-sections parallel to dentinal tubule axis after 14 d lactic acid immersion: (A, C) control, (B, D) PAMAM, (E, G) NACP, and (F, H) PAMAM+NACP. Collagen fibrils were visible in tubules in (A-D). PAMAM+NACP achieved the most dentin remineralization in lactic acid solution.
The EDS maps and the atomic percentages of Ca and P elements in dentin are shown in Fig. 6. The PAMAM and control groups showed no Ca and P peaks, which indicated that the dentin surface were completely demineralized. Moderate Ca and P peaks appeared in the NACP group. The PAMAM+NACP group had the strongest Ca and P peaks (p < 0.05), which demonstrated that PAMAM+NACP achieved the greatest dentin remineralization.
Figure 6.

EDS maps of demineralized dentin after treatments with: (A) control group, (B) PAMAM group, (C) NACP group, (D) PAMAM+NACP group. (E) Atomic percentages of Ca and P elements of dentin surfaces of the four groups (mean ± SD; n = 6). Dissimilar letters indicate significantly different values (p < 0.05).
Dentin hardness values are plotted in Fig. 7 (mean ± sd; n = 6). The hardness of sound healthy dentin was 0.55 GPa. After acid-etching, the hardness decreased to 0.31 GPa. After immersed in lactic acid for various time periods, for PAMAM and control groups, the dentin hardness decreased to 0.15 GPa at 14 d, and 0.05 GPa at 28 d. NACP group had higher dentin hardness than PAMAM and control (p < 0.05). PAMAM+NACP increased dentin hardness to 0.47 GPa at 14 d and 0.56 GPa at 28 d, which approached that of sound dentin (p > 0.1).
Figure 7.

Dentin hardness (mean ± SD; n = 6). The demineralized dentin hardness was measured after 14 and 28 d of lactic acid immersion for: Control group, PAMAM, NACP, and PAMAM+NACP. The hardness of sound healthy dentin and acid-etched dentin was also measured. Dissimilar letters indicate significantly different values (p < 0.05).
DISCUSSION
To date, no report has achieved successful tooth remineralization by placing the sample in an acidic solution at pH 4 without any initial Ca and P ions. Previous studies on tooth remineralization used saliva-like solutions with Ca and P ions and neutral pH.23–32 For the first time, the present study investigated PAMAM, NACP adhesive, and PAMAM+NACP for remineralization in a pH 4 solution with no initial Ca and P ions, and demonstrated that the novel PAMAM+NACP adhesive strategy induced complete dentin remineralization. The hypotheses were proven that the conventional remineralization agent (PAMAM) failed to remineralize the demineralized dentin in lactic acid; NACP adhesive released Ca and P ions and neutralized the acid, but only achieved a low level of remineralization; the novel PAMAM+NACP method yielded the greatest dentin remineralization. It completely returned the pre-demineralized dentin hardness to that of sound healthy dentin.
The composite-dentin bonded interface is the weak link in the restoration.4 HL plays an important role in dentin bonding.2–4 However, microcaps could form at the composite-dentin interfaces, allowing for the invasion of bacteria and enzyme.55,56 Bacterial acids could lead to secondary caries at the margins,55,56 and endogenous matrix metalloproteinases (MMPs) could cause hydrolysis and degradation of the collagen matrix in the HL, resulting in bond failure.57 A promising approach for the longevity of composite-dentin bonding and for inhibiting caries is to promote remineralization and prevent demineralization. The regenerated minerals may help repair nanometer-sized voids such as nanoleakages in the HL. The minerals could also preclude MMPs from penetrating and deteriorating the collagen fibrils.58 In addition, the remineralized HL is more resistant to degradation in the oral environment and able to resist and neutralize biofilm acids.7,8
Demineralized dentin consists of collagen fibrils and small quantities of remnant apatite crystallites.31 Although some believe that the pure collagen matrix cannot initialize crystal nucleation and growth during mineralization,23 more evidence supports that collagen fibril alone can induce apatite nucleation and mineral deposit through their carboxyl and carbonyl groups.27,59 However, the nucleation rate via collagen fibrils is extremely slow without the help of other remineralization materials.24 Although apatite in demineralized dentin could serve as seeds, their small quantities limit the deposit rate.60 Hence, the natural dentin remineralization is too weak to resist acids from cariogenic bacteria. Therefore, two strategies were employed to promote dentin remineralization: Applying nucleation templates to the demineralized dentin, and creating high concentrations of Ca and P ions.23,24,31,51
Previous tooth remineralization studies were mainly carried out in saliva-like solutions, which had Ca and P ions and a neutral pH.23–32 The saliva-like solutions simulate the mineralization function of human saliva.23,30–32 Several saliva-like solutions were used. For example, a simulated body fluid (SBF), with a pH of 6.8 and 2.5 mmol/L of Ca and 1 mmol/L of P ions, was used for enamel remineralization.32 A calcification solution with a pH of 7.6 and 2.58 mmol/L of Ca (CaCl2·2H2O) and 1.55 mmol/L of P ions (KH2PO4) was used to remineralize the demineralized dentin.23 Another remineralization solution had a pH of 7.3 and contained Portland cement (Ca resource), 8.3 mmol/L Na2HPO4 and 1.25 mmol/L KH2PO4.31 In addition, artificial saliva with a pH of 7, 1.5 mmol/L of Ca and 0.9 mmol/L of P ions was widely used for the remineralization of dental hard tissues.30,34,37
However, oral cavity often suffers from acid challenges by biofilms resulting in a low pH locally.9 Hence, studies used cyclic remineralization and demineralization regimens.52,61 In a previous study, PAMAM and NACP adhesive induced remineralization of demineralized dentin in a cyclic artificial saliva/lactic acid regimen.48 PAMAM macromolecules could adhere to dentin through electrostatic interactions, which is important for remineralization in a fluid-flowing oral environment.37 PAMAM can grab cationic Ca ions through electrostatic interactions via its abundant anionic carboxylic groups.62 On the other hand, NACP adhesive can neutralize the acids and release Ca and P ions.48 Therefore, the combination of PAMAM with NACP adhesive achieved the double benefits of remineralization-promotion and demineralization-prevention. In the previous study,48 the remineralization relied on the artificial saliva which had a neutral pH and large amounts of Ca and P ions.30
Unfortunately, while cyclic artificial saliva/lactic acid regimens simulate the common oral condition, it does not represent oral environments of dry mouth and with low pH locally. The present study showed that, indeed, the traditional remineralization agent PAMAM produced no dentin remineralization in the lactic acid solution without Ca and P ions. Even the NACP adhesive induced only a low level of remineralization. Strikingly, the PAMAM+NACP adhesive approach effectively remineralized the demineralized dentin in a lactic acid solution of pH 4 without any initial Ca and P ions. This was the first time where dentin remineralization was achieved in an acid solution without initial Ca and P ions, without the support of a saliva-like solution. This provides a new and much-needed remineralization method for patients having severe saliva reduction with extremely challenging oral environments where the pH is constantly acidic, lacking saliva to provide Ca and P ions. Of course, this method is expected to be even more highly effective in less-challenging oral environments with saliva to provide additional Ca and P ions.
In the present study, a pH 4 lactic acid was used to immerse the dentin specimens to simulate an extremely challenging oral environment. Three points should be noted. First, lactic acid accounts for the majority of acids produced by cariogenic bacteria but not all the acids; other biofilm acids include acetic, propionic and formic acids, etc.63 Second, demineralization solutions of pH 4 were widely used in previous studies as an accelerated laboratory testing model.61 However, plaque pH can indeed reach 4.5 or even 4.64 For example, a previous study showed that the pH of oral biofilms reached 4.65 Another study showed that the S. mutans plaque pH reached 3.7 ± 0.3.66 Third, the use of a lactic acid solution with a pH of 4 and without Ca and P ions, without using a cyclic remineralization/demineralization regimen, simulates the most extreme saliva reduction situation: a complete saliva deficiency condition providing no Ca and P ions in a long-term acidic oral environment. This was observed in patients taking large doses of head and neck radiation therapies.18–21 In the present study, after immersing in lactic acid for 14 d, as expected, control dentin showed more demineralization. The remnant apatite crystals in demineralized dentin were continually dissolved by acid, leading to the exposure of collagen fibrils and a further dentin hardness reduction. The dentin of PAMAM group also had further demineralization, with hardness loss similar to control group. The NACP group showed a low level of remineralization. When immersed in lactic acid with pH 4, NACP adhesive rapidly raised the pH to a safe pH of above 6, and released Ca and P ions which could turn the lactic acid into a saliva-like solution. It has been reported that there is a critical pH, above which remineralization dominates, and below which demineralization occurs.67 The critical pH was around 5.5 for human tooth, and the critical pH value is inversely proportional to the Ca and P ion concentrations in the solution.67 Previous study reported that remineralization of dentin occurred in a solution with Ca and P ion concentrations of 2.2 mmol/L at pH 6.68 Hence, the NACP adhesive could turn the lactic acid into a saliva-like solution. This effect of NACP adhesive could be durable and long-term, as a recent study showed that the PEHB-NACP adhesive can be repeatedly recharged to have sustained Ca and P ion release.46 However, the remineralization induced by using NACP adhesive alone was relatively weak in the lactic acid solution due to the weak nucleation ability of demineralized dentin.
In contrast, the PAMAM+NACP adhesive group induced the most remineralization, and showed the greatest dentin hardness, due to a synergy between PAMAM and NACP. When immersed in lactic acid, after NACP adhesive released Ca and P ions and increased the pH, PAMAM then had its chance to fulfill its nucleation function. PAMAM macromolecules immobilized on demineralized dentin rapidly absorbed Ca and P ions to form minerals in the demineralized dentin, yielding much greater remineralization than NACP adhesive alone. The PAMAM+NACP synergy achieved triple benefits: (1) Excellent nucleation templates; (2) a large Ca and P ion source; and (3) acid-neutralization to raise the pH. In the present study, SEM was used to examine the remineralization, and only the regenerated interfibrillar minerals were observed. Since the gap zone between collagen fibrils is only 67 nm, the minerals in the collagen fibril gap zone was too small to be observed by SEM. Further study should employ TEM to examine the extent, if any, of intrafibrillar mineralization.31
Regarding clinical applications, improving the longevity of the composite-dentin bond, inhibiting caries and protecting tooth structures for patients with reduced saliva, especially with severe saliva reduction and radiation caries, represent a great need in dentistry. The novel PAMAM+NACP approach is a promising, and based on a literature search, the only method to meet this need. After tooth cavity preparation and before bonding, a PAMAM solution could be first coated on the acid-etched dentin, and then NACP adhesive could be applied to bond the composite. When serve in vivo, the regenerated minerals could protect the HL from the invasion of biofilm acids and MMPs. This strategy could also be applicable to senior patients with gingival recession, reduced saliva flow, and root caries, to neutralize biofilm acids and remineralize the roots to protect the tooth structures. This method could also be used for parts of the population with a higher risk for caries, such as people in inner cities and developing countries where oral hygiene may be poor and oral plaque acids may be more prevalent. Furthermore, this method is also promising to be useful for the general population with composite restorations to reduce secondary caries at the margins, which is a primary reason for restoration failures. Further studies are needed to improve the longevity of the composite-dentin bond, to strengthen tooth structures via this method, to remineralize under clinically-relevant conditions, and to prevent caries for patients with saliva reduction.
CONCLUSIONS
This study investigated PAMAM, NACP adhesive, and PAMAM+NACP adhesive strategies for dentin remineralization in lactic acid without initial Ca and P ions for the first time. The novel PAMAM+NACP achieved the greatest dentin remineralization in this extremely challenging environment. This could prolong the composite-dentin bond and inhibit caries for patients with dry mouth where the local pH is often acidic, lacking saliva with Ca and P ions. PAMAM failed to induce remineralization in lactic acid. NACP adhesive released Ca and P ions, raised the pH 6.5, and achieved slight remineralization in this challenging environment. PAMAM+NACP achieved synergy where NACP provided the needed Ca and P ions and raised the pH, enabling PAMAM to fulfill its nucleation template function. This yielded the greatest remineralization and returned the hardness of demineralized dentin to that of healthy dentin. The method of PAMAM+NACP is promising for patients with reduced saliva and radiation caries, as well as for a wide range of dental restorations, to prolong the bonded interface, inhibit secondary caries, and remineralize and protect the tooth structures.
Supplementary Material
Appendix Figure 1. Schematic of the chemical structure of PAMAM. The blue arrow shows the central core. The purple arrow shows the branch structure. the red arrow shows the ending function groups.
Appendix Figure 2. Illustration on how to place three bars of NACP adhesive on the top of a dentin sample.
Acknowledgments
This work was supported by NIH R01 DE17974 (HX), National Natural Science Foundation of China 81670977 and 81170958 (JYL), International Science and Technology Cooperation Program of China 2014DFE30180, China Scholarship Council (KL), University of Maryland School of Dentistry bridging fund (HX) and University of Maryland seed grant (HX).
References
- 1.Satterthwaite JD, Maisuria A, Vogel K, Watts DC. Effect of resin-composite filler particle size and shape on shrinkage-stress. Dent Mater. 2012;28:609–14. doi: 10.1016/j.dental.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt K. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F. Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu RR, Fang M, Zhang L, Tang CF, Dou Q, Chen JH. Anti-proteolytic capacity and bonding durability of proanthocyanidin-biomodified demineralized dentin matrix. Int J Oral Sci. 2014;6:168–74. doi: 10.1038/ijos.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. doi: 10.1016/j.dental.2013.08.201. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osorio R, Osorio E, Medina-Castillo A, Toledano M. Polymer nanocarriers for dentin adhesion. J Dent Res. 2014;90:1258–63. doi: 10.1177/0022034514551608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Featherstone JD. Remineralization, the natural caries repair process–the need for new approaches. Adv Dent Res. 2009;21:4–7. doi: 10.1177/0895937409335590. [DOI] [PubMed] [Google Scholar]
- 10.Ten Cate J, Featherstone J. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2:283–96. doi: 10.1177/10454411910020030101. [DOI] [PubMed] [Google Scholar]
- 11.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida PDV, Gregio A, Machado M, De Lima A, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9:72–80. [PubMed] [Google Scholar]
- 13.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthetic Dent. 2001;85:162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 14.Lenander-Lumikari M, Loimaranta V. Saliva and dental caries. Adv Dent Res. 2000;14:40–7. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
- 15.Bardow A, Nyvad B, Nauntofte B. Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition, and the rate of tooth demineralization in situ. Arch Oral Biol. 2001;46:413–23. doi: 10.1016/s0003-9969(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 16.Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134:61–9. doi: 10.14219/jada.archive.2003.0018. [DOI] [PubMed] [Google Scholar]
- 17.Curzon M, Preston A. Risk groups: nursing bottle caries/caries in the elderly. Caries Res. 2003;38:24–33. doi: 10.1159/000074359. [DOI] [PubMed] [Google Scholar]
- 18.Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001;61:271–4. doi: 10.1016/s0167-8140(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 19.Dirix P, Nuyts S, Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer. Cancer. 2006;107:2525–34. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 20.Silva AR, Alves FA, Berger SB, Giannini M, Goes MF, Lopes MA. Radiation-related caries and early restoration failure in head and neck cancer patients. A polarized light microscopy and scanning electron microscopy study. Support Care Cancer. 2010;18:83–7. doi: 10.1007/s00520-009-0633-3. [DOI] [PubMed] [Google Scholar]
- 21.Liang X, Zhang J, Peng G, Li J, Bai S. Radiation caries in nasopharyngeal carcinoma patients after intensity-modulated radiation therapy: A cross-sectional study. J Dent Sci. 2016;11:1–7. doi: 10.1016/j.jds.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahnel S, Behr M, Handel G, Burgers R. Saliva substitutes for the treatment of radiation-induced xerostomia–a review. Support Care Cancer. 2009;17:1331–43. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YZ, Cao Y, Liu W, Chu CH, Li QL. Polydopamine-induced tooth remineralization. ACS Appl Mater Int. 2012;4:6901–10. doi: 10.1021/am302041b. [DOI] [PubMed] [Google Scholar]
- 24.Liang K, Xiao S, Shi W, Li J, Yang X, Gao Y, Hao L, He L, Cheng L, Xu X, Zhou X, Li J. 8DSS-promoted remineralization of demineralized dentin in vitro. J Mater Chem B. 2015;3:6763–72. doi: 10.1039/c5tb00764j. [DOI] [PubMed] [Google Scholar]
- 25.Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent. 2007;35:695–8. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Guo B, Que KH, Jing Y, Wang B, Liang QQ, Xie HH. Effect of Galla chinensis on the remineralization of two bovine root lesions morphous in vitro. Int J Oral Sci. 2012;4:152–6. doi: 10.1038/ijos.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Wang XM, Tian LL, Cheng ZJ, Cui FZ. In situ remineralizaiton of partially demineralized human dentine mediated by a biomimetic non-collagen peptide. Soft Matter. 2011;7:9673. [Google Scholar]
- 28.Gu LS, Kim YK, Liu Y, Takahashi K, Arun S, Wimmer CE, Osorio R, Ling JQ, Looney SW, Pashley DH, Tay FR. Immobilization of a phosphonated analog of matrix phosphoproteins within cross-linked collagen as a templating mechanism for biomimetic mineralization. Acta Biomater. 2011;7:268–77. doi: 10.1016/j.actbio.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Jiang T, Sauro S, Wang Y, Thompson I, Watson TF, Sa Y, Xing W, Shen Y, Haapasalo M. Dentine remineralization induced by two bioactive glasses developed for air abrasion purposes. J Dent. 2011;39:746–56. doi: 10.1016/j.jdent.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Ten Cate J, Duijsters P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201–10. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 31.Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–37. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Hsu CC, Chung HY, Yang JM, Shi W, Wu B. Influence of 8DSS peptide on nano-mechanical behavior of human enamel. J Dent Res. 2011;90:88–92. doi: 10.1177/0022034510381904. [DOI] [PubMed] [Google Scholar]
- 33.Sim CP, Wee J, Xu Y, Cheung YB, Soong YL, Manton DJ. Anti-caries effect of CPP-ACP in irradiated nasopharyngeal carcinoma patients. Clin Oral Invest. 2015;19:1005–11. doi: 10.1007/s00784-014-1318-y. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Yang J, Li J, Chen L, Liang K, Wu W, Chen X, Li J. Bioinspired intrafibrillar mineralization of human dentine by PAMAM dendrimer. Biomaterials. 2013;34:6738–47. doi: 10.1016/j.biomaterials.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Liang K, Gao Y, Li J, Liao Y, Xiao S, Lv H, He L, Cheng L, Zhou X, Li J. Effective dentinal tubule occlusion induced by polyhydroxy-terminated PAMAM dendrimer in vitro. RSC Adv. 2014;4:43496–503. [Google Scholar]
- 36.Jia R, Lu Y, Yang CW, Luo X, Han Y. Effect of generation 4.0 polyamidoamine dendrimer on the mineralization of demineralized dentinal tubules in vitro. Arch Oral Biol. 2014;59:1085–93. doi: 10.1016/j.archoralbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Liang K, Yuan H, Li J, Yang J, Zhou X, He L, Cheng L, Gao Y, Xu X, Zhou X, Li J. Remineralization of Demineralized Dentin Induced by Amine-Terminated PAMAM Dendrimer. Macromol Mater Eng. 2015;300:107–17. [Google Scholar]
- 38.Wang T, Yang S, Wang L, Feng H. Use of multifunctional phosphorylated PAMAM dendrimers for dentin biomimetic remineralization and dentinal tubule occlusion. RSC Adv. 2015;5:11136–44. [Google Scholar]
- 39.Liang K, Gao Y, Li J, Liao Y, Xiao S, Zhou X, Li J. Biomimetic mineralization of collagen fibrils induced by amine-terminated PAMAM dendrimers - PAMAM dendrimers for remineralization. J Biomater Sci Polym Ed. 2015;26:963–74. doi: 10.1080/09205063.2015.1068606. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Yang J, Liang K, Li J, He L, Yang X, Peng S, Chen X, Ding C, Li J. Effective dentin restorative material based on phosphate-terminated dendrimer as artificial protein. Colloids Surf B. 2015;128:304–14. doi: 10.1016/j.colsurfb.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 41.Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Delivery Rev. 2012;64:102–15. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K, Cheng L, Weir MD, Bai Y-X, Xu HH. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int J Oral Sci. 2016;8:45–53. doi: 10.1038/ijos.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dental Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melo MA, Cheng L, Weir MD, Hsia RC, Rodrigues LK, Xu HH. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J Biomed Mater Res B Appl Biomater. 2013;101:620–9. doi: 10.1002/jbm.b.32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C, Weir MD, Cheng L, Lin NJ, Lin-Gibson S, Chow LC, et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dental Mater. 2014;30:891–901. doi: 10.1016/j.dental.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Weir MD, Hack G, Fouad AF, Xu HH. Rechargeable dental adhesive with calcium phosphate nanoparticles for long-term ion release. J Dent. 2015;43:1587–95. doi: 10.1016/j.jdent.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang SP, Ge Y, Zhou XD, Xu HHK, Weir MD, Zhang KK, Wang Hh, Hannig M, Rupf S, Li Q, Cheng Lei. Effect of anti-biofilm glass–ionomer cement on Streptococcus mutans biofilms. Int J Oral Sci. 2016;8:76–83. doi: 10.1038/ijos.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang K, Weir MD, Reynolds MA, Zhou X, Li J, Xu HHK. Poly (amido amine) and nano-calcium phosphate bonding agent to remineralize tooth dentin in cyclic artificial saliva/lactic acid. Mater Sci Eng C. 2017;72:7–17. doi: 10.1016/j.msec.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–32. [Google Scholar]
- 50.Chen L, Liang K, Li J, Wu D, Zhou X, Li J. Regeneration of biomimetic hydroxyapatite on etched human enamel by anionic PAMAM template in vitro. Arch Oral Biol. 2013;58:975–80. doi: 10.1016/j.archoralbio.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental Mater. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res B Appl Biomater. 2011;98:80–8. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YR, Du W, Zhou XD, Yu HY. Review of research on the mechanical properties of the human tooth. Int J Oral Sci. 2014;6:61–9. doi: 10.1038/ijos.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Filho LR, Accorinte M, van Dijken J. Influence of adhesive systems on interfacial dentin gap formation in vitro. Oper Dent. 2006;31:431–41. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 56.Duarte S, Jr, Lolato AL, de Freitas CRB, Dinelli W. SEM analysis of internal adaptation of adhesive restorations after contamination with saliva. J Adhes Dent. 2005;7:51–56. [PubMed] [Google Scholar]
- 57.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR. Strategies to prevent hydrolytic degradation of the hybrid layer—a review. Dent Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, Zhang K, Pashley DH, Tay FR. Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices–implications in the aging of resin-dentin bonds. Acta Biomater. 2010;6:3729–39. doi: 10.1016/j.actbio.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W, Liao S, Cui F. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem Mater. 2003;15:3221–6. [Google Scholar]
- 60.Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, Pashley DH, Tay FR. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater. 2010;6:2740–50. doi: 10.1016/j.actbio.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langhorst S, O’Donnell J, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dental Mater. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lungu A, Rusen E, Butac L, Stancu I. Epoxy-mediated immobilization of PAMAM dendrimers on methacrylic hydrogels. Dig J Nanomater Biostruct. 2009;4:97–107. [Google Scholar]
- 63.Featherstone J. The continuum of dental caries—evidence for a dynamic disease process. J Dent Res. 2004;83:C39–C42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 64.Deng Dt, Ten Cate J. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res. 2004;38:54–61. doi: 10.1159/000073921. [DOI] [PubMed] [Google Scholar]
- 65.Wijeyeweera R, Kleinberg I. Acid-base pH curves in vitro with mixtures of pure cultures of human oral microorganisms. Arch Oral Biol. 1989;34:55–64. doi: 10.1016/0003-9969(89)90046-0. [DOI] [PubMed] [Google Scholar]
- 66.Kashket S, Yaskell T, Lopez L. Prevention of sucrose-induced demineralization of tooth enamel by chewing sorbitol gum. J Dent Res. 1989;68:460–2. doi: 10.1177/00220345890680030401. [DOI] [PubMed] [Google Scholar]
- 67.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–5. [PubMed] [Google Scholar]
- 68.Ten Cate J, Damen J, Buijs M. Inhibition of dentin demineralization by fluoride in vitro. Caries Res. 1998;32:141–7. doi: 10.1159/000016444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Schematic of the chemical structure of PAMAM. The blue arrow shows the central core. The purple arrow shows the branch structure. the red arrow shows the ending function groups.
Appendix Figure 2. Illustration on how to place three bars of NACP adhesive on the top of a dentin sample.


