Abstract
Objective
Detail the characteristic traits of children with fetal alcohol spectrum disorders (FASD) and maternal risk factors in a Southeastern U.S. County.
Methods
Independent samples were drawn from two different cohorts of first grade students. All consented children (49.8%) were measured for height, weight, and head circumference, and those ≤ 25th centile entered the study along with a random sample drawn from all enrolled students. Study children were examined for physical growth, dysmorphology, neurobehavior and their mothers were interviewed.
Results
Total dysmorphology scores discriminated well the physical traits of children across the FASD continuum: fetal alcohol syndrome (FAS) =15.8, partial FAS (PFAS) = 10.8, alcohol-related neurobehavioral disorder (ARND) = 5.2 and typically-developing controls = 4.4. Additionally, a neurobehavioral battery distinguished children with each FASD diagnosis from controls. Behavioral problems qualified more children for FASD diagnoses than cognitive traits. Significant proximal maternal risk variables were: reports of pre-pregnancy drinking, drinking in any trimester, and co-morbid use of other drugs in lifetime and during pregnancy, especially alcohol and marijuana (14.9% among mothers of children with FASD vs. 0.4% for controls). Distal maternal risks included reports of: other health problems (e.g., depression), living unmarried with a partner during pregnancy, and a lower level of spirituality. Controlling for other drug use during pregnancy, having a child diagnosed with a FASD was 17.5 times greater for women who reported usual consumption of three drinks per drinking day prior to pregnancy than for non-drinking mothers (p<.001, 95% CI = 5.1 – 59.9). There was no significant difference in prevalence of FASD by race, Hispanic ethnicity or socioeconomic status. The prevalence of FASD was not lower than 17.3 per 1,000 and weighted estimated prevalence was 49.0 per 1,000 or 4.9%.
Conclusion
This site had the second lowest rate in the CoFASP study, yet children with FASD are prevalent.
Keywords: fetal alcohol spectrum disorders, prevalence, alcohol use and abuse, women, prenatal alcohol use, children with FASD, maternal risk traits for FASD
INTRODUCTION
Previous Epidemiology Studies of Children with FASD
The specific traits of children with fetal alcohol syndrome (FAS) were first delineated by Jones and Smith (1973) from a dozen clinical cases, and in the next decade other clinical case descriptions of FAS appeared in the literature. A United States (U.S.) Institute of Medicine (IOM) study (Stratton et al., 1996) put forth guidelines for diagnosing four clinically-significant outcomes of prenatal alcohol exposures: FAS, partial fetal alcohol syndrome (PFAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD). These diagnoses were referred to as a continuum and later named fetal alcohol spectrum disorders (FASD) as an umbrella term (Calhoun et al., 2006). Several diagnostic systems were developed in North America and used globally with minor variations (Astley and Clarren, 2000; Chudley et al., 2005; Cook et al., 2016; Hoyme et al., 2005). The strengths and weaknesses of these systems have been described, debated, and evaluated (Coles et al., 2016), but all utilize similar: physical features and measurements, anomalous features, and neurobehavioral traits and assessments to evaluate children for FASD. The categories within FASD, names of the categories, and cut-off points for particular domains and traits have varied over the years. While some differences exist, all agree on the concept of a spectrum of damage, which is primarily attributed to quantity, frequency, and timing of prenatal alcohol exposure (May et al., 2013b).
Prior to 2000, few publications provided a population-based understanding of the prevalence of FAS or FASD, and therefore, no firm understanding of prevalence and variation of FASD physical and neurobehavioral traits among the general public. Active case ascertainment (ACA) methods emerged as a promising method for prevalence studies, for surveillance through registries and most clinic-based studies were found to be gross undercounts of total cases, were selective in whom was studied, and understanding of the prevalence and characteristic traits of children on the full continuum of FASD had not emerged (May et al., 2009). ACA studies in elementary schools emerged as an effective approach to fill this knowledge gap. Studies of whole communities were published from South Africa, Italy, Croatia, and Canada (May et al., 2017a, 2016a, 2016b, 2013a, 2011a, 2007, 2006, 2000; Okulicz-Kozaryn et al., 2017; Petkovic and Barisic, 2010, 2013; Popova et al., 2019; Urban et al., 2008, 2015; Viljoen et al., 2002, 2005). Rates of FAS and FASD varied in these studies by county and over time. However, total FASD was consistently found to be higher than older estimates of 1% and ranged from 1-5% in Croatia, Canada, and the United States to highs of 23-28% in five South African communities.
When samples in schools participating in ACA studies are representative, they establish the lower bound (lowest possible) FASD prevalence, an estimate of the true prevalence of FASD, and a representative description of the common traits (and their variability) of children with FASD. Furthermore, these studies can also establish the traits of typically-developing children in a population. In such studies few children are identified who were previously diagnosed with FASD (Clarren et al., 2001; May et al., 2018), indicating that FASD is under diagnosed (Chasnoff et al., 2015).
Associating Specific Maternal Risk Factors to Children with FASD
Identifying maternal risk factors for FAS and FASD has a long history. From clinical studies (Aase, 1994; Abel and Hannigan, 1995; Blume, 1985; Esper and Furtado, 2014; Jones and Smith, 1973) to basic science investigations (Balaraman et al., 2016; Sulik, 2014) a number of maternal risk factors have been identified in drinking prevalence studies (Denny et al., 2019, 2009; Hasin et al., 2019). Furthermore, an understanding of drinking quantity and frequency reported by women of childbearing age and pregnant women has been well covered (Denny et al., 2019; Grant et al., 2017; Green et al., 2016; Hasin et al., 2019; Keyes et al., 2011; Keyes and Miech, 2013; Roozen et al., 2018; Shmulewitz and Hasin, 2019). However, in such studies drinking patterns or quantities cannot be matched to specific child outcomes, because they do not have the link to a formal diagnosis of children on the continuum of FASD as do ACA studies.
Since it is standard procedure to interview all mothers of study children in ACA studies, maternal traits are matched with child outcomes (e.g., diagnosis and traits) (Ceccanti et al., 2014; May et al., 2013b, 2011b, 2009, 2008, 2005; Viljoen et al., 2002). A major goal is to understand particular quantity (Q), frequency (F), and gestational timing (T) of exposures associated with specific outcomes and diagnoses (May et al., 2013b).
The Collaboration on FASD Prevalence (CoFASP) was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in 2010 to provide solid prevalence data on FASD in the U.S. (May et al., 2018). CoFASP investigators sought to expand knowledge of the full continuum of effects of prenatal alcohol consumption on children. Population-based research was undertaken in four sites, each in different regions of the U.S.: Rocky Mountain, Midwest, Southwestern Pacific, and Southeast. This paper describes the Southeast regional site, a county that is representative of this large and populous region. It is a comprehensive summary and analysis of the domains covered in a CoFASP study: physical growth, dysmorphology, neurobehavior, and maternal risk factors.
The Study Community
One-hundred-sixty thousand persons reside in the study county. The county is designated by the U.S. Census as part of a Standard Metropolitan Area that is among the 15 most populous in the country and has been growing steadily for decades. The County has several major, incorporated urban areas (cities and towns), suburban bedroom communities, and rural areas where a variety of small agriculture enterprises are practiced. Small manufacturing and distribution businesses are prevalent, as are health care clinics, a major tertiary care hospital, and retail businesses. The population of the county was 68.2% White, non-Hispanic, 17.8% Black, non-Hispanic, and 10.8% Hispanic (U.S. Census, 2015) (see Table 1). The residents had per capita income of $25,544 and median household income of $47,694, both slightly below U.S. averages. Fewer people were below the poverty level than the U.S. average (U.S. Census, 2015). Religious survey data (Pew Research Center, 2015) indicate that 77% of the people in this state identify themselves as Christian, 3% Jewish, Muslim, Buddhist or Hindu, and 20% unaffiliated (“nones”), higher than U.S. averages. Also, 84% said that religion is very, or somewhat important, in their lives. Per capita alcohol consumption in this state was 2.02 gallons (7.65 liters) of ethanol per year in 2009, lower than the U.S. average of 2.3 gallons (8.71 liters) (LaVallee and Yi, 2011). The health ranking of this state falls between 30 and 34th of 50 states (America’s Health Rankings Annual Report, 2015). Almost 14% of adults in this county reported binge drinking, and excessive drinking was 15.1% in the state, both lower than the U.S. average (vs. 17.4%) (Tan et al., 2015).
Table 1.
Demographic Indicators for the Southeastern US County compared to the United States Averages
| Demographic Indicator | Southeastern County | United States |
|---|---|---|
| Population (7/2015)1 (percentage of US population) | 206,392 (0.06%) | 321,418,820 (100%) |
| Population change (%) since 20101 | 9.4% | 4.1% |
| Race/Hispanic Ethnicity (2010)1 | ||
| White, non-Hispanic | 68.2% | 63.7% |
| Black, non-Hispanic | 17.8% | 12.6% |
| American Indian and Alaskan Native | 0.4% | 0.9% |
| Asian | 1.6% | 4.8% |
| Two or more races | 2.3% | 2.9% |
| Hispanic or Latino | 10.8% | 16.3% |
| Foreign born persons1 | 7.5% | 13.1% |
| Age – years (median) | 36.4 | 37.2 |
| Housing1 | ||
| Median household value | $167,700 | $176,700 |
| Education1 | ||
| High School graduate or higher, % ages ≥25 years | 84.6% | 86.3% |
| Bachelor’s degree or higher, % ages ≥25 years | 22.1% | 29.3% |
| Economy1 | ||
| Per capita income in past 12 months (2014 dollars) | $25,544 | $28,555 |
| Median household income | $47,694 | $53,482 |
| Persons in poverty | 12.2% | 14.8% |
| Religion5 | ||
| Composition | ||
| Christian | 77% | 70.6% |
| Non-Christian | 3% | 5.9% |
| Unaffiliated (“nones”) | 20% | 22.8% |
| Importance of Religion | ||
| Very important | 62% | 58% |
| Somewhat important | 22% | 24% |
| Not too important/not at all | 16% | 16% |
| Health Behavior | Median 25 (Range 1-50) |
|
| Overall state health Rank in US2 | 30-34 | |
| Alcohol Use | ||
| Binge drinking^ state %, (US rank)2 | 13.6% (9) | 16.8% (25) |
| Excessive drinking+, state % (US rank)2 | 15.1% (9) | Median = 17.4% Mean = 16.8% |
| Excessive drinking, county3 | 16.0% | |
| Heavy drinking#, city3 | 4.9% | |
| State per capita ethanol consumption (2009), volume per person 14 years and older4 | 2.02 gallons | 2.30 gallons |
| 7.65 liters | 8.71 liters |
Sources:
(“U.S. Census Bureau QuickFacts: United States,” n.d.) US Census
United Health Foundation, America’s Health Rankings, 2015; comprised of scores on behaviors, community and environment, policy and clinical care; scores are ranked for each of the 50 states with better scores resulting in a higher rank among the 50 states; ranges indicate that different rankings are provided for each of the four domains named above
(“CDC - BRFSS,” n.d.) BRFSS (Behavioral Risk Factory Survey data of the CDC. Reported in local city and county statistical reports
La Valle and Yi, NIAAA Surveillance Report #92
Pew Research Center. America’s Changing Religion Landscape, 2015. Online. www.pewresearch.org.
Binge drinking defined as: during the past 30 days, the consumption of 5 or more drinks for men or 4 or more drinks for females on an occasion
Heavy drinking is defined as males having more than two drinks per day and females having more than one drink per day
Excessive drinking of alcohol is defined as both binge drinking (above) and chronic drinking also referred to as heavy drinking (above)
METHODS
Diagnostic Procedures
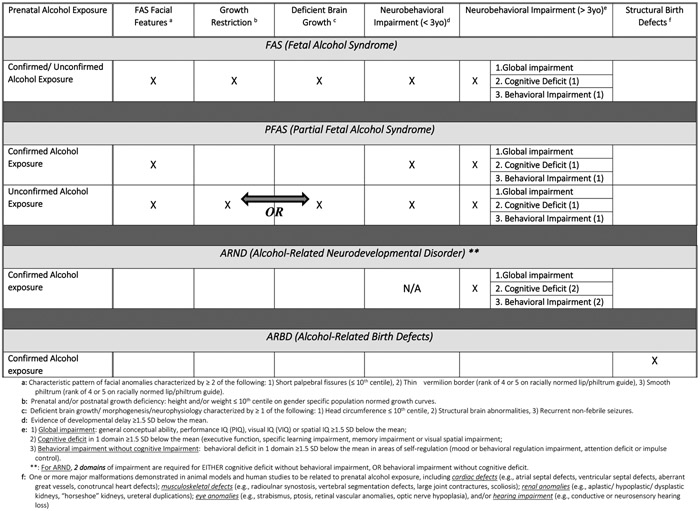
Revised IOM diagnostic guidelines for FASD (Hoyme et al., 2005) were used and consensus cut-off values were established by the investigators and CoFASP advisory group (Hoyme et al., 2016). Classification of children was based on blinded assessments of: (1) physical growth, (2) dysmorphology, (3) cognitive and behavioral assessments, and (4) maternal risk interviews of the participant’s mothers. Other recognizable malformation syndromes were ruled out, and final diagnoses were made for each child in formal, data-driven case conferences.
Each of the four diagnostic categories of FASD was carefully considered for the CoFASP study, and specific cut-off criteria are summarized in Figure 1. Diagnosis of FAS and PFAS without a confirmed history of alcohol exposure is permitted by the original and revised IOM criteria (Hoyme et al., 2016; Stratton et al., 1996) when other anomalies with similar phenotypes are ruled out. Other evidence of alcohol exposure of prenatal drinking is often used in clinical work, but the diagnosis of a FASD in epidemiology studies is rarely made without direct maternal reports of alcohol use reported: immediately prior to pregnancy, prior to pregnancy recognition, in any trimester, or collateral reports. In many populations a substantial number of women may underreport alcohol use or their levels of drinking during pregnancy (Alvik et al., 2006; Bakhireva et al., 2017; Wurst et al., 2008); yet in other populations, accurate reports of drinking and levels of drinking are the norm (Fortin et al., 2017; May et al., 2016b). An ARND diagnosis always requires direct confirmation of prenatal alcohol use in the index pregnancy.
Figure 1.
Collaboration on FASD Prevalence (CoFASP) Consensus Clinical Diagnostic Guidelines
Sampling of First Grade Children
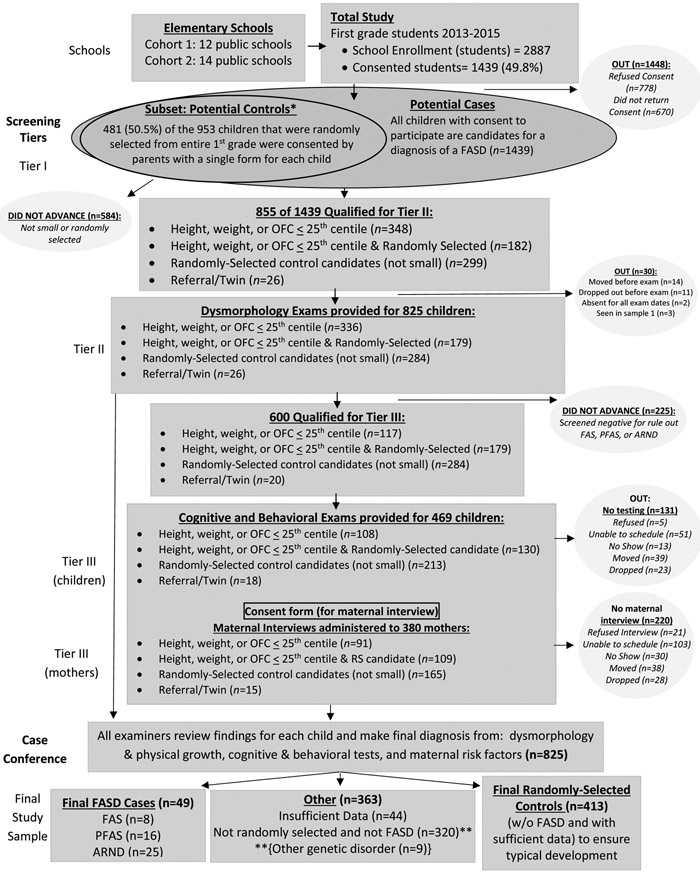
The sampling process and combined numbers of children and mothers participating from two independent cohorts are documented in Figure 2. There were two public school districts with a total of 24 elementary schools in this county. Consent forms were sent to the parents/guardians of all first-grade students in all five elementary schools in the independent city school district for two consecutive cohorts (2013 – 2014 and 2014 – 2015). In the county school district, 7 (cohort 1) and 9 (cohort 2) schools were chosen by random selection from the 19 schools in this administrative district. Therefore, in Cohort Sample 1, 12 schools participated and in Cohort Sample 2, 14 schools were studied. No schools refused participation, and total consent for both Cohorts was 49%.
Figure 2. Sampling Methodology for Prevalence of FASD in a Southeastern City and County: Cohorts 1 and 2 Combined.
*All potential controls were randomly-selected and became part of the control group if found to be not-FASD and within parameters of typical development. **Children not-randomly-selected or found to have another disorder did not default to the control groups.
Consented children entered the study primarily via one or both of two criteria: 1) oversampling of all children who were ≤25th centile on height or weight or head circumference and 2) selection by a simple random sample drawn from the entire first grade class roles (50.5% of the randomly-selected children were consented). Children selected at random and confirmed by the research team as not FASD (or as having another known anomaly) constituted the final comparison/control group. Additional children entered the study (n=26) either because a teacher or parent had concerns about their development or they were a twin to a participating child. Children entering the study via non-random selection routes and determined not FASD, did not default to the control group. Identical exams and testing were performed on all children who participated through all of the study (Figure 2).
Study Procedures: Screening in Tiers I and II
In Tier I, the research team measured all consented first grade children in both cohort samples (n=1,439) on height, weight, and occipitofrontal (head) circumference (OFC). Any consented child ≤25th centile on OFC or height or weight, or referred by teachers, or twins of a selected child, or selected randomly was included in Tier II physical exams (Figure 2). Four teams, each headed by a pediatric dysmorphologist, provided brief, structured examinations blinded to background information about the child or mother. Examinations assessed: growth, multiple anthropometric measurements, and minor anomalies of the craniofacies, limbs, skin, hair, hands, and heart. Each child was then assigned a “dysmorphology score,” an objective quantification of growth deficiency and minor anomalies (Figure E1) (Hoyme et al., 2016). Although not directly used for an assignment of FASD diagnoses, the score is a useful research tool, correlating well with extent of maternal drinking and learning/behavior difficulties in affected children (Ervalahti et al., 2007). Inter-rater reliability of key measurements required by the revised IOM criteria has been good when tested. Evaluation in previous studies produced acceptable correlation coefficients and Cronbach’s alpha coefficients of: 0.993 for OFC, 0.957 for inner canthal distance (ICD), 0.951 for palpebral fissure length (PFL), and 0.928 for philtrum length (May et al., 2011b, 2000; Viljoen et al., 2005). This examination and assessment system balances sensitivity and specificity in order to capture the full continuum of FASD for epidemiological studies (Hoyme et al., 2016).
After dysmorphology findings were reviewed for each child, a preliminary diagnosis was assigned: a) not-FASD, b) diagnosis deferred – rule out a specific FASD diagnosis or a related disorder, or c) probable FAS or PFAS. All randomly-selected children and those in categories b and c were advanced to Tier III.
Study Procedures: - Tier III: Neurobehavioral Testing and Maternal Risk Questionnaires
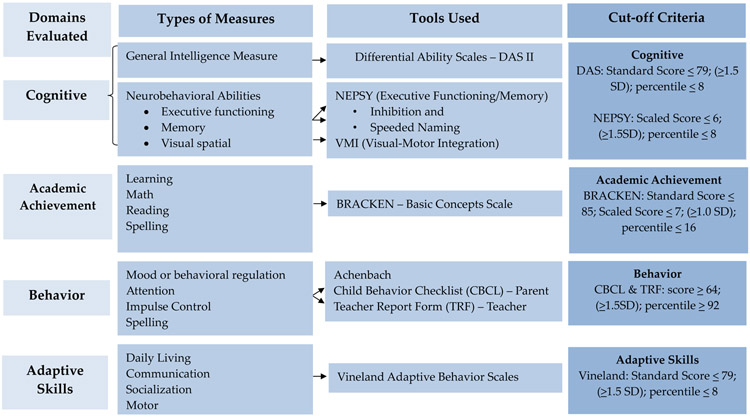
Development and behavior were assessed by licensed school psychologists with the CoFASP-endorsed battery which took 1.5 - 2 hours to administer (Figure 3). The battery evaluated the following domains: cognitive, academic achievement, behavior, and adaptive skills. Instruments included were: Differential Abilities Scale (DAS-II) (Elliott, 2007) to assess general intelligence; NEPSY-II (Korkman et al., 2007) to assess executive functioning, memory, and visual spatial integration; Developmental Test of Visual-Motor Integration (VMI) (Beery and Beery, 2004) to assess eye-hand coordination; and Bracken Basic Concepts Scale (Bracken, 1998) to assess basic concept development in math, reading, and spelling. Teachers and parents also completed behavior assessments: Achenbach (Achenbach and Rescorla, 2001) Child Behavior Checklist (CBCL) and Teachers Report Form (TRF); and Vineland Adaptive Behavior Scales (Sparrow et al., 2005).
Figure 3.
CoFASP Cut-Off Criteria: Neurobehavioral Testing Battery
All consenting mothers of children in Tier III (potential cases and potential controls), who could be scheduled successfully, were provided face-to-face interviews by experienced project staff. Sequencing of questions was designed to maximize accurate reporting of: general health, reproduction, nutrition, alcohol and drug use, socioeconomic status (SES), and maternal height, weight, and OFC were measured. Drinking questions employed a timeline, follow-back sequence, (Sobell et al., 2001, 1988) and Vessels alcohol quantity methodology for accurate calibration of standard alcohol units (Kaskutas and Graves, 2001, 2000; Kaskutas and Kerr, 2008). The American “Standard Drink” was used, where one drink was equal to consuming 14 grams of absolute alcohol: 12oz. (350mL at 5% alcohol by volume) of beer; 5oz. (150mL) of wine (12% by volume); and 1.5oz. (44mL of 40% alcohol by volume) liquor (“What Is A Standard Drink? ∣ National Institute on Alcohol Abuse and Alcoholism (NIAAA),” n.d.). Current alcohol consumption for the week preceding the interview was embedded into dietary intake questions (King, 1994) to aid accurate calibration of drinking quantity, frequency, and timing of alcohol use before and during the index pregnancies (Alvik et al., 2006; May et al., 2013b, 2008, 2005). Retrospective reports of alcohol use have been found to be accurate in some populations when designed and administered properly (Czarnecki et al., 1990; Fortin et al., 2017; Hannigan et al., 2010; May et al., 2018).
Maternal risk data were gathered for 380 mothers in the cohorts combined (see Figure 2). Drinking during pregnancy was confirmed with the CoFASP criteria if at least one of these measures were reported: a) six or more standard drinks per week for two or more weeks during pregnancy; b) a binge of 3 or more drinks per occasion on two or more occasions during pregnancy; or c) documentation of social or legal problems in proximity to the index pregnancy (e.g. treatment of alcohol abuse or infractions of driving under the influence). These criteria were not intended to reflect a threshold for damage associated with FASD. Rather cut-off levels were based on previous experience with responses in previous self-reported drinking surveys that were associated with dysmorphology and neurobehavioral impairment characteristic of a FASD.
Multidisciplinary Case Conferences for Final Diagnoses
Following data collection, final diagnoses were made in confidential, multidisciplinary case conferences. The findings for each child in each domain were discussed in a structured manner where summary results were presented by the research team members who produced them. While findings were being presented and discussed, two-dimensional, digital photos of the child’s face (frontal and profile views) were projected to contextualize the discussion. Findings from each domain and examiner were weighed throughout the presentation and the final diagnosis was made by the examining dysmorphologist with the consensus of the group. In rare cases there was lack of agreement among participants; the final diagnosis was delayed until clarification or additional data were brought to the group.
In classifying children, consistency and quality assurance were enhanced by strict application of the CoFASP criteria preparing for and during case conferences. Final diagnoses were double-checked for consistency and accuracy by the data management teams headquartered at the University of North Carolina, University of California, San Diego, and the University of New Mexico. Classifications were then triple-checked by CoFASP investigative teams by reciprocal exchange of diagnostic data for all cases and a sample of non-cases. Each team was blinded to the other team’s classification and was asked to determine whether criteria had been applied accurately.
Data Analysis and Final Prevalence Rates
Data analyses were performed with Excel (Microsoft Excel, 2016) and SPSS (IBM, 2017). Child physical, cognitive/behavioral, and maternal risk findings were compared across diagnostic groups using chi square, t-tests, and one-way analysis of variance. With statistically significant ANOVAs, post-hoc analyses were performed using Dunnett’s correction pairwise comparisons (α=.05).
In calculating the partial correlations, transformations were undertaken for most measures due to skewness. Transformations were undertaken for most measures due to skewness. Logarithmic transforms, square root transformations, and other appropriate data analysis techniques were applied where appropriate to individual variables. Logarithmic transformations were applied to usual number of drinks per drinking day before pregnancy (DDD), number of weeks before mother’s recognition of the index pregnancy, and the teacher’s reports of rule-breaking and attention problems. Square root transformations were applied to the child’s total dysmorphology and general abilities scores. Although highly unbalanced, transformations could not be applied to “yes/no” items: maternal reports of drinking during pregnancy trimesters, and the covariate, whether mother had used drugs during the index pregnancy. Use of pairwise deletion ensured that all available data were included. A statistical criterion of p<.0017 was set to control for Type I familywise error rate.
The lower prevalence rates reported in this paper represent the minimum prevalence possible given the total children meeting CoFASP criteria (numerator) at this site, over a denominator of total children enrolled in these first grade cohorts (consented or not). The higher prevalence rates employed a weighted correction factor for each specific diagnosis based on the proportion of diagnoses made within the subsample of randomly-selected entrants. These corrections were projected to the unconsented portion of the students for a final estimated prevalence. Calculation methods employed in the CoFASP initiative were published as an e-appendix of May et al. (2018).
RESULTS
Child Demographic, Growth and Cardinal Features of FASD, and Other Minor Anomalies
Final cases diagnosed in the two cohorts are presented in Table 2. There were eight children with FAS, 16 with PFAS, 25 with ARND, and 413 randomly-selected, typically developing controls. ARBD has been found to be rare in any population (May et al., 2016b, 2015, 2014; May and Gossage, 2001), and no cases of ARBD were found at this site. Racial composition at this site was diverse: 46.7% were non-Hispanic White, 21.9% were of Hispanic ethnicity, 23.9% were non-Hispanic African American, and 7.5% were Other. Racial make-up did not differ significantly between the children with FASD and the randomly-selected controls, whether analyzed by specific diagnosis (χ2(9) = 8.204, p=0.514) or by FASD vs. controls (χ2(3) = 4.252, p=0.235).
Table 2.
Distribution of FASD Cases and Randomly-Selected Controls by Racial and Hispanic Ethnicity Categories: Southeastern US County
| Southeastern | FAS | PFAS | ARND | RS controls |
X2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||
| White- non-Hispanic | 213 | 46.7 | 4 | 50.0 | 8 | 50.0 | 12 | 48.0 | 190 | 46.6 | ||
| Hispanic | 100 | 21.9 | 1 | 12.5 | 4 | 25.0 | 1 | 4.0 | 94 | 23.0 | ||
| African American-non-Hispanic | 109 | 23.9 | 2 | 25.0 | 3 | 18.8 | 8 | 32.0 | 96 | 23.5 | ||
| Other | 34 | 7.5 | 1 | 12.5 | 1 | 6.3 | 4 | 16.0 | 28 | 6.9 | 8.204 | 0.514 |
| FASD | RS controls |
X2 | p-value | |||||||||
| n | % | n | % | n | % | |||||||
| White- non-Hispanic | 213 | 46.7 | 24 | 49.0 | 190 | 46.6 | ||||||
| Hispanic | 100 | 21.9 | 6 | 12.2 | 94 | 23.0 | ||||||
| African American- non-Hispanic | 109 | 23.9 | 13 | 26.5 | 96 | 23.5 | ||||||
| Other | 34 | 7.5 | 6 | 12.2 | 28 | 6.9 | 4.252 | 0.235 | ||||
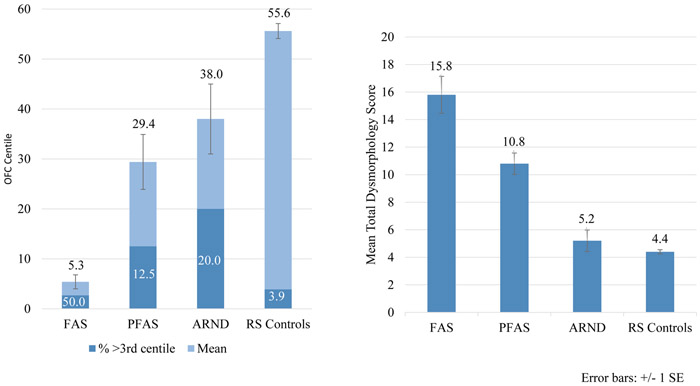
In Table 3, no significant difference in age was found by diagnosis. A difference in sex was due primarily to excess males with ARND, and all eight cases of children with FAS were female. Even though the mean values of the diagnostic groups fell within the normal range, virtually all physical variables differed significantly across diagnostic categories, indicating the discriminatory power of the CoFASP criteria. Child birthweight, height, weight, and OFC/head circumference centiles were all significantly different among groups, with post-hoc analyses indicating significant pairwise differences: FAS groups vs. controls on mean birthweight; FAS different than PFAS, ARND, and controls on height; FAS different than ARND and controls on weight; and FAS and PFAS different than all other groups on OFC. Mean OFC percentile of children with FASD was 5.3, and 50% of children with FAS were below the 3rd centile. Twelve and one-half percent (12.5%) of children with PFAS, and 20% of children with ARND were below 3rd centile (see Figure 3). The FAS group had the lowest body mass index (BMI). In keeping with diagnostic criteria, palpebral fissure length (PFL) centile differed by group, with significant post-hoc analysis differences among FAS and ARND, and FAS vs. controls. A higher frequency of smooth philtrum was confirmed among children with FAS than among children with PFAS, ARND, and controls. The vermilion border rank of the upper lip was also significantly different between all FASD groups and controls. Other minor anomalies found to differ significantly among groups were: inner pupillary distance, outer canthal distance, maxillary arc, mandibular arc, ptosis (droopy eyelid(s)), and hypoplastic fingernails. Post-hoc analyses indicated smaller maxillary arcs for children with FAS vs. ARND and FAS vs. controls and smaller mandibular arcs for FAS vs. controls. Finally, all groups differed significantly by mean total dysmorphology score (Figure 4). The children with FAS had the highest mean (15.8), followed by PFAS (10.8), ARND (5.2), and controls (4.4). In post-hoc analysis, total dysmorphology score significantly discriminated the individual FASD groups from one another and controls except for FAS vs. PFAS and ARND vs. controls.
Table 3.
Demographic, Physical Growth, Cardinal FAS Features, Other Minor Anomalies and Total Dysmorphology Scores for in a Southeastern US County
| All Children* (n=2558) |
Children with FAS (n=8) |
Children with PFAS (n=16) |
Children with ARND (n=25) |
Randomly- Selected Control Children (n=413) |
Test- score |
p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth and Cardinal Features | ||||||||||||
| Sex (% Male) | -- | 0.0 | 50.0 | 60.0 | 52.8 | χ2=9.433 | .024 | |||||
| Current Age (months) - Mean (SD) | -- | -- | 82.0 | (4.2) | 81.8 | (5.0) | 81.1 | (3.5) | 81.6 | (4.6) | F=.117 | .950 |
| Preterm (% Yes)+ | -- | 12.5 | 14.3 | 36.0 | 12.6 | χ2=10.029 | .022 | |||||
| Birth weight (grams) – Mean (SD) + | -- | -- | 2834 | (250) | 3335 | (780) | 2801 | (990) | 3225 | (596) | 4.249 | .006C |
| Height Centile – Mean (SD) | 52.3 | (29.0) | 8.0 | (9.8) | 34.9 | (26.5) | 43.4 | (26.7) | 52.8 | (28.2) | F=9.221 | <.001A,B,C |
| Weight Percentile – Mean (SD) | 59.6 | (28.9) | 15.6 | (19.4) | 46.3 | (29.7) | 51.2 | (35.6) | 58.6 | (28.3) | F=7.000 | <.001B,C |
| Child's BMI Percentage – Mean (SD) | -- | -- | 38.8 | (32.0) | 57.1 | (34.1) | 56.8 | (34.5) | 61.3 | (27.7) | F=1.937 | .123 |
| Occipitofrontal Circumference (OFC) Centile – Mean (SD) | 55.9 | (30.2) | 5.3 | (3.9) | 29.4 | (22.0) | 38.0 | (35.1) | 55.8 | (29.9) | F=13.50 | <.001A,B,C,E |
| OFC ≤3rd centile | -- | 50.0 | 12.5 | 20.0 | 3.9 | χ2=41.652 | <.001 | |||||
| OFC ≤10th centile | -- | 100.0 | 25.0 | 36.0 | 8.7 | χ2=77.163 | <.001 | |||||
| Palpebral Fissure Length (PFL) Percentile – Mean (SD) | -- | 8.6 | (9.9) | 18.8 | (19.1) | 33.5 | (13.4) | 32.7 | (15.3) | F=10.935 | <.001B,C | |
| PFL ≤3rd centile | -- | 25.0 | 25.0 | 0.0 | 2.2 | χ2=38.490 | <.001 | |||||
| PFL ≤10th centile | -- | 75.0 | 50.0 | 0.0 | 7.0 | χ2=77.409 | <.001 | |||||
| Smooth Philtrum (% Yes) | -- | 87.5 | 62.5 | 16.0 | 14.8 | χ2=51.020 | <.001 | |||||
| Narrow Vermilion (% Yes) | -- | 87.5 | 93.8 | 12.0 | 13.6 | χ2=96.409 | <.001 | |||||
| Other Minor Anomalies | ||||||||||||
| Inner Pupillary Distance (IPD) Percentile – Mean (SD) | -- | 36.9 | (24.6) | 47.7 | (24.3) | 50.3 | (25.8) | 57.3 | (25.8) | F=2.694 | .046 | |
| Outer Canthal Distance (OCD) Percentile – Mean (SD) | -- | 18.8 | (14.7) | 24.4 | (15.1) | 31.3 | (17.5) | 34.6 | (20.2) | F=3.025 | .029 | |
| Maxillary Arc (in cm) – Mean (SD) | -- | 23.0 | (0.6) | 24.0 | (1.1) | 24.0 | (1.3) | 24.6 | (1.2) | F=7.323 | <.001B,C | |
| Mandibular Arc (in cm) – Mean (SD) | -- | 24.1 | (1.0) | 24.8 | (1.2) | 25.1 | (1.4) | 25.6 | (1.4) | F=5.819 | .001C | |
| Ptosis (% Yes) | 37.5 | 25.0 | 12.0 | 8.0 | χ2=11.144 | .011 | ||||||
| Hypoplastic Nails (% Yes) | 0.0 | 6.3 | 0.0 | 0.5 | χ2=8.170 | .043 | ||||||
| Total Dysmorphology Score – Mean (SD) | -- | 15.8 | (3.8) | 10.8 | (3.1) | 5.2 | (3.9) | 4.4 | (3.1) | F=52.060 | <001B,C,D,E | |
Post-hoc significant difference between:
FAS & PFAS
FAS & ARND
FAS & Controls
PFAS & ARND
PFAS & Controls
ARND & Controls
As reported in the maternal questionnaire.
All children consented to initial screening, tests of significance only compared the children with FASD and controls.
Bonferroni adjusted significance level for Growth and Cardinal Features = 0.005; for other minor anomalies = .007
Figure 4.
Occipitofrontal Circumference (OFC) and Total Dysmorphology Score by FASD Diagnosis, Southeastern County
Child Performance on Neurobehavioral Measures
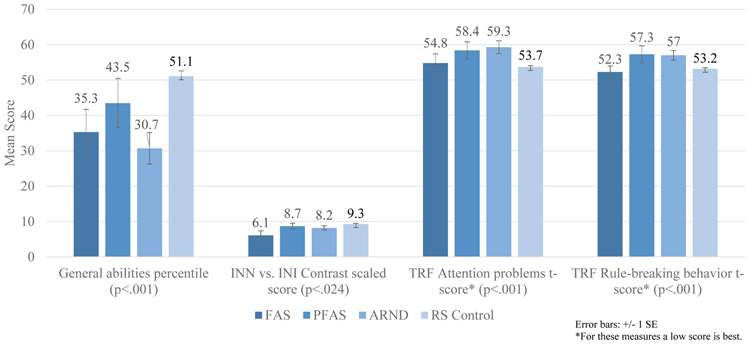
Performance centiles on virtually every cognitive and behavioral measure in Table 4 were significantly lower for children with a FASD diagnosis than controls. Highlighted in Figure 5 are four of the specific tests by specific FASD diagnoses. On the general abilities test, the children with ARND performed the worst, children with FAS next, then PFAS, and controls performed the best. The INN score refers to a naming task where the child must name shapes or the direction of arrows as quickly as possible. The INI task is an inhibition task that requires a child to say the opposite shape name or arrow direction as quickly as possible. The INN vs. INI Contrast Scaled Score compares the naming speed score and the inhibition score. On this difficult task the children with FAS performed most poorly, ARND next, then PFAS, and controls the best. For the Teacher Report Form (TRF) on attention problems, control children had the best attention scores and children with ARND the poorest, followed by children with FAS and PFAS also poorer than controls. Children with FAS and controls had the fewest problems with rule-breaking behavior and children with ARND and PFAS had the most. For the breakdown of neurobehavioral measure by specific diagnosis see Table E1 in the Appendix.
Table 4.
Neurocognitive Findings Among Children with FASD and Randomly-Selected Controls in a Southeastern County
| Children with FASD (n=49) |
Randomly- Selected Control Children (n=444) |
t-test | p-value | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |||
| Intellectual Domain | (n=49) | (n=302) | ||||
| General Abilities Percentile | 35.6 | (23.8) | 51.1 | (26.0) | −3.910 | <.001** |
| Verbal Cluster Percentile | 42.3 | (26.1) | 54.0 | (27.5) | −2.790 | .006** |
| Nonverbal Reasoning Cluster Percentile | 32.4 | (27.1) | 44.3 | (26.0) | −2.694 | .003** |
| Spatial Cluster Percentile | 40.0 | (24.3) | 54.9 | (24.3) | −3.970 | <.001** |
| Executive Function | (n=49) | (n=302) | ||||
| INN (Naming) combined scaled score | 8.2 | (4.2) | 9.7 | (3.6) | 2.628 | .009** |
| INN vs. INI Contrast Scaled Score | 8.0 | (3.3) | 9.3 | (3.3) | −2.461 | .014* |
| INI (Inhibition) combined scaled score | 7.5 | (3.3) | 9.3 | (3.4) | −3.338 | .001** |
| INS (Switching) combined scaled score | 8.0 | (3.0) | 9.0 | (3.0) | −2.062 | .040* |
| Speeded Naming Combined scaled score | 7.8 | (3.0) | 9.2 | (3.1) | −2.884 | .004** |
| Learning1 | (n=43) | (n=249) | ||||
| BBCS School Readiness Composite Scaled Score | 10.3 | (2.9) | 11.3 | (2.6) | −2.322 | .021** |
| BBCS Readiness Composite Standard Score | 101.5 | (14.5) | 106.4 | (12.9) | −2.297 | .022** |
| Visual Spatial | (n=49) | (n=302) | ||||
| VMI Standard Score | 93.6 | (7.6) | 95.4 | (10.6) | −1.105 | .270 |
| Visuomotor Precision Combined scaled score | 7.9 | (3.2) | 9.4 | (3.3) | −2.950 | .003** |
| Mood Regulation2 | ||||||
| CBCL Anxious/depressed t-score | 55.2 | (6.1) | 53.2 | (5.1) | 2.289 | .023* |
| TRF Anxious/depressed t-score | 54.2 | (7.2) | 52.0 | (4.6) | 2.084 | .042* |
| CBCL Withdrawn/depressed t-score | 56.1 | (7.9) | 53.4 | (5.2) | 2.095 | .041* |
| TRF Withdrawn/depressed t-score | 56.1 | (7.7) | 52.2 | (4.6) | 3.461 | .001** |
| CBCL Internalizing Problems t-score | 53.7 | (11.0) | 48.5 | (9.7) | 3.132 | .002** |
| TRF Internalizing Problems t-score | 49.7 | (11.4) | 44.5 | (8.7) | 3.022 | .004** |
| CBCL Externalizing Problems t-score | 54.2 | (10.8) | 48.0 | (10.0) | 3.690 | <.001** |
| TRF Externalizing Problems t-score | 53.8 | (11.0) | 48.9 | (8.4) | 2.968 | .004** |
| CBCL Affective problems t-score | 58.5 | (8.3) | 53.7 | (5.1) | 3.614 | .001** |
| TRF Affective problems t-score | 56.2 | (7.7) | 52.2 | (4.9) | 3.475 | .001** |
| CBCL Anxiety problems t-score | 55.3 | (6.7) | 53.7 | (5.6) | 1.617 | .107 |
| TRF Anxiety problems t-score | 54.9 | (6.7) | 52.2 | (5.0) | 2.675 | .010* |
| Attention2 | ||||||
| CBCL Attention problems t-score | 59.3 | (9.2) | 55.2 | (7.6) | 3.135 | .002** |
| TRF Attention problems t-score | 58.1 | (8.9) | 53.6 | (6.5) | 3.298 | .002** |
| CBCL Attention deficit/hyperactivity problems t-score | 58.3 | (8.5) | 55.1 | (7.3) | 2.345 | .023* |
| TRF Attention deficit/hyperactivity problems t-score | 57.9 | (8.2) | 54.2 | (7.3) | 2.895 | .005** |
| Impulse Control2 | ||||||
| CBCL Rule-breaking behavior t-score | 55.0 | (6.2) | 53.8 | (5.0) | 1.410 | .160 |
| TRF Rule-breaking behavior t-score | 56.4 | (7.5) | 53.2 | (5.4) | 2.797 | .007* |
| CBCL Aggressive behavior t-score | 57.8 | (9.1) | 53.3 | (5.6) | 3.116 | .003** |
| TRF Aggressive behavior t-score | 56.7 | (9.3) | 52.9 | (5.6) | 2.768 | .008* |
| CBCL Oppositional defiant problems t-score | 58.0 | (8.2) | 54.3 | (5.9) | 2.824 | .007* |
| TRF Oppositional defiant problems t-score | 55.8 | (7.5) | 52.9 | (5.5) | 2.528 | .015* |
| CBCL Conduct problems t-score | 55.5 | (7.2) | 53.3 | (5.2) | 1.918 | .061 |
| TRF Conduct problems t-score | 56.5 | (8.2) | 52.9 | (5.4) | 2.9928 | .005** |
| Adaptive Function3 | ||||||
| Vineland (Parent) VABS Communication Standard Score | 90.9 | (16.0) | 105.1 | (17.5) | −3.722 | <.001** |
| Vineland (Teacher) VABS Communication Standard Score | 91.5 | (14.9) | 99.7 | (14.6) | −3.439 | .001** |
| Vineland (Parent) VABS Daily Living Skills Standard Score | 99.3 | (21.6) | 106.0 | (16.9) | −1.746 | .083 |
| Vineland (Teacher) VABS Daily Living Skills Standard Score | 93.0 | (14.8) | 100.3 | (14.3) | −3.142 | .002** |
| Vineland (Parent) VABS Socialization Standard Score | 97.7 | (22.3) | 102.9 | (17.1) | −1.328 | .186 |
| Vineland (Teacher) VABS Socialization Standard Score | 96.2 | (17.5) | 105.4 | (15.7) | −3.382 | .001** |
Children less than 7 at time evaluation did not complete a BRACKEN.
For CBCL: n=43 for FASD; n=232 for Controls. For TRF: n=47 for FASD; n=370 for Controls.
For Vineland (Parent): n=24 for FASD; n=150 for Controls. For Vineland (Teacher): n=43 for FASD; n=342 for Controls.
Significant at <0.05
Significant at the Bonferroni-adjusted level of significance: Intellectual: 0.0125; Executive Function: 0.01; Learning: 0.025; Visual Spatial: 0.025; Mood Regulation: 0.004; Attention: 0.0125; Impulse Control: 0.006; Adaptive Function: 0.008
Figure 5.
Selected Cognitive and Behavioral Measures by FASD Diagnoses, Southeastern Site
Maternal Risk Factors – Proximal
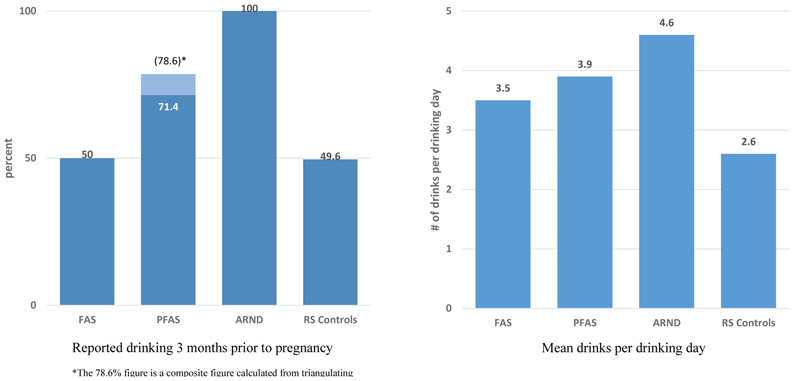
While 50% of the mothers of typically-developing children (controls) reported drinking three months prior to pregnancy, significantly more mothers of children with FASD reported drinking pre-pregnancy (83%) (Table 5). Mothers of children with FASD consumed significantly more drinks per drinking day (DDD) pre-pregnancy than controls. As presented in Appendix Table E2, only half of the mothers of children with FAS reported drinking pre-pregnancy, and mothers of children with FASD diagnoses reported higher mean DDD prior to pregnancy than controls: (4.3 vs. 2.6), with mothers of children with ARND reporting the highest average (4.6). Median drinking values in Table 5 did not differ much from the mean values, indicating minimal skewing from extremely high values reported. Mothers of children with FASD drank more frequently prior to pregnancy than controls, with 71.5% drinking once a week or more and 23.7% reporting drinking three times a week or more compared to 37.2% and 13.2% for controls. Mothers of children with a FASD were more likely to report drinking during pregnancy than controls: 24.4% vs. 4.4%, 17.6% vs. 1.6%, and 17% vs. 3.2%, respectively, in first, second and third trimesters. Mothers of children with ARND were most likely to report drinking in each trimester: 39%, 26%, and 24% in each. Among those mothers who drank during pregnancy, DDD did not differ significantly between groups in any trimester, although control mothers reported a higher DDD than mothers of children with FASD in each trimester, but lower percentage of control mothers drank at all.
Table 5.
Proximal Maternal Risk Factors for FASD: Alcohol and Drug Use a Southeastern County
| Children with FASD (n=47) |
Randomly- Selected Control Children (n=251) |
χ2 or t-test |
p-value | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |||
| Alcohol Use – Before and During Pregnancy | ||||||
| Drank before pregnancy (% Yes) | 83.0 | 49.6 | 17.784 | <.001** | ||
| # of drinks consumed on usual drinking day before pregnancy1 | 4.3 | (2.1) | 2.6 | (2.8) | 3.314 | .001** |
| Mdn = 4.0 | Mdn = 2.0 | — | -- | |||
| Usual frequency – before pregnancy1 | ||||||
| Everyday or almost everyday | 13.2 | 7.4 | ||||
| 3-4 times per week | 10.5 | 5.8 | ||||
| 1-2 times per week | 47.4 | 24.0 | ||||
| 3 times per month | 18.4 | 16.5 | ||||
| 1 time per month or less | 10.5 | 46.3 | 16.865 | .002** | ||
| Days drank more than usual – before pregnancy1 (% Yes) | 22.7 | 15.8 | 1.284 | .257 | ||
| Drank in 1st trimester (% Yes) | 24.4 | 4.4 | 22.080 | <.001A,** | ||
| # of drinks on usual drinking day1 -1st | 3.4 | (2.9) | 5.2 | (6.7) | −0.718 | .483 |
| Usual frequency1 – 1st | ||||||
| Everyday or almost everyday | 18.2 | 10.0 | ||||
| 3-4 times per week | 18.2 | 10.0 | ||||
| 1-2 times per week | 27.3 | 10.0 | ||||
| 2-3 times per month | 0.0 | 0.0 | ||||
| 1 time per month or less | 36.4 | 70.0 | 2.443 | .486 | ||
| Drank in 2nd trimester (% Yes) | 17.8 | 1.6 | 25458 | <.001A,** | ||
| # of drinks on usual drinking day1 -2nd | 1.7 | (1.0) | 6.3 | (9.2) | −0.872 | .474 |
| Usual frequency1 – 2nd | ||||||
| Everyday or almost everyday | 25.0 | 33.3 | ||||
| 3-4 times per week | 12.5 | 0.0 | ||||
| 1-2 times per week | 25.0 | 33.3 | ||||
| 2-3 times per month | 0.0 | 0.0 | ||||
| 1 time per month or less | 37.5 | 33.3 | .497 | .920 | ||
| Drank in 3rd trimester (% Yes) | 17.0 | 3.2 | 14.660 | <.001A,** | ||
| # of drinks on usual drinking day1 - 3rd | 1.1 | (0.4) | 2.4 | (3.5) | −0.922 | .373 |
| Usual frequency1 – 3rd | ||||||
| Everyday or almost everyday | 0.0 | 12.5 | ||||
| 3-4 times per week | 0.0 | 12.5 | ||||
| 1-2 times per week | 0.0 | 0.0 | ||||
| 2-3 times per month | 0.0 | 0.0 | ||||
| 1 time per month or less | 100.0 | 75.0 | 3.013 | .556 | ||
| Alcohol Use - Current | ||||||
| Drink in past 30 days (% Yes) | 59.1 | 52.7 | .617 | .432 | ||
| Binge 5+ in past month (% Yes) | 14.0 | 10.3 | .494 | .482 | ||
| Why usually drink: because others drink | 25.0 | 16.3 | 1.967 | .161 | ||
| Why usually drink: to feel less anxious | 11.4 | 7.3 | .837 | .360 | ||
| Recovering drinker (% Yes) | 0.0 | 1.0 | .362 | .547 | ||
| Other Drug Use | ||||||
| Used tobacco – during pregnancy (% Yes) | 38.3 | 13.2 | 17.521 | <.001** | ||
| Used any drugs in pregnancy (% Yes) | 17.0 | 4.0 | 11.562 | .001A,** | ||
| Abused prescription drugs – in pregnancy | 8.5 | 1.6 | 7.125 | .024A,* | ||
| Used marijuana – during pregnancy (% Yes) | 14.9 | 2.8 | 12.663 | .002A,** | ||
| Used marijuana & alcohol – during pregnancy (% Yes) | 14.9 | 0.4 | 30.660 | <.001A** | ||
| Used cocaine - during pregnancy (% Yes) | 8.9 | 0.8 | 12.346 | .006A,* | ||
| Used tobacco | ||||||
| Yes, within last 30 days | 40.4 | 18.5 | ||||
| Yes, in lifetime | 25.5 | 32.1 | ||||
| Never | 34.0 | 49.4 | 13.658 | .001** | ||
| Used any drug in lifetime (% Yes) | 70.2 | 42.3 | 12.331 | <.001** | ||
| Used marijuana – in lifetime (% Yes) | 70.2 | 41.7 | 12.913 | <.001** | ||
| Used methamphetamine – in lifetime (% Yes) | 6.7 | 2.0 | 3.056 | .110 | ||
| Used heroin – in lifetime (% Yes) | 8.9 | 1.2 | 9.531 | .012A,* | ||
| Used club drugs – in lifetime (% Yes) | 30.4 | 7.8 | 19.813 | <.001A,** | ||
| Used crack/cocaine – in lifetime (% Yes) | 19.6 | 5.3 | 11.422 | .003A,** | ||
| Abused pain killers – in lifetime (% Yes) | 17.0 | 5.6 | 7.409 | .013* | ||
Among women who drank in that specific time period.
Fisher’s Exact Test
Significant at <0.05
Bonferroni-adjusted significant levels: alcohol use -before and during pregnancy = 0.004; alcohol use – current = 0.01; other drug use = 0.004.
Per CoFASP criteria, maternal alcohol use during the index pregnancy was confirmed for all children diagnosed with ARND. For more dysmorphic forms of FASD, direct alcohol use confirmation is sought, but not required. At this site, 50% of FAS cases and 78.6% of PFAS cases were confirmed by direct maternal reports on one or more of the multiple alcohol variables (Figure 6).
Figure 6.
Pre-Pregnancy Drinking by Percentage and Drinks per Drinking Day by Child’s Diagnostic Group
Other drug use distinguished significantly the two maternal groups; drug use was uniformly and significantly higher for mothers of children with FASD for all categories of drugs. In Table 5, mothers of children with FASD report a significantly higher frequency of use during pregnancy for any drug use, tobacco, prescription drugs, marijuana, co-morbid use of alcohol and marijuana, and cocaine. For lifetime use, any drug use, tobacco, marijuana, cocaine, heroin, club drugs, crack cocaine, and pain killers were all higher for mothers of children with FASD than controls. Co-morbid use of alcohol and marijuana during pregnancy was significantly higher among mothers of children with any FASD (14.9%) than controls (0.4%), especially for mothers of children with ARND (28%).
Maternal Risk Factors - Distal
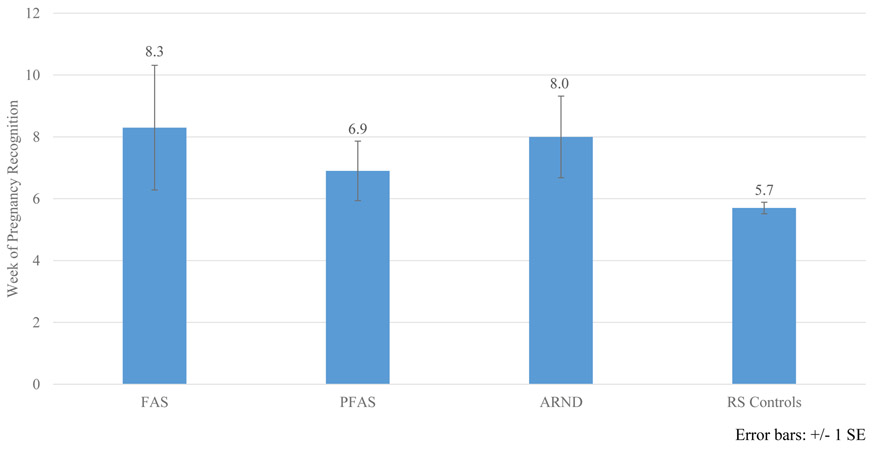
There were no significant differences in maternal physical variables among the groups: age, height, weight, BMI, or head circumference (Table 6). Mothers of children with FASD reported more negative lifetime health indicators than did mothers of controls: depression, liver problems, and neurological conditions. Mothers of children with FASD reported significantly later recognition of pregnancy than controls (p=.022). Average recognition of the index pregnancy was 7.7 weeks gestation for mothers of all children with FASD compared to 5.7 weeks for mothers of typically-developing controls. For mothers of children with FAS and ARND, the average was 8.3 and 8.0 weeks respectively (Figure 7).
Table 6.
Distal Maternal Risk Factors for FASD: Physical, Demographic, and Childbearing Variables in a Southeastern County
| Children with FASD (n=47) |
Randomly- Selected Control Children (n=251) |
χ2 or t-test |
p-value | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |||
| Physical | ||||||
| Age at pregnancy (yrs) | 27.6 | (5.8) | 28.5 | (5.8) | 0.675 | .335 |
| Height at interview (cm) | 161.7 | (6.1) | 163.2 | (8.0) | −1.117 | .240 |
| Weight at interview (kg) | 73.9 | (17.2) | 78.2 | (20.0) | −1.304 | .193 |
| Body Mass Index (BMI) | 28.2 | (6.3) | 29.3 | (7.0) | −0.936 | .350 |
| Head circumference | 55.2 | (1.9) | 55.6 | (2.4) | −1.072 | .284 |
| Health indicators | ||||||
| Weight before pregnancy (in kg) | 65.2 | (17.5) | 67.3 | (15.8) | 0.763 | .401 |
| Asthma – in lifetime (% Yes) | 22.2 | 12.1 | 3.313 | .069 | ||
| Stomach ulcers – in lifetime (% Yes) | 4.5 | 3.6 | 0.083 | .675A | ||
| Neurological conditions/ epilepsy - lifetime | 15.6 | 5.6 | 5.622 | .027A* | ||
| Liver problems / hepatitis – in lifetime | 11.1 | 2.4 | 7.915 | .016A* | ||
| Depression – in lifetime (% Yes) | 70.2 | 41.9 | 12.702 | <.001** | ||
| Childbearing | ||||||
| Gravidity | 3.7 | (2.8) | 3.2 | (1.7) | 1.114 | .270 |
| Parity | 2.7 | (1.1) | 2.6 | (1.2) | 0.419 | .676 |
| Miscarriages | 0.9 | (2.3) | 0.5 | (1.1) | 1.161 | .251 |
| Abortions | 0.1 | (0.5) | 0.1 | (0.3) | 0.666 | .508 |
| Stillbirths | 0.0 | (0.2) | 0.0 | (0.1) | 0.940 | .348 |
| Birth order of index child | 2.0 | (1.1) | 2.0 | (1.2) | 0.408 | .880 |
| Week of pregnancy recognition | 7.7 | (5.6) | 5.7 | (3.1) | 2.355 | .022 |
| Prenatal Care | ||||||
| Once knew pregnant, take vitamins (% Yes) | 93.3 | 95.9 | 0.603 | .432 | ||
| # of times seen by healthcare provider | ||||||
| Never | 2.3 | 0.4 | ||||
| 1-5 times | 0.0 | 1.2 | ||||
| More than 5 times | 97.7 | 98.4 | 2.441 | .323 | ||
| When first seen by healthcare provider | ||||||
| 1st trimester | 84.4 | 93.6 | ||||
| 2nd trimester | 11.1 | 4.8 | ||||
| 3rd trimester | 2.2 | 0.8 | ||||
| Delivery only | 2.2 | 0.8 | 4.500 | .183 | ||
| Other health problems - during pregnancy | 30.4 | 25.1 | 0.574 | .466 | ||
| Accidents/injury – during pregnancy (% Yes) | 8.7 | 10.8 | 0.190 | .798A | ||
| Postpartum depression (% Yes) | 27.3 | 20.2 | 1.101 | .319 | ||
| Postnatal Variables | ||||||
| Child’s Birth weight (grams) | 2970 | (864) | 3225 | (596) | −1.921 | .060 |
| Estimated gestation age at birth (in weeks) | 37.7 | (3.8) | 38.7 | (2.2) | −1.735 | .089 |
| COI had problem(s) at birth (% Yes) | 59.6 | 50.0 | 1.450 | .266A | ||
| Breastfed (% Yes) | 55.3 | 76.0 | 8.531 | .005* | ||
| Duration of breastfeeding (in months)1 | 5.5 | (6.0) | 7.6 | (7.2) | −1.292 | .198 |
| Supplement with formula1 | 76.0 | 72.0 | 0.181 | .814 | ||
| Consumed alcohol in breastfeeding period | 8.3 | 13.2 | 0.461 | .746 | ||
| Pump and dump (%Yes)1 among mothers who breastfed | 20.0 | 28.0 | .292 | .723A | ||
| Pump and dump (% Yes)2 among mothers who drank during period of breastfeeding | 100.0 | 79.2 | 0.516 | 1.00A | ||
| Age of biological father (in years) | 28.7 | (7.0) | 30.4 | (6.4) | −1.635 | .103 |
| Child lives with biological mother (% Yes) | 76.6 | 93.6 | 13.749 | .001A* | ||
| Child lives with: | ||||||
| Foster/Adopted/Relative | 9.5 | 4.0 | ||||
| Biological mother | 26.2 | 19.0 | ||||
| Biological father | 4.8 | 1.6 | ||||
| Biological mother and father | 59.5 | 75.3 | 8.957 | .023A** | ||
| Partner ever had a drinking problem | ||||||
| Never | 75.6 | 84.1 | ||||
| In the past, but not currently | 14.6 | 8.4 | ||||
| Currently | 0.0 | 1.3 | ||||
| Both past and currently | 9.8 | 6.3 | 2.923 | .404 | ||
| Years of Education completed | 13.5 | (2.8) | 13.8 | (3.4) | −0.578 | .561 |
| Household yearly income - during pregnancy | 45549 | (42587) | 58256 | (40656) | −1.839 | .067 |
| Household yearly income – at interview | 51125 | (41147) | 67398 | (52400) | −1.942 | .053 |
| Marital Status – Current | ||||||
| Married | 52.2 | 68.5 | ||||
| Divorced/Widowed/Separated/Single | 30.4 | 17.3 | ||||
| Living with partner | 17.4 | 14.1 | 5.294 | .073 | ||
| Marital Status – During Pregnancy | ||||||
| Married | 40.4 | 68.4 | ||||
| Divorced/Widowed/Separated/Single | 17.0 | 7.6 | ||||
| Living with partner | 42.6 | 24.0 | 13.758 | .002A** | ||
| Spirituality: none [0] to high [10]1 | 5.6 | (2.2) | 6.9 | (2.3) | −3.598 | <.001** |
Among women who breastfed.
Among women who breastfed and consumed alcohol in the breastfeeding period.
Among women who reported drinking in the specific time period.
Pump and dump is the colloquial name for expressing breastmilk after drinking alcohol and disposing of it.
Fisher’s Exact Test
Significant at 0.05
Significant at the Bonferroni-adjusted level: physical = 0.01; health indicators = 0.01; childbearing = 0.007; prenatal care = 0.001; postnatal = 0.004.
Figure 7.
Week When Pregnancy Was First Recognized by Diagnosis, Southeastern Site
There were no differences in prenatal care variables. Compared to controls, children with FASD were significantly lower in birth weight (p=.005) and gestational age at birth when measured across the four specific diagnostic groups (Table E3). Children with FASD were also less likely to have been breastfed or live with the biological mother, and more likely to receive baby formula supplementation. A common practice in the U.S. today among alcohol-using mothers is expression and disposal of breastmilk when drinking, a practice called “pump and dump”. All (100%) mothers of children with FASD who drank during the breastfeeding period report having used “pump and dump” with the index child, but none of the mothers of a child with FAS reported drinking while breastfeeding or us of “pump and dump” use (Table E3).
Some socioeconomic variables did not discriminate significantly among maternal groups on any variable: education completed or household income at pregnancy or at interview. Marital status during the index pregnancy was significantly different between groups: mothers of children with FASD were less likely to be married and more likely to be living with a partner. Spirituality was reported to be significantly higher among the mothers of controls (6.9 v. 5.6), but memberships in a formal religion (Christian, Jewish, or Muslim) was not different among groups nor was frequency of service attendance (not in Tables).
Correlation Analyses – Linking Selected Child and Maternal Traits to a FASD Diagnosis
Partial correlation analysis measured associations between maternal and cognitive/behavioral measures, FASD diagnosis, and total dysmorphology scores after adjusting for whether mother had used drugs other than alcohol during the index pregnancy (Table E4). All four measures of maternal alcohol use correlated significantly with FASD diagnosis: usual DDD consumed three months prior to pregnancy, and whether the mother drank during any of the trimesters. None of these statistically significant partial correlations were particularly strong once adjusted for drug use, with absolute values of r ranging from .11 to .36. Thus, each maternal variable accounted for no more than about 13% of the variance in the child’s diagnosis. Note, however, that correlations may be attenuated due to non-normality remaining even after transformation and to highly unbalanced frequencies in dichotomous yes/no categories. Thus, there was a suggestion of a link between mother’s late recognition of pregnancy and lower general abilities score. Also, late recognition of pregnancy may have been associated with lowered INN vs. INI (naming vs inhibition) Contrast Scaled Score. Drinking in the third trimester may also have been linked with teacher reports of more attention problems. There also was an unexpected possible link between drinking in the first trimester and lower total dysmorphology score.
Further correlation analysis was undertaken to define the association of alcohol use to final diagnosis via binary logistic regression. Adjustments were again made to control for any tobacco and illicit drug use during pregnancy. Table 7 presents adjusted results for reported DDD three months prior to pregnancy to a FASD diagnosis. Reporting of three DDD prior to pregnancy is significantly associated (p<0.001) with an odds ratio of 17.5 (95% CI = 5.1-59.9). Therefore, the likelihood of an FASD diagnosis in this community, controlling for other diagnosis, is approximately 18 times greater for a child of a woman who drinks three drinks or more prior to pregnancy. Furthermore, the probability is estimated to be 12.7 times greater (95% CI = 3.4 – 46.5) for women who reported drinking four or more DDD, and 7.8 (95% CI = 2.6 – 24.1) for those reporting five or more DDD prior to pregnancy. The decline in odds for the higher DDD amounts is likely due to under-reporting by respondents who consumed substantially more (e.g., five DDD) yet reported three DDD.
Table 7.
Adjusted Binary Logistic Regression Analysis of FASD Diagnosis as a Function of Usual Number of Drinks per Drinking Day 3 Months Prior to Pregnancy: Pooled Over 25 Imputations – Southeastern County
| B | S.E. | Sig. | Odds Ratio |
95% CI | Fraction Missing Info. |
Relative Increase Variance |
Relative Efficiency |
||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| 1 drink per drinking day | −0.425 | 0.821 | 0.605 | 0.654 | 0.131 | 3.278 | 0.183 | 0.221 | 0.993 |
| 2 drinks per drinking day | 0.311 | 0.635 | 0.624 | 1.365 | 0.393 | 4.738 | 0.043 | 0.044 | 0.998 |
| 3 drinks per drinking day | 2.866 | 0.626 | 0.000 | 17.559 | 5.148 | 59.889 | 0.032 | 0.033 | 0.999 |
| 4 drinks per drinking day | 2.540 | 0.663 | 0.000 | 12.679 | 3.454 | 46.536 | 0.040 | 0.041 | 0.998 |
| 5+ drinks per drinking day | 2.060 | 0.573 | 0.000 | 7.845 | 2.551 | 24.130 | 0.031 | 0.032 | 0.999 |
| Covariates | |||||||||
| Used tobacco during pregnancy | 0.579 | 0.459 | 0.207 | 1.784 | 0.726 | 4.383 | 0.016 | 0.017 | 0.999 |
| Used any illicit drugs during pregnancy | 1.126 | 0.668 | 0.092 | 3.084 | 0.833 | 11.419 | 0.094 | 0.103 | 0.996 |
| Constant | −2.778 | 0.364 | 0.000 | 0.062 | 0.030 | 0.127 | 0.022 | 0.023 | 0.999 |
Reference group: non-drinkers
Prevalence of FASD
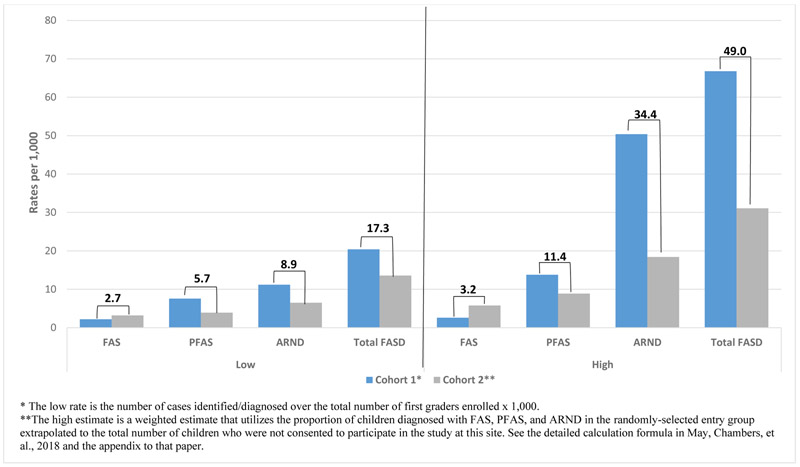
Figure 8 presents the Southeastern site prevalence based on the combined findings (mean) from both cohort samples. FAS prevalence cannot be calculated lower than 2.7 per 1,000 from diagnosed with CoFASP criteria, for this low rate is calculated as the number of cases as the numerator over the total children enrolled in first grade classes. The rate is more accurately estimated as 3.2 per 1,000 first grade children in this county (see formula and weighing techniques in May et al. (2018)). PFAS is not lower than 5.7 and is estimated to be 11.4 per 1,000. ARND is at least 8.9 per 1,000 and estimated at 34.4 per 1,000. Total FASD in this county is not lower than 17.3 per 1,000 but is more accurately estimated as 49.0 per 1,000.
Figure 8.
Southeastern Site Prevalence of FASD, Low and High Estimates from Cohorts 1 and 2 Combined
DISCUSSION
ACA epidemiology of FASD studies provide much data to digest and summarize. But for prevalence, the combined cohort rate for total FASD was estimated as 4.9% of the first-grade population in this County. This is the second lowest rate of FASD of the four CoFASP sites.
Physical Growth and Development
By definition, and as applied here, average growth and physical traits of the children with FASD differed significantly from controls on all cardinal criteria and many other minor anomalies at this site. Revised IOM diagnostic criteria and CoFASP cut-off criteria differentiated the physical attributes of the cases well from the randomly-selected, typically-developing, community comparison group. The physical traits form a continuum on most variables in which children with FAS have the most suppressed growth and more characteristic traits of FASD, followed by children with PFAS, ARND, and controls. Interestingly, all cases (n=8) of FAS were females and males were overreported among cases of ARND (see May et al. 2017b).
The neurobehavioral test battery also discriminated well FASD cases among diagnostic groups and children with any FASD from controls. Total dysmorphology, specific neurobehavioral tests and final diagnosis are associated with various alcohol exposure variables. We suspect that there is substantial under reporting of the quantity of alcohol use, especially the quantity consumed during pregnancy. This may be reflected by the logistic regression where the drinking data clustered to produce a higher odds ratio for a FASD diagnosis at three DDD than at higher DDD (four DDD and above). Nevertheless, reported pre-pregnancy quantity of DDD and drinking in each trimester were significantly different between mothers of children with FASD and controls indicating risk to the fetus for both structural growth and dysmorphology in the first trimester and for brain development throughout pregnancy. Furthermore, mothers of children with FASD reported recognizing that they were pregnant significantly later, on average, than other mothers, indicating possible exposure in the most critical period for structural effects that occur in the first 45 to 90 days of fetal development (Lipinski et al., 2012; Parnell et al., 2014; Sulik et al., 1981; Sulik, 2014). By definition, all children diagnosed with FAS and PFAS met the facial criteria for FAS with at least two of the three cardinal features (palpebral fissure ≤10th centile, smooth philtrum and/or thin vermilion border of the upper lip), and they had significantly smaller heads and BMI than normal controls. Although the physical growth of children with ARND was similar to that of other first graders, they have a higher occurrence of suppressed head growth and a non-statistically significant higher total dysmorphology score than controls. Minor anomalies other than the cardinal FAS features also play an important role in identifying affected children, as reflected in the dysmorphology score differences among the diagnostic groups. Total dysmorphology scores reflect a continuum of FASD diagnoses, for children with FAS had a total score of 15.8, PFAS = 10.8, ARND = 5.2 and controls a score of 4.4.
Neurobehavioral Characteristics
Although there were significant differences in virtually all areas of testing between children with a FASD vs. controls, intellectual, executive functioning, visual precision, and behavioral deficits were most markedly seen between the children diagnosed with ARND and controls. In the cognitive domain, significant differences were seen in general intellectual ability (GCA percentile), nonverbal reasoning and spatial skills percentiles (as assessed using the DAS-II). Similarly, executive functioning abilities were significantly lower for the ARND group compared with randomly selected controls in Naming and Inhibition (using the NEPSY-II). Behaviorally, the most significance between ARND and control children was seen in externalizing problems, including conduct problems, assessed by the Achenbach Teacher Report Form. While children with ARND are less affected by prenatal alcohol exposure in growth and dysmorphology, this study (and other CoFASP samples) indicate they are often as impaired or more impaired in the neurobehavioral domain as the other FASD groups.
Maternal Risk
Over 80% of mothers with children with FASD reported drinking prior to pregnancy, drinking 4.3 DDD. But only 24% of the mothers of a child with FASD directly reported first trimester drinking and 18% and 17% reported second and third trimester drinking. Average pregnancy recognition was at 7.7 weeks gestation among mothers of children with FASD significantly different from 5.7 for mothers of typically-developing controls. And fewer mothers of children with FASD report for prenatal care in the first trimester (although this later prenatal care is not statistically significant).
Other drug use was common among all groups of mothers, even controls. Thirty-eight percent (38%) of mothers of children with FASD smoked tobacco during the index pregnancy, compared to 13.2% of mothers of controls. And 17% used other drugs during the index pregnancy, compared to 4% of the mothers of controls. After tobacco, marijuana was the most frequently used other drug, used by 28% of mothers of a child with FASD and 2.8% of controls. Co-morbid use of alcohol and marijuana during pregnancy was 14.9% for mothers of children with FASD, and 28% of the mothers of children with ARND. Other drug use in pregnancy was highest among the mothers of children with ARND and higher lifetime use was highest among mothers of children with FAS and PFAS. The former use may account for the poorer neurobehavioral performance of the ARND group (Fish et al., 2019).
Reports of drinking three months prior to pregnancy were the most accurate measures in the domains relevant to FASD: quantity and frequency. Given the diagnostic outcomes of the children, direct reports of drinking during pregnancy may be unreliable. For example, alcohol use reported for first trimester seem to be much less accurate when compared with reports of pre-pregnancy drinking. By triangulating quantity and frequency of drinking prior to pregnancy with gestational week of pregnancy recognition, the reports of drinking during first trimester, more accurate estimates of maternal risk may be ascertained in this population. Therefore, it is not surprising that reports of three drinks or more prior to pregnancy and late recognition of pregnancy best predict risk for FASD in this population, even when smoking and other drug use are controlled in regression analysis.
Making Sense of the Prevalence Findings
The prevalence of total FASD found in this Southeastern site, both the low and the high estimates, exceed the old estimate of 1% (Sampson et al., 1997), and recent estimates from meta-analyses (Lange et al., 2017; Roozen et al., 2016). ACA methods were used consistently and recorded higher rates of FASD, in this case with revised IOM criteria and CoFASP cut-off criteria. Chasnoff and colleagues (2015) reported that 80% to 87% of children with FASD may be undiagnosed or mis-diagnosed in the first years of life, and therefore ACA brings forth a higher prevalence than other methods of prevalence estimations.
Unlike our studies of less economically developed communities in South Africa where a larger percentage of cases are full-blown FAS, the large proportion of PFAS and ARND cases to FAS cases in this population is notable. It may indicate that in this U.S. community many children are negatively affected by prenatal drinking by three DDD both in the critical period of the first trimester, but throughout pregnancy. The high proportion of less dysmorphic cases (PFAS and ARND) diagnosed seems indicative of: 1) a community with a range of drinking styles, as opposed to a dominance of chronic alcohol consumption, 2) a population that has norms allowing, or encouraging, drinking, at least until official recognition of pregnancy, and 3) co-morbid drug use before and during the index pregnancies. And if similar trends are at work in this community to that reported for the U.S. in the past 20 years, these FASD rates may be higher than 20 years ago (Grant et al., 2015; Hasin et al., 2019; Shmulewitz and Hasin, 2019). Furthermore, the rates of alcohol use reported by controls at this site parallel those of the U.S. Centers for Disease Control (Denny et al., 2009; Tan et al., 2015) where 50 to 54% of women of childbearing age drank alcohol, 18% binge drank and 7% of pregnancies were alcohol exposed. Therefore, a rate of FASD of 4.9% in this site is generally supported by other maternal drinking data. Furthermore, SES variables of maternal risk in this community did not differentiate significantly among the FASD and control groups. The risk of FASD is spread throughout various SES strata of the community. The CDC and others also report that half of all pregnancies are not planned in the U.S. population today (Green et al., 2016), which further supports the findings of late pregnancy recognition and continued pre-pregnancy drinking patterns.
One unique variable that distinguished mothers of children with FASD from controls, at this site, was spirituality, where the former group rated themselves as 5.6 on a scale of spirituality compared to 6.9 for the latter (p<.001). Furthermore ecological data presented in Table 1 indicate that this state is one: with a high percentage of the population that is affiliated with a formal religion (Christian, Jewish, or Muslim = 80%), that rates religion as very or somewhat important (84%), and the percentage reporting “no formal religion” is lower than the overall U.S. population (20% vs. 22.8%) (Pew Research Center, 2015). This community is in the changing, but historical “Bible Belt” of the U.S. The community with the lowest prevalence rate in CoFASP had a similar spirituality profile (Midwest site), and the two CoFASP sites with the highest rates had the highest percentage of “unaffiliated” people /those who report no religious affiliation, who are referred to as “nones” (Rocky Mountain, 30% and Pacific Southwest, 27%). Each of the three formal religions most practiced in America have historical normative orientation which encourages abstinence from alcohol, or at least moderation of use (Fjær et al., 2016; Meyers et al., 2017; Room et al., 2016). Norms emanating from a formal affiliation with an established religion promote abstinence or moderation of drinking during pregnancy. This may play a role in regulating the prevalence of FASD at this site.
Limitations
The consent rate for this site was slightly lower (50%) than other samples that we have collected in the U.S.; but it is similar to samples we completed in Italy (May et al., 2011a, 2006), and higher than most attempted elsewhere by other researchers in Europe and North America. Often when one or two teachers or key administrators in one or more schools are not enthusiastic, lower consent rates result. But there was also a relatively high non-completion rate from maternal interviews. Scheduling issues for two income families and that was encountered as the deadline for completing this study approached, emphasis of the staff was focused on completing interviews for those with preliminary diagnosis of “rule out” an FASD. A second limitation is suspected underreporting of prenatal drinking. The experienced interviewers did an exceptional job of interviewing all but two mothers of the children diagnosed with a FASD, at this site, but only 50% of the mothers of the children with FAS directly admitted to drinking during the pregnancy, 12.5% reported drinking during the first trimester, and none reported drinking during the second and third trimesters. Similarly, drinking reports of quantity, frequency, and timing from mothers of children with PFAS seem quite low given the amount of dysmorphology recorded in their children. Even though blinded, the interviewers had estimated at interview completion, that at least three-fourths of the mothers of a child with FAS and PFAS were not fully forthcoming regarding their quantity or frequency of alcohol use in pregnancy. Third, by initiating this study in both cohorts with screening of child physical growth and head circumference, the number of children with ARND (less dysmorphic) may have been under identified, especially given the reluctance of some mothers to report prenatal alcohol use. Therefore, the rate of ARND may be somewhat higher than reported here. ARND cases may be most accurately estimated from methods that utilize a very large random sample and can obtain more accurate data on alcohol use in the index pregnancy.
CONCLUSION
This site had the second lowest rate of FASD of the CoFASP sites. There was no difference in the prevalence of FASD by race, Hispanic ethnicity, or socioeconomic status. Over 80% of all mothers with children with FASD reported drinking prior to pregnancy at a usual level of pre-pregnancy drinking of 4.3 DDD. Pregnancy recognition was later among mothers of children with FASD than controls, and co-morbid use of other drugs were common during pregnancy and lifetime, especially alcohol and marijuana during pregnancy for mothers of children with FASD.
Supplementary Material
What’s Known on This Subject: There are few studies of the characteristics of children with FAS and FASD and their mothers within the general population of the United States. Additionally, most studies of FASD prevalence and maternal and child characteristics have been undertaken using passive methods of case ascertainment or methods which are selective and underestimate the rates of FASD. Furthermore, most clinical and epidemiological studies of FASD do not provide a detailed description and overview of child physical and neurodevelopmental traits. Furthermore, maternal risk factors are rarely associated with fully diagnosed children with FASD. Here, children with FASD are described and compared to normally-developing children and their mothers in the same population.
What This Study Adds: Using active case ascertainment (ACA) methods among children in a representative, ethnically and racially diverse county in the Southeastern United States, child traits of all diagnoses within the continuum of FASD are described and compared to typically-developing children from the same community and cohorts. The results of two ACA cohort samples in two different cohorts of first grade students from the same county are presented here. The traits provide clear differentiation of the diagnostic groups. The prevalence of FASD in this community was found to be substantially higher than most previous estimates for the general U.S. population, and lower than two other sites in the same study in the USA.
ACKNOWLEDGEMENTS
This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grant UO1 AA019894. Marcia Scott, Ph.D., Kenneth Warren, Ph.D., Faye Calhoun, D.P.A., and the late T-K Li, M.D. of NIAAA provided intellectual guidance, encouragement, and support for prevalence studies of FASD for many years. Our deepest thanks are extended to the superintendent of schools, Boards of Education, administrators, principals, psychologists, and teachers of the school system in the study communities who have hosted and assisted in the research process over the years. Their professional support, guidance, and facilitation have been vital to the success of this study. We are also grateful for the advice and participation in the planning and implementation of the project by the CoFASP Advisory Committee members who were led by Marcia Scott, Ph.D., NIAAA Project Officer: Judith Arroyo, Ph.D., Michael Charness, M.D., William Dunty, Ph.D., Daniel Falk, Ph.D., Dale Herald, M.D., Ph.D., and Edward Riley, Ph.D. We dedicate this paper to our deceased colleague, Jason Blankenship, Ph.D. who invested much effort into this project before his untimely death in October 2013.
Protocols and consent forms were approved by The University of New Mexico School of Medicine, HRRC #96-209, #06-199; #10-342; and the University of North Carolina, #11-0717. Active consent for children to participate was obtained from parents/guardians. Maternal interviews required a separate consent form.
Funding Source: This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grant UO1 AA019894 as part of the Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP) consortium. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ACA
active case ascertainment
- ARBD
alcohol-related birth defects
- ARND
alcohol-related neurodevelopmental disorder
- BMI
Body Mass Index
- CDC
Centers for Disease Control and Prevention
- CoFASP
Collaboration on Fetal Alcohol Spectrum Disorders Prevalence
- DDD
drinks per drinking day
- FASD
fetal alcohol spectrum disorders
- FAS
fetal alcohol syndrome
- ICD
inner canthal distance
- IPD
inter pupillary distance
- IOM
Institute of Medicine
- OFC
occipitofrontal (head) circumference
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PFAS
partial fetal alcohol syndrome
- PFL
palpebral fissure length
- SES
socioeconomic status
Footnotes
Financial Disclosure: The authors have no financial relationship relevant to this article to disclose.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Aase JM (1994) Clinical Recognition of FAS: Difficulties of Detection and Diagnosis. Alcohol Health Res World 18:5–9. [PMC free article] [PubMed] [Google Scholar]
- Abel EL, Hannigan JH (1995) Maternal risk factors in fetal alcohol syndrome: Provocative and permissive influences. Neurotoxicol Teratol 17:445–462. [DOI] [PubMed] [Google Scholar]
- Achenbach T, Rescorla L (2001) Manual For The ASEBA School-Age Forms And Profiles. Burlington, VT, University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Alvik A, Haldorsen T, Lindemann R (2006) Alcohol consumption, smoking and breastfeeding in the first six months after delivery. Acta Paediatr 95:686–693. [DOI] [PubMed] [Google Scholar]
- America’s Changing Religious Landscape ∣ Pew Research Center (n.d.). Available at: https://www.pewforum.org/2015/05/12/americas-changing-religious-landscape/ Accessed November 15, 2019.
- America’s Health Rankings Annual Report (2015) . Minnetonka, Minnesota. [Google Scholar]
- Astley SJ, Clarren SK (2000) Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol 35:400–410. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC (2017) Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol Clin Exp Res 41:1004–1011. [DOI] [PubMed] [Google Scholar]
- Balaraman S, Schafer JJ, Tseng AM, Wertelecki W, Yevtushok L, Zymak-Zakutnya N, Chambers CD, Miranda RC (2016) Plasma miRNA Profiles in Pregnant Women Predict Infant Outcomes following Prenatal Alcohol Exposure. PLoS One 11:e0165081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE, Beery NA (2004) The Beery-Buktenica Development Test of Visual-Motor Integration, Fifth Edit. ed. San Antonio, TX, Pearson Assessment. [Google Scholar]
- Blume SB (1985) Is social drinking during pregnancy harmless? There is reason to think not. Adv Alcohol Subst Abuse 5:209–19. [DOI] [PubMed] [Google Scholar]
- Bracken BA (1998) Braken Basic Concept Scale - Revised. San Antonio, Texas. [Google Scholar]
- Calhoun F, Attilia ML, Spagnolo PA, Rotondo C, Mancinelli R, Ceccanti M (2006) National Institute on Alcohol Abuse and Alcoholism and the study of fetal alcohol spectrum disorders. The International Consortium. Ann Ist Super Sanita 42:4–7. [PubMed] [Google Scholar]
- CDC - BRFSS (n.d.). Available at: https://www.cdc.gov/brfss/ Accessed November 15, 2019.
- Ceccanti M, Fiorentino D, Coriale G, Kalberg WO, Buckley D, Hoyme HE, Gossage JP, Robinson LK, Manning M, Romeo M, Hasken JM, Tabachnick B, Blankenship J, May PA (2014) Maternal risk factors for fetal alcohol spectrum disorders in a province in Italy. Drug Alcohol Depend 145:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L (2015) Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics 135:264–70. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N (2005) Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ 172:S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderson M, Fineman RM (2001) Screening for fetal alcohol syndrome in primary schools: A feasibility study. Teratology 63:3–10. [DOI] [PubMed] [Google Scholar]
- Coles CD, Gailey AR, Mulle JG, Kable JA, Lynch ME, Jones KL (2016) A Comparison Among 5 Methods for the Clinical Diagnosis of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res 40:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, LeBlanc N, Loock CA, Lutke J, Mallon BF, McFarlane AA, Temple VK, Rosales T, Canada Fetal Alcohol Spectrum Disorder Research Network (2016) Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. Can Med Assoc J 188:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D (1990) Five-year reliability of self-reported alcohol consumption. J Stud Alcohol 51:68–76. [DOI] [PubMed] [Google Scholar]
- Denny CH, Acero CS, Naimi TS, Kim SY (2019) Consumption of Alcohol Beverages and Binge Drinking Among Pregnant Women Aged 18–44 Years — United States, 2015–2017. MMWR Morb Mortal Wkly Rep 68:365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CH, Tsai J, Floyd FL, Green PP (2009) Alcohol use among pregnant and nonpregnant women of childbearing age - United States, 1991–2005 MMWR Morb Mortal Wkly Rep 58:529–532. [PubMed] [Google Scholar]
- Elliott CD (2007) Differential Ability Scales-II (DAS-II). San Antonio, Texas, Harcourt Assessment. [Google Scholar]
- Ervalahti N, Korkman M, Fagerlund Å, Autti-Rämö I, Loimu L, Hoyme HE (2007) Relationship between dysmorphic features and general cognitive function in children with fetal alcohol spectrum disorders. Am J Med Genet Part A 143A:2916–2923. [DOI] [PubMed] [Google Scholar]
- Esper LH, Furtado EF (2014) Identifying maternal risk factors associated with Fetal Alcohol Spectrum Disorders: a systematic review. Eur Child Adolesc Psychiatry 23:877–889. [DOI] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Zhang C, Boschen KE, Boa-Amponsem O, Mendoza-Romero HN, Tarpley M, Chdid L, Mukhopadhyay S, Cole GJ, Williams KP, Parnell SE (2019) Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction. Sci Rep 9:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjær EG, Pedersen W, von Soest T, Gray P (2016) When is it OK to be drunk? Situational and cultural variations in the acceptability of visible intoxication in the UK and Norway. Int J Drug Policy 29:27–32. [DOI] [PubMed] [Google Scholar]
- Fortin M, Muckle G, Jacobson SW, Jacobson JL, Belanger RE (2017) Alcohol use among Inuit pregnant women: Validity of alcohol ascertainment measures over time. Neurotoxicol Teratol 64:73–78. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013. JAMA Psychiatry 74:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder. JAMA Psychiatry 72:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PP, McKnight-Eily LR, Tan CH, Mejia R, Denny CH (2016) Vital Signs : Alcohol-Exposed Pregnancies — United States, 2011–2013. MMWR Morb Mortal Wkly Rep 65:91–97. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V (2010) A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 44:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Shmulewitz D, Keyes K (2019) Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18–44: 2002–2017. Drug Alcohol Depend 205:107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme H, May P, Kalberg W, Kodituwakku P, Gossage J, Trujillo P, Buckley D, Miller J, Aragon A, Khaole N, Viljoen D, Jones K, Robinson L (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM; (2017) IBM SPSS Statistics for Windows. [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet (London, England) 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2001) Pre-pregnancy drinking: how drink size affects risk assessment. Addiction 96:1199–1209. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2000) An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse 12:67–78. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Kerr WC (2008) Accuracy of Photographs to Capture Respondent-Defined Drink Size. J Stud Alcohol Drugs 69:605–610. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS (2011) Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res 35:2101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Miech R (2013) Age, period, and cohort effects in heavy episodic drinking in the US from 1985 to 2009. Drug Alcohol Depend 132:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC (1994) Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Public Health 84:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S (2007) NEPSY (NEPSY-II), Second ed. San Antonio, Texas, Pearson Assessment. [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr 171:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallee RA, Yi H (2011) Surveillance Report #92: Apparent per alcohol consumption: national, state, and regional trends, 1977–2009.
- Lipinski RJ, Hammond P, O’Leary-Moore SK, Ament JJ, Pecevich SJ, Jiang Y, Budin F, Parnell SE, Suttie M, Godin EA, Everson JL, Dehart DB, Oguz I, Holloway HT, Styner MA, Johnson GA, Sulik KK (2012) Ethanol-Induced Face-Brain Dysmorphology Patterns Are Correlative and Exposure-Stage Dependent. PLoS One 7:e43067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, De Vries M, Marais A-S, Kalberg W, Buckley D, Adnams C, Hasken J, Tabachnick B, Robinson L, Manning M, Bezuidenhout H, Adam M, Jones K, Seedat S, Parry C, Hoyme H (2017a) Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa. Int J Environ Res Public Health 14:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE (2014) Prevalence and Characteristics of Fetal Alcohol Spectrum Disorders. Pediatrics 134:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais A-S, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S (2013a) Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res 37:818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDH, Hoyme HE, Tabachnick B, Seedat S (2013b) Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend 133:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D (2000) Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health 90:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Daniel F, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018) Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA - J Am Med Assoc 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, de Vries MM, Marais AS, Kalberg WO, Adnams CM, Hasken JM, Tabachnick B, Robinson LK, Manning MA, Jones KL, Hoyme D, Seedat S, Parry CDH, Hoyme HE (2016a) The continuum of fetal alcohol spectrum disorders in four rural communities in south africa: Prevalence and characteristics. Drug Alcohol Depend 159:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Coriale G, Kalberg WO, Eugene Hoyme H, Aragón AS, Buckley D, Stellavato C, Phillip Gossage J, Robinson LK, Jones KL, Manning M, Ceccanti M (2011a) Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: New estimated rates are higher than previous estimates. Int J Environ Res Public Health 8:2331–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Phillip Gossage J, Kalberg WO, Eugene Hoyme H, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M (2006) Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res 30:1562–1575. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP (2001) Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Heal 25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-S, Hendricks LS, Croxford JA, Viljoen DL (2005) Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health 95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE (2009) Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 15:176–192. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL (2007) The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend 88:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL (2008) Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res 32:738–753. [DOI] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Gossage JP, Robinson LK, Manning M, Hoyme HE (2015) Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend 155:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Marais AS, de Vries MM, Kalberg WO, Buckley D, Hasken JM, Adnams CM, Barnard R, Joubert B, Cloete M, Tabachnick B, Robinson LK, Manning MA, Jones KL, Bezuidenhout H, Seedat S, Parry CDH, Hoyme HE (2016b) The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend 168:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick B, Hasken JM, Marais AS, de Vries MM, Barnard R, Joubert B, Cloete M, Botha I, Kalberg WO, Buckley D, Burroughs ZR, Bezuidenhout H, Robinson LK, Manning MA, Adnams CM, Seedat S, Parry CDH, Hoyme HE (2017b) Who is most affected by prenatal alcohol exposure: Boys or girls? Drug Alcohol Depend 177:258–267. [DOI] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, Manning M, Buckley D, Hoyme HE (2011b) Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend 119:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Brown Q, Grant BF, Hasin D (2017) Religiosity, race/ethnicity, and alcohol use behaviors in the United States. Psychol Med 47:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft Excel (2016).
- Okulicz-Kozaryn K, Borkowska M, Brzozka K (2017) FASD Prevalence among Schoolchildren in Poland. J Appl Res Intellect Disabil 30:61–70. [DOI] [PubMed] [Google Scholar]
- Parnell SE, Holloway HE, Baker LK, Styner MA, Sulik KK (2014) Dysmorphogenic effects of first trimester-equivalent ethanol exposure in mice: a magnetic resonance microscopy-based study. Alcohol Clin Exp Res 38:2008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic G, Barisic I (2013) Prevalence of fetal alcohol syndrome and maternal characteristics in a sample of schoolchildren from a rural province of Croatia. Int J Environ Res Public Health 10:1547–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic G, Barisic I (2010) FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod Toxicol 29:237–241. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Poznyak V, Chudley AE, Shield KD, Reynolds JN, Murray M, Rehm J (2019) Population-based prevalence of fetal alcohol spectrum disorder in Canada. BMC Public Health 19:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room R, Callinan S, Dietze P (2016) Influences on the drinking of heavier drinkers: Interactional realities in seeking to “change drinking cultures”. Drug Alcohol Rev 35:13–21. [DOI] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Koek G, Curfs L (2018) Systematic literature review on which maternal alcohol behaviours are related to fetal alcohol spectrum disorders (FASD). BMJ Open 8:e022578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L (2016) Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res 40:18–32. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM (1997) Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology 56:317–326. [DOI] [PubMed] [Google Scholar]
- Shmulewitz D, Hasin DS (2019) Risk factors for alcohol use among pregnant women, ages 15–44, in the United States, 2002 to 2017. Prev Med (Baltim) 124:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M (2001) Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse 36:313–331. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI (1988) The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol 49:225–232. [DOI] [PubMed] [Google Scholar]
- Sparrow SA, Cicchetti D V, Balla DA (2005) Vineland Adaptive Behavior Scales, Second ed. Bloomington, Minnesota. [Google Scholar]
- Stratton K, Howe C, Battaglia F (1996) Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington DC. [Google Scholar]
- Sulik K, Johnston M, Webb M (1981) Fetal alcohol syndrome: embryogenesis in a mouse model. Science (80- ) 214:936–938. [DOI] [PubMed] [Google Scholar]
- Sulik KK (2014) Fetal alcohol spectrum disorder In: Handbook of Clinical Neurology , pp 463–475. [DOI] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D (2015) Alcohol use and binge drinking among women of childbearing age - United States, 2011–2013. MMWR Morb Mortal Wkly Rep 64:1042–1046. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau QuickFacts: United States (n.d.). Available at: https://www.census.gov/quickfacts/fact/table/US/PST045218 Accessed November 15, 2019.
- Urban M, Chersich MF, Fourie L-A, Chetty C, Olivier L, Viljoen D (2008) Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. S Afr Med J 98:877–882. [PubMed] [Google Scholar]
- Urban MF, Olivier L, Viljoen D, Lombard C, Louw JG, Drotsky L-M, Temmerman M, Chersich MF (2015) Prevalence of fetal alcohol syndrome in a South African city with a predominantly Black African population. Alcohol Clin Exp Res 39:1016–1026. [DOI] [PubMed] [Google Scholar]
- Viljoen D, Croxford J, Gossage JP, Kodituwakku PW, May PA (2002) Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: a case control study. J Stud Alcohol 63:6–17. [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole NCO, Kodituwakku P, Asante KO, Findlay R, Quinton B, Marais A-S, Kalberg WO, May PA (2005) Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol 66:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- What Is A Standard Drink? ∣ National Institute on Alcohol Abuse and Alcoholism (NIAAA) (n.d.). Available at: https://www.niaaa.nih.gov/what-standard-drink Accessed November 18, 2019.
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundstrom Poromaa I (2008) Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT-a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol 198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.