Abstract
Natural gas extraction (NGE) has expanded rapidly in the United States in recent years. Despite concerns, there is little information about the effects of NGE on air quality or personal exposures of people living or working nearby. Recent research suggests NGE emits polycyclic aromatic hydrocarbons (PAHs) into air. This study used low-density polyethylene passive samplers to measure concentrations of PAHs in air near active (n=3) and proposed (n=2) NGE sites. At each site, two concentric rings of air samplers were placed around the active or proposed well pad location. Silicone wristbands were used to assess personal PAH exposures of participants (n=19) living or working near the sampling sites. All samples were analyzed for 62 PAHs using GC-MS/MS, and point sources were estimated using the fluoranthene/pyrene isomer ratio. ∑PAH was significantly higher in air at active NGE sites (Wilcoxon rank sum test, p < 0.01). PAHs in air were also more petrogenic (petroleum-derived) at active NGE sites. This suggests that PAH mixtures at active NGE sites may have been affected by direct emissions from petroleum sources at these sites. ∑PAH was also significantly higher in wristbands from participants who had active NGE wells on their properties than from participants who did not (Wilcoxon rank sum test, p < 0.005). There was a significant positive correlation between ∑PAH in participants’ wristbands and ∑PAH in air measured closest to participants’ homes or workplaces (simple linear regression, p < 0.0001). These findings suggest that living or working near an active NGE well may increase personal PAH exposure. This work also supports the utility of the silicone wristband to assess personal PAH exposure.
Capsule:
Living or working near an active natural gas extraction well may increase an individual’s PAH exposure.
Keywords: monitoring, organic contaminant, air pollution, fracking
Introduction
Natural gas extraction (NGE) from shale has expanded rapidly in the United States in the last 15 years. This is largely due to technological improvements to hydraulic fracturing and horizontal drilling (colloquially known as “fracking”), which liberate previously inaccessible gas reserves from shale (EIA 2011).
There is a need for data that directly assesses the environmental and public health impacts of NGE (Adgate, Goldstein et al. 2014, Goldstein, Brooks et al. 2014, Penning, Breysse et al. 2014). Some studies have acknowledged that reduced air quality may be the most significant risk to communities near NGE (McKenzie, Witter et al. 2012, Litovitz, Curtright et al. 2013, Adgate, Goldstein et al. 2014, Bunch, Perry et al. 2014, Colborn, Schultz et al. 2014, McKenzie, Guo et al. 2014, Roy, Adams et al. 2014, Shonkoff, Hays et al. 2014, Boyle, Payne-Sturges et al. 2016, Fawole, Cai et al. 2016, Paulik, Donald et al. 2016, Rasmussen, Ogburn et al. 2016). There is evidence that NGE emits methane (Brandt, Heath et al. 2014, Brantley, Thoma et al. 2014), volatile organic compounds (VOCs) (McKenzie, Witter et al. 2012, Pétron, Frost et al. 2012, Macey, Breech et al. 2014, Roy, Adams et al. 2014, Marrero, Townsend-Small et al. 2016) and semi-volatile organic compounds (SVOCs) (Colborn, Schultz et al. 2014, Paulik, Donald et al. 2016). Recent studies have concluded that exposure to NGE emissions may pose health risks, but that many important data gaps remain (Shonkoff, Hays et al. 2014, Ward, Eykelbosh et al. 2016).
One class of SVOCs that has been measured in air near NGE is polycyclic aromatic hydrocarbons (PAHs) (Colborn, Schultz et al. 2014, Paulik, Donald et al. 2016, Elliott, Trinh et al. 2017). PAHs are pervasive environmental pollutants that are commonly associated with fossil fuel production (Ana, Sridhar et al. 2012). PAHs are also commonly associated with and adverse health outcomes such as increased cancer risk (Menzie, Potocki et al. 1992, Baird, Hooven et al. 2005), respiratory distress (Miller, Garfinkel et al. 2004, Padula, Balmes et al. 2015), and developmental effects (Perera, Li et al. 2009, Perera and Herbstman 2011).
PAHs exist in two states in air: freely dissolved in the “gas phase” and bound to particles in the “particulate phase”. While much research on health effects of inhaling PAHs has focused only on PAHs measured in the particulate phase, a growing body of evidence suggests that PAHs in the gas phase also contribute to the toxicity of inhaled PAH mixtures (Tsai, Shieh et al. 2002, Liu, Tao et al. 2007, Samburova, Zielinska et al. 2017).
While carcinogenic potencies are typically higher for individual PAHs with higher molecular weights, lower molecular weight PAHs are often present at significantly higher concentrations in the gas phase; this can increase the contribution of gas phase PAHs to the total carcinogenic potency of a PAH mixture (Liu, Tao et al. 2007). In a recent review, Samburova et al. evaluated 13 studies and concluded that only measuring particulate phase PAHs significantly underrepresented the carcinogenic potency of PAH mixtures compared to measuring both the gas and particulate phases (Samburova, Zielinska et al. 2017). Samburova et al. made the recommendation that “gas-phase PAHs be included because of their strong contribution to the total [carcinogenic potency]” (Samburova, Zielinska et al. 2017). These findings provide rationale for measuring exposure to the fraction of PAHs in the gas phase, even if data for the particulate fraction is not available.
Increased understanding of the environmental fate of PAHs from NGE would answer questions about the potential environmental health impacts of these emissions.
As air quality sampling moves toward more cost-effective and user-friendly techniques (Snyder, Watkins et al. 2013), passive air sampling is becoming increasingly more relevant. Low-density polyethylene (LDPE) passive samplers sequester freely dissolved lipophilic compounds through passive diffusion in a time-integrated manner (Huckins, Petty et al. 2006, Anderson, Sethajintanin et al. 2008, Lohmann 2012, O’Connell, McCartney et al. 2014, Paulik, Smith et al. 2016, Tidwell, Paulik et al. 2017). Since the development of LDPE as an air sampler in the 1990s, many studies have demonstrated its ability to measure gas phase PAHs from air (Petty, Huckins et al. 1993, Prest, Huckins et al. 1995, Bartkow, Hawker et al. 2004, Khairy and Lohmann 2012, Paulik, Donald et al. 2016). In this study, stationary LDPE passive air samplers were used to perform spatial assessments of PAH concentrations in air at 5 NGE sites: 3 sites with active NGE wells and 2 proposed NGE sites. This sampling design allowed for assessment of emissions from the point sources and spatial assessment of PAHs in air at these sites.
In addition to questions surrounding the environmental fate of PAHs emitted from NGE, there is concern regarding human health and personal exposure to PAHs emitted from NGE (Penning, Breysse et al. 2014, Werner, Vink et al. 2015). Some studies have used data from stationary monitors to estimate community-level health impacts (McKenzie, Witter et al. 2012, Bunch, Perry et al. 2014, Colborn, Schultz et al. 2014, Marrero, Townsend-Small et al. 2016, Paulik, Donald et al. 2016), while others have used health records or questionnaire responses to approximate individual health impacts of NGE (Brasier, Filteau et al. 2011, Bamberger and Oswald 2014, McKenzie, Guo et al. 2014, Rabinowitz, Slizovskiy et al. 2015, Rasmussen, Ogburn et al. 2016, Tustin, Hirsch et al. 2016). Still others have predicted exposures associated with NGE from emissions inventories or known toxicity information of chemicals reportedly used in NGE (Colborn, Kwiatkowski et al. 2011, Roy, Adams et al. 2014, Boyle, Payne-Sturges et al. 2016, Elliott, Trinh et al. 2017). Personal monitoring is an effective tool for assessing individuals’ contaminant exposures, as personal monitors yield more accurate exposure estimates than approximating exposure from questionnaires or extrapolating exposure from stationary monitoring data (Bohlin, Jones et al. 2007, Paulik and Anderson In press). To date, no study has directly measured personal PAH exposures of people living or working near active NGE wells.
Personal exposure to PAHs and other SVOCs has previously been assessed by active and passive personal monitors (Perera, Rauh et al. 2003, Bohlin, Jones et al. 2007, Bohlin, Jones et al. 2010, Zhu, Wu et al. 2011, Herbstman, Tang et al. 2012). The silicone wristband (hereafter “wristband”), is a novel personal sampler that absorbs VOCs and SVOCs (O’Connell, Kincl et al. 2014, Donald, Scott et al. 2016, Kile, Scott et al. 2016, Bergmann, North et al. 2017, Dixon, Scott et al. 2018). The wristband is lightweight, small, and easy to use, and it does not require a motor or batteries. In this study, 23 participants living or working near the 5 stationary air sampling sites wore wristbands to assess their personal PAH exposures.
This study combined stationary and personal passive sampling techniques to: a) compare PAH concentrations in air at active and proposed NGE sites, b) compare sources of PAHs at active and proposed NGE sites, and c) assess the contribution of active NGE wells to personal PAH exposure.
Materials and Methods
Site Description
This study was conducted in Carroll County and bordering counties of rural eastern Ohio, in the United States. This region has been heavily affected by the U.S. natural gas boom, as it sits atop natural gas and oil reserves in both the Utica and Marcelles shale formations. In 2014 Carroll County had the highest number of active wells in Ohio (Carlton, Little et al. 2014). This historically rural region was expected to have limited pre-existing anthropogenic sources of pollution, relative to an industrial area or a city. The sampling was conducted on individual residential properties. The exact sampling locations are therefore not provided to protect the confidentiality of the participants. Landowners for stationary sampling and participants for personal sampling were identified through collaboration with a local community group. This study was approved by the Institutional Review Boards (IRB) at the University of Cincinnati (UC) and Oregon State University (OSU); UC was the IRB of record.
Sampling Design
Stationary passive LDPE air samplers (hereafter referred to as LDPE) were deployed at five sites that were permitted for NGE activity. At the time of the study three of those sites had active NGE well pads at the sites, with NGE activity occurring on the well pads during the sampling period (hereafter referred to as “active” sites, labeled as sites A1-A3). These sites also had small service roads leading to the NGE well pads. The remaining two sites had neither well pads nor NGE activity occurring at the time of sampling (hereafter referred to as “proposed” sites, labeled as sites P1-P2). Sites were selected from a prior air sampling campaign(Paulik, Donald et al. 2016) based on their NGE status. Landowners from each site agreed to have air samplers and communicate with study team regarding activity on the sites (e.g., farming activity, new NGE activity, planting/grazing needs). During sampler deployment, researchers trained landowners in protocols necessary to maintain sample integrity while retrieving and mailing LDPE to OSU for analysis.
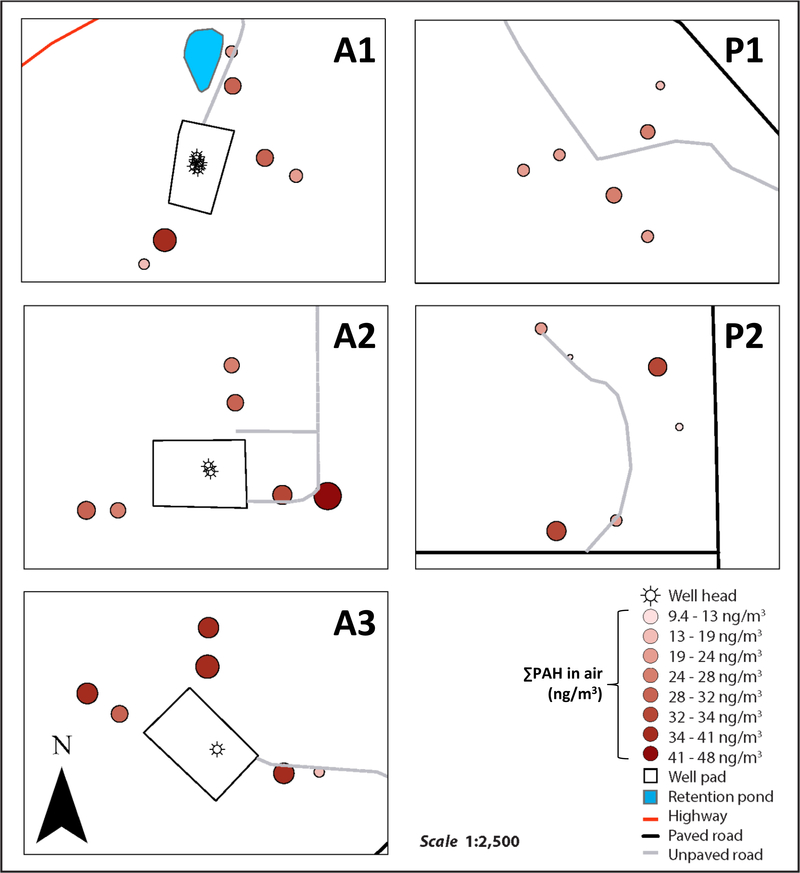
At each site, six (6) stationary air sampler cages were arranged in two (2) concentric rings (each containing 3 LDPE samplers) around either the active NGE well pad or the proposed NGE well pad location (Figure 1). This design yielded a total of 30 samplers. The inner and outer rings of samplers were 55–60 m and 112–122 m, respectively, from the edges of the active or proposed well pads at each site. Researchers worked with landowners to choose specific locations for sampling cages within each site. Care was taken, as much as possible, to minimize both a) inputs from potentially confounding PAH sources and b) inconvenience to landowners. Sampling occurred from May to June, 2014 and the average air temperature was 20±0.67°C.
Figure 1.
Schematic of spatial sampling design at each of the five stationary LDPE air sampling sites. Each circle (n=6) represents one sampling cage, each containing 3 LDPE passive air sampling strips. Sampling cages were arranged in two approximately concentric circles surrounding either the well pad, or the proposed well pad location, at each of the 5 stationary air sampling sites.
Using a targeted recruitment approach, 23 participants wore wristbands during the same air sampling period. Each participant wore one wristband throughout the three (3) week sampling period. During deployment, researchers trained participants in protocols to preserve sample integrity while wearing the wristbands and returning them to OSU for analysis. This technique of training participants to complete sampling protocols has been previously used (Rohlman, Syron et al. 2015, Paulik, Donald et al. 2016).
Participants were also asked to complete a daily exposure log in order to identify other PAH exposure sources, such as cigarette smoking, exhaust from heavy machinery, and wood smoke. Summary statistics were calculated for each participant to gauge exposure to these other common sources of PAHs.
Sample Preparation, Cleaning, and Extraction
LDPE and wristband samplers were transported in airtight polytetrafluoroethylene bags. LDPE was cleaned before deployment using hexanes as described in Anderson et al. 2008 (Anderson, Sethajintanin et al. 2008). Each LDPE strip was infused with performance reference compounds (PRCs) to enable calculation of in situ sampling rates and time-integrated air concentrations, as described in Sower and Anderson and references therein (Sower and Anderson 2008). PRCs used in this study were fluorene-d10, pyrene-d10 and benzo[b]fluoranthene-d12. PRCs were spiked into LDPE at 1–19 μg per strip. Samplers were cleaned after deployment in two isopropanol baths and subsequently extracted using two dialyses with n-hexane at room temperature (Anderson, Sethajintanin et al. 2008). Silicone wristband samplers were prepared as previously described. After deployment, wristbands were cleaned in 18 MΩ∗cm water to remove any debris from the surface and then quickly rinsed in isopropanol (O’Connell, Kincl et al. 2014). Wristbands extracted using two dialyses with ethyl acetate at room temperature (O’Connell, Kincl et al. 2014).
Prior to extraction, all samples were spiked with deuterated PAHs extraction surrogate standards (specified in SI Table S1), all concentrations are surrogate corrected. All extracts were quantitatively concentrated to 1 mL using TurboVap closed cell evaporators, transferred to amber chromatography vials, and stored at –20°C until analysis.
Chemical Analysis
Samples were analyzed for 62 PAHs using an Agilent 7890A gas chromatograph interfaced with an Agilent 7000 GC/MS-MS. An Agilent Select PAH column was used and each PAH was calibrated with a curve of at least five points, with correlations ≥ 0.99 (Anderson, Szelewski et al. 2015). Limits of detection (LODs) in air were ≤0.50 ng/m3, and LODs in wristbands ranged from 0.050 to 1.4 ng/g wristband, specified in SI Table S1.
Air Concentration Calculations for LDPE
Gas phase air concentrations (ng/m3) of PAHs measured in LDPE were calculated using PRCs. In situ sampling rates (RS) for each PAH were calculated as described by Huckins et al (Huckins, Petty et al. 2006) incorporating deployment time, the temperature-corrected sampler-air partition coefficient (Ksa(T)), logKoa, and initial amount of PRCs. LogKoa values and the selected PRC for each compound are in Table S2. Temperature-corrected Ksa values (Ksa(T)) were calculated using a modified van ‘t Hoff equation (Khairy and Lohmann 2012). Average PRC concentrations of fluorene-d10, pyrene-d10 and benzo[b]fluoranthene-d12 retained in LDPE samplers after deployment were 0.05, 44 and 61% of the initial concentrations, respectively. Previous studies have suggested that sampling rates are estimated most precisely when the fraction of PRCs retained in the samplers after deployment is between 20 and 80% (Söderström and Bergqvist 2004, Booij and Smedes 2010). Therefore pyrene-d10 and benzo[b]fluoranthene-d12 were used to calculate all air concentrations. Further explanation of the air concentration calculations is included in the SI as Equations S1-S9. Air concentrations of individual PAHs measured at the five stationary air sampling sites are provided in SI Tables S3-S7.
PAH Sourcing
A PAH isomer ratio was used to determine source signatures of PAH mixtures. Fluoranthene and pyrene are an isomer pair that is often used to diagnose whether a PAH mixture is predominantly petrogenic (petroleum-derived) or pyrogenic (combustion-derived) (Budzinski, Jones et al. 1997, Wang, Fingas et al. 1999, Yunker, Macdonald et al. 2002, Fabbri, Vassura et al. 2003, Pies, Hoffmann et al. 2008, Zhang, Zhang et al. 2008, Tobiszewski and Namieśnik 2012). Fluoranthene/pyrene ratios > 1.0 indicate pyrogenic sources, while ratios < 1.0 indicate petrogenic sources (Budzinski, Jones et al. 1997, Wang, Fingas et al. 1999, Fabbri, Vassura et al. 2003). While using more than one isomer ratio can help strengthen sourcing inferences, other PAHs that are commonly used in sourcing ratios were not consistently detected in this study.
Natural Gas Production Data
Natural gas production data was obtained from the Ohio Department of Natural Resources’ database (DNR). Average daily production rates were calculated from amounts of natural gas produced in the quarter in which this sampling campaign occurred.
Distance Comparisons
Active and proposed NGE well locations were obtained from permit records from the Ohio Department of Natural Resources’ division of Oil and Gas Production (DNR). ESRI ArcGIS version 10.2.2 was used to produce stationary sampling maps, used in SI Figures S1 and S2, and to measure distances between sampling locations and NGE wells.
Wristband participants’ reference locations (home or workplace) were geocoded, and then distances were measured from each reference location to the nearest active NGE well within 10 km. Previous research suggests that NGE influences SVOC exposures most heavily within ~1–3 km from active wells (McKenzie, Witter et al. 2012, Paulik, Donald et al. 2016). Therefore 10 km was chosen as a conservative cutoff for inclusion criteria for analyses of wristbands. There were 2 wristband participants who had no active wells within 10 km of their reference locations. These 2 participants were therefore excluded from all analyses presented here. There were an additional 2 participants whose wristbands were excluded from all analyses due to lack of compliance with protocol. Excluding these 4 wristbands yielded a total of 19 wristbands that were included in all analyses. These 19 wristbands were divided into 3 participant groups, as follows: within 0.75 km of an active NGE well (“Well on Property”, n=3), between 0.75 and 2.0 km of an active NGE well (“Well Nearby”, n=4), and 2–10 km from the nearest active NGE well (“No Well Nearby”, n=12).
Statistical Analyses
Wilcoxon rank sum tests were performed to assess statistical differences between PAHs measured in stationary air samples active and proposed NGE wells, and in inner and outer rings at each site, and in wristbands of participants living or working at various distances from active NGE wells. Signed-rank tests were used to compare average fluoranthene/pyrene source signatures at each air sampling site to 1.0. These tests were used to assess whether PAH mixtures in air at each sites were predominantly petrogenic or pyrogenic. The statistical software R (version 2.15.3) and JMP PRO (v12) were used to perform these statistical analyses and comparisons. A Spearman’s rho correlation was used to explore correlations between PAHs and production of natural gas; further detailed in the SI. For all comparisons, results were deemed significantly different when α < 0.05.
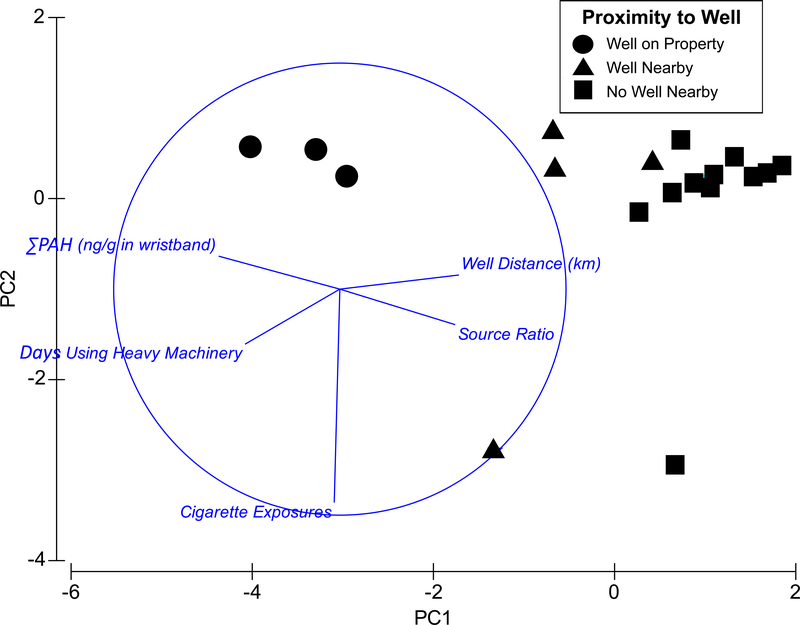
Principal component analysis (PCA) was used to compare ∑PAH in wristbands, the distance from participants’ reference locations to the nearest active NGE wells, reported cigarette exposures, reported days exposed to exhaust from heavy machinery, and the petrogenic or pyrogenic source signature of PAH mixtures in wristbands. Cigarette exposure is reported as the sum of the number of cigarettes each participant reported smoking over the 3 week period. PCA was performed using Primer version 6.1.1.3. Pearson’s correlation coefficients for all pairwise correlations between the variables included in the PCA are presented in Table 1.
Table 1.
Pearson’s correlation coefficients representing pairwise correlations between the variables included in the PCA presented in Figure 4. Significant correlations are highlighted with bold text.
| Pearson Correlation Coefficients | |||
|---|---|---|---|
| Distance from home or work to active NGE well | ∑PAH in Wristband (ng/g) | ∑Cigarette Exposures | |
| Distance from home or work to active NGE well | |||
| p-value | |||
| ∑PAH in Wristband (ng/g) | −0.76 | ||
| p-value | 0.00010 | ||
| ∑Cigarette Exposures | −0.10 | −0.071 | |
| p-value | 0.67 | 0.61 | |
| Days Using Heavy Machinery | −0.57 | 0.61 | 0.17 |
| p-value | 0.005 | 0.0027 | 0.76 |
| Source Ratio | 0.79 | 0.82 | −0.40 |
| p-value | <0.00001 | <0.00001 | 0.043 |
PAH concentrations that were below LODs were treated as zeros in all data analyses.
Quality Control (QC) and Results
Quality control samples included passive sampler preparation blanks, trip blanks, extraction blanks, instrument blanks, and continue calibration verifications (CCVs). Approximately 50% of analyzed samples were QC. Perylene-d12 was used as an internal standard. The majority of PAHs were below LOD in all blank QC samples. If present, concentrations in blank QC samples were averaged and subtracted from sample concentrations. Average recoveries of individual extraction surrogate standards (Table S1) ranged from 46 to 97% for LDPE and from 50 to 87% for wristbands, averaging 69% in all extractions PAHs were within ±20% of the true value for >80% of PAHs in all of the CCVs.
Results and Discussion
Sample Retrieval and Participant Compliance
Participants mailed the stationary LDPE air samplers and wristbands back to OSU after deployment with over 97% and 91% compliance, respectively. Compliance rates were determined by assessing whether participants followed sampling protocols to prepare and return the samples to the laboratory via USPS mail. The high rates of compliance suggest that passive sampling is a robust and fit-for-purpose technology that can be reliably deployed in collaboration with community members.
The high compliance rate with the wristband is of note. Traditional personal sampling tools can be noisy, cumbersome, and require power; these factors may reduce participant compliance (Bohlin, Jones et al. 2007). Previous studies that have incorporated the wristband have also observed high participant compliance rates (Donald, Scott et al. 2016, Kile, Scott et al. 2016, Bergmann, North et al. 2017, Dixon, Scott et al. 2018).
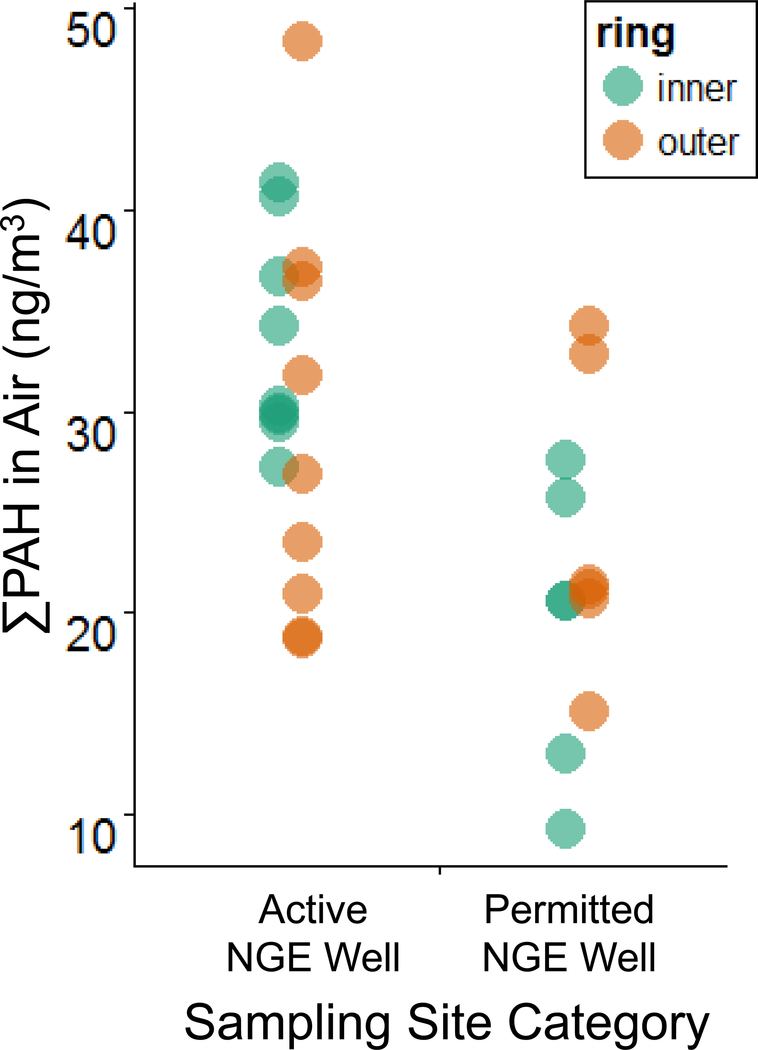
PAHs at Active and Proposed Well Sites
Overall, average ∑PAH concentrations in air were significantly higher at active than proposed well sites (Wilcoxon rank sum test, p = 0.0058) (Figure 2, Table S8). Median ∑PAH in in inner rings of air samples were significantly higher at active than proposed NGE sites (Wilcoxon rank sum test, p = 0.00080). Stronger spatial trends between ∑PAH in air and NGE activity were observed in inner rings of stationary air samples than outer rings (Figure S1, Table S9). In contrast to inner rings, ∑PAH in the outer rings of samples were not significantly different between active and proposed NGE sites (Wilcoxon rank sum test, p = 0.46) (Figure S1, Table S9). This suggests that PAH emissions are elevated near active NGE sites, but that these elevated PAH concentrations dissipate quickly at ground level.
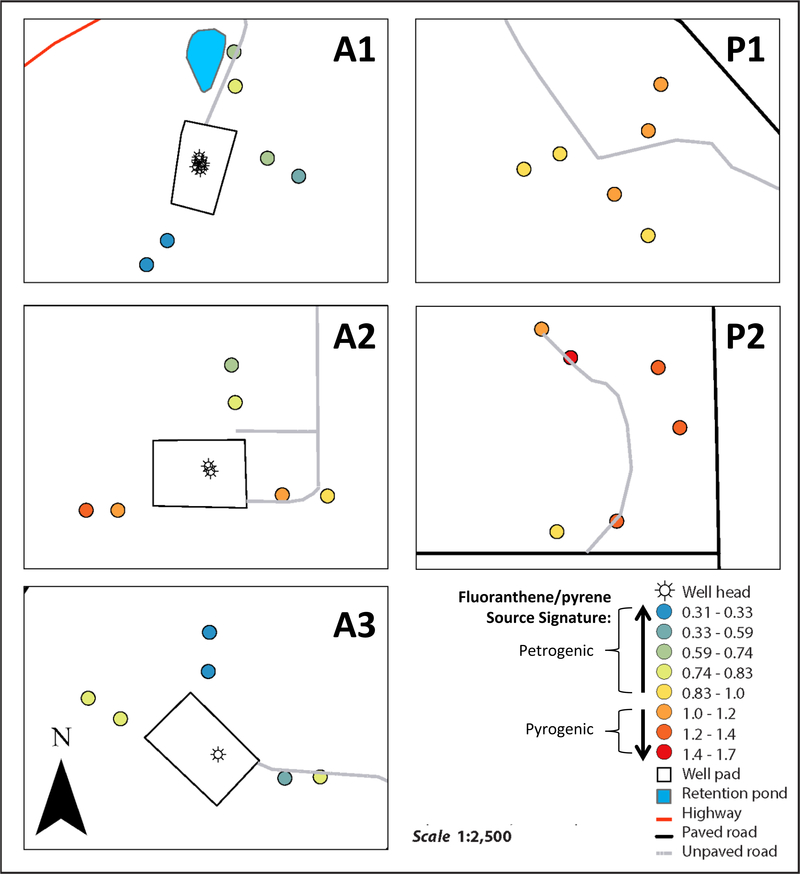
Figure 2.
Average ∑PAH and average source signature measured in air at the active (A1-A3) and proposed (P1-P2) NGE sites. Source signature was measured using the fluoranthene/pyrene isomer ratio. Values of this ratio < 1.0 indicate a petrogenic source; these values are shaded blue. Values of this ratio > 1.0 indicate a pyrogenic source; these values are shaded red. Note from Brian on this version (as of Dec2916): “Using all data. I don’t know why labels run off the field of view to the left. The data were scaled by dividing by standard deviation which can affect small data sets like this if a few points affect variation. There are a couple of other things I’ll send along soon that I have been looking at. Please send questions comments.”
In addition to natural gas, sites A1 and A3 were producing oil during the deployment period;(DNR) this could contribute to the spatial trends of PAHs observed. At site A2, ∑PAH was highest in air samplers closest to a service road leading to the well pad (Figure S1), suggesting that service road activity was an additional source of PAHs at site A2. At the proposed NGE sites there were generally no ∑PAH trends between the inner and outer air samples (Figure S1). This is consistent with the absence of known PAH point sources at the proposed NGE sites.
Air PAH Sourcing
Fluoranthene/pyrene isomer ratios less than 1.0 suggest petrogenic, petroleum-derived, sources while ratios greater than 1.0 suggest pyrogenic, combustion-derived sources (Budzinski, Jones et al. 1997, Wang, Fingas et al. 1999, Fabbri, Vassura et al. 2003). Stronger trends were observed between ∑PAH and PAH source signature in inner rings of stationary air samples than outer rings (Figure S2, Table S9). In the inner rings of air samples, higher ∑PAH was associated with more petrogenic PAH mixtures, and lower ∑PAH was associated with more pyrogenic PAH mixtures (Simple linear regression, R2 = 0.77, p < 0.001) (Figure 3). In the outer rings of air samples, there was no correlation between ∑PAH concentration and source signature (Simple linear regression, R2 = 0.01, p = 0.66) (Figure 3). This is further evidence suggesting that PAH emissions from NGE diffused quickly, affecting only inner rings of stationary air samples.
Figure 3.
∑PAH vs. the flouranthene/pyrene source signature in individual air samples from all 5 stationary air sampling sites (active and proposed). Data are separated into the inner and outer rings of air samplers. There was a negative correlation between ∑PAH and the source signature for air samples in the inner rings of air samplers (simple linear regression, R2 = 0.77, p < 0.001). In contrast, there was no correlation between ∑PAH and the source signature for air samples in the outer rings of air samplers (simple linear regression, R2 = 0.01, p = 0.66).
Overall, PAH mixtures in air were more petrogenic at active NGE sites (A1-A3) (Figure 2). At active NGE sites A1 and A3, average source signatures were significantly below 1.0, indicating petrogenic sources (signed-rank tests, p = 0.016 and 0.016, respectively). At the A2 site the average source signatures were not significantly different than 1 (signed-rank test, p > 0.05). Fugitive emissions of hydrocarbons from oil would also have petrogenic signatures, and would contribute to the petrogenic signatures measured at sites A1 and A3. These comparisons indicate that, on average, PAH mixtures in air were petrogenic or mixed at active NGE sites (Figure 2, Table S8).
Overall, PAH mixtures in air were more pyrogenic at proposed NGE sites (P1-P2) (Figure 2). At proposed NGE site P2, the average source signature was significantly higher than 1.0, indicating a pyrogenic source (signed-rank test, p = 0.031). At site P1, the average source signature was not significantly different than 1.0 (signed-rank test, p > 0.05).
The relationship between the source signature and daily natural gas production was also used to assess the impact of NGE on PAHs in air. There was a significant negative correlation between the fluoranthene/pyrene ratios from inner rings of air samples, and the daily natural gas production at the nearest active NGE site (Spearman rho correlation = –0.58, p = 0.0028). Thus, stronger petrogenic profiles were found in air near NGE wells that were producing more natural gas during this study. This is consistent with observations made in a previous study, where petrogenic PAH signatures were observed in air within 0.1 mile (160m) of NGE wells (Paulik, Donald et al. 2016).
Comparison of PAH Concentrations in Air to Previous Research
In the present study, average ∑PAH measured in air at sites with active NGE wells (sites A-C) was 31 ng/m3. In a previous study, average ∑PAH measured in air within 160 m of active NGE wells was 8.3 ng/m3 (Paulik, Donald et al. 2016). Stationary air samplers on sites with active NGE wells in the present study were closer to active NGE wells than stationary air samplers closest to active NGE wells were in the previous study. Specifically, air samplers closest to active NGE sites in the present study were 55 m from the NGE well pads. In contrast, air samplers closest to active NGE in the previous study were within 160 m from active NGE well pads. Therefore, higher ∑PAH in this study may be due in part to the samplers being closer to the well pads.
The 3.7-fold increase in average ∑PAH levels in air in the present study may also be due in part to the 20°C increase in temperature compared to the previous study. This would be consistent with Huckins et al.’s suggestion that a 2 to 4-fold increase in vapor phase PAH concentrations in air is observed with each 10°C increase in air temperature (Huckins, Petty et al. 2006). Other studies have commonly observed about a 2-fold increase in PAH concentrations in air with each 10°C increase in temperature (Motelay-Massei, Harner et al. 2005, Ravindra, Bencs et al. 2006, Khairy and Lohmann 2012). Seasonal differences in NGE activity, or in other intermittent activities in the region, could also affect PAH levels in air.
PAHs in Wristbands
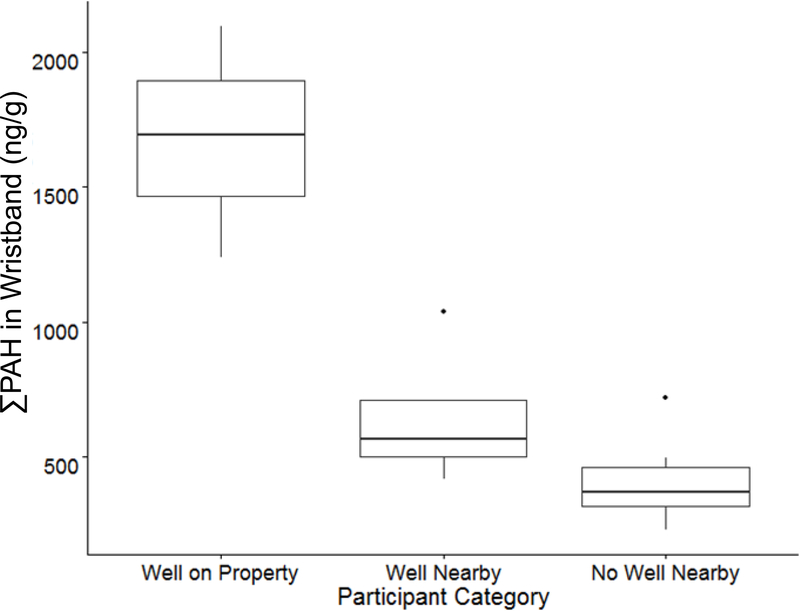
The highest ∑PAH concentrations were found in wristbands worn by participants in the Well on Property group (n=3) (Figure 4, Figure 5, Table S10). Median ∑PAH in wristbands of Well on Property participants was 5-fold higher than in wristbands of participants in the No Well Nearby group (n=12); this difference was significant (Wilcoxon rank sum test, p = 0.0044) (Figure 5, Table S10, Table S11). There was a significant negative correlation between ∑PAH in a participant’s wristband and the distance from that participant’s home or work location to the nearest active NGE well (Pearson’s correlation = –0.76, p = 0.00010) (Figure 4, Table 1). PCA showed participants grouping together based on both the proximity of their reference locations to active NGE wells, and the PAH concentrations measured in their wristbands. The Well on Property wristbands clustered farthest to the left, indicating that the highest ∑PAH concentrations were measured in these wristbands. In contrast, the No Well Nearby wristbands clustered farthest to the right, indicating that the lowest ∑PAH concentrations were measured in these wristbands (Figure 4). Given that wristbands can act as surrogates for participants’ personal chemical exposures, this suggests that living or working closer to active NGE wells was associated with elevated personal PAH exposures in this study.
Figure 4.
Principal Components Analysis (PCA) comparing ∑PAH in participants’ wristbands, the distance from participants’ home or workplaces to the nearest NGE well, the petrogenic or pyrogenic signature measured in wristbands (assessed by the fluoranthene/pyrene isomer ratio, small values suggest petrogenic sources), and participants’ self-reported exposures to cigarette smoke and to exhaust from heavy machinery during the studystudy.The shape of the symbol represents each participant’s group,indicating the proximity of their home or workplace to the nearest active NGE well. Pearson’s correlation coefficients for all pairwise correlations between the variables included in this PCA are provided in Table 1.
Figure 5.
∑PAH concentrations in participants’ wristbands (ng/g wristband). Participant groups describe the proximity of a participant’s home or workplace to the nearest active NGE well. Horizontal lines inside each box represent the median ∑PAH concentration in each participant group. Notes: This is not currently included in the main text or SI. I made it a while back while we were exploring the data. I added it here for consideration, in response to comments on the call last week about the mapping figures perhaps not making it super easy to see the PAH concentration trends among active and permitted well sites. We could consider adding something like this (either into Figure 1, or as an additional figure). I think it would require some beautification and modification if wanted to do that. Also, we could also consider adding visual representation of the medians to this figure (such as horizontal lines as we’ve done before) Maybe try to make this into a summary graphic to add to and/or add into F1 – at least SI? Might be more helpful if could add line for avgs or something – see cfish paper for example of R code.. PAHs measured by stationary air samplers in May 2014 in Ohio (F14–15), >>PAH levels are significantly higher at sites with wells in the inner ring of samplers (Wilcoxon Rank sum test, p = 0.000799). However, PAH levels are not significantly different between sites with and without a well in the outer ring of samplers (Wilcoxon Rank sum test, p = 0.456), LBP, 5/5/16 >>so, perhaps we should use the inner ring of samplers when we compare air levels at the stationary sites to wristband levels for participants matched to these sites. Because the inner ring levels seem to vary more with site.
∑PAH concentrations in Well Nearby wristbands (n=4) were more similar to No Well Nearby wristbands than to Well on Property wristbands (Figure 4, Figure 5, Table S10, Table S11). This is consistent with the stationary sampler data that indicated that PAH concentrations dissipate quickly. Specifically, median ∑PAH in Well Nearby wristbands was 3.0-fold smaller than in Well on Property wristbands, and was 1.7-fold larger than ∑PAH in No Well Nearby wristbands (Figure 5). The difference between ∑PAH in wristbands from the Well on Property group and the Well Nearby group was just above the significance level (Wilcoxon rank sum test, p = 0.057). This suggests that NGE-related PAH exposures of people living or working nearby to a well may be more similar to exposures of people far from NGE wells, than to people with wells on their property. This comparison indicates that, in this study, PAHs emitted from NGE wells diffused quickly, and had relatively little impact on personal PAH exposures of participants who did not have NGE wells directly on their home or work properties.
Wristband Sourcing
The highest PAH concentrations in wristbands were primarily found at locations where fluoranthene/pyrene isomer ratios indicated there were petrogenic PAH sources (Pearson’s correlation = – 0.82, p < 0.00010) (Table 1). These findings are consistent with participants who lived or worked closer to active NGE wells having been exposed to greater proportions of PAHs from petroleum-derived sources in this study.
There was no correlation between cigarette exposure and ∑PAH in the wristbands (Pearson’s correlation = –0.071, p = 0.61) (Figure 4, Table 1). This indicates that the number of cigarettes a participant smoked was not a driver of ∑PAH in the wristbands. Mean days using gas-powered machinery was higher in participants with a NGE well on their property (18.7 days; range: 16–20 day)) compared to participants with a well nearby (5.0 days; range: 0–16) or participants without a well near their property (5.8 days; range: 0–19). There was a significant positive correlation between the number of days participants reported using heavy machinery and ∑PAH measured in participants’ wristbands (Pearson’s correlation = 0.61, p = 0.0027) (Figure 4, Table 1). Exposure to exhaust while using heavy machinery is therefore a potential confounding factor that may have increased ∑PAH in wristbands. While un-combusted gasoline is not a source of PAHs, combustion of fuel in heavy machinery produces PAHs. Thus, it is not surprising that exposure to exhaust from heavy machinery was correlated with higher PAH concentrations in wristbands. PCA also revealed that the distance from a participant’s reference location to the nearest active NGE well was significantly negatively correlated with the number of days that participant used heavy machinery (Pearson’s correlation = –0.57, p = 0.0051) (Figure 4, Table 1). This demonstrates that participants who lived or worked closer to active NGE wells also used more heavy machinery. Exposure to wood smoke was an additional consideration. However, only 3 participants (no well nearby) reported an average value of 1 day exposed. Therefore, the predominantly petrogenic signatures in wristbands of Well on Property participants suggest that these participants’ PAH exposures were more heavily influenced by petroleum-derived emissions than by the combustion-derived PAHs in exhaust from heavy machinery (Figure 4).
Comparison between PAHs in Wristbands and Stationary Air Samples
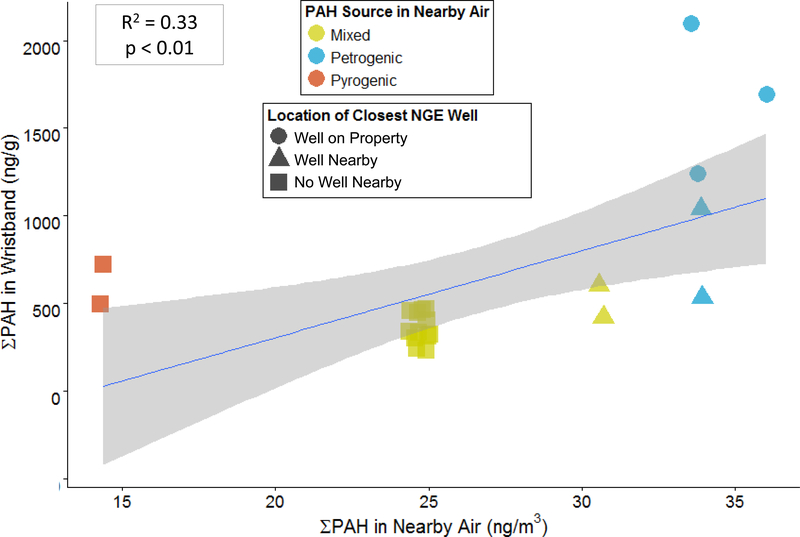
There was a significant positive correlation between ∑PAH in participants’ wristbands and in the stationary air samples deployed closest to each participant’s home or work location (simple linear regression, R2 = 0.64, p < 0.0001) (Figure 6). The significant correlation between PAH concentrations in wristbands and in nearby air is compelling. Individuals are mobile, and PAHs are pervasive pollutants that come from many sources. While there is potential for multiple exposure pathways to contribute PAHs to wristbands, participants’ home and work locations appeared to affect their overall PAH exposures as measured by wristbands in this study. This correlation provides additional evidence supporting the ability of the wristband to assess personal exposure to semi-volatile chemicals in the environment, such as PAHs (O’Connell, Kincl et al. 2014, Donald, Scott et al. 2016, Kile, Scott et al. 2016).
Figure 6.
∑PAH in each participant’s wristband (ng/g wristband) vs. ∑PAH in air measured at the nearest stationary air sampling site to each participant’s home or workplace (ng/m3). There is a significant positive correlation between ∑PAH in a participant’s wristbands and ∑PAH in the inner ring of air samplers at the nearest stationary air sampling site closest to participants’ home or workplaces (simple linear regression, R2 = 0.64, p < 0.0001). Notes: This is currently Figure S2…could consider adding to PCA figure in main text as a/b, or adding as its own figure in main text? (I would argue that it needs some beautification if we’re going to do that)
Limitations
Participants were selected for personal sampling from a group of volunteers who lived or worked near the stationary air sampling stations. Therefore they do not represent a random sample, and findings may not be directly applicable to the entire population or to other regions affected by NGE. Additionally, the sample sizes for the Well on Property and Well Nearby participant groups were small (n=3 and 4, respectively), due to the study taking place in a sparsely populated rural area. In personal sampling analyses, participants’ reported homes or workplaces were used as reference locations. Reference locations were used to spatially relate participants’ exposures to nearby NGE activity and to data from the stationary air sampling campaign. It is unknown exactly how much time participants spent each day at their reference locations. This is a source of uncertainty in the interpretation of how participants’ personal PAH exposures are related to emissions from NGE activity. The PAH sourcing analysis presented in this study relies on one ratio between a pair of isomers, fluoranthene and pyrene. Using more than one isomer ratio can help strengthen sourcing inferences. However, other PAHs that are commonly used in sourcing ratios were not consistently detected in this study.
Conclusions
∑PAH in air was significantly higher at active NGE sites than proposed NGE sites. ∑PAH in air quickly dissipated with distance from active NGE sites. ∑PAH was significantly higher in wristbands worn by participants who lived or worked closer to active NGE wells. PAH mixtures in both air and wristbands were more petrogenic closer to active NGE sites. There was a significant positive correlation between ∑PAH in wristbands and ∑PAH in air near participants’ homes or workplaces. This correlation further affirms the utility of the wristband to assess personal exposure to semi-volatile contaminants, such as PAHs. This work suggests that NGE emits PAHs into air, and that living or working closer to an active NGE well may increase personal PAH exposure.
Supplementary Material
Highlights:
-
∑
PAH in air was higher at active NGE sites than proposed sites
-
∑
PAH mixtures were more petrogenic at active NGE sites than proposed sites
-
∑
PAH exposures were higher if participants lived/worked closer to active NGE sites
-
∑
PAH were correlated in wristbands and air near participants’ homes or workplaces
Acknowledgments
This work was funded by NIEHS grants to OSU: P30-ES000210 and to UC: P30-ES06096. We thank Glenn Wilson, Jorge Padilla, and Gary Points of the OSU Food Safety and Environmental Stewardship Program for help with analysis. Thank you to Carey Donald, Jamie Minick, Alan Bergmann, and Holly Dixon for help with data interpretation and analysis. Thank you to Sarah Elam of the UC Environmental Health Sciences Center Community Outreach and Engagement Core, Jody Alden and Delores Silverthorn of UC, and Paul Feezel of Carroll Concerned Citizens, all for assistance with participant recruitment, training, and communication. Thank you to Heidi Sucharew of Cincinnati Children’s Hospital Medical Center for help with statistical analyses, and to Sean Carver for illustrating the graphical abstract. Finally, thank you to the participants in Ohio for making this study possible.
Footnotes
Conflict of Interest
Kim A. Anderson, Kevin A. Hobbie, and Diana Rohlman disclose a financial interest in MyExposome, Inc., which is marketing products related to the research being reported here. The terms of this arrangement have been reviewed and approved by Oregon State University in accordance with its policy on research conflicts of interest.
References
- Adgate JL, Goldstein BD and McKenzie LM (2014). “Potential public health hazards, exposures and health effects from unconventional natural gas development.” Environmental Science & Technology 48(15): 8307–8320. [DOI] [PubMed] [Google Scholar]
- Ana GREE, Sridhar MKC and Emerole GO (2012). “Polycyclic aromatic hydrocarbon burden in ambient air in selected Niger Delta communities in Nigeria.” Journal of the Air & Waste Management Association 62(1): 18–25. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Sethajintanin D, Sower G and Quarles L (2008). “Field trial and modeling of uptake rates of in situ lipid-free polyethylene membrane passive sampler.” Environmental Science & Technology 42: 4486–4493. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Szelewski MJ, Wilson G, Quimby BD and Hoffman PD (2015). “Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses.” Journal of Chromatography A 1419: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird WM, Hooven LA and Mahadevan B (2005). “Carcinogenic polycyclic aromatic hydrocarbon‐DNA adducts and mechanism of action.” Environmental and molecular mutagenesis 45(2‐3): 106–114. [DOI] [PubMed] [Google Scholar]
- Bamberger M and Oswald RE (2014). “Unconventional oil and gas extraction and animal health.” Environmental Science: Processes & Impacts 16: 1860–1865. [DOI] [PubMed] [Google Scholar]
- Bartkow ME, Hawker DW, Kennedy KE and Müller JF (2004). “Characterizing uptake kinetics of PAHs from the air using polyethylene-based passive air samplers of multiple surface area-to-volume ratios.” Environmental Science & Technology 38(9): 2701–2706. [DOI] [PubMed] [Google Scholar]
- Bergmann AJ, North PE, Vasquez L, Bello H, del Carmen Gastañaga Ruiz M and Anderson KA (2017). “Multi-class chemical exposure in rural Peru using silicone wristbands.” Journal of Exposure Science and Environmental Epidemiology 27: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin P, Jones KC, Levin J-O, Lindahl R and Strandberg B (2010). “Field evaluation of a passive personal air sampler for screening of PAH exposure in workplaces.” Journal of Environmental Monitoring 12(7): 1437–1444. [DOI] [PubMed] [Google Scholar]
- Bohlin P, Jones KC and Strandberg B (2007). “Occupational and indoor air exposure to persistent organic pollutants: A review of passive sampling techniques and needs.” Journal of Environmental Monitoring 9(6): 501–509. [DOI] [PubMed] [Google Scholar]
- Booij K and Smedes F (2010). “An improved method for estimating in situ sampling rates of nonpolar passive samplers.” Environmental Science & Technology 44(17): 6789–6794. [DOI] [PubMed] [Google Scholar]
- Boyle MD, Payne-Sturges DC, Sangaramoorthy T, Wilson S, Nachman KE, Babik K, Jenkins CC, Trowell J, Milton DK and Sapkota A (2016). “Hazard ranking methodology for assessing health impacts of unconventional natural gas development and production: the Maryland case study.” PLOS ONE 11(1): e0145368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Heath G, Kort E, O’Sullivan F, Pétron G, Jordaan S, Tans P, Wilcox J, Gopstein A and Arent D (2014). “Methane leaks from North American natural gas systems.” Science 343(6172): 733–735. [DOI] [PubMed] [Google Scholar]
- Brantley HL, Thoma E, Squier W, Guven B and Lyon D (2014). “Assessment of methane emissions from oil and gas production pads using mobile measurements.” Environmental science & technology. [DOI] [PubMed] [Google Scholar]
- Brasier KJ, Filteau MR, McLaughlin DK, Jacquet J, Stedman RC, Kelsey TW and Goetz SJ (2011). “Residents’ perceptions of community and environmental impacts from development of natural gas in the Marcellus Shale: a comparison of Pennsylvania and New York cases.” Journal of Rural Social Sciences 26(1): 32–61. [Google Scholar]
- Budzinski H, Jones I, Bellocq J, Pierard C and Garrigues P (1997). “Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary.” Marine Chemistry 58(1): 85–97. [Google Scholar]
- Bunch A, Perry C, Abraham L, Wikoff D, Tachovsky J, Hixon J, Urban J, Harris M and Haws L (2014). “Evaluation of impact of shale gas operations in the Barnett Shale region on volatile organic compounds in air and potential human health risks.” Science of the Total Environment 468: 832–842. [DOI] [PubMed] [Google Scholar]
- Carlton AG, Little E, Moeller M, Odoyo S and Shepson PB (2014). “The data gap: can a lack of monitors obscure loss of clean air act benefits in fracking areas?” Environmental Science & Technology 48: 893–894. [DOI] [PubMed] [Google Scholar]
- Colborn T, Kwiatkowski C, Schultz K and Bachran M (2011). “Natural gas operations from a public health perspective.” Human and Ecological Risk Assessment 17(5): 1039–1056. [Google Scholar]
- Colborn T, Schultz K, Herrick L and Kwiatkowski C (2014). “An exploratory study of air quality near natural gas operations.” Human and Ecological Risk Assessment 20(1): 86–105. [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl L, Waters K, Camann D, Calafat A, Herbstman J and Anderson KA (2018). “Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods.” Analytical & Bioanalytical Chemistry In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNR. “Ohio oil & gas well locator.” from http://oilandgas.ohiodnr.gov/well-information/oil-gas-well-locator.
- Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC and Anderson KA (2016). “Silicone wristbands detect individuals’ pesticide exposures in West Africa.” Royal Society Open Science 3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIA, U. S. (2011). Review of Emerging Resources: U.S. Shale Gas and Shale Oil Plays, Energy Information Administration, U.S. Department of Energy [Google Scholar]
- Elliott EG, Trinh P, Ma X, Leaderer BP, Ward MH and Deziel NC (2017). “Unconventional oil and gas development and risk of childhood leukemia: Assessing the evidence.” Science of The Total Environment 576: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri D, Vassura I, Sun C-G, Snape CE, McRae C and Fallick AE (2003). “Source apportionment of polycyclic aromatic hydrocarbons in a coastal lagoon by molecular and isotopic characterisation.” Marine Chemistry 84(1): 123–135. [Google Scholar]
- Fawole OG, Cai XM and MacKenzie AR (2016). “Gas flaring and resultant air pollution: A review focusing on black carbon.” Environmental Pollution 216: 182–197. [DOI] [PubMed] [Google Scholar]
- Goldstein BD, Brooks BW, Cohen SD, Gates AE, Honeycutt ME, Morris JB, Penning TM, Orme-Zavaleta J and Snawder J (2014). “The role of toxicological science in meeting the challenges and opportunities of hydraulic fracturing.” Toxicological Sciences 139(2): 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Tang D, Zhu D, Qu L, Sjodin A, Li Z, Camann D and Perera FP (2012). “Prenatal exposure to polycyclic aromatic hydrocarbons, benzo [a] pyrene-DNA adducts, and genomic DNA methylation in cord blood.” Environmental Health Perspectives 120(5): 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins JN, Petty JD and Booij K (2006). Monitors of Organic Chemicals in the Environment New York, Springer. [Google Scholar]
- Khairy MA and Lohmann R (2012). “Field validation of polyethylene passive air samplers for parent and alkylated PAHs in Alexandria, Egypt.” Environmental Science & Technology 46(7): 3990–3998. [DOI] [PubMed] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M and Anderson KA (2016). “Using silicone wristbands to evaluate preschool children’s exposure to flame retardants.” Environmental Research 147: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovitz A, Curtright A, Abramzon S, Burger N and Samaras C (2013). “Estimation of regional air-quality damages from Marcellus Shale natural gas extraction in Pennsylvania.” Environmental Research Letters 8(1): 014017. [Google Scholar]
- Liu Y, Tao S, Dou H, Zhang T, Zhang X and Dawson R (2007). “Exposure of traffic police to polycyclic aromatic hydrocarbons in Beijing, China.” Chemosphere 66(10): 1922–1928. [DOI] [PubMed] [Google Scholar]
- Lohmann R (2012). “Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler.” Environmental Science & Technology 46(2): 606–618. [DOI] [PubMed] [Google Scholar]
- Macey G, Breech R, Chernaik M, Cox C, Larson D, Thomas D and Carpenter D (2014). “Air concentrations of volatile compounds near oil and gas production: a community-based exploratory study.” Environmental Health 13(82): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero JE, Townsend-Small A, Lyon DR, Tsai TR, Meinardi S and Blake DR (2016). “Estimating emissions of toxic hydrocarbons from natural gas production sites in the Barnett Shale region of Northern Texas.” Environmental Science & Technology 50(19): 10756–10764. [DOI] [PubMed] [Google Scholar]
- McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS and Adgate JL (2014). “Birth outcomes and maternal residential proximity to natural gas development in rural Colorado.” Environmental Health Perspectives 122(4): 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie LM, Witter RZ, Newman LS and Adgate JL (2012). “Human health risk assessment of air emissions from development of unconventional natural gas resources.” Science of the Total Environment 424: 79–87. [DOI] [PubMed] [Google Scholar]
- Menzie CA, Potocki BB and Santodonato J (1992). “Exposure to carcinogenic PAHs in the environment.” Environmental Science & Technology 26(7): 1278–1284. [Google Scholar]
- Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM and Kinney PL (2004). “Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort.” Chest 126(4): 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelay-Massei A, Harner T, Shoeib M, Diamond M, Stern G and Rosenberg B (2005). “Using passive air samplers to assess urban-rural trends for persistent organic pollutants and polycyclic aromatic hydrocarbons. 2. Seasonal trends for PAHs, PCBs, and organochlorine pesticides.” Environmental Science & Technology 39(15): 5763–5773. [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD and Anderson KA (2014). “Silicone wristbands as personal passive samplers.” Environmental science & technology 48(6): 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, McCartney MA, Paulik LB, Allan SE, Tidwell LG, Wilson G and Anderson KA (2014). “Improvements in pollutant monitoring: Optimizing silicone for co-deployment with polyethylene passive sampling devices.” Environmental Pollution 193: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Balmes JR, Eisen EA, Mann J, Noth EM, Lurmann FW, Pratt B, Tager IB, Nadeau K and Hammond SK (2015). “Ambient polycyclic aromatic hydrocarbons and pulmonary function in children.” J Expos Sci Environ Epidemiol 25(3): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik LB and Anderson KA (In press). Considerations for measuring exposure to chemical mixtures. Chemical Mixtures and Combined Chemical and Nonchemical Stressors: Exposure, Toxicity, Analysis and Risk. Rider C and Simmons JE, Springer. [Google Scholar]
- Paulik LB, Donald CE, Smith BW, Tidwell LG, Hobbie KA, Kincl L, Haynes EN and Anderson KA (2016). “Emissions of polycyclic aromatic hydrocarbons from natural gas extraction into air.” Environmental Science & Technology 50(14): 7921–7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik LB, Smith BW, Bergmann AJ, Sower GJ, Forsberg ND, Teeguarden JG and Anderson KA (2016). “Passive samplers accurately predict PAH levels in resident crayfish.” Science of The Total Environment 544: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Breysse PN, Gray K, Howarth M and Yan B (2014). “Environmental health research recommendations from the inter-environmental health sciences core center working group on unconventional natural gas drilling operations.” Environmental Health Perspectives 122(11): 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F and Herbstman J (2011). “Prenatal environmental exposures, epigenetics, and disease.” Reproductive Toxicology 31(3): 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D and Rauh V (2009). “Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years.” Pediatrics 124(2): e195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai W-Y, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu Y-H and Diaz D (2003). “Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population.” Environmental health perspectives 111(2): 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétron G, Frost G, Miller BR, Hirsch AI, Montzka SA, Karion A, Trainer M, Sweeney C, Andrews AE and Miller L (2012). “Hydrocarbon emissions characterization in the Colorado Front Range: A pilot study.” Journal of Geophysical Research: Atmospheres 117(D4). [Google Scholar]
- Petty JD, Huckins JN and Zajicek JL (1993). “Application of semipermeable membrane devices (SPMDs) as passive air samplers.” Chemosphere 27(9): 1609–1624. [Google Scholar]
- Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA and Hofmann T (2008). “Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils.” Chemosphere 72(10): 1594–1601. [DOI] [PubMed] [Google Scholar]
- Prest HF, Huckins JN, Petty JD, Herve S, Paasivirta J and Heinonen P (1995). “A survey of recent results in passive sampling of water and air by semipermeable membrane devices.” Marine Pollution Bulletin 31(4): 306–312. [Google Scholar]
- Rabinowitz PM, Slizovskiy IB, Lamers V, Trufan SJ, Holford TR, Dziura JD, Peduzzi PN, Kane MJ, Reif JS and Weiss TR (2015). “Proximity to natural gas wells and reported health status: results of a household survey in Washington County, Pennsylvania.” Environmental Health Perspectives 123(1): 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Ogburn EL, McCormack M, Casey JA, Bandeen-Roche K, Mercer DG and Schwartz BS (2016). “Association between unconventional natural gas development in the Marcellus Shale and asthma exacerbations.” JAMA Internal Medicine 176(9): 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra K, Bencs L, Wauters E, De Hoog J, Deutsch F, Roekens E, Bleux N, Berghmans P and Van Grieken R (2006). “Seasonal and site-specific variation in vapour and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities.” Atmospheric Environment 40(4): 771–785. [Google Scholar]
- Rohlman D, Syron L, Hobbie K, Anderson KA, Scaffidi C, Sudakin D, Peterson ES, Waters KM, Haynes E and Arkin L (2015). “A community-based approach to developing a mobile device for measuring ambient air exposure, location, and respiratory health.” Environmental Justice 8(4): 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AA, Adams PJ and Robinson AL (2014). “Air pollutant emissions from the development, production, and processing of Marcellus Shale natural gas.” Journal of the Air & Waste Management Association 64(1): 19–37. [DOI] [PubMed] [Google Scholar]
- Samburova V, Zielinska B and Khlystov A (2017). “Do 16 polycyclic aromatic hydrocarbons represent PAH air toxicity?” Toxics 5(3): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff SB, Hays J and Finkel ML (2014). “Environmental public health dimensions of shale and tight gas development.” Environmental Health Perspectives 122(8): 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EG, Watkins TH, Solomon PA, Thoma ED, Williams RW, Hagler GSW, Shelow D, Hindin DA, Kilaru VJ and Preuss PW (2013). “The changing paradigm of air pollution monitoring.” Environmental Science & Technology 47(20): 11369–11377. [DOI] [PubMed] [Google Scholar]
- Söderström HS and Bergqvist P-A (2004). “Passive Air Sampling Using Semipermeable Membrane Devices at Different Wind-Speeds in Situ Calibrated by Performance Reference Compounds.” Environmental Science & Technology 38(18): 4828–4834. [DOI] [PubMed] [Google Scholar]
- Sower GJ and Anderson KA (2008). “Spatial and temporal variation of freely dissolved polycyclic aromatic hydrocarbons in an urban river undergoing superfund remediation.” Environmental science & technology 42(24): 9065–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell LG, Paulik LB and Anderson KA (2017). “Air-water exchange of PAHs and OPAHs at a superfund mega-site.” Science of The Total Environment 603: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiszewski M and Namieśnik J (2012). “PAH diagnostic ratios for the identification of pollution emission sources.” Environmental Pollution 162: 110–119. [DOI] [PubMed] [Google Scholar]
- Tsai P-J, Shieh H-Y, Lee W-J and Lai S-O (2002). “Characterization of PAHs in the atmosphere of carbon black manufacturing workplaces.” Journal of hazardous materials 91(1–3): 25–42. [DOI] [PubMed] [Google Scholar]
- Tustin AW, Hirsch AG, Rasmussen SG, Casey JA, Bandeen-Roche K and Schwartz BS (2016). “Associations between unconventional natural gas development and nasal and sinus, migraine headache, and fatigue symptoms in Pennsylvania.” Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fingas M, Shu Y, Sigouin L, Landriault M, Lambert P, Turpin R, Campagna P and Mullin J (1999). “Quantitative characterization of PAHs in burn residue and soot samples and differentiation of pyrogenic PAHs from petrogenic PAHs-the 1994 mobile burn study.” Environmental Science & Technology 33(18): 3100–3109. [Google Scholar]
- Ward H, Eykelbosh A and Nicol A-M (2016). “Addressing uncertainty in public health risks due to hydraulic fracturing.” Environmental Health Review 59(2): 57–61. [Google Scholar]
- Werner AK, Vink S, Watt K and Jagals P (2015). “Environmental health impacts of unconventional natural gas development: A review of the current strength of evidence.” Science of The Total Environment 505: 1127–1141. [DOI] [PubMed] [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D and Sylvestre S (2002). “PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition.” Organic Geochemistry 33(4): 489–515. [Google Scholar]
- Zhang W, Zhang S, Wan C, Yue D, Ye Y and Wang X (2008). “Source diagnostics of polycyclic aromatic hydrocarbons in urban road runoff, dust, rain and canopy throughfall.” Environmental Pollution 153(3): 594–601. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu X, Jung KH, Ohman-Strickland P, Bonanno LJ and Lioy PJ (2011). “Ambient concentrations and personal exposure to polycyclic aromatic hydrocarbons (PAH) in an urban community with mixed sources of air pollution.” Journal of Exposure Science and Environmental Epidemiology 21(5): 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.