Prevention and management of hypertension represent a global public health challenge. Therefore, the identification of new therapeutic strategies is of growing interest. The American Heart Association defines as “alternative approaches,” the non‐pharmacological treatments able to lower blood pressure, classifying them into three main categories: behavioral therapies, non‐invasive procedures, and exercise‐based regimens.1 In the last decades, several studies have revealed that exercise and fitness produce beneficial effects in the general population, reducing the relative risk of death by 20%‐35%,2, 3 particularly death caused by cardiovascular disease.4, 5
The definition of “exercise” given by the American College of Sports Medicine reads “Any and all activity involving generation of force by the activated muscle(s) that results in disruption of a homeostatic state.” Behind this general definition, different categories of exercise are recognized, which differ for type, intensity, and duration. As the success of pharmacological therapies is linked to the optimal dose, also for “exercise” the potential therapeutic effect strongly depends on the “dose,” resulting from optimal intensity and duration. This critical point has opened an extensive research aimed at considering exercise training in therapeutic plans for the management of systemic disorders like diabetes and hypertension. Substantial evidence in literature supports the efficacy of fitness on hypertension, suggesting that physical activity lowers blood pressure, thereby preventing the development and progression of hypertension.
In this issue of the Journal, Wellman and colleagues have shown in an elegant study performed in adolescents that engaging in physical activity is associated with lower odds of having blood pressure in the hypertensive range.6 The exact molecular basis of the beneficial effects that physical activity produces on blood pressure is not completely understood, probably because the regulation of blood pressure is complex and multifactorial, including neuro‐hormonal, hemodynamic, and metabolic mechanisms. Starting from this multifaceted substrate, exercise training can affect blood pressure acting on different processes (Figure 1), only in part known.
Figure 1.
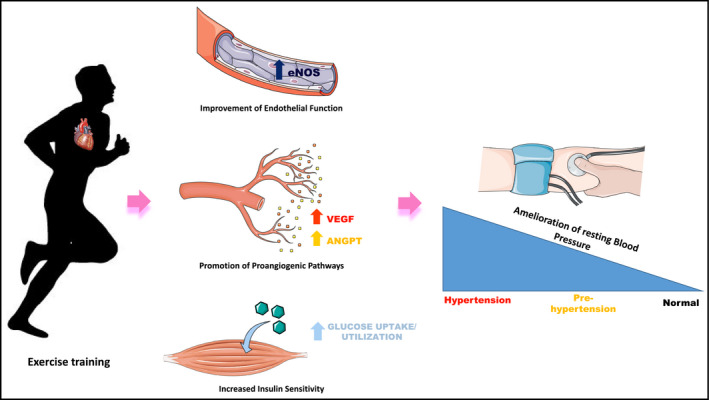
Exercise training activates adaptive mechanisms: (a) improvement of endothelial function increasing NO production, (b) induction of pro‐angiogenic pathways, and (c) increased insulin sensitivity. These mechanisms produce a significant reduction in blood pressure. The beneficial effects are more pronounced as higher as the starting blood pressure. VEGF: Vascular endothelial growth factor; ANGPT: Angiopoietin.
1. PHYSICAL ACTIVITY AND ENDOTHELIAL FUNCTION
A foremost mechanism by which physical exercise can affect blood pressure is the regulation of endothelial function. Indeed, vascular homeostasis depends on the activity of the endothelium, which is a fundamental regulator of the vasomotor responses, modulating the health and resistance of the vessels. Nitric oxide (NO) is a key mediator of endothelial function, and both clinical and preclinical studies have confirmed the ability of exercise to improve NO‐dependent endothelial vasodilation.7, 8, 9, 10, 11, 12 Physiologically, high NO production occurs in response to increased blood flow to compensate the shear stress by vasodilation.13, 14, 15 Exercise training results in repeated exposure to shear stress, thus improving the bioavailability of NO.16 In rats, a 2‐ to 4‐week exercise training not only stimulates the NO production in the arterioles of the skeletal muscle, but also markedly improves the vasodilator response to acetylcholine.17 A regular aerobic exercise can improve endothelial function also in aged population, in which the endothelium is compromised. Indeed, exercise training has been shown to attenuate endothelial dysfunction during aging,18 probably increasing NO synthesis to counteract ROS generation.19 A direct evidence of the improvement of endothelial function as a pivotal mechanism underlying the antihypertensive effect of physical exercise comes from the SEFRET study (Study of endothelial function response to exercise training in hypertensive individuals).20 In this report, the authors unveil two crucial points: (a) physical activity ameliorates the endothelium‐dependent vasodilation in a hypertensive population; and (b) the magnitude of the improvement of endothelial function depends on the type of exercise training (aerobic, resistance, or concurrent training). This evidence strengthens the concept that the prescription of a specific exercise program is fundamental in the therapeutic approach of hypertensive patients.
2. EXERCISE TRAINING AND ANGIOGENESIS
Hypertension is characterized by microvascular rarefaction caused by impaired angiogenesis.21 Constant physical exercise has been shown to induce vessel adaptation, augmenting blood flow reserves.22 These morphological changes in the vascular tree result from exercise‐induced angiogenesis, which increases the size and number of blood vessels.23 The formation of new microvessels and the increase of vessel networks are processes regulated by vascular endothelial growth factor (VEGF) and other mediators; released in response to exercise training, VEGF produces a mitotic effect on endothelial cells, induces endothelial cell migration, and promotes chemotaxis.24 Other factors able to regulate angiogenesis are released in response to exercise, including angiopoietins and metalloproteinases, which initiate the degradation of the extracellular matrix, a process essential for the formation of vascular networks.25 An interesting research by Oliveira and colleagues demonstrated that exercise training prevents the microvascular rarefaction in hypertensive rats.26 Interestingly, this effect seems to be mediated by specific microRNAs implicated in angiogenetic process.27, 28 This new mechanism again supports the potential therapeutic application of exercise training in vascular disease, mainly in hypertension.
3. PHYSICAL ACTIVITY AND INSULIN SENSITIVITY
A seminal study demonstrated that the hypertensive phenotype induced by high‐fructose diet was strongly attenuated by spontaneous physical activity in rodents, accompanied by a significant attenuation of insulin resistance,29 strongly suggesting a pathophysiologic link between essential hypertension and hyperinsulinemia.30
More recently, in an animal model of metabolic syndrome, exercise training of moderate intensity has been shown to play a preventive role, playing an important role on the regulation of blood pressure and insulin sensitivity.31 The recurrent association between exercise training and recovery of insulin sensitivity strongly supports the hypothesis that physical activity may prevent hypertension development and progression through its action on insulin responsiveness. Direct evidence about such a link comes from a number of studies: Chisholm and colleagues demonstrated that exercise training induces a significant increase in whole‐body insulin sensitivity, in particular acting on glucose uptake and oxidation by the skeletal muscle.32 The increased glucose uptake in response to exercise is essentially due to GLUT4 translocation on the membrane, which is independent from insulin receptor; indeed, insulin receptor knockout animals displayed the same response to exercise in terms of muscular glucose uptake.33 Other not fully understood pathways could be involved in the increased glucose uptake during muscle contraction: epigenetics, oxidative stress, and intracellular Ca2+ dynamics.34, 35, 36, 37, 38
4. EXERCISE TRAINING AS EFFECTIVE THERAPEUTIC TOOL IN HYPERTENSIVE PATIENTS
One of the first clinical studies showing the antihypertensive effect of physical activity was published in 1968. The research demonstrated that in men that self‐reported more than 5 hours/week of physical exercise the incidence of hypertension was significantly reduced.39 In five decades, this finding has been reproduced by large prospective studies, confirming that physical activity inversely correlates with hypertension.40, 41, 42
Interventional studies have corroborated the direct effects of physical activity on blood pressure, providing information about the more responsive target populations. A training program of 2 days for week lowered blood pressure in both hypertensive and normotensive subjects43; another study demonstrated that the effect of physical exercise on blood pressure is more pronounced in hypertensive patients than in normotensive individuals.44 Nevertheless, if on one hand the major effect of exercise training is recorded in hypertensive conditions, on the other hand the training inhibits the progression from normal to pre‐hypertension and from pre‐hypertension to hypertension.43, 45, 46, 47, 48 Therefore, physical exercise represents a powerful therapeutic tool to prevent the development of hypertension, especially in high‐risk populations like diabetic patients or individuals with family history of hypertension.
5. CONCLUSIONS
Physical exercise has been shown to have beneficial effects on blood pressure, through means of adaptation mechanisms which culminate in both hemodynamic and metabolic changes. Several studies demonstrate that the effects of physical exercise on blood pressure are dose‐dependent.49, 50, 51 Moreover, the beneficial effects depend on the type of exercise training.52, 53, 54, 55, 56, 57, 58 Importantly, resistance training is contraindicated in the presence of instable cardiovascular conditions, including uncontrolled severe hypertension.59, 60 Therefore, it is imperative to emphasize that a specific training plan including the specific type and dose of exercise training has to be delineated for each individual. Accordingly, a stress testing before starting exercises should be performed. Physical activity remains a potential therapeutic and prevention tool, which needs further investigations to support a fully personalized therapeutic program.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The Santulli's laboratory is supported by the National Institutes of Health (R01‐HL146691, R01‐DK123259, R00‐DK107895, P30‐DK020541, and R01‐DK033823 to GS) and by the American Heart Association (AHA‐20POST35211151 to JG).
Gambardella J, Morelli MB, Wang X‐J, Santulli G. Pathophysiological mechanisms underlying the beneficial effects of physical activity in hypertension. J Clin Hypertens. 2020;22:291–295. 10.1111/jch.13804
This Invited Commentary refers to JCH‐19‐0421: “Intensity and Frequency of Physical Activity and High Blood Pressure in Adolescents: A Longitudinal Study”
REFERENCES
- 1. Brook RD, Appel LJ, Rubenfire M, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013;61:1360‐1383. [DOI] [PubMed] [Google Scholar]
- 2. Macera CA, Hootman JM, Sniezek JE. Major public health benefits of physical activity. Arthritis Rheum. 2003;49:122‐128. [DOI] [PubMed] [Google Scholar]
- 3. Stamatakis E, Gale J, Bauman A, et al. Sitting Time, Physical activity, and risk of mortality in adults. J Am Coll Cardiol. 2019;73:2062‐2072. [DOI] [PubMed] [Google Scholar]
- 4. Myers J, Kaykha A, George S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117:912‐918. [DOI] [PubMed] [Google Scholar]
- 5. Golbidi S, Laher I. Exercise and the aging endothelium. J Diabetes Res. 2013;2013:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wellman RJ, Marie‐Pierre Sylvestre MP, Abi Nader P, et al. Intensity and frequency of physical activity and high blood pressure in adolescents: a longitudinal study. J Clin Hypertens. 2020;22:283‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santulli G, Ciccarelli M, Trimarco B, Iaccarino G. Physical activity ameliorates cardiovascular health in elderly subjects: the functional role of the beta adrenergic system. Front Physiol. 2013;4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moraes‐Silva IC, Mostarda CT, Silva‐Filho AC, Irigoyen MC. Hypertension and exercise training: evidence from clinical studies. Adv Exp Med Biol. 2017;1000:65‐84. [DOI] [PubMed] [Google Scholar]
- 9. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493‐503. [DOI] [PubMed] [Google Scholar]
- 10. Lefferts WK, DeBlois JP, Receno CN, et al. Effects of acute aerobic exercise on arterial stiffness and cerebrovascular pulsatility in adults with and without hypertension. J Hypertens. 2018;36:1743‐1752. [DOI] [PubMed] [Google Scholar]
- 11. Pal S, Radavelli‐Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7:494‐506. [DOI] [PubMed] [Google Scholar]
- 12. Montero D, Roche E, Martinez‐Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre‐ and hypertensive subjects: a systematic review and meta‐analysis. Int J Cardiol. 2014;173:361‐368. [DOI] [PubMed] [Google Scholar]
- 13. Hutcheson IR, Griffith TM. Release of endothelium‐derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol. 1991;261:H257‐H262. [DOI] [PubMed] [Google Scholar]
- 14. Koller A, Seyedi N, Gerritsen ME, Kaley G. EDRF released from microvascular endothelial cells dilates arterioles in vivo. Am J Physiol. 1991;261:H128‐H133. [DOI] [PubMed] [Google Scholar]
- 15. Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652‐1660. [DOI] [PubMed] [Google Scholar]
- 16. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium‐derived nitric oxide function in humans. J Physiol. 2004;561:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun D, Huang A, Koller A, Kaley G. Short‐term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol. 1994;76:2241‐2247. [DOI] [PubMed] [Google Scholar]
- 18. DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation. 2000;102:1351‐1357. [DOI] [PubMed] [Google Scholar]
- 19. Taddei S, Galetta F, Virdis A, et al. Physical activity prevents age‐related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896‐2901. [DOI] [PubMed] [Google Scholar]
- 20. Pedralli ML, Waclawovsky G, Camacho A, et al. Study of endothelial function response to exercise training in hypertensive individuals (SEFRET): study protocol for a randomized controlled trial. Trials. 2016;17:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei T, Huang G, Gao J, et al. Sirtuin 3 deficiency accelerates hypertensive cardiac remodeling by impairing angiogenesis. J Am Heart Assoc. 2017;6. 10.1161/JAHA.117.006114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol. 1989;67:1140‐1149. [DOI] [PubMed] [Google Scholar]
- 23. White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85:1160‐1168. [DOI] [PubMed] [Google Scholar]
- 24. Santulli G. Angiogenesis: Insights from a Systematic Overview. New York: Nova; 2013. [Google Scholar]
- 25. Morelli MB, Chavez C, Santulli G, et al. Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: focus on lipid disorders. Expert Opin Ther Targets. 2020. 10.1080/14728222.2020.1707806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernandes T, Magalhaes FC, Roque FR, Phillips MI, Oliveira EM. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs‐16, ‐21, and ‐126. Hypertension. 2012;59:513‐520. [DOI] [PubMed] [Google Scholar]
- 27. Santulli G. MicroRNAs and endothelial (Dys) function. J Cell Physiol. 2016;231:1638‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santulli G. microRNAs distinctively regulate vascular smooth muscle and endothelial cells: functional implications in angiogenesis, atherosclerosis, and in‐stent restenosis. Adv Exp Med Biol. 2015;887:53‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reaven GM, Ho H, Hoffman BB. Attenuation of fructose‐induced hypertension in rats by exercise training. Hypertension. 1988;12:129‐132. [DOI] [PubMed] [Google Scholar]
- 30. Lembo G, Napoli R, Capaldo B, et al. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. J Clin Invest. 1992;90:24‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moraes‐Silva IC, Mostarda C, Moreira ED, et al. Preventive role of exercise training in autonomic, hemodynamic, and metabolic parameters in rats under high risk of metabolic syndrome development. J Appl Physiol. 2013;114:786‐791. [DOI] [PubMed] [Google Scholar]
- 32. James DE, Kraegen EW, Chisholm DJ. Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Investig. 1985;76:657‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wojtaszewski JF, Higaki Y, Hirshman MF, et al. Exercise modulates postreceptor insulin signaling and glucose transport in muscle‐specific insulin receptor knockout mice. J Clin Investig. 1999;104:1257‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanford KI, Goodyear LJ. Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv Physiol Educ. 2014;38:308‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Climie RE, Boutouyrie P, Perier MC, et al. Association between occupational, sport, and leisure related physical activity and baroreflex sensitivity: the Paris Prospective Study III. Hypertension. 2019;74:1476‐1483. [DOI] [PubMed] [Google Scholar]
- 36. de Andrade LH, de Moraes WMAM, Matsuo EH, et al. Aerobic exercise training improves oxidative stress and ubiquitin proteasome system activity in heart of spontaneously hypertensive rats. Mol Cell Biochem. 2015;402:193‐202. [DOI] [PubMed] [Google Scholar]
- 37. Liu X, Wang Y, Gao R, Xing Y, Li X, Wang Z. Serum metabolomic response to exercise training in spontaneously hypertensive rats. J Am Soc Hypertens. 2017;11:428‐436. [DOI] [PubMed] [Google Scholar]
- 38. Roque FR, Briones AM, García‐Redondo AB, et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol. 2013;168:686‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paffenbarger RS Jr, Thorne MC, Wing AL. Chronic disease in former college students. VIII. Characteristics in youth predisposing to hypertension in later years. Am J Epidemiol. 1968;88:25‐32. [DOI] [PubMed] [Google Scholar]
- 40. Carnethon MR, Evans NS, Church TS, et al. Joint associations of physical activity and aerobic fitness on the development of incident hypertension: coronary artery risk development in young adults. Hypertension. 2010;56:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15:659‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyer JL, Kasch FW. Exercise therapy in hypertensive men. JAMA. 1970;211:1668‐1671. [PubMed] [Google Scholar]
- 44. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882‐1888. [DOI] [PubMed] [Google Scholar]
- 45. Kim SJ, Lee J, Jee SH, et al. Cardiovascular risk factors for incident hypertension in the prehypertensive population. Epidemiol health. 2010;32:e2010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zheng L, Sun Z, Zhang X, et al. Predictors of progression from prehypertension to hypertension among rural Chinese adults: results from Liaoning Province. Eur J Cardiovasc Prev Rehabil. 2010;17:217‐222. [DOI] [PubMed] [Google Scholar]
- 47. Faselis C, Doumas M, Kokkinos JP, et al. Exercise capacity and progression from prehypertension to hypertension. Hypertension. 2012;60:333‐338. [DOI] [PubMed] [Google Scholar]
- 48. Bakker EA, Sui X, Brellenthin AG, Lee DC. Physical activity and fitness for the prevention of hypertension. Curr Opin Cardiol. 2018;33:394‐401. [DOI] [PubMed] [Google Scholar]
- 49. Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. A systematic review of the evidence for Canada's Physical Activity Guidelines for Adults. Int J Behav Nutr Phys Act. 2010;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pavey TG, Peeters G, Bauman AE, Brown WJ. Does vigorous physical activity provide additional benefits beyond those of moderate? Med Sci Sports Exerc. 2013;45:1948‐1955. [DOI] [PubMed] [Google Scholar]
- 51. Boutcher YN, Boutcher SH. Exercise intensity and hypertension: what's new? J Hum Hypertens. 2017;31:157‐164. [DOI] [PubMed] [Google Scholar]
- 52. MacDonald HV, Johnson BT, Huedo‐Medina TB, et al. Dynamic resistance training as stand‐alone antihypertensive lifestyle therapy: a meta‐analysis. J Am Heart Assoc. 2016;5. 10.1161/JAHA.116.003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pagonas N, Vlatsas S, Bauer F, et al. Aerobic versus isometric handgrip exercise in hypertension: a randomized controlled trial. J Hypertens. 2017;35:2199‐2206. [DOI] [PubMed] [Google Scholar]
- 54. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60:653‐658. [DOI] [PubMed] [Google Scholar]
- 56. Pescatello LS, MacDonald HV, Ash GI, et al. Assessing the existing professional exercise recommendations for hypertension: a review and recommendations for future research priorities. Mayo Clin Proc. 2015;90:801‐812. [DOI] [PubMed] [Google Scholar]
- 57. Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta‐analysis. Mayo Clin Proc. 2014;89:327‐334. [DOI] [PubMed] [Google Scholar]
- 58. Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NCL, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta‐analysis to optimize benefit. Hypertens Res. 2016;39:88‐94. [DOI] [PubMed] [Google Scholar]
- 59. Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta‐analysis. J Hypertens. 2013;31:639‐648. [DOI] [PubMed] [Google Scholar]
- 60. Pescatello LS, MacDonald HV, Lamberti L, Johnson BT. Exercise for hypertension: a prescription update integrating existing recommendations with emerging research. Curr Hypertens Rep. 2015;17:87. [DOI] [PMC free article] [PubMed] [Google Scholar]


