Summary
Pediatric low-grade gliomas (pLGG) are frequently driven by genetic alterations in the RAS/MAPK pathway yet show unexplained variability in their clinical outcome. To address this, we characterized a cohort of >1,000 clinically annotated pLGG. 84% of cases harbored a driver alteration, while those without an identified alteration also often exhibited up-regulation of the RAS/MAPK pathway. pLGG could be broadly classified based on their alteration type. Rearrangement-driven tumors were diagnosed at a younger age, enriched for WHO grade I histology, infrequently progressed, and rarely resulted in death as compared to SNV-driven tumors. Further sub-classification of clinical-molecular correlates stratified pLGG into risk categories. These data highlight the biological and clinical differences between pLGG subtypes and opens avenues for future treatment refinement.
Keywords: Low-grade glioma, pediatric, RAS/MAPK pathway, brain tumor, molecular diagnostics, risk stratification, neurooncology
Graphical Abstract

Introduction
Pediatric low-grade gliomas (pLGG) are the most frequent brain tumors in children, accounting for −30% of all cases (Ostrom et al., 2015). pLGG encompasses a broad range of glial, neuronal and mixed glioneuronal entities in the World Health Organization’s (WHO) classification of central nervous system (CNS) tumors (Louis et al., 2016). Unlike lower grade gliomas in adults, which primarily arise in the cerebral hemispheres and inevitably transform to higher grade glioma, pLGG can occur throughout the CNS and rarely transforms (Broniscer et al., 2007; Mistry et al., 2015). Nevertheless, outcome and response to therapy is highly variable. If complete surgical resection is possible, 10-year progression-free survival (PFS) exceeds 85%, but drops below 50% if there is radiologically visible residual tumor (Wisoff et al., 2011). In deep-seated midline locations, gross total resection (GTR) is not often achievable and adjuvant therapy may be required, often with unsatisfactory tumor control and/or long-term morbidity (Krishnatry et al., 2016; Nageswara & Packer, 2014). Which patients require these therapies and who will benefit from them is not well understood.
Over the last decade, molecular profiling studies have incrementally identified key genetic events in pLGG that converge on the RAS-mitogen-activated protein kinase (RAS/MAPK) pathway. Most commonly, these are somatic events involving BRAF or germline NF1 alterations (Collins et al., 2015; Jones et al., 2008; Schindler et al., 2011; Dougherty et al., 2010; Lassaletta et al., 2017; Listernick et al., 1999; Uusitalo et al, 2016; Seminog et al., 2013). In addition to these common pLGG alterations, rarer alterations affecting RAS/MAPK signalling, including those involving FGFR1/2/3, NTRK2, RAF1, ALK, and ROS1 (Zhang et al., 2013; Jones et al., 2013; Qaddoumi et al., 2016; Guerreiro Stucklin et al., 2019), as well as non-RAS/MAPK alterations such as MYB, MYBL1, IDH1, and H3F3A (Qaddoumi et al., 2016, Tatevossian et al., 2010; Ramkissoon et al., 2013; Bandopadhayay et al., 2016; Ryall et al., 2016; Hartmann et al., 2009) have been identified in small numbers of cases. However, several key issues remain undefined: 1. Are all NF1, BRAF fused and BRAF mutant tumors the same? 2. What is the mechanism of tumorigenesis in pLGG without an identifiable genetic alteration? 3. What are the clinical features of the rare alterations in pLGG and is their outcome unique? 4. Can molecular alterations help provide biological insights for disease stratification?
To answers to these questions and provide a population-based resource for the pediatric neurooncology community, we molecularly characterized >1000 pLGG with comprehensive clinical data. This enabled us to provide a statistically robust, annotated resource which includes representation of the rarest pLGG molecular entities and their clinical features.
Results
Patient Cohort
Our population-based cohort of pLGG consisted of 976 patients (<19 years) followed and treated at the Hospital for Sick Children (Toronto, ON, Canada) from 1986–2017 (Table S1). For each patient we collected demographic, treatment and outcome data.
At the population-level, tumors were distributed equally between the diencephalon (n=313, 32%; n=124, 13% were from Neurofibromatosis Type 1 [NF1] patients), cerebral hemispheres (n=265, 27%) and the cerebellum (n=252, 26%), whereas pure brainstem (n=92, 9.4%), spinal cord (n=41, 4.2%) and extensively disseminated tumors (n=13, 1.3%) were less frequent (Figure 1A). Upon pathologic review of non-NF1 cases (n=843), pilocytic astrocytoma (n=303, 36%) was the most common diagnosis (excluding LGG, not otherwise specified [NOS]) across tumor locations (Figure 1B) with the exception of the cerebral hemispheres, which was histologically diverse (Figure 1C). The median age of diagnosis was 7.6 years (range 0–18.7 years), with pLGG in the cerebral hemispheres being diagnosed at a later age (median=10.7 years) as compared to other tumor locations (p<0.0001, ANOVA) (Figure 1D). There was a significant association between tumor location, PFS, and overall survival (OS) (p<0.0001, log-rank test) (Figure 1E, Figure S1), with 10-year PFS and OS best for patients with tumors in the cerebellum (89% and 99%, respectively), and worst for those with extensively disseminated disease (0% and 67%, respectively). Importantly, only 7.5% of patients succumbed to their disease (median time to death=3.9 years, median OS follow-up=15.9 years) despite 33% experiencing tumor progression (median time to progression=2.3 years, median PFS follow-up=5.9 years) (Figure 1F).
Figure 1. pLGG Cohort Details.
(A) Anatomical location of all pLGG within the cohort (n=976).
(B) The histological spectrum of all non-NF1 pLGG (n=843). PA: pilocytic astrocytoma LGG, NOS: low grade glioma, not otherwise specified, GG: ganglioglioma, DNET: dysembryoplastic neuroepithelial tumor, PXA: pleomorphic xanthoastrocytoma, GNT: glioneuronal tumor, DA: diffuse astrocytoma, AG: angiocentric glioma, ODG: oligodendroglioma, DIA/DIG: Desmoplastic infantile astrocytoma/ganglioglioma.
(C) Histological distribution of samples based on tumor location of all non-NF1 pLGG (n=843).
(D) Boxplot showing age at diagnosis separated by tumor location of the entire pLGG cohort (n=976). The thick line within the box represents the median, the lower and upper limits of the box represent the first and third quartiles and the whiskers the min. and max. values. Adjusted p value for all pairwise comparisons, t-test.*<0.05, **<0.01, ***<0.001, ****<0.0001, NS=not significant.
(E) Progression-free survival of the pLGG cohort segregated by tumor location. Adjusted p value, log-rank test.
(F) Progression-free and overall survival of the entire pLGG cohort (n=976). p value calculated via the log-rank test.
Characteristics of NF1-driven pLGG
Patients diagnosed with the genetic pre-disposition disorder NF1 are done so by a series of clinical observations and tests indicative of a germline NF1 mutation (Gutmann et al., 2017). As such, pLGG arising in these patients are primarily diagnosed via imaging and clinical observation, rather than their genetics. Therefore, we examined this group of tumors separately, prior to our extensive molecular profiling of somatic pLGG (Table S2).
Although NF1-driven pLGG are generally thought to have a favorable clinical course, analysis of a large cohort revealed important risk groups. In our study, NF1-driven pLGG accounted for 14% of cases (n=133). While most NF1 pLGG occur as optic pathway glioma (OPG), 19% (n=25) arose outside of this location. Patients with NF1 tumors arising outside the optic pathway had significantly worse OS and PFS compared to those arising in the optic pathway (p=0.0011, p=0.0029, respectively, log-rank test) (Figure S2A–B). Furthermore, of the high risk, recurrent and biopsied NF1 pLGG, 20% were found to harbor mutations in other molecular drivers including BRAF p.V600E, FGFR1, and/or H3F3A (H3.3) p.K27M. All of these patients experienced multiple progressions with two succumbing to their disease post-chemoradiation after 15.5 and 13.7 years, respectively. These observations, although preliminary, suggest that non-OPG NF1 tumors may require specialized management, including an early biopsy and molecular profiling in agreement with recent reports (D’Angelo et al., 2018).
The molecular landscape of non-NF1 pLGG
To determine the true frequency of molecular alterations in pLGG we analyzed 540 tumors from 2000–2017 where material quality was sufficient to utilize our tiered profiling approach (Figure S3A). In total, 88% (n=477/540) had sufficient material for molecular profiling.
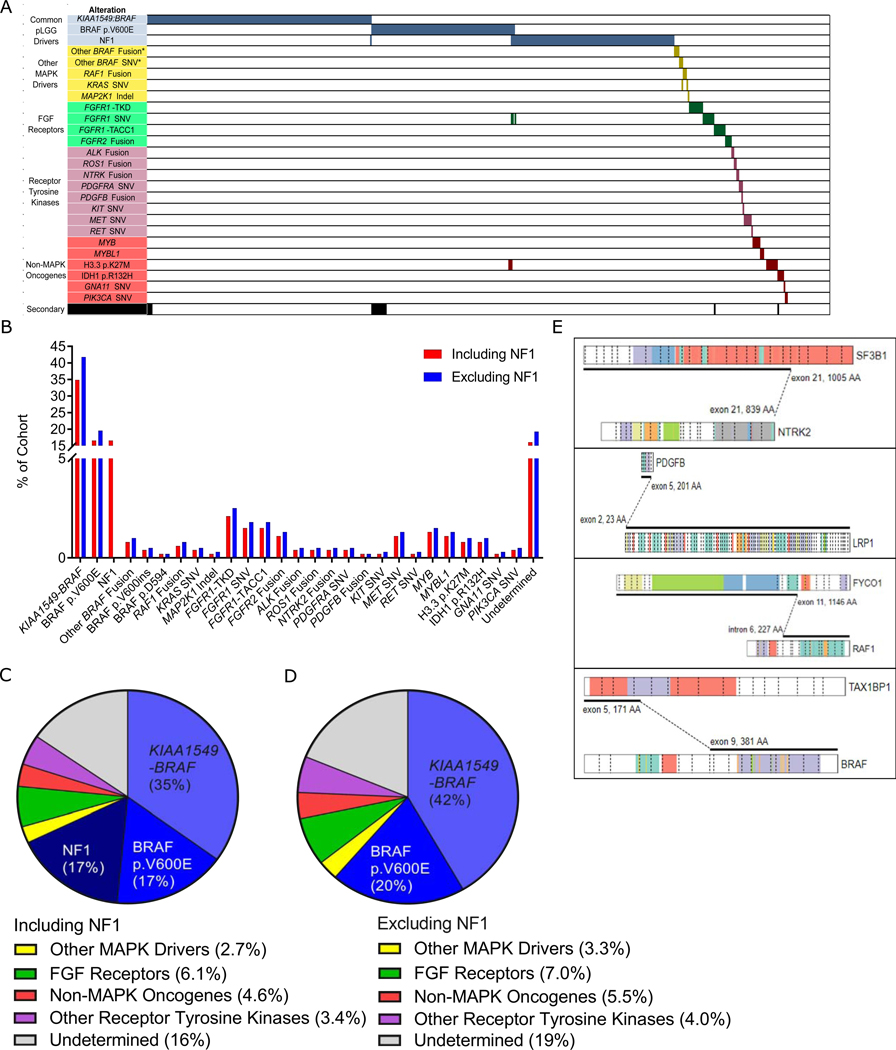
Together, KIAA1549-BRAF (n= 1 66), BRAF p.V600E (n=79), and germline NF1 mutations (n=79) accounted for 68% (n=324) of tumors (Figure 2A–C). Rare alterations accounted for an additional 17% of cases. These included non-canonical BRAF alterations, such as fusions partnered with genes others than KIAA1549 (n=4), 2 insertion events at position 600 (p.V600ins) and 1 SNV at position 594 (p.D594N) (Figure 2A-C,E). A further 1.3% (n=6) of cases contained alterations in other direct members of the RAS/MAPK pathway, including 3 RAF1 fusions, 2 KRAS mutations and one patient with a short deletion in MAP2K1 (Figure 2A-C,E). The next most frequent alterations were those impacting receptor tyrosine kinases (RTK) (n=45, 9.4%) (Figure 2A–C) and included two categories: FGFR and other RTK. Alterations in FGFR most frequently involved FGFR1/2 (n=29, 6.1%) and included FGFR1-TACC1 fusions (n=7, 1.5%), FGFR1 tyrosine kinase domain duplications (TKD, n=10, 2.1%), FGFR2 fusions (n=5, 1.0%), and hotspot mutations in FGFR1 (n=7, 1.5%) (Figure 2A-C). Alterations in other RTK (n=16, 3.4%) included mutations in MET (n=5, 1.0%) or PDGFRA (n=l, 0.2%), as well as fusions involving ALK (n=2, 0.4%), ROS1 (n=2, 0.4%), and NTRK2 (n=2, 0.4%) (Figure 2A–C,E). Lastly, 4.6% (n=22) of cases contained alterations in genes with seemingly no direct impact on the RAS/MAPK pathway (Figure 2A-C). These included mutations in H3F3A (n=4, 0.8%), IDH1 (n=4, 0.8%) and rearrangements involving MYB (n=6, 1.3%) or MYBL1 (n=5, 1.0%) (Figure 2A-C). Altogether, we identified a driver mutation in 84% of pLGG. Incidences of molecular alterations excluding NF1 patients (n=398) are seen in Figures 2B,D and enrichment across tumor location and pathology in Figure S3B,C.
Figure 2. The Molecular Landscape of pLGG.
(A) Oncoprint representation of the molecular alterations and their associated categories in 610 pLGG. Samples are arranged in columns with genes & gene categories labelled along the row. *denotes that these BRAF SNVs and fusions are not the canonical KIAA1549-BRAF or p.V600E.
(B) Bar graph of all recurrent somatic mutations across all 477 cases diagnosed from 2000–2017 for which sufficient material for molecular testing was available, in order of frequency and colored based on the inclusion (blue) or exclusion (red) of NF1 patients.
(C) Pie chart depicting the frequency of alterations per molecular category in a population-based cohort of pLGG diagnosed from 2000–2017 (n=477).
(D) Pie chart depicting the frequency of alterations per molecular category in non-NF1 pLGG diagnosed from 2000–2017 (n=397).
(E) Schematic representation of the rare and novel fusions identified in this study. Figures were derived using the Protein Paint feature of the St. Jude PeCan website (https://pecan.stjude.cloud/proteinpaint).
See also Figures S2, S3, Table S2.
The RAS/MAPK pathway is up-regulated across pLGG regardless of underlying mutation
The predilection of NF1 patients for developing pLGG together with the identification of KIAA1549-BRAF and BRAF p.V600E as molecular drivers in pLGG led to the hypothesis that up-regulation of the RAS/MAPK pathway may be the primary driver for tumor formation (Collins et al., 2015; Northcott et al., 2015; Jones et al., 2012; Zhang et al., 2013). However, how broadly this applies to pLGG has not been tested and is of major importance due to the increasing use of RAS/MAPK-pathway targeting agents in the clinic.
We therefore asked whether the 16% of pLGG without identified mutations nonetheless resulted in up-regulation of the RAS/MAPK pathway. To test this hypothesis, we first analyzed a series of pLGG with non-BRAF alterations including FGFR alterations, rare RTK, RAF1 fusions, KRAS mutations and MYB or MYBL1 rearrangements and compared their phosphorylated ERK (ppERK) levels to KIAA1549-BRAF and BRAF p.V600E tumors. Interestingly, all tumors had significantly increased ppERK as compared to normal brain controls (p<0.0001, ANOVA) (Figure 3A). Importantly, increased ppERK was also seen in MYB and MYBL1 altered tumors, which would not themselves be expected to directly signal via the RAS/MAPK pathway. To further explore whether RAS/MAPK pathway up-regulation is a unifying event in pLGG even in the absence of an activating genetic event, we examined a subset of pLGG in which a molecular driver was not identified. Utilizing RNA sequencing, we performed single sample gene set enrichment analysis (ssGSEA) focussing on the RAS/MAPK pathway. We observed that genes known to be up or down-regulated by RAS/MAPK activation were significantly enriched in these tumors as compared to normal brain controls (Figure 3B,C). Furthermore, when compared against samples with known RAS/MAPK pathway alterations, the activation signature was indiscernible between the two (p=0.4103, ANOVA) (Figure 3D), indicating similar levels of RAS/MAPK up-regulation in pLGG lacking a clear molecular driver.
Figure 3. RAS/MAPK Pathway Up-regulation in Non-canonical and Molecularly Undetermined pLGG.
(A) Boxplot showing the ppERK/ERK protein levels, separated by molecular alteration. The thick line within the box represents the median, the lower and upper limits of the box represent the first and third quartiles and the whiskers the min. and max. values. Adjusted p value for all pairwise comparisons, t-test.*<0.05, **<0.01, ***<0.001, ****<0.0001, NS=not significant.
(B) Pre-ranked gene set enrichment analysis (GSEA) of the RAS/MAPK pathway activation signature in molecularly undetermined pLGG. NES: normalized enrichment score; FDR: false-discovery rate.
(C) Single sample gene set enrichment analysis (ssGSEA) of RAS/MAPK activation for normal brain controls and molecularly undetermined pLGG. The thick line within the box represents the median, the lower and upper limits of the box represent the first and third quartiles and the whiskers the min. and max. values. Adjusted p value for all pairwise comparisons, Mann-Whitney test.*<0.05, **<0.01, ***<0.001, ****<0.0001, NS=not significant.
(D) RAS/MAPK ssGSEA scores for known RAS/MAPK mutant and molecularly undetermined pLGG compared with normal brain. The thick line within the box represents the median, the lower and upper limits of the box represent the first and third quartiles and the whiskers the min. and max. values. Adjusted p value for all pairwise comparisons, t-test.*<0.05, **<0.01, ***<0.001, ****<0.0001, NS=not significant.
Alteration type predicts pLGG outcome
In order to further interrogate the impact of rare pLGG alterations, we collected data from an additional 61 patients in which a rare molecular alteration had been previously identified from St. Jude Children’s Hospital (Memphis, TN, USA), Children’s Hospital of Philadelphia (Philadelphia, PA, USA), and Memorial Sloan Kettering Cancer Center (New York, NY, USA) (Table S3). This yielded a total of 1037 pLGG. With these additional cases and detailed clinical and molecular information, we asked which features were predictive of clinical outcome in pLGG. Interestingly, patient outcome was significantly associated with the type of alteration (rearrangement versus SNV), and not exclusively on which particular gene was altered (Figure 4A,B, Table 1). Patients with rearrangement-driven pLGG had good long-term outcome with very few deaths (n=7, 2.6%) and fewer progressions (n=67, 27%) (Figure 4B–D, Table 1). In contrast, patients with SNV-driven pLGG were significantly more likely to succumb to their disease (n=24, 13%, p<0.0001, Fisher’s exact test versus rearrangement-driven) and/or progress (n=80, 44%, p<0.0001, Fisher’s exact test versus fusion-driven) (Table 1, Figure 4B–D). Furthermore, rearrangement-driven pLGG were diagnosed at a significantly younger age (median 6.6 versus 10.9 years, p<0.0001, t-test) and were enriched for WHO grade I histology (p<0.0001, Fischer Exact Test) (Figure 4B, Table 1). This pattern is evident in BRAF, where tumors with KIAA1549-BRAF have superior outcome to BRAF p.V600E (5-year PFS of 69% for KIAA1549-BRAF versus 52% for BRAF p.V600E, p=0.0058, log-rank test). When investigating BRAF in grade I tumors alone, the pattern remained (5-year PFS of 72% and 56% for KIAA1549-BRAF and BRAF p.V600E, respectively, p=0.0176). Although the numbers are too small to allow statistical comparisons between SNVs and fusions for other genes, the same trend is evident. For example, for FGFR1, patients with FGFR1-TACC1 or FGFR1 TKD tumors had similar outcome to those with KIAA1549-BRAF fusions (5-year PFS of 69% for all). Patients with FGFR1 SNVs, on the other hand, were more similar to BRAF p.V600E (5-year PFS of 53% and 52% for FGFR1 SNV and BRAF p.V600E, respectively. Importantly, where FGFR1-TACC1 and FGFR1-TKD tumors did not contain additional alterations, FGFR1 SNVs often did, sometimes resulting in late deaths.
Figure 4. Rearrangement versus SNV-driven pLGG.
(A) Pie charts depicting the molecular alteration breakdown of rearrangement (top) (n=265) and SNV (bottom)-driven (n=182) pLGG.
(B) Rearrangement versus SNV-driven pLGG as compared across several clinical features. * Adjusted p < 0.05, Fisher’s exact test. GTR: gross total resection.
(C) Kaplan-Meier plot of overall survival of cases separated by rearrangement- or SNV-driven status, p value calculated via the log-rank test.
(D) Kaplan-Meier plot of progression-free survival of cases separated by rearrangement- or SNV-driven status, p value calculated via the log-rank test.
See also Table S3.
Table 1.
| Characteristic | pLGG Subtype | p-value | ||
|---|---|---|---|---|
| Rearrangement | SNV | |||
| Number | ||||
| N | 265 | 182 | ||
| Grade | ||||
| I | 216 (88%)* | 97 (66%)* | <0.0001 | |
| II | 30(12%)* | 50 (34%)* | ||
| Sex | ||||
| Male | 130 (49%) | 98 (54%) | 0.337 | |
| Female | 135 (51%) | 84 (46%) | ||
| Age at Diagnosis | ||||
| Under 10 | 185 (70%) | 83 (46%) | <0.0001 | |
| Over 10 | 80 (30%) | 99 (54%) | ||
| Mean | 7.6 ±4.8 | 10.1 ±5.1 | ||
| Median | 6.6(0.5–18.9) | 10.9(0.2–18.9) | ||
| Extent of Resection | ||||
| GTR | 137 (52%) | 76 (44%) | 0.078 | |
| NoGTR | 127 (48%) | 96 (56%) | ||
| Location | ||||
| Cerebral Hemisphere | 86 (32%) | 97 (53%) | <0.0001 | |
| Midline | 75 (28%) | 72 (40%) | ||
| Cerebellum | 104 (40%) | 13 (7%) | ||
| Progression | ||||
| Progressed | 67 (27%) | 80 (46%) | <0.0001 | |
| Stable | 184 (73%) | 94 (54%) | ||
| 5-year PFS | 70.6% | 51.4% | ||
| 10-year PFS | 59.8% | 30.0% | ||
| Outcome | ||||
| Dead | 7 (3%) | 24(13%) | <0.0001 | |
| Alive | 249 (97%) | 155 (87%) | ||
| 5-year OS | 97.8% | 91.2% | ||
| 10-year OS | 97.8% | 88.1% | ||
Values are displayed as raw counts and the percentage of the group.
denotes categories with omitted samples (LGG, NOS was not assigned a histological grade).
Characteristics of fusion-driven pLGG
BRAF Fusions:
KIAA1549-BRAF was the most frequent alteration in pLGG (35%) and was almost exclusively a single-event driver (n=175/180, 97%), with 4 cases also having a CDKN2A deletion and 1 present in a patient with NF1 (Figure 2A). These 5 rare cases are still alive (median follow-up=7.5 years). KIAA1549-BRAF was significantly enriched in pilocytic astrocytoma (n=150/180, 83%, p<0.0001, Fisher’s exact test), and in cerebellar tumors (n=100/180, 56%, p=0.0002, Fischer Exact) (Figure 5A).
Figure 5. Clinicopathologic Features of Rearrangement-driven pLGG.
Schematic representation of key clinical features and outcomes for (A) KIAA1549-BRAF. (B) FGFR1-TACC1, (C) FGFR1 TKD, (D) FGFR2 Fusions, (E) MYB, and (F) MYBL1. PA: pilocytic astrocytoma LGG, NOS: low grade glioma, not otherwise specified, GG: ganglioglioma, DNET: dysembryoplastic neuroepithelial tumor, PXA: pleomorphic xanthoastrocytoma, GNT: glioneuronal tumor, DA: diffuse astrocytoma, AG: angiocentric glioma, ODG: oligodendroglioma, DIA/DIG: Desmoplastic infantile astrocytoma/ganglioglioma, Dx: diagnosis, GTR: gross total resection.
Due to the large number of tumors, we could sub-stratify the BRAF fusions into subgroups based on their breakpoints (Figure S4A). The most common KIAA1549-BRAF fusion involved exon 16 in KIAA1549 and 9 in BRAF (16:09). Like all KIAA1549-BRAF fusions, 16:09 was significantly enriched in pilocytic astrocytoma (n=73/83, 88%, p<0.0001, Fisher’s exact test) and in cerebellar tumors (n=60/83, 72%, p<0.0001, Fisher’s exact test) (Figure S4B,C). Interestingly, the only KIAA1549-BRAF fusion seen in hemispheric tumors was 15:09 (Figure S4B). 15:09 was also the primary fusion seen in tumors with extensive dissemination (n=5, 83%, p<0.0001, Fisher’s exact test). 15:09 was associated with a worse PFS as compared to the other fusion subtypes (p=0.0003, log-rank test, Figure S4D), with a 5-year PFS of 59% as compared to 77%–100% for other fusion subtypes. BRAF fusions not involving KIAA1549 occurred exclusively in adolescents with no progression events (median follow-up=3.7 years), while 15:11 was only observed in 2 infants who rapidly experienced tumor progression and died (Figure S4D–E). Identifying the specific fusion breakpoints of KIAA1549-BRAF will be important to properly ascertain their propensity for certain clinical features and impact on outcome.
FGFR1/2 Fusions & FGFR1 TKD:
FGFR fusions/TKD were observed in 6.1% of the cohort (Figure 2B). FGFR1-TACC1 pLGG were often cystic lesions, most commonly pilocytic astrocytoma (n=7/14, 50%) and occurred throughout the CNS, most commonly in the cerebral hemispheres (n=6/14, 43%) (Figure 5B). FGFR1 TKD and FGFR2 fused pLGG were primarily glioneuronal or oligodendroglial in origin, respectively and were restricted to the cerebral hemispheres (Figure 5C-D). FGFR2 fusions included FGFR2-INA, FGFR2-CTNNA3, and FGFR2-ERC1 (Figure S5). While progressions were seen in some cases (5-year PFS of 69%, 69%, and 88% for FGFR1-TACC1, FGFR1 TKD and FGFR2 fused cases, respectively), importantly none of these tumors resulted in patient death with a median follow-up of 11.3, 11.7, and 7 1 vears resnectivelv fFieure 5B–DY
ALK, R0S1, NTRK, and PDGFB Fusions:
Fusions in other RTK were rare in pLGG (3.4%) and included novel events as well as some previously described in pediatric gliomas (Wu et al., 2014; Aghajan et al., 2016; Kiehna et al., 2017; Guerreiro Stucklin et al., 2019). These included CCDC88A-ALK, PPP1CB-ALK, GOPC-ROS1 and NTRK2-MID1 in addition to novel NTRK2-SF3B1 and PDGFB-LRP1 fusions (Figure 2E). These fusions were restricted to the cerebral hemispheres with the exception of ROS1 fusions which were both seen in the intraventricular space (Table S4). Interestingly, ALK fusions were exclusively observed in infants (0.9 and 1.1 years). No patients harboring these alterations succumbed to their disease after a median follow-up of 4.9 years and only a single ALK and ROSl fused patient progressed. A clinical summary of these rare fusions is included in Table S4.
MYB & MYBL1 rearrangements:
MYB and MYBL1 alterations were histologically enriched for angiocentric glioma (n=14, 100%, p<0.0001, Fisher’s exact test) and diffuse astrocytoma (n=5, 83%, p<0.0001, Fisher’s exact test), respectively, with both primarily arising in the cerebral hemispheres (n=13/14, 92% and n=5/6, 83%, respectively, p<0.0001, Fisher’s exact test) (Figure 5E,F). All patients harboring either MYB or MYBL1 rearrangements are alive with median follow-up of 8.1 and 5.3 years, respectively. However, while progressions were rare in MYB altered tumors (n=2/10, 20%), they were more frequent in those with MYBL1 (n=2/6, 33%), resulting in a 5-year PFS of 90 and 67%, respectively (Figure 5E,F). Our data suggests that the clinical differences between MYB and MYBL1 altered tumors merit further investigation.
Characteristics of SNV-driven pLGG
BRAF p.V600E:
BRAF p.V600E was the second most common alteration in pLGG (17%) and was frequently associated with additional alterations, most commonly deletion of CDKN2A (n=13, 9.6%) (Figure 2A). BRAF p.V600E also co-occurred with several other SNVs including those in NF1, FGFR1, KRAS and H3F3A, but never with a fusion event (Figure 2A). Unlike KLAA1549-BRAF, tumors with BRAF p.V600E were histologically diverse and included ganglioglioma (n=36, 31%), diffuse astrocytoma (n=16, 14%), and pleomorphic xanthoastrocytoma (n=12, 10%) (Figure 6A). Both ganglioglioma and pleomorphic xanthoastrocytoma were more likely to harbor BRAF p.V600E than other tumor types (p=0.0028, p=0.0048, respectively, Fisher’s exact test, Figure S3C). BRAF p.V600E cases occurred most frequently in the cerebral hemispheres (n=64, 56%) but were also common in the diencephalon (n=33, 29%) and in contrast to KLAA1549-BRAF, were rare in the cerebellum (n=6, 5.2%) (Figure 6A). BRAF p.V600E pLGG had worse OS and PFS than those with KIAA1549-BRAF(10-year OS 97% versus 89%, p=0.0416 and 10-year PFS of 64% versus 30%, p=0.0058, respectively, log-rank test, Figure S6A-B). BRAF p.V600E tumors with pleomorphic xanthoastrocytoma histology had a worse outcome than those without (5-year PFS of 14% versus 58%, respectively, p=0.0328, log-rank test Figure S6C), although OS was not significantly different (p=0.1892, Figure S6D). The same was not the case for BRAF p.V600E tumors with co-occurring CDKN2A deletions (5-year PFS of 34% versus 55%, respectively, p=0.1157, log-rank test, Figure S6E), although OS was significantly different (p=0.0100, log-rank test, Figure S6F). However, both non-pleomorphic xanthoastrocytoma (5-year PFS of 55%) and CDKN2A balanced (5-year PFS of 55%) BRAF p.V600E tumors had inferior PFS to KIAA1549-BRAF (5-year PFS of 69%) fused tumors (p=0.0139 and p=0.0356, respectively, log-rank test, Figure S7A,B), despite their OS not being significantly different (p=0.1169 and 0.1888, respectively, log-rank test) (Figure S7C,D).
Figure 6. Clinicopathologic Features of SNV-Driven pLGG.
Schematic representation of key clinical features and outcomes for (A) BRAF p.V600E, (B) FGFR1 SNVs, (C) IDH1 p.R132H, and (D) H3.3 p.K27M. PA: pilocytic astrocytoma LGG, NOS: low grade glioma, not otherwise specified, GG: ganglioglioma, DNET: dysembryoplastic neuroepithelial tumor, PXA: pleomorphic xanthoastrocytoma, GNT: glioneuronal tumor, DA: diffuse astrocytoma, AG: angiocentric glioma, ODG: oligodendroglioma, DIA/DIG: Desmoplastic infantile astrocytoma/ganglioglioma, Dx: diagnosis, GTR: gross total resection.
FGFR1 Mutations:
FGFR1 point mutations were observed in 1.5% of pLGG and primarily consisted of p.N546K and p.K656E. Histologically, these tumors were most frequently dysembryoplastic neuroepithelial tumors (n=13, 41%) or pilocytic astrocytoma (n=9, 28%), diagnosed in older children (p=0.032, Fisher’s exact test), and often (n=16, 50%) co-occurred with other genetic alterations including NF1 (n=7, 22%) or additional RAS/MAPK pathway mutations (n=11, 34%) (Figure 2A, 6B). Interestingly, in some cases, multiple point mutations in FGFR1 were seen (n=6, 19%). Of those not lost to follow-up, 43% (n=12) progressed rapidly (median of 2.2 years, 5-year PFS of 53%). Despite this, only two cases had late deaths after 13.7 and 15.5 years, respectively, both of which had additional alterations.
IDH1 p.R132H:
IDH1 mutations are common in adult lower grade gliomas, arising in approximately 70% of grade II and III gliomas (Parsons et al., 2008; Yan et al., 2009, Balss et al., 2008). In pLGG, IDH1 p.R132H mutations were extremely rare, accounting for only 0.8% of cases (Figure 2B). Most IDH1 p.R132H patients presented with a prolonged history of seizures, sometimes years before the biopsy was performed. All tumors were restricted to the cerebral hemispheres and were either oligodendroglioma or diffuse astrocytoma (Figure 6C). Patients harboring IDH1 p.R132H were diagnosed in late childhood (median: 15.7 years), with the youngest patient being diagnosed at 8.9 years (Figure 6C). 50% of IDH1 p.R132H pLGG progressed within a median of 5.1 years (5-year PFS of 56%) despite only one succumbing to their disease (Figure 6C).
H3.3 p.K27M:
H3F3A mutations are common in childhood high grade gliomas and DIPG and confer a dismal outcome (Khuong-Quang et al., 2012; Buczkowicz et al., 2014). In our pLGG series, all H3F3A mutations were p.K27M, with no p.G34R/V alterations identified (Table S1). These cases were restricted to the midline (diencephalon [n=8] and brainstem [n=4]), enriched for diffuse astrocytomas (n=8, 67%, p=0.0011, Fischer Exact), and like other SNVs, often co-occurred with other alterations (25%), most often with BRAF p.V600E (Figure 2A, 6D). Although morphologically and clinically different than midline HGG, H3.3 p.K27M patients progressed early (median time to progression=0.8 years), with all patients eventually succumbing to their disease (Figure 6D). These data support the role of H3.3 p.K27M as a marker of aggressive behavior regardless of the initial morphology and presentation.
Molecular based risk stratification for pLGG
Based on the above data, we define a risk stratification for children with pLGG (Figure 7A). pLGG harboring gene fusions or germline NF1 mutations comprise the low risk group. These tumors progress less frequently and often eventually stop growing with very few progressions seen after 10 years and almost no deaths at 20 years follow-up (10-year PFS of 67% and OS of 98%, 20-year PFS and OS of 58% and 96%, respectively) (Figure 7B–C). These tumors require conservative management as therapy may carry higher long-term morbidity than the tumor itself.
Figure 7. Risk Stratification of pLGG.
(A) Donut plot representing assigned risk portfolio of pLGG and their associated biomarkers. Risk assignment is based on the incidence of progression and/or death. In samples harboring multiple alterations, the highest potential risk group was assigned. Alterations appearing in <5 samples are not assigned a risk group.
(B) Kaplan-Meier plot of overall survival of cases separated by risk, p value calculated via the log-rank test.
(C) Kaplan-Meier plot of progression-free of cases separated by risk, p value calculated via the log-rank test.
The intermediate risk group of pLGG includes tumors with BRAF p.V600E without CDKN2A deletion, FGFR1 SNV, IDH1 p.R132H or MET mutations. Intermediate risk tumors had a 10-year PFS and OS of 35% and 90%, respectively (Figure 7B–C). However, in contrast to the low risk tumors, these tumors continue to progress with a 20-year PFS of 27% and 20-year OS of 81%. Further, they have a propensity for acquiring additional alterations, which may result in the need to refine treatment over time. These patients may therefore require multiple treatment courses and longer-term follow-up than the low risk patients due to the risk of late death.
High risk pLGG include those with H3.3 p.K27M, or BRAF p.V600E with CDKN2A deletion. These tumors almost invariably progress (10-year PFS of 0%) and these patients often succumb to their disease (10-year OS of 41%) (Figure 7B,C). Patients with H3.3 p.K27M do worse than those with BRAF p.V600E and CDKN2A deletion (10-year PFS and OS was 0% and 35% and 0% and 60%, respectively), but both do far worse than low and intermediate risk patients. Although H3.3 p.K27M tumors are more likely to result in patient death, both H3.3 p.K27M and BRAF p.V600E and CDKN2A deletions result in rapid progression, indicating a need for immediate, aggressive treatment and the introduction of novel, targeted agents.
Finally, pLGG with an undetermined molecular alteration (and hence risk category) showed PFS and OS trends consistent with representation of both low and intermediate risk (10-year PFS and OS of 51% and 92%, respectively and 20-year PFS and OS of 34% and 89%) (Figure S8A–B).
In multivariate analysis including tumor location (midline), age at diagnosis, sex, extent of resection (GTR), and histological grade, risk group was determined to be the most significant predictor of both progression (Hazard ratio 4.030 [2.030–7.998], p<0.0001, Cox proportional hazards model) and death (Hazard ratio 16.547 [4.556–59.958], p<0.0001, Cox proportional hazards model) (Table S5).
Discussion
Molecular studies of pLGG over the last decade have uncovered oncogenic drivers shown to activate the RAS/MAPK pathway (Collins et al., 2015; Jones et al., 2008; Schindler et al., 2011; Dougherty et al., 2010; Lassaletta et al., 2017; Listernick et al., 1999; Uusitalo et al, 2016; Seminog et al., 2013). However, despite these advances, the extent of molecular diversity, the frequency of alterations in a population-based setting, and the clinical significance of these diverse alterations are poorly understood. In this study we perform combined morphological, imaging, clinical and molecular profiling of pLGG on a population-based cohort with extensive clinical follow-up. This allowed us to comprehensively investigate the molecular underpinnings and provide comprehensive clinical insights for some of the rarest of pLGG molecular subtypes. Further, we introduce a robust risk stratification system for pLGG, which has the potential to significantly influence the management of these tumors.
RAS/MAPK activation and pLGG were first linked due to the appearance of OPGs in patients with NF1 (Listernick et al., 1999) and was further supported upon the discovery of KIAA1549-BRAF and BRAF p.V600E in these tumors (Jones et al, 2008; Schindler et al., 2011). Here 378 tumors from 2000–2017 had an identifiable alteration impacting the RAS/MAPK pathway with an additional 10 showing up-regulation via ssGSEA analysis of RNA sequencing data. Therefore, of those we could exhaustively profile (including RNA sequencing), 95% (388/410) showed up-regulation of the RAS/MAPK pathway. Thus, pLGG patients may benefit from RAS/MAPK pathway inhibitors even if no genomic alteration in the pathway is identified. Indeed, this is supported by recent work showing favourable responses to MEK inhibitors in pLGG (Fangusaro et al., 2019). Future work will inevitably look to investigate the alternative mechanisms of RAS/MAPK activation in molecularly silent cases which may include alternative splicing (Siegfried et al., 2013), epigenetic changes, or miRNA alterations (Paroo et al., 2009). However, understanding the specifics of these mechanisms need not hinder the adoption of updated treatment protocols that exploit the RAS/MAPK dependence of these tumors.
Our comprehensive approach to profiling these tumors included morphologic, clinical, and molecular interrogation. In utilizing these approaches, we were able to provide insights into the clinical features of the rarest molecular entities of pLGG (Figure 5B–F and 6B–D) and provide disease stratification based on the type of molecular alteration driving the tumor (Figure 4C,D and 7B,C). In our dataset, rearrangement-driven pLGG had a younger age of onset, were enriched for WHO grade I histology, and had a less-aggressive clinical course (Figure 4B–D). This suggests that these oncogenic alterations may occur early in development, promoting tumor initiation in a developmental context permissive for one-hit tumorigenesis. Previous work identified that BRAF fusions promoted gliogenesis in region specific neural stem cell populations, while having little effect in differentiated astrocytes (Kaul et al., 2012). When originally identified, KIAA1549-BRAF was shown to have higher kinase activity than BRAF p.V600E (Jones et al., 2008). This may help explain why many KIAA1549-BRAF pLGG undergo spontaneous growth arrest; an environment where too much RAS/MAPK up-regulation promotes senescence and too little fails to initiate tumor growth (Jacob et al., 2011; Raaba et al., 2011). In comparison, SNV-driven pLGG were more commonly diagnosed at a later age, consistent with these alterations being acquired later in development. Furthermore, SNV-driven pLGG co-occurred with additional SNVs, but never co-harbored fusion events; displaying a pattern of mutual exclusivity as seen across many cancer types (Gao et al., 2018). Lastly, SNV-driven pLGG were associated with poorer outcome. Future work will need to compare the mechanisms behind rearrangements and SNVs in pLGG to elucidate why the observed clinical differences occur.
These results enable us to develop a risk-based stratification system for pLGG (Figure 7A). Genetic rearrangements, including all fusions and duplications, as well as germline NF1 inactivation are considered low-risk. As these tumors are rarely fatal, we propose they should be managed expectantly, with careful consideration of additional treatment post-surgery and radiation excluded from all post-operative treatment. For example, for asymptomatic NF1 -driven pLGG, surveillance is justified. However, if patients display progressive symptoms, most often as vision loss, treatment with chemotherapy (Mahoney et al., 2000; Packer et al., 1997) or targeted inhibitors (Banerjee et al., 2017; Fangusaro et al., 2019) can be beneficial. Beyond 10 years these tumors are much less likely to recur and frequency of follow-up may potentially be reduced. Intermediate risk pLGG are SNV-driven tumors, including those with BRAF or FGFR1 SNVs. These frequently harbor more than one alteration and have a higher risk of recurrence which extends beyond 10 years. These patients may require multiple treatments and longer follow-up than low risk patients. Importantly, compared with high risk patients, intermediate risk tumors rarely die of their disease, so efforts should focus on mitigating clinical progression. Finally, high risk tumors harboring H3.3 p.K27M or BRAF p.V600E and CDKN2A deletion may require new approaches to improve survival including the development of novel agents as well as combination therapies to promote synthetic lethality in these difficult tumor entities. Importantly, as the era of novel targeted therapies such as BRAF and MEK inhibitors inevitably arrives, the risk stratifications of these high and intermediate risk pLGG may change. Nevertheless, significant long-term follow-up is required to determine if the above is indeed true. The large number of patients in this cohort, long term clinical follow-up data and the similarity between subgroups suggest that these findings are robust and provide reliable information of critical importance to clinicians today.
In conclusion, this comprehensive molecular landscape of the clinical and molecular features of pLGG provides clinicians with an invaluable resource for the management of common and rare molecular pLGG subtypes. These data can guide diagnostic protocols and treatment approaches while aiding in expediting clinical trials for new, better targeted therapies for these children in the near future.
STAR Methods
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Cynthia Hawkins (cynthia.hawkins@sickkids.ca). This study did not generate new unique reagents.
Experimental Model and Subject Details
Patient Samples:
Tumor specimens and clinical information were collected according to protocols approved by the Research Ethics Board at all participating institutions. All pLGG diagnosed at the Hospital for Sick Children (SickKids) from 1985–2017 are included in this study. SickKids is the only reference center for children in a population of 5 million people and 97.0% of patients remain in the province qualifying this as a population-based study (Krishnatry et al, 2016; Mistry et al, 2015). For patients older than 18 at the time of clinical data collection, information was extracted from the Pediatric Oncology Group of Ontario Network Information System (POGONIS) (Greenberg et al, 2003). Pathology was reviewed by two neuropathologists (CH and CD). A full description of the samples included are provided in Table S1 including patient sex and age at diagnosis.
To determine the clinical implications of rare alterations, additional cases for which a rare molecular alteration was identified at its originating institute (n=61) with accompanying clinicopathologic and outcome data were included from three participating centers: St. Jude Children’s Research Hospital, Children’s Hospital of Philadelphia, and Memorial Sloan Kettering Cancer Center. A full description of these samples is provided in Table S2 including patient sex and age at diagnosis.
Method Details
Nucleic Acid Extraction
DNA/RNA was extracted from 3–5 10 μM thick scrolls obtained from formalin-fixed paraffin embedded (FFPE) tissue. Extractions were completed using the QIAamp DNA FFPE Tissue Kit or RNeasy FFPE Kit (Qiagen, Valencia, CA). If available, fresh frozen tissue rather than FFPE was used to extract DNA and RNA with the DNeasy Blood and Tissue Kit or RNeasy Mini Kit, respectively being used (Qiagen, Valencia, CA). DNA/RNA was quantified with the Qubit Fluorometer V2.0 using the dsDNA or RNA Broad Range Assay Kit (Thermo Scientific, Waltham, MA). All assay kits were used under the manufacturer’s guidelines. Material used for tier 3 molecular profiling underwent further quantification/qualification testing with the 2100 Bioanalyzer using RNA 6000 NanoKit (Agilent, Santa Clara, CA) under manufacturer’s specifications.
Droplet Digital PCR
Reactions consisted of IX ddPCR Supermix for probes (no dUTP) (Bio-Rad, Hercules, CA), 900 nM of HPLC-purified forward and reverse primers, 250 nM of target-specific mutant and wild-type probes, and 10–20 ng of genomic DNA in 20 μl of total volume. Each reaction was mixed with 70 μl of Droplet Generation Oil (Bio-Rad, Hercules, CA), partitioned into a minimum of 10,000 droplets on the QX200 droplet generator (Bio-Rad, Hercules, CA), transferred to a 96-well plate and sealed prior to PCR amplification. PCR cycling conditions were as follows unless otherwise specified:
95°C for 10min
39 cycles of 94°C for 30 s followed by 55°C for 60 s (with a 2°C s-1 ramp rate)
98°C for 10min
Held at 4°C
Fluorescent intensity was measured with the QX200 Droplet Reader (Bio-Rad, Hercules, CA) and data analysis performed with the QuantaSoft droplet reader software (Bio-Rad, Hercules, CA). Positive and negative droplet populations were detected on a two-dimensional graph and variant allele frequency (VAF) calculated based on the total number of fluorescent-positive droplets. All samples were run in duplicate to ensure validity. Samples were considered positive if a minimum 1% VAF was detected in both duplicate runs and a minimum threshold of 50 fluorescent-positive droplets were detected.
BRAF p.V600E: BRAF p.V600E detection was completed as previously described (Lassaletta et al, 2017). PrimePCR ddPCR mutation assay BRAF wild-type/p.V600E for p.V600E, Human (unique assay ID: dHsaCP2000027/28) was used (Bio-Rad, Hercules, CA).
H3F3A p.K27M: H3F3A p.K27M detection was completed as previously described (Ryall et al, 2016). PrimePCR ddPCR mutation assay H3F3A wild-type/K28M for p.K28M, Human (unique assay ID: dHsaCP2500510/l 1) was used (Bio-Rad, Hercules, CA).
H3F3A p.G34R: H3F3A p.G34R detection was completed as previously described (Ryall et al, 2016). PrimePCR ddPCR mutation assay H3F3A wild-type/G35R for p.G35R, Human (unique assay ID: dHsaMDS720957813) was used (Bio-Rad, Hercules, CA).
FGFR1 TKD: FGFR1 TKD detection was completed as previously described (Fina et al, 2017). Custom primer and probe sequences used for this target are previously described (Fina et al, 2017). PCR cycling conditions were as described above. As described, a ratio value of 1.125 for exon 16 relative to exon 8 were called duplicated.
CDKN2A Deletion: CDKN2A deletion detection was completed as previously described (Lassaletta et al, 2016). PCR ddPCR copy number assay CDKN2A (unique assay ID: dHsaCPl 000581) (Bio-Rad, Hercules, CA) and a reference prime PCR ddPCR copy number assay APB31 (unique assay ID: dHsaCP2500348) (Bio-Rad, Hercules, CA) were used.
IDH1 p.R132H: IDH1 p.R132H detection was completed as described above. PrimePCR ddPCR mutation assay BRAF wild-type/p.R132H for p.R132H, Human (unique assay ID: dHsaCP2000055/56) was used (Bio-Rad, Hercules, CA).
FGFR1 p.N546K, p. K656E: FGFR1 p. N546K, p. K656E detection was completed as described above. Custom primer and probe sequences used for this target are as follows:
N546KFP: 5’-TGATGAAGATGATCGGGAAGC-3’
N546KRP: 5’-CACCCACCATCCTGCGT-3’
N546K wild-type Probe: AATATCATCAACCTGCTGG
N546K Probe: AATATCATCAAACTGCTGG
K656E FP: 5’-CACGGGACATTCACCACATC-3’
K656ERP: 5’-CACAGGGCGGCCTTGTC-3’
K656E wild-type Probe: CTACTATAAAAAGACAACCAA
K656E Probe: TACTATAAAGAGACAACCAA
NanoString nCounter Analysis
Probes that targeted KIAA1549-BRAF fusion variants were designed in collaboration with NanoString (Seattle, WA) and samples tested as previously described (Ryall et al, 2017). 200–500 ng of RNA was added to the nCounter Elements TagSet and hybridized for 20 h before being loaded on the NanoString nCounter system (Seattle, WA). Samples were processed on the nCounter Preparation Station and the cartridge scanned at 555 fields of view on the nCounter Digital Analyzer. Raw NanoString counts were background adjusted with a Poisson correction based on the negative control spikes included in each run. This was followed by a technical normalization using the 4 housekeeping transcripts included in each run (ABCF1, ALAS1, CLTC, and HPRT1). Data is viewed using a box plot and the extreme statistical outlier (3X the interquartile range (IQR)) method was used to detect the presence of an expressed fusion. A second probe set targeting less common fusion variants in pLGG was used in tier 2 under the identical protocol (Guerreiro Stucklin et al., 2019).
NanoString nCounter Vantage 3D for Protein Analysis
RNA and protein were simultaneously analyzed on the NanoString nCounter system. RNA in addition to one slide (10 μm) of FFPE was used for protein analysis. Samples were deparaffinized, processed according to the NanoString protocol and incubated with the antibodies of interest overnight. The RNA (50–100 ng) and the protein lysate were hybridized with the TagSet for 16–24 h at 67°C and loaded on the nCounter Prep Station, where each DNA oligonucleotide is UV cleaved and recognized by a unique reporter probe that contains a fluorescent barcode. All analytes are imaged and counted simultaneously by the nCounter Analysis System to provide a direct, digital readout of RNA, and protein expression in tandem.
Fluorescent in situ Hybridization
Fluorescent in situ hybridization was designed to detect BRAF, MYB, MYBL1, and FGFR2 fused transcripts by using bacterial artificial chromosome clones located over each respective gene target. Probes were obtained from The Center for Applied Genomics (HSC, Toronto, Canada) and are available upon request. Clones were directly labeled with Spectrum Green or Spectrum Orange fluorochrome. Paraffin fluorescent in situ hybridization analysis was performed on 4-μm tumor sections. Slides were baked overnight to fix the section to the slide and were pretreated by using a paraffin pretreatment kit (Abbott, Chicago IL). Sections were dehydrated before slide/probe co-denaturation on thermobrite (Intermedico, Markham, ON, Canada). Denaturation conditions used for paraffin-embedded slides/probes were as follows:
85°C for 7 min
37°C overnight
Slides were washed in x0.4 side scatter/0.3% NP-40 at 73°C for 30 s, followed by x2 side scatter/0.1% NP-40 at room temperature for 30 s. Slides were counterstained with DAPI. Nuclei were analyzed by using an Axioplan2 epifluorescence microscope (Zeiss, Jena, Germany). Images were captured by an Axiocam MRm Camera (Imaging Associates, Bicester, United Kingdom) and analyzed by using an imaging system with Isis Software (Version 5.1.110; MetaSystems, Boston, MA).
Immunohistochemistry
Detection of BRAF p.V600E and H3.3 p.K27M by immunohistochemistry was performed on a Benchmark Ventana Machine (Tucson, AZ) using the Optiview detection kit (Tucson, AZ). CC1 was used for heat retrieval for 40 min and slides were incubated for 36 min with the respective target antibody. Antibodies used were as follows:
Mouse Anti-Human BRAF p.V600E Monoclonal Antibody from Spring Bioscience (El9290, Pleasanton, CA).
Rabbit Anti-Histone H3F3A p.K27M Polyclonal Antibody from Millipore (ABE419, Burlington, MA)
Casein was used for 8 min to help lessen background staining and haematoxylin counterstain was used for 12 min.
SNP Array
Samples were hybridized to the Genome-Wide Human SNP Array 6.0 from Affymetrix (Santa Clara, CA, USA). The sample preparation, including DNA extraction, digestion, labelling and hybridization, was performed as directed by the manufacturer. Data were analyzed using Partek Genomics Suite v6.4 (Partek Incorporated, St. Louis, MO, and Genotyping Console 4.1 (Affymetrix), GISTIC2.0 (Broad Institute, Cambridge, MA, USA).
Targeted DNA Sequencing
Samples with sufficient material and negative for our targeted testing protocol had their DNA constructed into DNA-sequencing libraries using the Illumina Ampliseq Comprehensive DNA Focus Panel Kit (Illumina, San Diego, CA), following the manufacturer’s guidelines utilizing the Illumina Library PLUS Kit (Illumina, San Diego, CA). Sequencing adapters were ligated to the fragments to allow for amplification DNA followed by a quality control validation step to ensure proper adapter ligation. Samples were next hybridized to Ampliseq CD indexes (Illumina, San Diego, CA) used to enrich for 52 cancer-associated genes outlined in the manufacturer’s documentation prior to amplification. Paired-end DNA-sequencing was performed using the Illumina MiSeq sequencing platform and the MiSeq Reagent Micro Kit V2 (Illumina, San Diego, CA). Raw sequencing data was converted to the fastq format and analyzed using the BaseSpace application (Illumina, San Diego, CA), with DNA-Seq Alignment V. 1.0.0. Variant calling was completed in BaseSpace using the Isaac Variant Caller.
Whole Transcriptome Sequencing
Samples with sufficient RNA quality and quantity were sent for whole transcriptome sequencing at The Center for Applied Genomic (HSC, Toronto, Canada). Library preparation was completed using the TruSeq RNA Library Prep Kit v2 (Illumina, San Diego, CA) using the rRNA depletion kit RiboZero Gold (Illumina, San Diego, CA) according to the manufacturer’s specifications. Paired-end sequencing was performed on the Illumina HiSeq 2500 platform to an average of 250 million paired reads. STAR (Dobin et al, 2013) was used to align the raw sequencing data to genome reference ‘Homo sapiens UCSC hgl9’ (RefSeq and Gencode gene annotations). Fusion events were independently called using 4 fusion callers:
DeFuse (McPherson et al, 2011)
TopHat (Kim & Salzberg, 2011)
Ericscript (Benelli et al, 2012)
FusionMap (Ge et al, 2011)
Variant calling from RNAseq was completed using Annovar (Wang et al, 2012).
Gene Set Enrichment Analysis
Gene expression was counted with HTSeq (Anders et al, 2015), and differential expression calculated with edgeR (Robinson et al, 2010). Genes were ranked by multiplying their fold-change sign with the –logl0 (adjusted p value) for pre-ranked GSEA (Subramanian et al, 2005). For ssGSEA, reads were aligned to the transcriptome using RSEM-vl.2 (Li & Dewey, 2011). Genes with mean FPKM < 1 were discarded, and genes with duplicated names were filtered to keep the most expressed gene.
Genetic Analysis at Collaborating Institutions
Molecular analysis of supplemental cases provided from our collaborators were determined based on institute-specific protocols (Surrey et al, 2019; Cheng et al, 2015; Rusch et al, 2018). The details of these protocols are available upon request.
Quantification and Statistical Analysis
Statistical analysis was carried out using a combination of R 3.3.1 (www.r-project.org), GraphPad Prism version 7.00 for Windows (La Jolla California USA, www.graphpad.com), and IBM SPSS Statistics for Windows, Version 25.0. (Armonk, NY: IBM Corp). Categorical comparisons of counts were carried out using Fisher’s exact test, comparisons between groups of continuous variables were completed using a Student’s t-test, Wilcoxon signed-rank test, ANOVA or Mann-Whitney U test (specified in the text). Differences in survival were analysed by the Kaplan-Meier method and significance determined by the log-rank test. Univariate and multivariate analysis was done using multivariate Cox proportional hazards models and significance testing (α=0.05) based on the Wald test. All tests were two-sided and a p value of less than 0.05 was considered significant.
Data and Code Availability
RNAseq data sets and Ampliseq DNA sequencing have been deposited in the European-Genome-Phenome Archive under ascension code EGAS00001004242. Additional data not infringing on ethics restrictions is available upon request to the corresponding authors.
Supplementary Material
Table S1. Clinical and molecular characteristics of the Hospital for Sick Children’s pLGG Cohort, related to Figure 1
Table S3. Clinical and molecular characteristics of the pLGG TaskForce pLGG Cohort, related to Figure 4.
Table S4. Clinical summary of rare molecular drivers in pLGG, related to Figure 5.
Significance.
Pediatric low-grade gliomas (pLGG) are the most common brain tumors affecting children. This integrated clinicopathologic and genomic analysis of >1,000 pLGG defines molecular subgroups with distinct biological drivers and clinical features. RAS/MAPK pathway is activated universally in pLGG, regardless of the presence of a clear genomic activator. Further, although many alterations converge on the RAS/MAPK pathway, clinical presentation and outcome are highly variable depending on the type of underlying alteration. This information helped define clinical risk groups of pLGG with different progression-free and overall survival which likely require different treatment strategies. As modernized treatment regimens utilize alteration-specific agents, we provide the framework for molecular classification of pLGG reflecting unique biological mechanisms driving the disease that likely promote different therapeutic susceptibilities.
Highlights.
KIAA1549-BRAF, BRAF p.V600E, and NF1 mutations account for 2/3 of pLGG.
Activation of the RAS/MAPK pathway is nearly universal in pLGG.
pLGG comprise two distinct clinical subgroups: rearrangement- or SNV-driven.
Risk stratification based on alteration type effectively predicts patient outcome.
Acknowledgments
This work was supported by endowed funds from the Government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-121); A Kid’s Brain Tumor Cure; Brain Tumour Research Assistance and Information Network; The Pediatric Low-Grade Astrocytoma Foundation; Meagan’s Walk; B.r.a.i.n.child Canada; Canadian Cancer Society (Grant # 702296); Canadian Institute of Health Research (Grant # 159805); Restracomp Scholarship and Fellowship funds from the Garron Family Chair in Childhood Cancer Research at The Hospital for Sick Children (S.R, M.Z, A.L, and K.F); Canadian Institute of Health Research (CGS-M) scholarship (S.R); Ontario Graduate Scholarship (S.R); Tokyo Children’s Cancer Study Group (TCCSG) scholarship of the Gold Ribbons Network of Japan (K.F); The Marie-Josee and Henry R. Kravis Center for Molecular Oncology; The National Cancer Institute Cancer Center Core Grant No. P30-CA008748; The American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital.
We thank colleagues Sergio Pereira and Jo-Anne Herbrick (both at the Centre for Applied Genomics at SickKids, Toronto, ON, Canada) for facilitating our work in addition to Paula Marrano, Famida Spatare, and Monte Borden (Department of Pathology, the Hospital for Sick Children, Toronto) and Cindy Zhang (Brain Tumor Research Center, The Hospital for Sick Children, Toronto). We further acknowledge the Members of the Molecular Diagnostics Service in the Department of Pathology (MSK, New York, USA.).
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajan Y, Levy ML, Malicki DM & Crawford JR. (2016) Novel PPP1CB-ALK fusion protein in a high-grade glioma of infancy. BMJ Case Rep. 16;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, & Huber W. (2015) HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics. 31(2): 166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balss J1, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. (2008). Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 116(6): 597–602. [DOI] [PubMed] [Google Scholar]
- Bandopadhayay P, Ramkissoon LA, Jain P, Bergthold G, Wala J, Zeid R, Schumacher SE, Urbanski L, O’Rourke R, Gibson WJ, et al. (2016). MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 48(3):273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolai des T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, et al. (2017). A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 19(8): 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli M, Pescucci C, Marseglia G, Severgnini M, Torricelli F, Magi A (2012). Discovering chimeric transcripts in paired-end RNA-seq data by using EricScript. Bioinformatics. 28(24):3232–9. [DOI] [PubMed] [Google Scholar]
- Broniscer A, Baker SJ, West AN, Fraser MM, Proko E, Kocak M, Dalton J, Zambetti GP, Ellison DW, Kun LE, et al. (2007). Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 25:682–689. [DOI] [PubMed] [Google Scholar]
- Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C. (2014). Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol. 128(4):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, et al. (2015). Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn.l7(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins VP, Jones DT, Giannini C (2015). Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 129(6):775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo F, Ceccarelli M, Tala, Garofano L, Zhang J, Frattini V, Caruso FP, Lewis G, Alfaro KD, Bauchet L, et al. (2019). The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med. 25(1): 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29(1): 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, Storm PB, Biegel JA. (2010). Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 12(7):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S, et al. (2019). Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 20(7): 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina F, Barets D, Colin C, Bouvier C, Padovani L, Nanni-Metellus I, Ouafik L, Scavarda D, Korshunov A, Jones DT, et al. (2017). Droplet digital PCR is a powerful technique to demonstrate frequent FGFR1 duplication in dysembryoplastic neuroepithelial tumors. Oncotarget. 8 (2): 2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Liang WW, Foltz SM, Mutharasu G, Jayasinghe RG, Cao S, Liao WW, Reynolds SM, Wyczalkowski MA, Yao L, et al. (2018). Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 23(l):227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Liu K, Juan T, Fang F, Newman M, Hoeck W (2011). FusionMap: detecting fusion genes from next-generation sequencing data at base-pair resolution. Bioinformatics. 27(14): 1922–8. [DOI] [PubMed] [Google Scholar]
- Greenberg ML, Barr RD, DiMonte B, McLaughlin E, Greenberg C. (2003). Childhood cancer registries in Ontario, Canada: lessons learned from a comparison of two registries. Int J Cancer. 105(1): 88–91. [DOI] [PubMed] [Google Scholar]
- Guerreiro Stucklin AS, Ryall S, Fukuoka K, Zapotocky M, Lassaletta A, Li C, Bridge T, Kim B5, Arnoldo A, Kowalski PE, Zhong Y, et al. (2019). Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun. 10(1):4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. (2017). Neurofibromatosis type 1. Nat Rev Dis Primers. 3:17004. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, et al. (2009). Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 118(4):469–474. [DOI] [PubMed] [Google Scholar]
- Jacob K, Quang-Khuong DA, Jones DT, Witt H, Lambert S, Albrecht S, Witt O, Vezina C, Shirinian M, Faury D, et al. (2011). Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 17(14):4650–60. [DOI] [PubMed] [Google Scholar]
- Jones DT, Gronych J, Lichter P, Witt O, Pfister SM. (2012). MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sei. 69(11): 1799–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Hutter B, Jäger N, Korshunov A, Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA, et al. (2013). Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Kocialkowski S, Liu L, Pearson DM, Bäcklund LM, Ichimura K, Collins VP (2008). Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 68(21): 8673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul A, Chen YH, Emnett RJ, Dahiya S, Gutmann DH. (2012). Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 26(23):2561–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, Bartels U, Albrecht S, Schwartzentruber J, Letourneau L, et al. (2012). K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 124(3):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehna EN, Arnush MR, Tamrazi B, Cotter JA, Hawes D, Robison NJ, Fong CY, Estrine DB, Han JH, Biegel JA. (2017). Novel GOPC(FIG)-ROSl fusion in a pediatric high-grade glioma survivor. JNeurosurg Pediatr. 20(1):51–55. [DOI] [PubMed] [Google Scholar]
- Kim D & Salzberg SL (2011). TopHat-Fusion: an algorithm for discovery of novel fusion transcripts. Genome Biol. 12(8): R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnatry R, Zhukova N, Guerreiro Stucklin AS, Pole JD, Mistry M, Fried I, Ramaswamy V, Bartels U, Huang A8, Laperriere N, et al. (2016). Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade gliomas. Cancer. 2016; 122(8): 1261–9. [DOI] [PubMed] [Google Scholar]
- Lassaletta A, Zapotocky M, Mstry M, Ramaswamy V, Honnorat M, Krishnatry R, Guerreiro Stucklin A, Zhukova N, Arnoldo A, Ryall S, et al. (2017). Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J Clin Oncol. 35(25):2934–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B & Dewey CN (2011) RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listernick R, Charrow J, Gutmann DH. Intracranial gliomas in neurofibromatosis type 1. Am J Med Genet. 1999;89(l):38–44. [DOI] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestier OD, Kleihues P, Ellison DW (2016). The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta. Neuropathol. 131(6):803–820. [DOI] [PubMed] [Google Scholar]
- Mahoney DH Jr, Cohen ME, Friedman HS, Kepner JL, Gemer L, Langston JW, James HE, Duffner PK, Kun LE (2000). Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol. 2(4): 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A Hormozdiari F Zayed A, Giuliany R, Ha G, Sun MG, Griffith M, Heravi Moussavi A, Senz J, Melnyk N, et al. (2011). deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS ComputBiol. 7(5), el001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, Stavropoulos J, Alon N, Pole JD, Ray PN, et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. (2015). J Clin Oncol. 20;33(9):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nageswara Rao AA & Packer RJ. Advances in the management of low-grade gliomas. Curr. Oncol. Rep. 2014;16: 398. [DOI] [PubMed] [Google Scholar]
- Northcott PA, Pfister SM, Jones DT. (2015). Next-generation (epi)genetic drivers of childhood brain tumors and the outlook for targeted therapies. Lancet Oncol. 16(6):e293–e302. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS (2015). Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16:(Suppl 10):xl–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RJ, Ater J, Allen J, Phillips P, Gey er R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, et al. (1997). Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997 May;86(5):747–54. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Ye X, Chen S, Liu Q. (2009) Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 139:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. (2008). An integrated genomic analysis of human glioblastoma multiforme. Science. 2008 26;321(5897):1807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J, et al. (2016). Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 131(6):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG, Nikkhah G, Maciaczyk J, Kahlert U, Jain D, Bar E, et al. (2011). BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 17(11):3590–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE, McKenna A, Lawrence MS, Bergthold G, Brastianos PK, et al. (2013). Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci USA. 110(20):8188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26(1): 139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch M, Nakitandwe J, Shurtleff S, Newman S, Zhang Z, Edmonson MN, Parker M, Jiao Y, Ma X, Liu Y, et al. (2018). Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat Commun. 9(1):3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall S, Arnoldo A, Krishnatry R, Mistry M, Khor K, Sheth J, Ling C, Leung S, Zapotocky M, Guerreiro Stucklin A, et al. (2017). Multiplex Detection of Pediatric Low-Grade Glioma Signature Fusion Transcripts and Duplications Using the NanoString nCounter System. J Neuropathol Exp Neurol. 76(7): 562–570. [DOI] [PubMed] [Google Scholar]
- Ryall S, Krishnatry R, Arnoldo A, Buczkowicz P, Mistry M, Siddaway R, Ling C, Pajovic S, Yu M, Rubin JB, et al. (2016). Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun. 4(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, et al. (2011). Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 121(3):397–405. [DOI] [PubMed] [Google Scholar]
- Seminog OO, Goldacre MJ (2013). Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 108(1): 193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried Zl, Bonomi S, Ghigna C, Kami R. (2013). Regulation of the Ras-MAPK and PI3K-mTOR Signalling Pathways by Alternative Splicing in Cancer. Int J Cell Biol. 568931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 25; 102(43): 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey LF, MacFarland SP, Chang F, Cao K, Rathi KS, Akgumus GT, Gallo D, Lin F, Gleason A, Raman P, et al. (2019). Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 11(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatevossian RG, Tang B, Dalton J, Forshew T, Lawson AR, Ma J, Neale G, Shurtleff SA, Bailey S, Gajjar A, et al. (2010). MYB upregulation and genetic aberrations in a subset of pediatric low-grade gliomas. Acta Neuropathol. 120(6): 731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo E, Rantanen M, Kallionpaa RA, Poyhonen M, Leppavirta J, Yla-Outinen H, Riccardi VM, Pukkala E, Pitkaniemi J, Peltonen S et al. (2016). Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol. 34(17): 1978–86. [DOI] [PubMed] [Google Scholar]
- Wang K, Li M, & Hakonarson H (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38(16):el64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE (2011). Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery. (6): 1548–54. [DOI] [PubMed] [Google Scholar]
- Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, Zhu X, Qu C, Chen X, Zhang J, et al. (2014). The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 46(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. (2009). IDH1 and IDH2 mutations in gliomas. N Engl J Med. 360(8):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, et al. (2013). Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical and molecular characteristics of the Hospital for Sick Children’s pLGG Cohort, related to Figure 1
Table S3. Clinical and molecular characteristics of the pLGG TaskForce pLGG Cohort, related to Figure 4.
Table S4. Clinical summary of rare molecular drivers in pLGG, related to Figure 5.
Data Availability Statement
RNAseq data sets and Ampliseq DNA sequencing have been deposited in the European-Genome-Phenome Archive under ascension code EGAS00001004242. Additional data not infringing on ethics restrictions is available upon request to the corresponding authors.