Abstract
Lateral flow assay (LFA) is a well-established platform for point-of-care (POC) testing due to its low cost and user friendliness. Conventional LFAs provide qualitative or semi-quantitative results, and require dedicated instruments for quantitative detection. Here we developed an “LFA Ruler” for quantitative and rapid readout of LFA results, using a 3D printed strip cassette and a simple, inexpensive microfluidic chip. Platinum nanoparticles are used as signal amplification reporters, which catalyze the generation of oxygen to push ink advancement in the microfluidic channel. The concentration of target is linearly correlated with the ink advancement distance. The entire assay can be completed within 30 minutes without external instrument and complicated operations. We demonstrated quantitative prostate specific antigen testing using LFA ruler, with a limit of detection of 0.54 ng/mL, linear range 0–12 ng/mL, and high correlation with clinical gold standard assay. The LFA ruler achieves low cost, quantitative, sensitive and rapid detection, which has great potential in POC testing and can be extended to quantify other disease biomarkers.
1. Introduction
Point-of-care (POC) diagnostics provide clinicians access to rapid and actionable diagnostic results at or near the site of patient care to facilitate quick treatment decision making.1–5 The major advantages of POC diagnostics are portability, low cost and user friendliness. Lateral flow assay (LFA) is a well-established platform for POC testing with proven utility for the detection of diverse analytes, such as proteins, nucleic acids, toxins, bacterial and viral pathogens.6–8 The entire immunoassay process, including washing and signal-reporting, can be integrated into one capillary paper-based strip starting with one drop of sample. Because of its speed of analysis, user-friendly format, low operation cost and long-term stability, LFA is one of the most successfully clinically translated POC testing platform with a global market size of USD 5.55 Billion in 2017 and an expected market size of USD 8.24 Billion by 2022.9
In conventional LFAs, qualitative or semi-quantitative results are generated by visual inspection in less than 30 min.7 Colloidal gold nanoparticles or other colored labels conjugated to detection antibodies are used as signal reporters, which are analyte-bridged to capture antibodies immobilized on the surface of an LFA strip.10,11 Direct visualization of colorimetric LFA readout in POC testing is very useful for clinicians to make an immediate medical decision. However, subjective judgment variation in visual interpretation may exist among end-users, caused by the differences of illumination setting and personal visual ability and other psychological factors. Thus, it could lead to controversial readouts, especially when the colorimetric signal is close to threshold. To achieve quantitative colorimetric readout of LFAs, various optical strip readers and image processing algorithms are specifically designed to measure the color intensities of test/control lines,6 which can be correlated with analyte concentrations. However, the sensitivity and quantification ability of LFA are intrinsically limited by the colorimetric signal readout. The need for additional readers also increases overall testing costs.
Quantitative LFAs utilizing fluorescence, magnetic or Raman reporters instead of colorimetric labels have also been developed.12–14 Although these strategies contribute to improvement in the sensitivity of LFAs and expand their applications, they all require additional dedicated and sophisticated instruments for readout and experienced operators for quantitative analysis. These factors render the above strategies unsuitable for POC testing in resource-limited settings. Recently, platinum nanoparticle (PtNP) has been used as a signal probe in immunoassays.15–24 These assays were used for facile visual quantitation by direct distance measurement,17–21 of which the manual washing and incubation steps can be greatly simplified in LFA.
In this paper, we present a 3D printed strip cassette and a simple, inexpensive microfluidic chip for LFA quantitation and rapid detection with distance-based readout, which is called “LFA Ruler”. After the conventional operation of PtNP-based LFA in the strip cassette, the entire test zone is cut by inserting a blade into the slots of strip cassette and added to the reaction chamber in LFA ruler. PtNP-catalyzed oxygen generation in H2O2 solution pushes colored ink to advance in the microfluidic channel. The ink advancement distance, read directly with naked eyes, is linearly correlated with the concentration of target. We applied the LFA ruler for quantification of prostate specific antigen (PSA) in clinical serum samples, and compared LFA results with commercial electro-chemiluminescence immunoassay (ECLIA). PSA is a protein produced mostly by cells of the prostate gland, and is used clinically as a prostate cancer screening biomarker.25 Globally, prostate cancer is the second most common type of cancers and the fifth leading cause of cancer-related deaths in men.26 Serum PSA concentration below 4 ng/mL in screening indicates low probability of prostate cancer; concentration above 10 ng/mL indicates possible presence of prostate cancer; concentration between 4 and 10 ng/mL is within grey zone and indicates further definitive testing.27 The LFA ruler enables low cost, quantitative, and rapid readout of PtNP-based PSA LFA strip, allowing clinical decision making with relation to the above thresholds, which is especially suitable for POC testing in resource-limited areas. Moreover, as a versatile platform, the LFA ruler has great potential to be applied to quantification of other disease biomarkers.
2. Methods
2.1. Materials and chemicals
Glass slides (75 × 50 × 1 mm3 and 75 × 25 × 1 mm3) were purchased from Corning, Inc. (Corning, NY, USA). Silicon wafers (100 mm) were purchased from University Wafer (Boston, MA, USA). KMPR-1050 photoresist and SU-8 developer were purchased from MicroChem Corp. (Newton, MA, USA). Polydimethylsiloxane (PDMS) elastomer kits (Sylgard™ 184) were purchased from Electron Microscopy Sciences (Hatfield, PA, USA). Platinum Nanoparticles (70 nm) were purchased from Nanocomposix, Inc. (San Diego, CA, USA). Bovine serum albumin (BSA, A7906–50G), Tween-20 (Molecular Biology Grade, P9416–100ML), 1H,1H,2H,2H-Perfluorooctyltrichlorosilane (97%), and prostate specific antigen (PSA, human seminal fluid, 539832) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). EZ-Link NHS-Biotin, (PI20217), Zeba™ spin desalting columns (89882), HABA (4’-hydroxyazobenzene-2-carboxylic acid, 28010), disposable standard biopsy punches (6mm, 12-460-412), hydrogen peroxide (30% in water, BP2633500), NeutrAvidin Protein (PI31000), sodium citrate (78–101-KG), red ink and sealing tape for 96-well plates (15036) were purchased from Thermo Fisher Scientific, Inc. (Rockford, IL, USA). Phosphate-buffered saline (PBS) tablets (T9181), pH 7.4, were purchased from Clontech Laboratories, Inc. (Mountain View, CA, USA). Mouse monoclonal anti-PSA antibodies (ABPSA-0405 and ABPSA-0406) were purchased from Arista Biologicals, Inc. (Allentown, PA, USA). Goat anti-mouse IgG (ABGAM-0500) was purchased from Arista Biologicals, Inc. (Allentown, PA, USA). Polyethylene glycol (PEG) 3350 was purchased from GoldBio, Inc. (St. Louis, MO, USA). Scotch tape was purchased from 3M (Maplewood, MN, USA). The glass fiber (G041) was obtained from EMD Millipore Corporation (Billerica, MA, USA). The Fusion 5 membrane, nitrocellulose membrane (FF 80HP) and absorbent paper (GB003) were purchased from GE Healthcare Life Sciences (Pittsburgh, PA, USA). The backing card was purchased from DCN Dx (Carlsbad, CA, USA).
2.2. Device design and fabrication
The strip cassette contains a substrate and a cover for the operation of LFA strip tests, which was fabricated by 3D printing technique (Biomedical Library of the University of Pennsylvania). The material was ABS plastic and the whole size was 50 × 80 × 15 mm3. After assembling the substrate and cover, the operation of LFA strip tests can start from loading sample solution into the sample chamber. After LFA completion, a blade is inserted into the slots to cut the test zone and/or control zone of LFA strips. Then the zone pad is added from the well of strip cassette to the reaction chamber in the LFA ruler. The microfluidic chip of LFA ruler was composed of one layer of PDMS bonded to a glass slide, fabricated with conventional soft lithography techniques. The inside of the microchannels was treated with the hydrophobic reagent (1H,1H,2H,2H-perfluorooctyltrichlorosilane in IPA, 1% v/v).
2.3. Preparation and conjugation of platinum nanoparticles
For the preparation of NeutrAvidin-conjugated PtNPs, 20 μL of 5 mg/mL NeutrAvidin were mixed with 1 mL of 0.05 mg/mL 70 nm PtNPs in citrate buffer, pH 7.2, and continuously mixed using a rotator (20 rpm) at 4 °C overnight. Then BSA was added to a final concentration of 1% and mixed on a rotator (20 rpm) at 4 °C overnight to block the PtNPs surface. Unconjugated NeutrAvidn was removed by centrifugation at 3000 g 6 times for 8 min each. Finally, the NeutrAvidin-conjugated PtNPs were suspended in 100 μL of PBS, pH 7.4, containing 1% BSA.
Biotinylation of monoclonal anti-PSA antibody (ABPSA-0406) with Pierce premium-grade NHS-Biotin was performed according to the manufacturer’s protocol. Briefly, the protein was mixed with NHS-Biotin (mole ratio, 1:20), and the reaction was allowed to occur at room temperature for 30 min. The uncoupled NHS-Biotin was removed with a Zeba™ desalting column (40 kDa molecular weight) according to the manufacturer’s protocol. The biotinylated antibodies were stored with 1% BSA in PBS pH 7.4 at 4°C.
For the preparation of antibody-platinum nanoparticle (Ab-PtNP) conjugates, 25 μL biotinylated antibody were mixed with 1 mL of NeutrAvidin-conjugated PtNPs in PBS buffer, pH 6.5, and continuously mixed using a rotator (20 rpm) at 4 °C overnight. BSA was added to a final concentration of 1% to block the PtNPs surface, and unconjugated antibody was removed via centrifugation. Finally, the antibody-conjugated PtNPs were suspended in 500 μL of PBS, pH 7.4, containing 1% BSA, and stored at 4 °C. NanoDrop™ One Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific, Inc., USA) was used to quantify the concentration of Ab-PtNP conjugates, calibrated with commercial PtNP standards (absorption intensity @500 nm).
2.4. Lateral flow strips preparation
The test strip was constructed with four main elements: the sample pad, the conjugate pad, the nitrocellulose membrane, and the absorbent pad. The four parts were pasted on a plastic backing one by one, with ends overlapping 2 mm. Then the strips with widths of 4 mm each were produced using a paper cutting machine. The Fusion 5 membrane was used as the sample pad because of its low non-specific binding. The glass fiber membrane was used as the conjugate pad, pretreated with 10% sucrose to improve the stability of Ab-PtNP conjugates. The conjugates were rinsed into the glass fiber, and air dried at room temperature. Monoclonal anti-PSA antibody (ABPSA-0405) and goat anti-mouse IgG were separately diluted in PBS buffer, pH 7.4. The diluted antibodies were applied onto the nitrocellulose membrane to generate the test zone and control zone, respectively. The strips were dried at 37 °C and relative humidity of 25 to 30% overnight and stored at room temperature in a sealed package with silica gel.
2.5. Using LFA ruler to quantify lateral flow assay results
Fifty microliters of LFA buffer (0.01 M PBS, pH 7.4; 0.1% Tween-20; 0.2% BSA; 0.1% PEG-3350) containing different concentrations of analytes was loaded into the sample chamber of strip cassette. After incubation for 15 min at room temperature, the test zone (4 × 4 mm2) was cut by inserting a blade into the slot and added to the reaction chamber of LFA ruler, and 3 μL of red ink was loaded into the ink chamber. Finally, 35 μL H2O2 (30%) was added into the reaction chamber. To seal the device, a piece of sealing tape (15 × 20 mm2) was gently pasted on top of the reaction chamber and another piece of Scotch tape was gently pasted on top of the ink chamber and the balance reservoir. After incubation for 12 min at room temperature, the ink advancement distances were read directly with the naked eye, and photos showing oxygen bubbles were captured using a cellphone with a uHandy Mobilephone Microscope (Aidmics Biotechnology Co., Taipei, Taiwan, China).
To compare the quantitative ability of LFA ruler and commercial PSA strip with scanner, the commercial strips (Instant-view® PSA Whole Blood/Serum Test) with different PSA concentrations (0, 1, 2, 4, 8, and 12 ng/mL, respectively) were scanned successively by laboratory Gel Doc™ XR+ System from Bio-Rad Laboratories, Inc. (Hercules, CA, USA) and MP C3004 office scanner from Ricoh USA, Inc. (Malvern, PA, USA). The intensity values of test line and reference line were analyzed using the Image Lab™ and Image J software, respectively. The ratio of test line and reference line was used for the quantitation of commercial PSA LFA strip. The experiment was repeated three times independently.
2.6. Clinical serum sample collection and analysis
All de-identified human serum samples were obtained from ARUP Laboratories (Salt Lake City, UT, USA). All serum samples were first analyzed using the commercial ECLIA method (Roche Elecsys Cobas Total PSA assay), then frozen till tested using the LFA ruler. The study was approved by the institutional IRB committee. For analysis using the LFA ruler, the samples were diluted with LFA buffer (0.01 M PBS, pH 7.4; 0.1% Tween-20; 0.2% BSA; 0.1% PEG-3350) and then analyzed as described above, in triplicate. The results are shown as mean ± standard error.
3. Results and discussion
3.1. Working principle of strip cassette and LFA ruler
The working principle of the strip cassette and LFA ruler is shown in Figure 1. As in classic LFA assays, the LFA strip is composed of a sample pad, a conjugate pad, a nitrocellulose membrane, and an absorbent pad, which are successively assembled on a plastic backing card. After assembling the LFA strips in the strip cassette, sample solution is applied into the sample chamber of strip cassette and flows toward the absorbent pad driven by capillary force. Target molecules in the sample solution bind to the pre-immobilized detection Ab-PtNP conjugates when flowing through the Ab-PtNP conjugation pad. The PtNPs labeled target molecules are captured by the target-specific capture antibody (Ab) in the test zone and the excess conjugates migrate further and bind to the anti-mouse capture Ab in the control zone. The entire test zone pad is then cut by inserting a blade into the slot of strip cassette and added to the reaction chamber in the microfluidic chip. The PtNPs captured in the pad catalyze the breakdown of H2O2 into water and oxygen. The generated oxygen is sealed in the chip and pushes the ink forward in the microchannel. The ink advancement distance of test zone within a specified time period is read directly with the naked eye, which is proportional to the amount of target molecules in the sample. Furthermore, the control zone pad can be tested in the same way as the test zone pad, and functions as internal quality control of the LFA strip. Unlike previous LFA quantitative readout methods, the LFA ruler achieves the direct visualization of quantitative results without the need for external instruments. Figure S1 shows photographs of the 3D printed strip cassette and LFA ruler.
Figure 1.
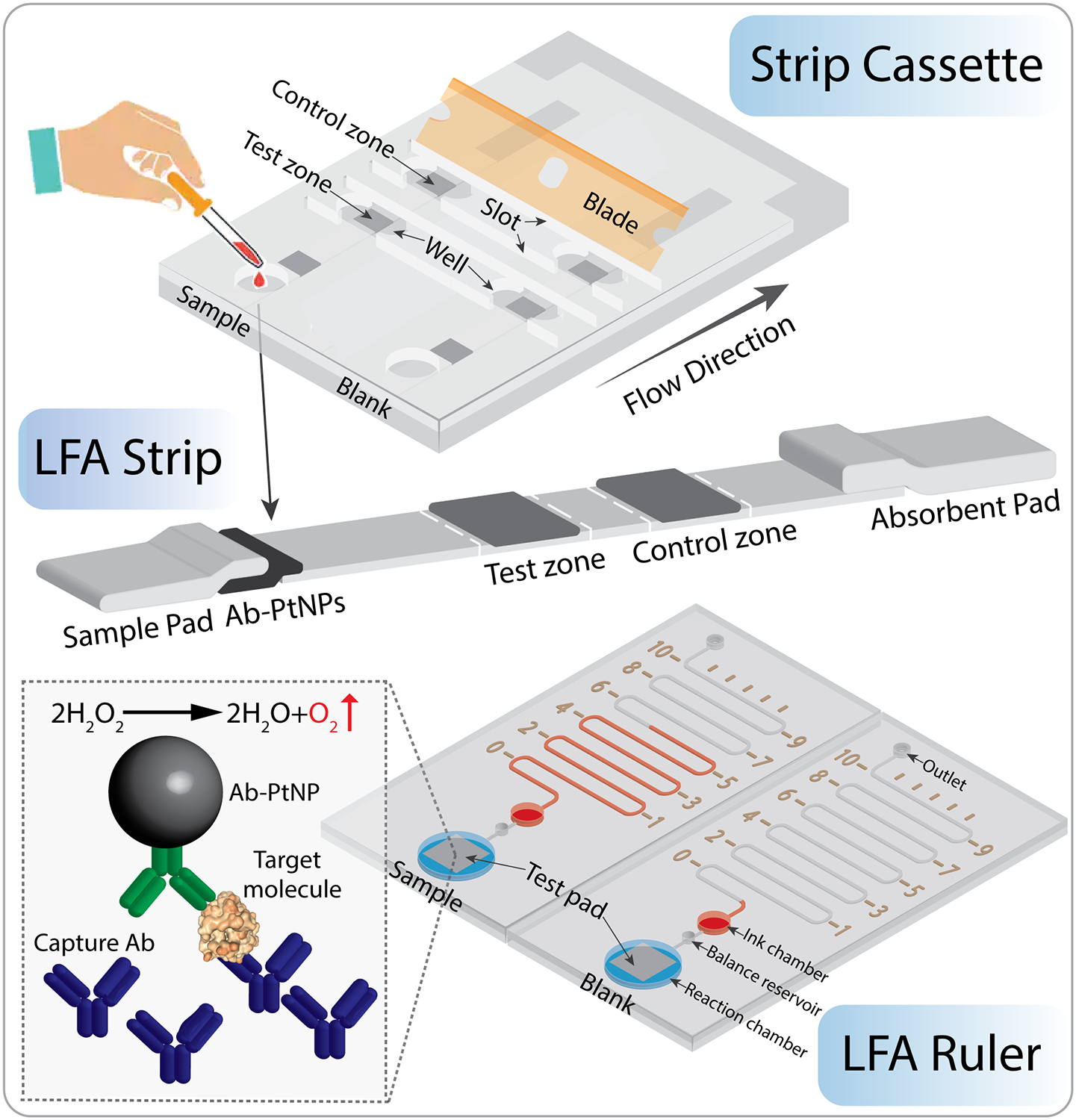
Working principle of the strip cassette and LFA ruler. First, LFA strips were placed into the strip cassette, and sample or blank control was loaded into sample chambers. After LFA completion (~15 min), a blade was inserted into the slots to cut the test zone of LFA strips. Then the pads were added from the well of strip cassette to the reaction chamber in the LFA ruler. The LFA ruler contains microchannel, distance markers, ink chamber, balance reservoir, reaction chamber, outlet and sealing tapes. The PtNPs captured in the test zone pad catalyze the breakdown of H2O2 into water and oxygen, which pushes the red ink forward in the microchannel. The ink advancement distance is proportional to the amount of target molecules in the sample. The LFA ruler achieves quantitative detection of biomarkers by the naked eye. Not to scale.
3.2. Feasibility of LFA ruler
The LFA ruler is based on a PDMS-glass hybrid microfluidic chip, which is low cost and easy to fabricate using conventional soft lithography techniques. To test if PDMS needs to be treated to enhance gas impermeability, a 3-μm-thick layer of low-permeability Parylene C (PC) membrane was deposited on the surface of the LFA ruler (LABCOTER®2, Specialty Coating Systems Inc., IN, USA), including the entire interior of the chambers (Figure 2A). Under the same experimental conditions, the distance of ink advancement in the device with PC membrane was only a little longer than that in the device without PC membrane (Figure 2B). This can be explained by the fact that the LFA ruler is an open-ended device, so the effect of gas permeability of PDMS is not significant. All subsequent experiments were conducted on the devices without PC membrane.
Figure 2.
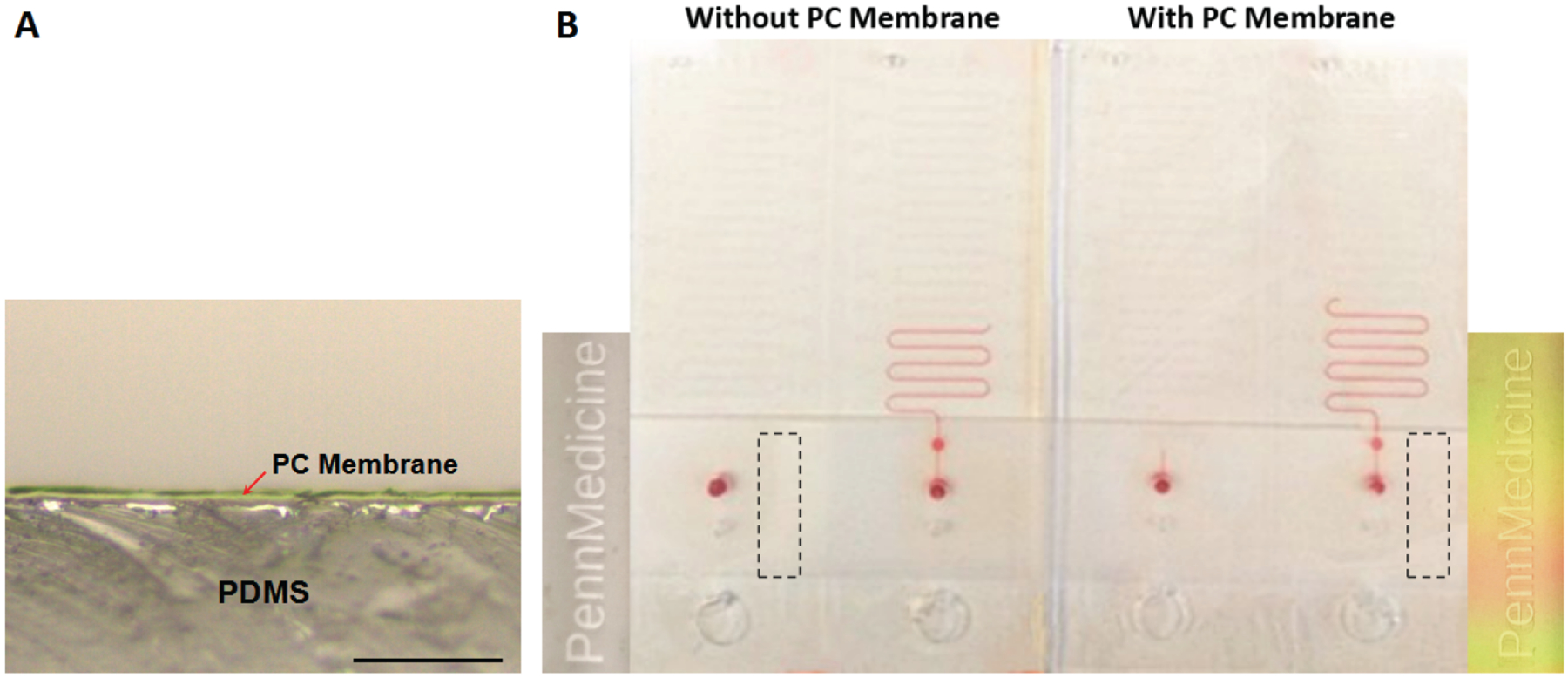
(A) Microscope image of a 3-μm-thick layer of low-permeability Parylene C (PC) membrane deposited on the surface of the LFA ruler. Scale bar, 50 μm. (B) The ink advancement distances in different LFA rulers with/without PC membrane, pushed by oxygen generated as a result of different numbers of PtNPs (0, 5.6 × 104; 0, 5.6 × 104, respectively) reacting with 30% H2O2. Illustrations on both sides are the enlarged views of the black dotted rectangles. Under a certain angle of illumination, the label on the device without PC membrane is gray; the label on the device with PC membrane is colored.
Furthermore, the elasticity of PDMS might change the internal pressure when the tape is applied to the surface to seal the chambers. To address this issue, a balance reservoir is added between the reaction chamber and the ink chamber, and the sealing process is changed to two-step method. When the reaction chamber is sealed, the balance reservoir can keep the internal pressure the same as the atmospheric pressure, eliminating interference caused by PDMS deformation. Then, the ink chamber and the balance reservoir are sealed successively by adding another tape.
To verify the working principle of LFA ruler and evaluate the relationship between ink advancement and PtNP concentration, PtNP solutions were directly loaded in the reaction chamber for an oxygen generation test. Figure 3A shows the ink advancement distances in the device, pushed by oxygen generated as a result of different numbers of PtNP reacting with H2O2 for 12 min. As the number of PtNP increases, the ink advancement distance in the device increases, which correlates with the density and size of bubbles in the reaction chamber. The plots of time-dependent ink advancement distances with different concentrations of PtNPs and H2O2 are shown in Figure S2. In Figure 3B, the ink advancement distance is linearly correlated with the number of PtNP in H2O2 at 12 min, (r2 = 0.99, three parallel measurements of each concentration). These indicate that the LFA ruler is sensitive and can detect as low as twenty thousand PtNPs, and also has a wide dynamic range. We compared the catalytic activity of PtNP and Ab-PtNP. At the same concentration, the catalytic activity of Ab-PtNP is approximately half that of PtNP (Figure S3).
Figure 3.
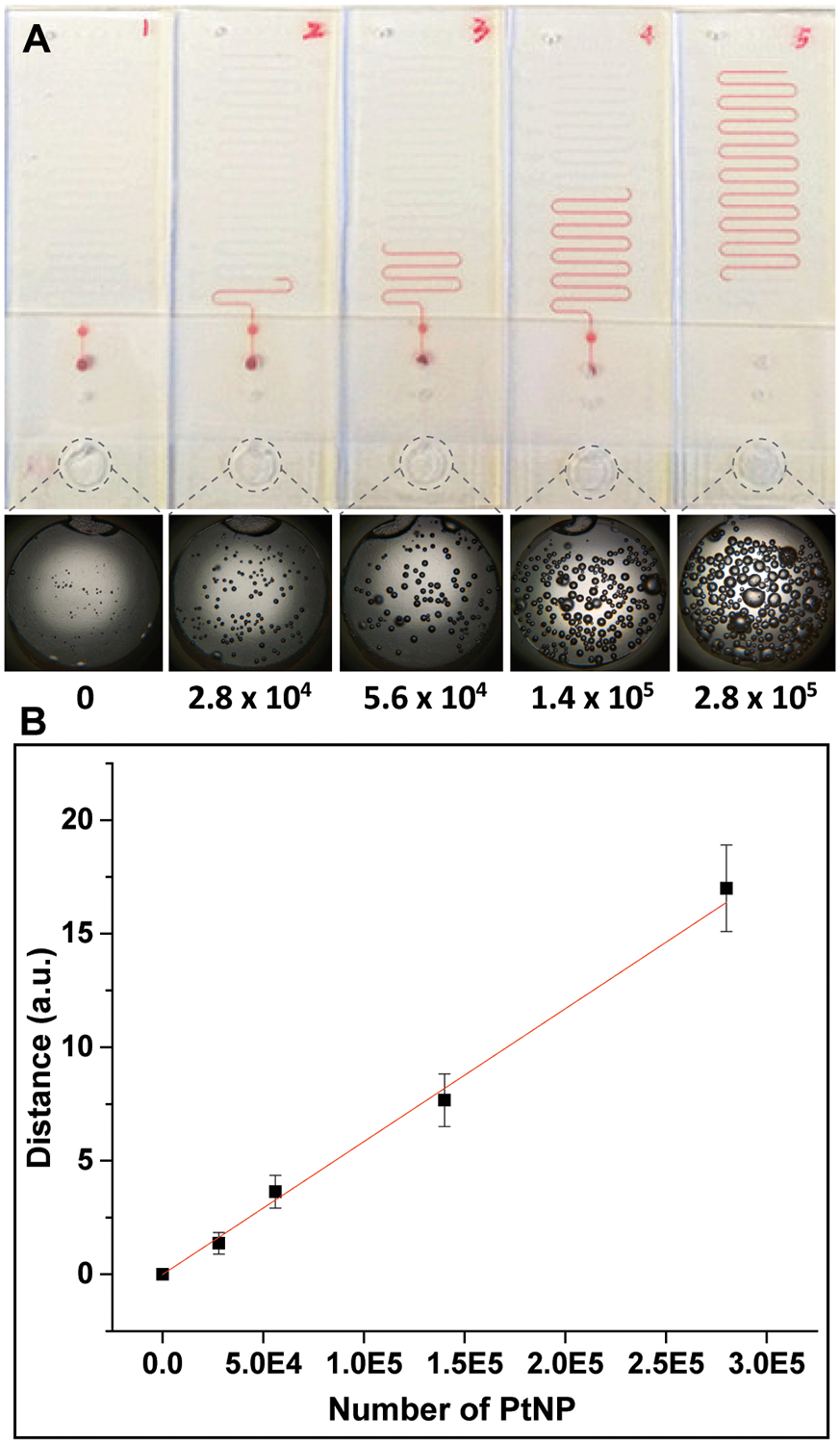
Correlation between number of PtNP and ink advancement distance on LFA ruler. (A) The ink advancement distances pushed by oxygen generated as a result of different numbers of PtNP (0, 2.8 × 104, 5.6 × 104, 1.4 × 105, and 2.8 × 105, respectively) reacting with 30% H2O2. The pictures at the bottom show the density and size of bubbles in the reaction chamber after 12 min of incubation. (B) Linear correlation plot of ink advancement distance with number of PtNP in 30% H2O2 (r2 = 0.99).
3.3. Quantitation of PSA lateral flow strips with LFA ruler
To demonstrate the feasibility of LFA ruler with distance-based readout for target quantitation, PSA was used as a model biomarker. All commercially available PSA lateral flow strips used in the POC settings generate only qualitative or semi-quantitative results. For example, “See Now” PSA Strip (Camp Medica, Romania), Accu-Tell® One Step PSA Serum Test (AccuBio Tec Co. Ltd., China), and Home Prostate Test (Home Health (UK) Ltd., UK) provide qualitative tests with a cut-off value of 4.0 ng/mL; One Step PSA Rapid Test (Biogate Laboratories Ltd., Canada) has a cut-off value and a reference value of 4 ng/mL; OnSite PSA Semi-quantitative Rapid Test (CTK Biotech Inc., USA) provides semi-quantitative tests with a cut-off value of 4 ng/mL and a reference value of 10 ng/mL. In order to meet the POC testing requirements for using PSA as a prostate cancer screening biomarker, there is an unmet need to overcome the shortcomings of colorimetric readout of LFA strips and generate quantitative PSA results in concentrations <4 ng/mL, 4–10 ng/mL, and >10 ng/mL, which places patients with respect to clinical decision thresholds but is currently only achievable in the central clinical laboratory setting. To achieve this with the LFA ruler, anti-PSA capture Ab and anti-mouse IgG Ab were pre-immobilized on the surface of the nitrocellulose membrane in the test zone and control zone, separately. The test zone pad and control zone pad from positive (8 ng/mL PSA) and blank strips were cut and simultaneously tested in LFA rulers (Figure S4). The ink advancement distance of test zone from positive strip is much longer than that from blank strip. Furthermore, there is no significant difference in ink advancement distance between the two control zone pads. Figure 4A shows scanning electron microscope images of the test zone pads from positive and blank strips, respectively. Some PtNPs are observed in the cavities of test zone pad from positive strip, which are identified by the green arrows in Figure 4A; and almost no PtNPs are observed in the cavities of test zone pad from blank strip (Figure 4B). The ink advancement distances in the LFA ruler with different PSA concentrations are shown in Figure 4C. The linear correlation between ink advancement distances with PSA concentrations is shown in Figure 4D, tested in three parallel measurements with optimized Ab-PtNP conjugates (optimization shown in Figure S5). The calibration equation was y = 0.99x + 0.04, linear range 0–12 ng/mL, with a correlation coefficient (r2) of 0.99. The limit of detection (LOD) was calculated to be 0.54 ng/mL, extrapolated by the mean concentration of blank samples (n = 3) plus three standard deviations. From the operation standpoint, the time length for reactions on the LFA strip is 15 min, and the subsequent time length on the LFA ruler is 12 min. Thus, the entire testing time is approximately 30 min, which is highly practical in the POC setting.
Figure 4.
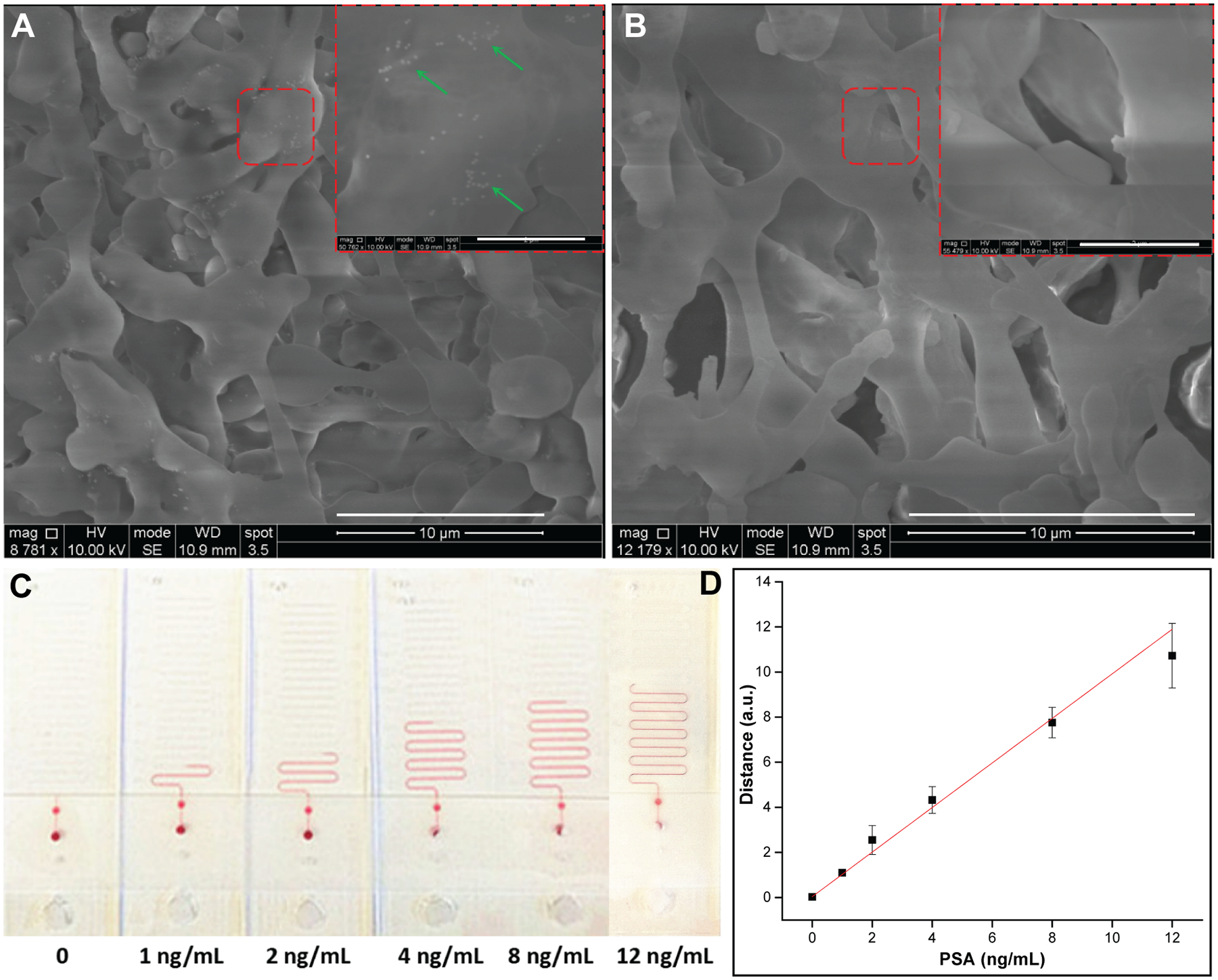
(A) Scanning electron microscope images of the test zone pads from positive and (B) blank strip, respectively. Scale bar, 10 μm. The insets in the top right corner show the enlarged view of the red dashed squares. Scale bar, 2 μm. The green arrows identify PtNPs in the cavities of nitrocellulose membrane. (C) Quantitation of PSA lateral flow strips with LFA ruler. Ink advancement distances in the LFA ruler with different PSA concentrations (0, 1, 2, 4, 8, and 12 ng/mL, respectively). (D) Linear correlation between ink advancement distance and PSA concentration, tested in triplicates (r2 = 0.99).
3.4. Validation of the LFA ruler against clinical standard using clinical serum samples
To validate the performance of the LFA ruler against gold-standard clinical assays, PSA concentrations in clinical serum samples (n = 30) were quantitated using both the LFA ruler and an FDA-approved ECLIA method (Roche Elecsys Cobas Total PSA assay). The comparison of the results is shown in Figure 5A. Compared to the clinical results, all of the LFA results remained within the same clinical decision zones (<4 ng/mL, 4–10 ng/mL and >10 ng/mL). Figure 5B shows a linear relationship between the two analysis methods with an r2 value of 0.92. (r2 = 0.95 in the inset, for PSA concentrations below 12 ng/mL). These data suggested that the LFA ruler shows excellent agreement with the clinical gold-standard method.
Figure 5.
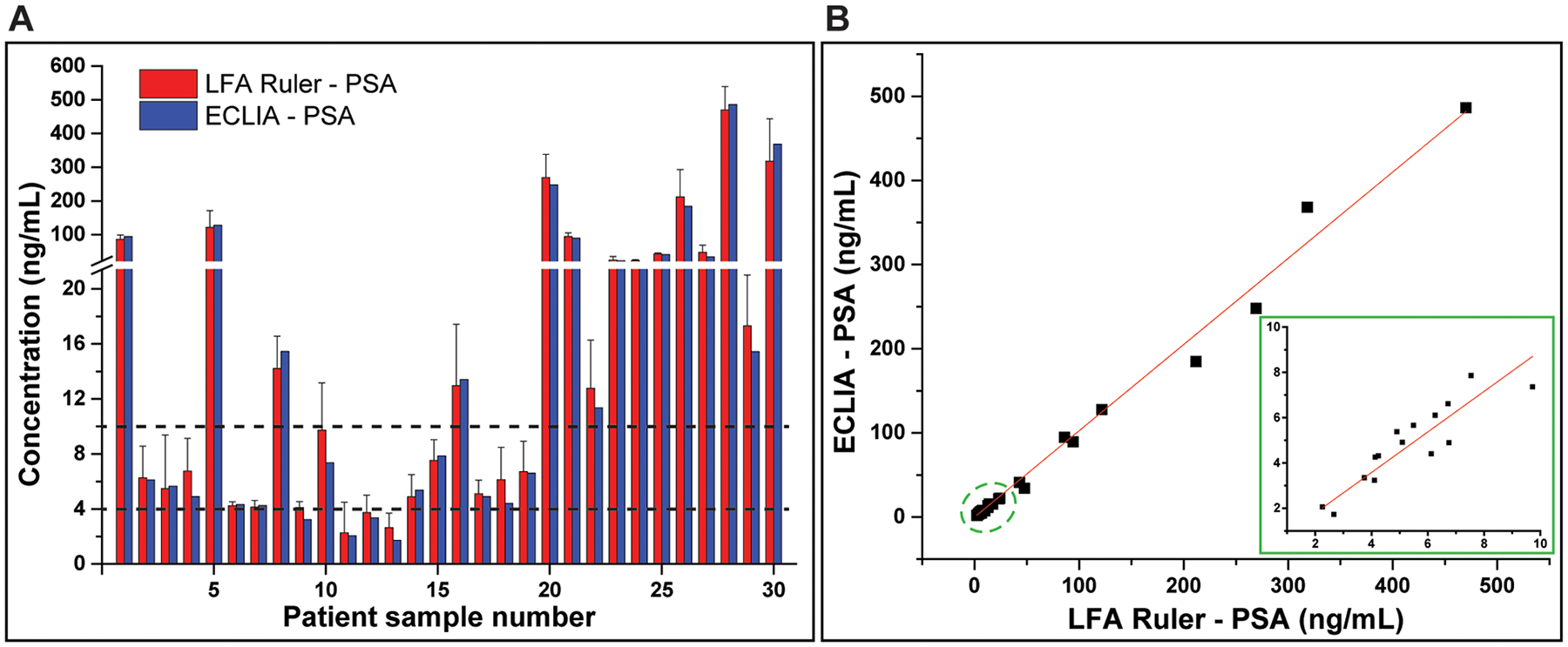
Validation of the LFA ruler against clinical gold standard PSA assay. (A) Histogram of the clinical serum sample test results generated by the LFA ruler (mean ± standard error) and the ECLIA assay (Roche Elecsys Cobas Total PSA). Two dashed lines represent the clinical cutoffs for PSA, 4 ng/mL and 10 ng/mL, respectively. (B) Linear relationship between the LFA ruler and standard clinical results with an r2 value of 0.92. (r2 = 0.95 in the inset, for PSA concentrations below 12 ng/mL).
The LFA ruler is the first time that ink advancement signal is used in LFA quantitation, with a simple, robust and portable microfluidic chip. Unlike previous LFA quantitative readout methods, the LFA ruler achieves direct visualized quantitation of assay results with no need for external instruments, such as optical strip reader, fluorescent reader, chemiluminescence reader, magnetic reader, or pressure meter, therefore much more convenient for quantitative and rapid POC testing. In addition, the relative wider test zone replaces the test line in traditional LFA, in which the thin test line is necessary for colorimetric need. The increased width of “test zone” would provide longer interaction time between target molecules and capture antibodies, which has a positive effect in increasing the capture efficiency and assay sensitivity. Coupled with PtNPs’ excellent catalytic ability for signal amplification, the sensitivity of this platform is comparable to or better than the best commercially available PSA LFA strips, where gold nanoparticles or other colored labels are used to obtain colorimetric signals for qualitative or semi-quantitative readout. For example, the Instant-view® PSA Whole Blood/Serum Test has an analytical sensitivity of 1 ng/mL and a reference value of 4 ng/mL. (Alfa Scientific Designs, Inc., CA, USA).28 We compared the quantitative ability of LFA ruler and the commercial Instant-view® PSA strips with scanners (Figure 6). The quantitative ability of LFA ruler is comparable to or better than the best commercially available PSA LFA strips with complicated and expensive laboratory instruments (Gel Doc™ XR+ System, Bio-Rad), followed by the tedious software analysis. Moreover, the office scanner (MP C3004, Ricoh) may not be suitable for quantitative analysis of LFA strips, which unadjusted high-intensity illumination will produce large deviations for the low-concentration strips.
Figure 6.
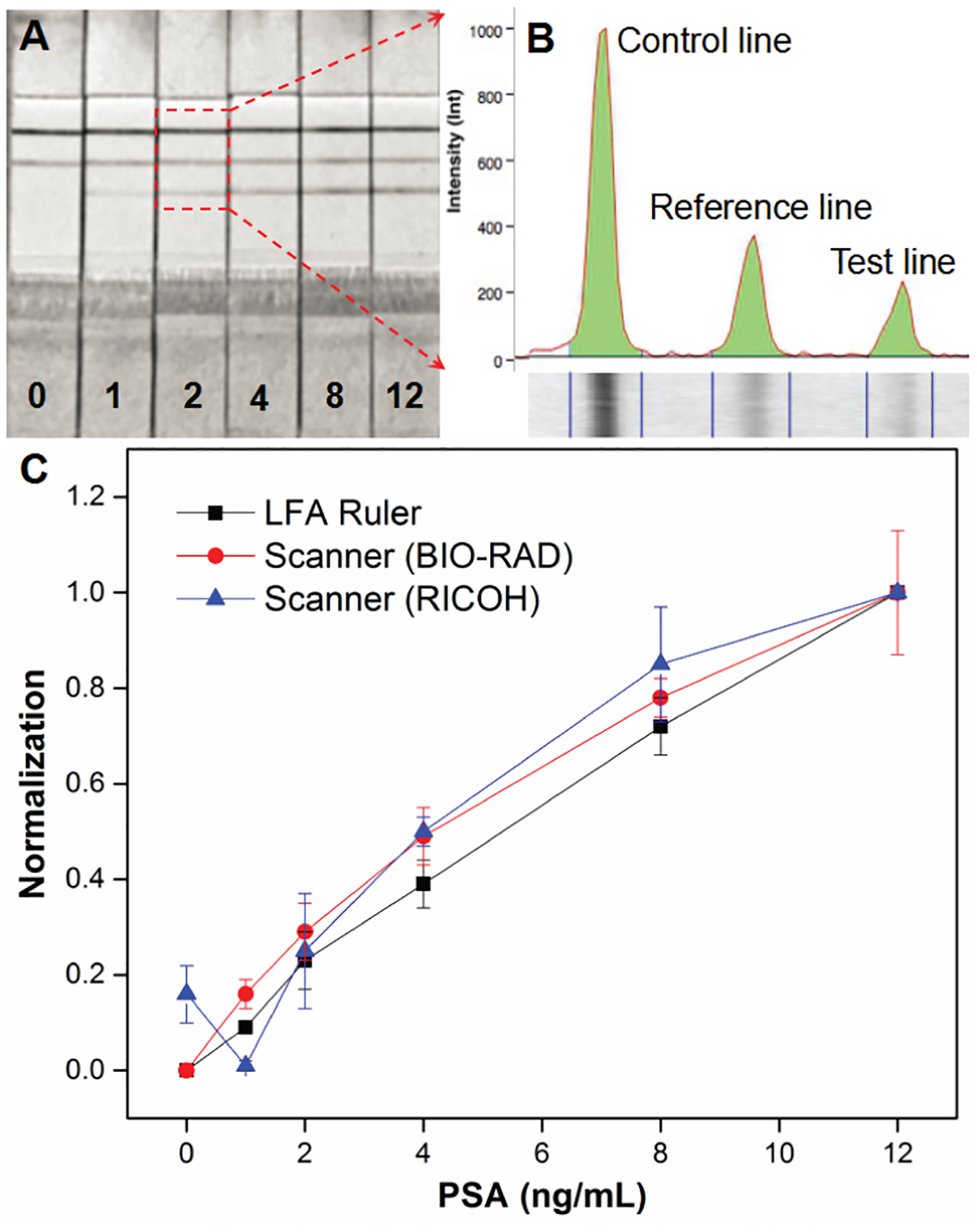
Comparison of quantitative ability of LFA ruler and commercial PSA strips with scanner. (A) The scanning images of commercial strips (Instant-view® PSA Whole Blood/Serum Test) with different PSA concentrations (0, 1, 2, 4, 8, and 12 ng/mL, respectively) using laboratory Gel Doc™ XR+ System (Bio-Rad). (B) The intensity of control line, reference line and test line were analyzed using the Image Lab™ software. (C) The normalization plots of LFA ruler (black square), commercial PSA strips with Bio-Rad scanner (red circle) and Ricoh MP C3004 office scanner (blue triangle).
The microfluidic chip of LFA ruler is inexpensive and easy to prepare based on common materials. The estimated cost of main materials is approximately $2.25 per device, including PDMS ($0.630), glass ($0.598), paper ($0.034), 3d-printing resin ($0.574), and reagents ($0.410). The total cost will be further reduced when the device is manufactured via mass production. The gas permeability of PDMS is not an issue for the open-ended device because the generated oxygen is eventually released through the opening outlet with minimum pressure. We have not observed swelling or detaching of the sealing tape from the PDMS surface, even in experiments with high concentrations of PtNP. Furthermore, we tested whether the substance, ascorbic acid (AA), in human blood samples affected the reaction between PtNP and H2O2.29,30 It was found that the catalytic reaction was not affected by AA, even at the concentrations that are much higher than the blood concentration of people who are supplemented with vitamin C (Figure S6).
The timing of recording the ink advancement is critical as the visual result is time-dependent (Figure S2). We chose 12 min as the readout time of LFA ruler due to three considerations. First, the linearity between the ink advancement distance at 12 min and the number of PtNP is the best compared to other readout times (r2 value is 0.99) (Figure 3B). It indicates that the LFA ruler has the best quantitative characteristics and a wide dynamic range at this readout time. Second, the ink advancement distances at 4 ng/mL and 10 ng/mL PSA (two clinical thresholds) are both quantifiable at 12 min (Figure 4C). Third, a longer readout time will improve the sensitivity of LFA ruler in the quantitative detection of PSA. However, the ink advancement distance of “blank” strip may also exceed “0” at longer readout time because of the non-specifically adsorbed PtNPs reacting with H2O2 (Figure S5). Balancing all above considerations, we chose a readout time of 12 min.
The LFA ruler offers the potential to quantitatively, sensitively and rapidly assess PSA in the POC settings without any other equipment, with accuracy comparable to clinical gold-standard method. This platform can also be extended to other applications. More biomarkers and more “ruler” channels could be added to the device to achieve multiplexed quantitation.31,32 To further improve the user interface and ease the operation in the POC settings, we generated a 3D-printed LFA ruler with a slider to transfer the pads conveniently without cutting, as shown in Figure S7. This prototype will be further validated in future studies to achieve the goal of multiplexed quantification and ease of use.
4. Conclusion
In summary, we have developed a 3D printed strip cassette and an “LFA Ruler” for the quantitative and rapid detection of LFA strips. The “LFA Ruler” is a PDMS-glass hybrid microfluidic chip with distance-based readout. This platform takes advantage of the convenience of LFA strips, the excellent catalytic ability of PtNP-based signal amplification reporter, as well as the high sensitivity of microfluidic chip. The prototype LFA ruler was capable of rapidly quantitating PSA within 30 min with an LOD of 0.54 ng/mL. The on-chip testing results showed good agreement with those confirmed by an ECLIA method. Compared with conventional LFA techniques, the LFA ruler enables quantitative and sensitive detection of biomarkers by the naked eye, without need for any instruments and complex operations, which is especially suitable for low-cost quantitation in clinical diagnostics, drug screening, food safety, and environmental monitoring.
Supplementary Material
Acknowledgements
This work was carried out in part at the Singh Center for Nanotechnology, which is supported by the NSF National Nanotechnology Coordinated Infrastructure Program under grant NNCI-1542153. 3D printed object printed courtesy of the Biomedical Library of the University of Pennsylvania.
Footnotes
Supporting Information
Platform photographs, time-dependent ink advancement, comparison of catalytic activity, test/control zone tests, and ascorbic acid experiment.
Competing Interests
The authors have declared that no competing interest exists.
References
- 1.Vashist SK; Luppa PB; Yeo LY; Ozcan A; Luong JHT Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [DOI] [PubMed] [Google Scholar]
- 2.Drain PK; Hyle EP; Noubary F; Freedberg KA; Wilson D; Bishai WR; et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014, 14, 239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang P; Kricka LJ Current and Emerging Trends in Point-of-Care Technology and Strategies for Clinical Validation and Implementation. Clin Chem. 2018, 64, 1439–1452. [DOI] [PubMed] [Google Scholar]
- 4.Chen H; Hagström AE; Kim J; Garvey G; Paterson A; Ruiz-Ruiz F; et al. Flotation Immunoassay: Masking the Signal from Free Reporters in Sandwich Immunoassays. Sci Rep. 2016, 6, 24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarei M; Advances in point-of-care technologies for molecular diagnostics. Biosens Bioelectron. 2017, 98, 494–506. [DOI] [PubMed] [Google Scholar]
- 6.Huang X; Aguilar ZP; Xu H; Lai W; Xiong Y Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens Bioelectron. 2016, 75, 166–80. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee R; Jaiswal A Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. Analyst. 2018, 143, 1970–1996. [DOI] [PubMed] [Google Scholar]
- 8.Sajid M; Kawde AN; Daud M Designs, formats and applications of lateral flow assay: A literature review. J Saudi Chem Soc. 2015, 19, 689–705. [Google Scholar]
- 9.[Internet] The MarketsandMarkets™ Online. Revised 30 Augest 2018 https://www.marketsandmarkets.com/PressReleases/lateral-flow-assay.asp.
- 10.Quesada-González D; Merkoçi A Nanoparticle-based lateral flow biosensors. Biosens Bioelectron. 2015, 73, 47–63. [DOI] [PubMed] [Google Scholar]
- 11.Syedmoradi L; Daneshpour M; Alvandipour M; Gomez FA; Hajghassem H; Omidfar K Point of care testing: The impact of nanotechnology. Biosens Bioelectron. 2017, 87, 373–387. [DOI] [PubMed] [Google Scholar]
- 12.Nargang TM; Runck M; Helmer D; Rapp BE Functionalization of paper using photobleaching: A fast and convenient method for creating paper‐based assays with colorimetric and fluorescent readout. Eng Life Sci. 2016, 16, 525–531. [Google Scholar]
- 13.Jacinto MJ; Trabuco JRC; Vu BV; Garvey G; Khodadady M; Azevedo AM; et al. Enhancement of lateral flow assay performance by electromagnetic relocation of reporter particles. PLoS One. 2018, 13, e0186782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stambach NR; Carr SA; Cox CR; Voorhees KJ Rapid Detection of Listeria by Bacteriophage Amplification and SERS-Lateral Flow Immunochromatography. Viruses. 2015, 7, 6631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D; Tian T; Chen X; Lei Z; Song Y; Shi Y; et al. Gas-generating reactions for point-of-care testing. Analyst. 2018, 143, 1294–1304. [DOI] [PubMed] [Google Scholar]
- 16.Liu D; Li X; Zhou J; Liu S; Tian T; Song Y; et al. A fully integrated distance readout ELISA-Chip for point-of-care testing with sample-in-answer-out capability. Biosens Bioelectron. 2017, 96, 332–338. [DOI] [PubMed] [Google Scholar]
- 17.Song Y; Zhang Y; Bernard PE; Reuben JM; Ueno NT; Arlinghaus RB; et al. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nat Commun. 2012, 3, 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y; Xuan J; Song Y; Wang P; Qin L A microfluidic platform with digital readout and ultra-low detection limit for quantitative point-of-care diagnostics. Lab Chip. 2015, 15, 3300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y; Wang Y; Qi W; Li Y; Xuan J; Wang P; et al. Integrative volumetric bar-chart chip for rapid and quantitative point-of-care detection of myocardial infarction biomarkers. Lab Chip. 2016, 16, 2955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y; Uddayasankar U; He B; Wang P; Qin L Fast, Sensitive, and Quantitative Point-of-Care Platform for the Assessment of Drugs of Abuse in Urine, Serum, and Whole Blood. Anal Chem. 2017, 89, 8273–8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y; Xuan J; Song Y; Qi W; He B; Wang P; et al. Nanoporous Glass Integrated in Volumetric Bar-Chart Chip for Point-of-Care Diagnostics of Non-Small Cell Lung Cancer. ACS Nano. 2016, 10, 1640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z; Fu Q; Yu S; Sheng L; Xu M; Yao C; et al. Pt@AuNPs integrated quantitative capillary-based biosensors for point-of-care testing application. Biosens Bioelectron. 2016, 85, 657–663. [DOI] [PubMed] [Google Scholar]
- 23.Cui X; Hu J; Choi JR; Huang Y; Wang X; Lu TJ; et al. A volumetric meter chip for point-of-care quantitative detection of bovine catalase for food safety control. Anal Chim Acta. 2016, 935, 207–12. [DOI] [PubMed] [Google Scholar]
- 24.Lin B; Guan Z; Song Y; Song E; Lu Z; Liu D; et al. Lateral flow assay with pressure meter readout for rapid point-of-care detection of disease-associated protein. Lab Chip. 2018, 18, 965–970. [DOI] [PubMed] [Google Scholar]
- 25.Adhyam M; Gupta AK A Review on the Clinical Utility of PSA in Cancer Prostate. Indian J Surg Oncol. 2012, 3,120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong MC; Goggins WB; Wang HH; Fung FD; Leung C; Wong SY; et al. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur Urol. 2016, 70, 862–874. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa AI; Castanheira AP; Edwards AD; Reis NM A lab-in-a-briefcase for rapid prostate specific antigen (PSA) screening from whole blood. Lab Chip. 2014, 14, 2918–28. [DOI] [PubMed] [Google Scholar]
- 28.[Internet] Instant-view. Revised 30 Augest 2018 http://www.surplusgolden.com/File/PSA%20Serum%20Test.pdf.
- 29.Padayatty SJ; Sun H; Wang Y; Riordan HD; Hewitt SM; Katz A; Wesley RA; Levine M Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004, 140, 533–7. [DOI] [PubMed] [Google Scholar]
- 30.Lin J; Li H; Wen Y; Zhang M Adjuvant Administration of Vitamin C Improves Mortality of Patients with Sepsis and Septic Shock: A Systems Review and Meta-Analysis. J. Intern. Med 2018, 8, 146–159. [Google Scholar]
- 31.Li J; Macdonald J Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosens Bioelectron. 2016, 83, 177–92. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Martinez J; Silvy M; Chiaroni J; Fournier-Wirth C; Roubinet F; Bailly P; et al. Multiplex Lateral Flow Assay for Rapid Visual Blood Group Genotyping. Anal Chem. 2018, 90, 7502–7509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


