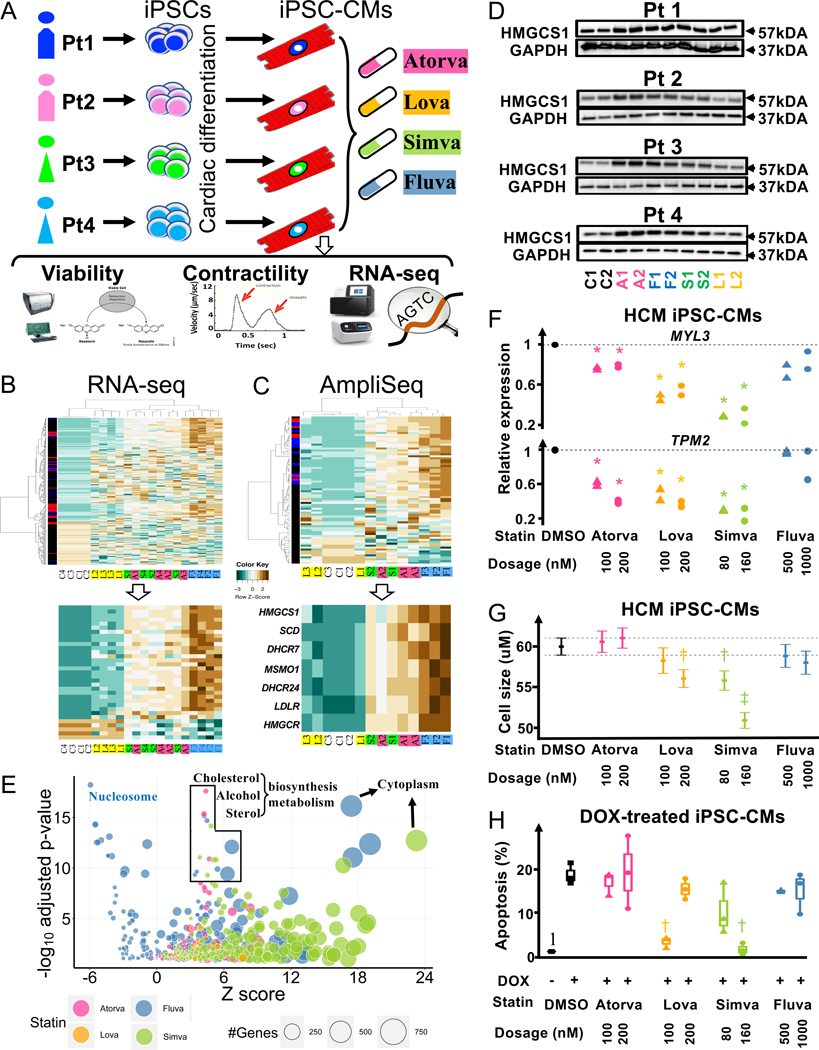
Statins prevent cardiac diseases via inhibition of cholesterol biosynthesis and mediation of pleiotropic effects on the cardiovascular system1. Statins may also act as anti-hypertrophic and anti-apoptotic agents to prevent cardiomyocyte injury. However, the effects of clinically relevant concentrations of statins (i.e., serum peak concentration (Cmax)) on cardiomyocytes remain largely unknown. In the current study, we investigate the class effects of atorvastatin, lovastatin, simvastatin, and fluvastatin applied at their respective Cmax, on the transcriptome and functional properties of human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs)2.
Human iPSCs from two male (Lines #1–2) and female (Lines #3–4) control individuals were differentiated to iPSC-CMs (Figure A) with higher than 80% efficiency, as measured by expression of cardiac troponin (data not shown). Statins mediated no effect on the viability (2 and 7 days) and contractile properties of iPSC-CMs (7 days) when tested in a dose-dependent manner (data not shown). To examine the transcriptional effects of statins, we performed comprehensive RNA sequencing (RNA-seq) analysis of each iPSC-CM line using the Cmax of each statin, corresponding to the clinical dosage of 40 mg. We focused on significantly differentially expressed genes (DEGs) compared to vehicle-treated cells (FDR adjusted p-value <0.05). Heatmap analysis revealed that fluvastatin (F1-F4) mediated the most potent effects on the iPSC-CM transcriptome, followed by atorvastatin (A1-A4), simvastatin (S1-S4), and lovastatin (L1-L4) (Figure B, upper panel). Thirty-three common DEGs were significantly affected (FC≥2) by atorvastatin, simvastatin, and fluvastatin (Figure B, lower panel). Many of the commonly regulated genes were related to cholesterol biosynthesis, such as methylsterol monooxygenase-1 (MSMO1), stearoyl-CoA desaturase (SCD), 3-hydroxy-3-methylglutaryl-CoA synthase-1 (HMGCS1), low-density lipoprotein receptor (LDLR), 24-dehydrocholesterol reductase (DHCR24), and 7-dehydrocholesterol reductase (DHCR7) (Figure B, lower panel). These results suggest that statins induce a molecular signature in a drug-specific manner independent of genetic background.
Figure: Statin class effects on the transcriptome and functional properties of iPSC-CMs.
(A) Schematic depiction of study design. (B) Upper panel: Heatmap of DEG fold-changes (FCs) from RNA-seq analysis. Red boxes (left side) indicate genes significantly affected by fluvastatin, atorvastatin, and simvastatin, blue indicates genes significantly affected by any two statins, and black indicates genes affected by only one drug. Genes with FDR adjusted p-value < 0.05 under likelihood ratio test were considered as significant. Lower panel: Heatmap of FCs of commonly affected DEGs by all drugs. C: control, A: atorvastatin, F: fluvastatin, S: simvastatin, L: lovastatin. 1–4: Lines 1–4. (C) Upper panel: Heatmap of AmpliSeq data showing FCs of DEGs in statin-treated iPSC-CMs from Line #2. Lower panel: Heatmap of Ampli-seq data showing FCs of DEGs regulated in common by atorvastatin, fluvastatin, simvastatin, and lovastatin in iPSC-CMs. Genes with FDR adjusted p-value < 0.05 under were considered as significant. C1–3: control, A1–3: atorvastatin, F1–3: fluvastatin, S1–2: simvastatin, L1–3: lovastatin. (D) Representative Western blot analysis of HMGCS1 protein expression in iPSC-CMs following statin treatment. (E) Bubble chart showing the top signaling pathways and cellular processes with the largest number of affected DEGs, acccording to transcriptomic analysis. (F) qRT-PCR quantification of HCM pathway genes in HCM MYBPC3 p.Val321Met iPSC-CMs treated with statins. (G) Cell size analysis of HCM MYBPC3 p.Val321Met iPSC-CMs following treatment with statins. Error bars indicate 95% confidence intervals and square dots represent mean values. Dash lines show 95% confidence interval in the control group. (H) Cell death analysis (apoptosis) of healthy iPSC-CMs (Linese #2 and #3) treated with four statins. *p<0.05, †p<1e-6, ‡p<1e-33 under paired t-test.
Additional transcriptomic profiling of three different iPSC-CM batches (Rep 1–3) derived from a single donor (Line #2) was performed to ensure reproducibility of the observed effects on iPSC-CM, independent of technical variability. Comprehensive assessment of these iPSC-CM transcriptomes following drug treatment was performed using the Ion AmpliSeq™ Human Transcriptome Kit. In accordance with the RNA-seq analysis, fluvastatin mediated the most potent effects on iPSC-CM transcriptome, followed by atorvastatin, simvastatin, and lovastatin (Figure C, upper panel). Further analysis revealed that fluvastatin, atorvastatin, and simvastatin regulate a common set of 6 DEGs related to cholesterol biosynthesis (MSMO1, SCD, LDLR, DHCR24, HMGCS1, and DHCR7) (Figure C, lower panel). This set of commonly regulated genes represents the core molecular signature of the effects of fluvastatin, atorvastatin, and simvastatin in iPSC-CMs. Our results correlate with the clinical efficacy of statins, with the exception of fluvastatin, which may have cardiac specific effects and needs to be further investigated in the future.
The induced protein level of HMGCS1 was confirmed by Western blot analysis in all iPSC-CMs following statin treatment (Figure D). Atorvastatin and fluvastatin mediated the strongest effects on HMGCS1 protein expression, followed by simvastatin and lovastatin. These results are in accordance with both RNA-seq and AmpliSeq analysis, further suggesting all statins regulate the expression of key regulators of the metabolic properties of iPSC-CMs in a drug-specific manner. Subsequently, we performed functional enrichment analysis to uncover the signaling pathways and cellular processes affected by statins in iPSC-CMs. All statins affected signaling pathways/cellular processes that are primarily involved in cholesterol metabolism, secondary alcohol biosynthesis, and sterol biosynthetic pathway, as shown by bubble chart analysis (Figure E). Although the hypertrophic cardiomyopathy (HCM) signaling pathway was not significanlty enriched, various genes were found to be significantly regulated. Significant down-regulation of two HCM pathway genes, TPM2 and MYL3, was observed in iPSC-CMs differentiated from a HCM patient (MYBPC3 p.Val321Met (c.961G>A))3 following treatment by atorvastatin, simvastatin, and lovastatin (Figure F). To confirm the anti-hypertrophic effects at the cellular level, we next treated the HCM iPSC-CMs with statins and found that simvastatin and lovastatin significantly reduced the cell size (Figure G). Finally, we also tested the anti-apoptotic effects of statins in two healthy iPSC-CM lines (Line #2–3) following treatment with Doxorubicin (0.1 uM DOX), as previously described4. Our data show that simvastatin and lovastatin significantly reduced the number of DOX-induced apoptosis in iPSC-CMs (Figure H), demostrating pro-survival effects for each of these drugs.
Overall, our study reveals that fluvastatin mediates the strongest effects on the transcriptome of healthy iPSC-CMs. On the other hand, simvastatin and lovastatin exerted stronger anti-hypertrophy effects in HCM iPSC-CMs and pro-survival effects in DOX-treated iPSC-CMs. When applied at physiologically relevant concentrations, statins primarily affect genes related to cholesterol and fatty acid homeostasis, which are in accordance with previous reports implicating long-chain polyunsaturated fatty acids in statin’s cardio-protective effects5. In addition, statins exert anti-hypertrophic and anti-apoptotic cellular processes in human iPSC-CMs in a drug-specific manner. These effects might be independent from the clinical action of statins on atherosclerosis, although further work is needed to confirm this observation.
Acknowledgments
SOURCES OF FUNDING
AO was supported by AHA 17SDG33660794; TK was supported by the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad; CL was supported by AHA 16POST30960020; SGO was supported by NIH R00 HL130416; MS was supported by the Dutch Heart Association and the Michaël Fonds; IK was supported by AHA 17IRG33410532; NS was supported by NIH K01 HL135455 and Stanford TRAM scholar award; and JCW was supported by NIH R01 HL123968, R01 HL126527, R01 HL145676, R01 HL146690, R01 HL130020, and P01 HL141084.
The data supporting the findings of this study are available from the corresponding author upon request. RNA-seq data is publicly available with GEO accession number GSE113546.
J.C. Wu is a co-founder of Khloris Biosciences but has no competing interests, as the work presented here is completely independent.
Footnotes
DISCLOSURES
Dr Wu is a cofounder of Khloris Biosciences but has no competing interests, because the work presented here is completely independent. The other authors report no conflicts.
REFERENCES
- 1.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109:III50–57. [DOI] [PubMed] [Google Scholar]
- 3.Ma N, Zhang JZ, Itzhaki I, Zhang SL, Chen H, Haddad F, Kitani T, Wilson KD, Tian L, Shrestha R, Wu H, Lam CK, Sayed N, Wu JC. Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation. 2018;138:2666–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burridge PW, Li YF, Matsa E, Wu H, Ong S, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC. Human induced pluripotent stem cell – derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn L V. Dietary Fats and Cardiovascular Disease: A presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23 [DOI] [PubMed] [Google Scholar]