Abstract
Background
Uterine leiomyosarcoma (uLMS) is a rare female malignancy with poor survival rates. The objective of this study was to construct prognostic nomograms for predicting the prognosis of women with uLMS.
Material/Methods
Patients with uLMS diagnosed between 2004 and 2015 were identified in the Surveillance, Epidemiology, and End Results (SEER) database. The essential clinical predictors were identified via univariate and multivariate Cox analysis models. Nomograms were constructed to predict the 3- and 5-year cancer-specific survival (CSS) and overall survival (OS) rates. Concordance index (C-index) and calibration plots were constructed to validate the predictive performance of nomograms.
Results
We enrolled 1448 patients with uLMS from the SEER database, with 1016 categorized into a training set and 432 categorized into a validation set. In multivariate analysis of the training set, predictors including age, disease stage, histological grade, tumor size, and surgery type were found to be associated with OS and CSS. Race and chemotherapy were only associated with OS. Construction of nomograms based on these predictors was performed to evaluate the prognosis of uLMS patients. The C-index and calibration curves also showed the satisfactory performance of these nomograms for prediction of prognosis.
Conclusions
The developed nomograms are useful tools for precisely analyzing the prognosis of uLMS patients, which could help clinicians in making personalized survival predictions and assessing individualized clinical options.
MeSH Keywords: Leiomyosarcoma, Nomograms, Prognosis, SEER Program, Uterine Neoplasms
Background
Uterine sarcomas are a series of rare, aggressively malignant diseases that accounts for only 3–7% of all uterine cancer cases [1,2]. They have various clinical courses and outcomes due to their histopathological diversity [3]. uLMS is the most common histological subtype of uterine sarcoma, accounting for approximately 30–70% of all cases [4]. The annual incidence of uLMS is low, at 0.36 per 100 000 women; however, it causes a significant proportion of all uterine cancer deaths [5]. uLMS is characterized by hematogenous metastasis and has a high recurrence rate of 45–71% [6,7]. Current treatments for uLMS are hysterectomy-based surgery, along with chemotherapy and/or radiotherapy for local recurrent control, and a new novel targeted immunotherapy [8]. The survival rate of uLMS remains poor, even when the disease is restricted to the uterus at the time of diagnosis [2,9]. The 5-year overall survival (OS) is estimated at 25–76% for local disease and 10–15% for metastatic disease [10].
There have been inconsistent reports of the predictive factors regarding patient age, race, tumor grade, clinical stage, tumor size, mitotic rate, vascular invasion, and treatment patterns [10–13]. However, the survival rate is affected by many variables, and few studies have incorporated all prognostic predictors to forecast the survival probability of patients with uLMS.
Nomograms are convenient predictive tools used to calculate the prognosis for many diseases by integrating several risk factors [14]. They have been widely used in many malignant cancers and are proven to be accurate [15,16]. In the present study, the clinical characteristics of uLMS patients were acquired from the SEER database from 2004 to 2015. The SEER database collects cancer information of 18 registries in the USA and accounts for approximately 28% of the USA population, which offers considerable data for detailed analysis [17]. Therefore, we performed an analysis of uLMS patients and constructed accurate prognostic nomograms for uLMS. We also sought to determine whether this model is reliable and which clinical characteristics are predictors for prognosis of patients with uLMS.
Material and Methods
Data source and patient selection
Study data were collected from the SEER database released on April 15, 2019, using SEER*Stat software (version 8.3.6; National Cancer Institute, USA). Since all patient information derived from the SEER database is publicly available online, this study was exempted from the requirement for approval by our Institutional Review Board. We had access to the SEER program information after obtaining permission from the US National Cancer Institute (username number: 17620-Nov2018).
The inclusion criteria were: (1) identified as leiomyosarcoma with ICD-O-3/WHO 2008 histology codes (8890, 8891, 8896); (2) tumor anatomic site codes (C54.0–C54.3, C54.8–54.9, C55.9); and (3) diagnosed between 2004 and 2015. The exclusion criteria were: (1) multiple primary cancers and (2) clinical information missing or unknown.
Prognostic variables
Prognostic factors were abstracted from the SEER program on uLMS patients, including age at diagnosis, race, year of diagnosis, tumor grade, American Joint Committee on Cancer (AJCC) stage, tumor size, treatment strategy, vital status, cause of death, and survival time (months).
We used the X-tile program (Yale University, New Haven, USA) to determine optimal cut-off points. Then, patient age was classified into 3 subgroups: <51 years, 51–58 years, and >58 years (Figure 1). Tumor size was classified as <70 mm, 70–140 mm, and >140 mm. Treatment strategy included surgery type, lymphadenectomy, radiotherapy, and chemotherapy. The definition of OS was the time from diagnosis to death for any reason, and CSS was the time from diagnosis to death due to uLMS. Patients were censored if alive at the last follow-up.
Figure 1.
Defining the optimal cut-off points of age and tumor size via the X-tile program (A–F). The black dot represents the optimal cut-off points of age and tumor size (A, D). The histogram (B, E) and survival curves (C, F) were demonstrated based on cut-off points. Optimal cut-off points of age and tumor size were 51 years and 58 years and 70 mm and 140 mm, respectively.
Statistical analysis
All qualified uLMS patients were randomly assigned to a training set and a validation set in a 7: 3 split ratio. The chi-square test was performed to compare the demographics and clinical statistics between the 2 sets. Survival analysis of different subgroups was performed using Kaplan-Meier curves. Based on Cox proportional-hazard regression models, we performed univariate and multivariate analysis to identify the prognostic variables.
Prognostic nomograms were constructed by combining all these predictors to predict 3- and 5-year OS and SCC. To validate these nomograms, we performed measurements both internally and externally. We used the C-index to assess the discrimination ability of the developed nomograms. A C-index of 0.5 indicates poor discrimination ability and 1.0 indicates excellent discrimination ability [18]. The calibration plots were applied using a bootstrap approach with 1000 resamples to show the consistency between observation and prediction.
SPSS software (version 21.0; IBM Corporation, NY, USA) and R software (version 3.6.1) were used to perform all statistical analyses. P value <0.05 was deemed statistically significant.
Results
Patients baseline information
Using the inclusion criteria, a total of 1448 eligible patients extracted from the SEER database were enrolled in this study. Then, they were classified into the training set (n=1016) and the validation set (n=432) (Table 1). There were no statistically significant differences in the demographic and clinical characteristics between the 2 sets. We found 38.0% of patients diagnosed with uLMS were under 51 years old and 71.0% were white. Regarding tumor features, uLMS patients tended to have high-grade (81.8%) and stage I disease (51.5%), and 47.0% of tumors were 70–140 mm. Most patients were initially treated with hysterectomy-based surgery (94.3%), 64.5% had no lymphadenectomy, 79.8% had not undergone radiotherapy, and 51.7% had not undergone chemotherapy.
Table 1.
Demographics and clinicopathologic characterisitcs of patients with uterine leiomyosarcoma.
| Cetegory | Trainning cohort (n=1016) | Validation cohort (n=432) | Total cohort (n=1448) | P |
|---|---|---|---|---|
| No. of patients (%) | No. of patients (%) | No. of patients (%) | ||
| Age(y) | 0,667 | |||
| <51 | 381 (37.5%) | 169 (39.1%) | 550 (38.0%) | |
| 51–58 | 257 (25.3%) | 113 (26.2%) | 370 (25.5%) | |
| >58 | 378 (37.2%) | 150 (34.7%) | 528 (36.5%) | |
| Race | 0,484 | |||
| White | 712 (70.1%) | 316 (73.1%) | 1028 (71.0%) | |
| Other* | 109 (10.7%) | 40 (9.3%) | 149 (10.3%) | |
| Black | 195 (19.2%) | 76 (17.6%) | 271 (18.7%) | |
| Year at diagnosis | 0,119 | |||
| 2004–2009 | 472 (46.5%) | 220 (50.9%) | 692 (47.8%) | |
| 2010–2015 | 544 (53.5%) | 212 (49.1%) | 756 (52.2%) | |
| Grade** | 0,854 | |||
| Low | 184 (18.1%) | 80 (18.5%) | 264 (18.2%) | |
| High | 832 (81.9%) | 352 (81.5%) | 1184 (81.8%) | |
| Stage | 0,239 | |||
| I | 506 (49.8%) | 239 (55.3%) | 745 (51.5%) | |
| II | 103 (10.1%) | 42 (9.7%) | 145 (10.0%) | |
| III | 106 (10.5%) | 43 (10.0%) | 149 (10.3%) | |
| IV | 301 (29.6%) | 108 (25.0%) | 409 (28.2%) | |
| Tumor size (mm) | 0,248 | |||
| <70 | 264 (26.0%) | 123 (28.5%) | 387 (26.7%) | |
| 70–140 | 473 (46.5%) | 208 (48.1%) | 681 (47.0%) | |
| >140 | 279 (27.5%) | 101 (23.4%) | 380 (26.3%) | |
| Surgery type | 0,45 | |||
| Hysterectomy | 953 (93.8%) | 412 (95.4%) | 1365 (94.3%) | |
| Pelvic exenteration | 16 (1.6%) | 4 (0.9%) | 20 (1.3%) | |
| No surgery | 47 (4.6%) | 16 (3.7%) | 63 (4.4%) | |
| Lymphadenectomy | 0,938 | |||
| Yes | 360 (35.4%) | 154 (35.6%) | 514 (35.5%) | |
| No | 656 (64.6%) | 278 (64.4%) | 934 (64.5%) | |
| Radiotherapy | 0,787 | |||
| Yes | 203 (20.0%) | 89 (20.6%) | 292 (20.2%) | |
| No | 813 (80.0%) | 343 (79.4%) | 1156 (79.8%) | |
| Chemotherapy | 0,386 | |||
| Yes | 498 (49.0%) | 201 (46.5%) | 699 (48.3%) | |
| No | 518 (51.0%) | 231 (53.5%) | 749 (51.7%) |
American Indian/Alaskan Native, Asian/Pacific Islander;
Low: Grade I (well differentiated) and Grade II (moderately differentiated); High: Grade III (poorly differentiated) and Grade IV (undifferentiated anaplastic);
Total/simple/pan/radical hysterectomy with or without removal of adnexa.
Independent predictors for patients with uterine leiomyosarcoma
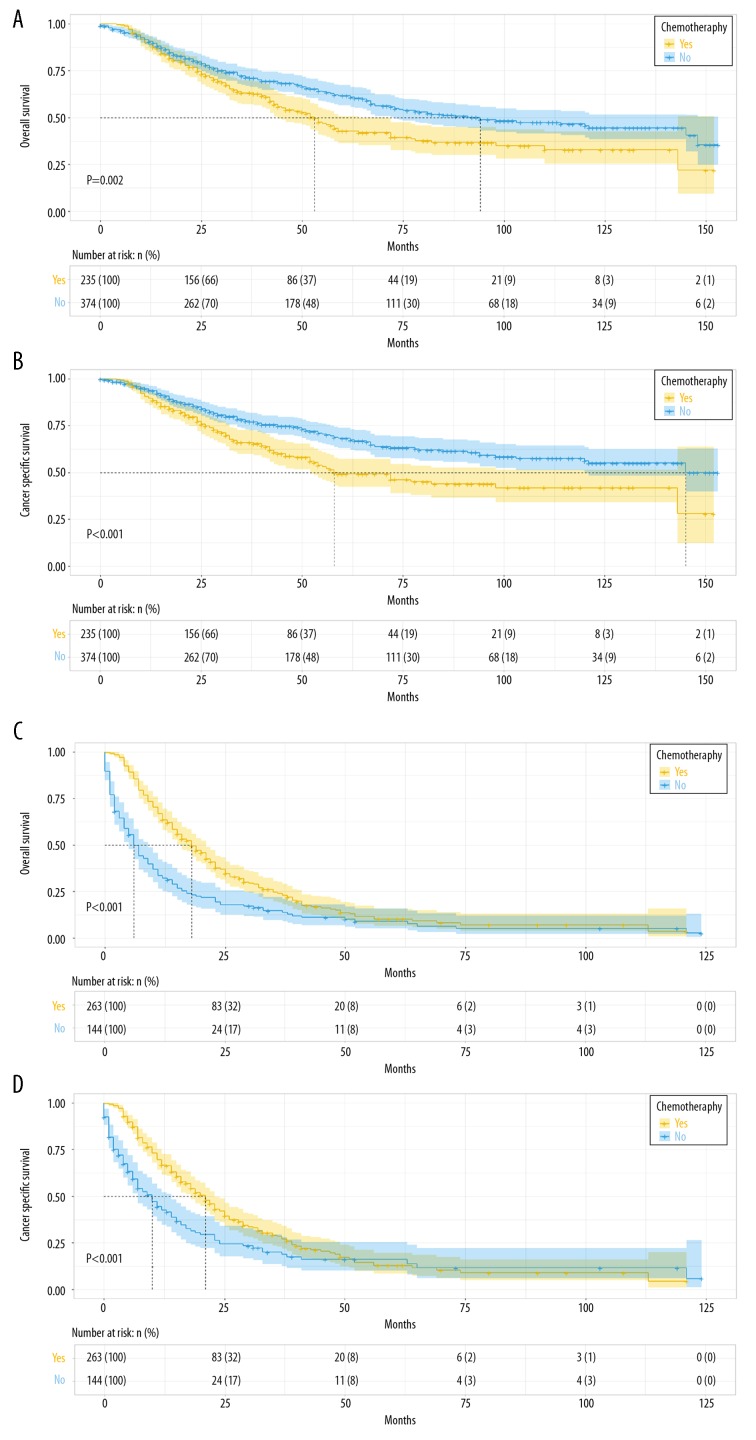
In the training set, all the variables associated with OS in the univariate analysis were further processed by multivariate analysis, revealing that age, race, disease stage, histological grade, tumor size, surgery type, and chemotherapy were independent predictors for OS (P<0.05) (Table 2). For CSS, we followed the same steps specified above, and multivariate analysis identified age, disease stage, histological grade, tumor size, and surgery type as independent predictors for CSS (P<0.05) (Table 3). Furthermore, to separately analyze chemotherapy based on the prognosis on uLMS, the training set was stratified into an early-stage subgroup (n=609) and an advanced-stage subgroup (n=407). In the early-stage subgroup, patients treated with chemotherapy tended to have worse prognosis of OS and CSS than patients with no chemotherapy, but in the advanced-stage subgroup chemotherapy was associated with better prognosis for uLMS patients (Figure 2).
Table 2.
Univariate and multivariate analysis for OS in trainning cohort.
| Cetegory | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P | Hazard ratio (95% CI) | P | |
| Age (y) | <0.001 | ||
| <51 | Reference | ||
| 51–58 | 1.272 (1.029–1.572) | 0.026 | |
| >58 | 2.032 (1.678–2.462) | <0.001 | |
| Race | 0.003 | ||
| White | Reference | ||
| Black | 1.278 (1.045–1.563) | 0.017 | |
| Other* | 1.187 (0.918–1.534) | 0.190 | |
| Year at diagnosis | 0.896 | ||
| 2004–2009 | |||
| 2010–2015 | |||
| Grade** | <0.001 | ||
| Low | Reference | ||
| High | 2.329 (1.796–3.021) | <0.001 | |
| Stage | <0.001 | ||
| I | Reference | ||
| II | 2.242 (1.836–3.168) | <0.001 | |
| III | 3.759 (2.878–4.911) | <0.001 | |
| IV | 4.539 (3.690–5.585) | <0.001 | |
| Tumor size (mm) | <0.001 | ||
| <70 | Reference | ||
| 70–140 | 1.376 (1.107–1.709) | 0.004 | |
| >140 | 2.029 (1.606–2.564) | <0.001 | |
| Lymphadenectomy | 0.254 | ||
| Yes | |||
| No | |||
| Surgery type | <0.001 | ||
| Hysterectomy** | Reference | ||
| Pelvic exenteration | 0.695 (0.387–1.247) | 0.222 | |
| No surgery | 2.439 (1.753–3.393) | <0.001 | |
| Radiotherapy | 0.068 | ||
| Yes | |||
| No | |||
| Chemotherapy | <0.001 | ||
| Yes | Reference | ||
| No | 1.280 (1.074–1.526) | 0.005 | |
CI – confidence interval.
American Indian/Alaskan Native, Asian/Pacific Islander;
Low: Grade I (well differentiated) and Grade II (moderately differentiated); High: Grade III (poorly differentiated) and Grade IV (undifferentiated anaplastic);
Total/simple/pan/radical hysterectomy with or without removal of adnexa.
Table 3.
Univariate and multivariate analysis for CSS in trainning cohort.
| Characteristic | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P | Hazard ratio (95% CI) | P | |
| Age (y) | <0.001 | ||
| <51 | Reference | ||
| 51–58 | 1.216 (0.969–1.524) | 0.09 | |
| >58 | 1.701 (1.379–2.097) | <0.001 | |
| Race | 0.106 | ||
| White | |||
| Black | |||
| Other* | |||
| Year at diagnosis | 0.490 | ||
| 2004–2009 | |||
| 2010–2015 | |||
| Grade** | <0.001 | ||
| Low | Reference | ||
| High | 2.591 (1.909–3.515) | <0.001 | |
| Stage | <0.001 | ||
| I | Reference | ||
| II | 2.387 (1.757–3.243) | <0.001 | |
| III | 3.661 (2.716–4.934) | <0.001 | |
| IV | 4.795 (3.818–6.022) | <0.001 | |
| Tumor size (mm) | <0.001 | ||
| <70 | Reference | ||
| 70–140 | 1.477 (1.155–1.890) | 0.002 | |
| >140 | 2.161 (1.659–2.812) | <0.001 | |
| Lymphadenectomy | 0.221 | ||
| Yes | |||
| No | |||
| Surgery type | <0.001 | ||
| Hysterectomy** | Reference | ||
| Pelvic exenteration | 0.814 (0.443–1.495) | 0.507 | |
| No surgery | 2.312 (1.603–3.335) | <0.001 | |
| Radiotherapy | 0.058 | ||
| Yes | |||
| No | |||
| Chemotherapy | <0.001 | ||
| Yes | Reference | ||
| No | 1.171 (0.965–1.423) | 0.109 | |
CI – confidence interval.
American Indian/Alaskan Native, Asian/Pacific Islander;
Low: Grade I (well differentiated) and Grade II (moderately differentiated); High: Grade III (poorly differentiated) and Grade IV (undifferentiated anaplastic);
Total/simple/pan/radical hysterectomy with or without removal of adnexa.
Figure 2.
Kaplan-Meier survival curves for patients based on the use of chemotherapy. In the early-stage subgroup, patients treated with chemotherapy had an unfavorable prognosis of OS (A) and CSS (B), while in the advanced-stage subgroup, patients treated with chemotherapy had a better prognosis of OS (C) and CSS (D).
Predictive nomograms
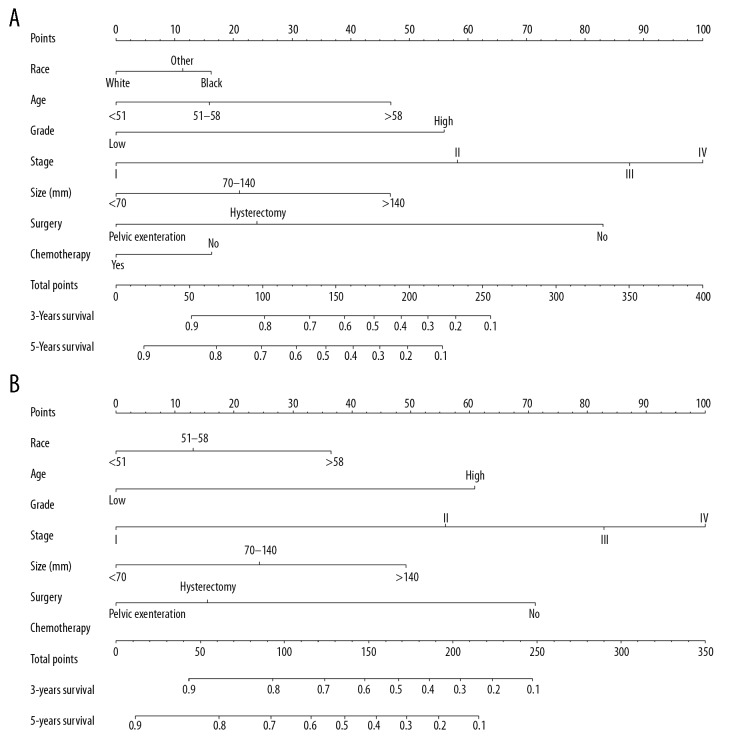
The nomograms were constructed by incorporating all predictors (Figure 3). The stage contributed most to the OS and CSS, followed by surgery type and grade. Patient age and tumor size had moderate effects on OS and CSS, while race had the least effect on OS. By summing up the specific points of each predictor, then measuring the total points of the OS and CSS, the individual survival probability can be easily calculated, as shown in the following example. A 49-year-old black woman was diagnosed with high-grade and stage I uLMS and had a primary tumor size of 80 mm. She was treated with primary hysterectomy surgery without receiving lymphadenectomy or adjuvant therapy. Calculating the total scores from each point of the nomogram variables resulted in 134 points for the OS and 100 points for the CSS. Therefore, her predicted 3-year OS and CSS probabilities were approximately 69% and 78%, respectively.
Figure 3.
Nomograms predicting 3- and 5-year OS (A) and CSS (B). The total points were calculated by adding the points of each predictor, and correspond to the possibilities of 3- and 5-year OS and CSS of patients with uLMS.
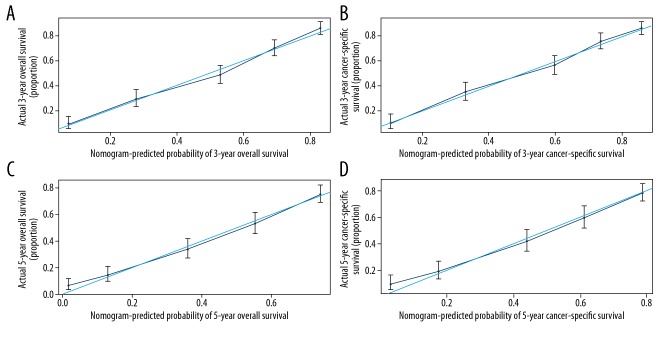
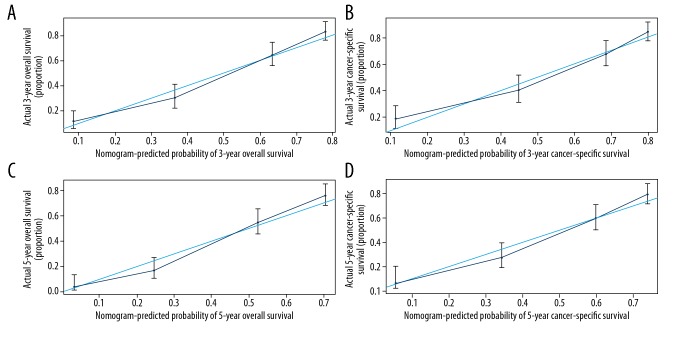
Validation of the nomograms was performed internally and externally. The C-index of internal validation for CSS and OS were 0.768 (95% CI: 0.748–0.787) and 0.769 (95% CI: 0.751–0.786), respectively; and the C-index of external validation were 0.756 (95% CI: 0.728–0.783) and 0.758 (95% CI: 0.732–0.783), respectively. The calibration curves also revealed strong consistency between the predicted and observed survival outcomes (Figures 4, 5).
Figure 4.
Internal calibration plots show the nomograms-predicted survival rate (X-axis) agreed well with actual survival rate (Y-axis), comprising the 3-year OS (A) and CSS (B) and the 5-year OS (C) and CSS (D).
Figure 5.
External calibration plots show the nomograms-predicted survival rate (X-axis) which agrees well with actual survival rates (Y-axis), comprising the 3-year OS (A) and CSS (B) and the 5-year OS (C) and CSS (D).
Discussion
We established practical nomograms to predict the survival outcomes of uLMS patients. Validation of the nomograms revealed excellent differentiation and calibration, both internally and externally. A previous study found that data on prognostic predictors of uLMS are still limited, and there is a lack of effective treatments to prolong survival benefits in uLMS patients [19]. Therefore, a practical tool for differentiating patient risk factors instead of simple tumor staging is needed. Nomograms provide a pictorial display of calculation models, which gives simple prognostic survival information by incorporating easily accessible clinical parameters [18]. Thus, nomograms can be used by clinicians to make precise and individualized medical decisions [20].
Several studies reported that black women have a higher incidence and worse prognosis of uLMS in comparison with other races. A study of population-based data from 2000 to 2012 revealed a higher incidence rate of uLMS in black women (1.61 per 100 000 African Americans vs. 0.86 per 100 000 for whites vs. 0.81 per 100 000 for other races; P<0.05) [21]. A retrospective analysis of patients with uLMS that included 1396 patients found that race was identified as an independent predictor of disease-specific survival, and the African American racial group had low survival compared with whites (14% vs. 65.7%, respectively, aHR=1.45, 95% CI: 1.09–1.94, P<0.05). Amant et al. suggested that genetics and socioeconomic factors can explain the poor prognosis of blacks [22].
Age is commonly considered as an independent factor of uLMS [4,21]. Major et al. reported that most cases of uLMS occur in women over 40 years of age, and the incidence is increasing rapidly during the perimenopausal years [2]. An analysis of 566 uLMS cases showed that OS significantly decreased in patients age ≥60 years compared with those age <60 years (aHR=1.55, 95% CI: 1.11–2.18, P=0.011) [23]. Kapp et al. found that 52 years of age was the best cut-off point, as patients aged ≤52 years had higher 5-year CSS rates compared with older patients (73.5% vs. 56.1%, respectively, P<0.001) [12]. Consistent with these previous studies, we found that 51 years and 58 years are the best cut-off points to make a precise grouping for OS and CSS, respectively, of uLMS patients.
We also found a positive correlation between tumor grade, stage, and survival outcomes. High grade (poorly or undifferentiated) was found to be a risk factor of mortality and had higher metastasis and recurrence rates than low grade (well or moderately differentiated) [24]. Disease stage was a strong predictor of OS and CSS. In a series of 208 uLMS patients, Giuntoli et al. reported that high grade and advanced stage of disease were correlated with worse CSS rates [1].
Tumor size is recognized as an essential predictor of uLMS [7,12]. We found that tumor size >70 mm is associated with poor prognosis. D’Angelo et al. suggested that tumors greater than or equal to 100 mm in diameter were associated with a worse prognosis than were smaller leiomyosarcomas [13]. A retrospective analysis of 819 patients revealed that tumor size is better than myometrial invasion and cervical involvement for uLMS risk stratification [25].
The current preferred treatment of uLMS is a multidisciplinary approach involving surgery, chemotherapy, and radiotherapy [8]. Several studies have analyzed the association between treatment and survival, but showed inconsistent results. Surgery remains the standard treatment for uLMS and confers a survival advantage [4], which was confirmed in our multivariate analysis. An early study found that myomectomy was sufficient to treat early-stage disease [26]; however, it is now commonly recognized that total abdominal hysterectomy with resection of all the visible tumor is the optimal initial surgical management for uLMS patients [27]. The role of regional lymphadenectomy in uLMS is still controversial because metastasis of lymph nodes occurs in 5–11% of patients [1,12]. Some authors reported that initial routine pelvic and optional periaortic lymphadenectomy can help to determine the stage and increase the possible need for adjuvant therapy [2,28]. In a retrospective study of 348 women with uLMS, Kapp et al. suggested that omitting lymphadenectomy is feasible [12]. In the present study, we also confirmed that there was no survival difference between patients with and without lymphadenectomy.
Although many uLMS patients with various stages received adjuvant pelvic radiation after surgery, the data to support radiotherapy in uLMS patients remains sparse. Proponents of radiotherapy have considered the evidence of local control in a small-sample study [26]; however, OS and progression-free survival (PFS) benefits were not been assessed in another large retrospective study [23]. The EORTC 55874 trial that assessed the utility of radiotherapy by performing a prospective randomized study of 103 completely resected, early-stage uLMS patients [29] and compared an adjuvant radiation group (patients received 5100 cGy external beam radiotherapy) with an observation group, finding no improvement in the local control and PFS rates in the adjuvant radiation group. In a meta-analysis, Chae et al. reported that uLMS patients tended to have early hematogenous spread; therefore, local radiation did not improve survival benefits for patients with uLMS [30].
In the present study, chemotherapy improved the survival outcomes in the advanced-stage subgroup, but adverse results were observed in the early-stage subgroup. After adjusting for confounding factors, our multivariate analysis confirmed that chemotherapy was a predictor for OS (aHR=1.208, 95%CI: 1.074–1.526, P=0.005). Similarly, in an observational cohort study from the 1998 to 2013 National Cancer Database, Seagle et al. reported that chemotherapy increased OS for patients with metastatic uLMS but not in early-stage uLMS [10]. Various chemotherapeutic agents have been tested for uLMS patients in previous studies, such as doxorubicin, ifosfamide, olaratumab, gemcitabine, and docetaxel [31–35], but the heterogeneity of the patient cohorts, small sample sizes, and lack of observation control arms restrict the analysis of data and the dependability of results. The combination of docetaxel and gemcitabine has been reported to be an effective regimen in patients with advanced-stage uLMS [36], but the question of whether chemotherapy improves survival for early-stage uLMS remains unanswered. A study assessing adjuvant gemcitabine plus docetaxel followed by doxorubicin in patients with early-stage uLMS revealed a 2-year PFS rate of 78% and a 3-year PFS rate of 58%, whereas the median PFS was not reached and exceeded 36 months [34]. Littell et al. performed a trial of the same regimen versus an observation control group in uLMS patients with FIGO stage I, showing no difference in survival and recurrence rates [35].
Previously, Zivanovic et al. established a nomogram from 270 uLMS patients in a monocentric cohort to predict 5-year OS probability, which had relatively weak discrimination in internal and external validation (the concordance index was 0.67 in both internal and external validation). Based on the multivariate analysis of a large population-based cohort, we established nomograms for predicting the 3- and 5-years OS and CSS rates for women with uLMS. A C-index greater that 0.7 is widely recognized as having relatively good discrimination, and calibration plots revealed that the prediction by the nomograms agreed perfectly with actual survival data.
It should be noted that our study has certain limitations. First, the SEER database has no information on the mitotic index, vascular invasion, or biologic markers (e.g., p53, p16, Ki67, and Bcl-2), which had also been examined as potential prognostic indicators, and more specific information such as the dose, type, and the course of treatment of adjuvant therapy were unavailable in the SEER database. Second, because the nomograms were established based on a retrospective study, some selection bias was unavoidable. Third, larger randomized controlled trials are needed to determine whether our findings are reliable and broadly applicable.
Conclusions
We constructed and validated nomograms to predict survival outcomes for uLMS patients. The developed nomograms showed good discrimination and calibration and can help clinicians by providing individual prognosis assessments and assist in clinical decision-making for treatment of women with uLMS. However, these nomograms require validation in other independent populations.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Giuntoli RL, 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89(3):460–69. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 2.Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71(4 Suppl):1702–9. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 3.Prat J, Mbatani Uterine sarcomas. Int J Gynaecol Obstet. 2015;131(Suppl 2):S105–10. doi: 10.1016/j.ijgo.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145(1):208–16. doi: 10.1016/j.ygyno.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Skorstad M, Kent A, Lieng M. Uterine leiomyosarcoma – incidence, treatment, and the impact of morcellation. A nationwide cohort study. Acta Obstet Gynecol Scand. 2016;95(9):984–90. doi: 10.1111/aogs.12930. [DOI] [PubMed] [Google Scholar]
- 6.Rose PG, Piver MS, Tsukada Y, Lau T. Patterns of metastasis in uterine sarcoma. An autopsy study. Cancer. 1989;63(5):935–38. doi: 10.1002/1097-0142(19890301)63:5<935::aid-cncr2820630525>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Mayerhofer K, Obermair A, Windbichler G, et al. Leiomyosarcoma of the uterus: A clinicopathologic multicenter study of 71 cases. Gynecol Oncol. 1999;74(2):196–201. doi: 10.1006/gyno.1999.5436. [DOI] [PubMed] [Google Scholar]
- 8.Roberts ME, Aynardi JT, Chu CS. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol Oncol. 2018;151(3):562–72. doi: 10.1016/j.ygyno.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 9.George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018;36(2):144–50. doi: 10.1200/JCO.2017.75.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: A National Cancer Database study. Gynecol Oncol. 2017;145(1):61–70. doi: 10.1016/j.ygyno.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118(3):660–69. doi: 10.1002/cncr.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: With emphasis on the impact of lymphadenectomy and oophorectomy. Cancer. 2008;112(4):820–30. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- 13.D’Angelo E, Espinosa I, Ali R, et al. Uterine leiomyosarcomas: tumor size, mitotic index, and biomarkers Ki67, and Bcl-2 identify two groups with different prognosis. Gynecol Oncol. 2011;121(2):328–33. doi: 10.1016/j.ygyno.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 15.Bianco FJ. Nomograms and medicine. Eur Urol. 2006;50(5):884–86. doi: 10.1016/j.eururo.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SL, Wang X, Li ZM, et al. Score for the overall survival probability of patients with first-diagnosed distantly metastatic cervical cancer: A novel nomogram-based risk assessment system. Front Oncol. 2019;9:1106. doi: 10.3389/fonc.2019.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health NIo. National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. 1975–2016. https://seer.cancer.gov/statfacts/html/corp.html.
- 18.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015;16(4):E173–80. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desar IME, Ottevanger PB, Benson C, van der Graaf WTA. Systemic treatment in adult uterine sarcomas. Crit Rev Oncol Hematol. 2018;122:10–20. doi: 10.1016/j.critrevonc.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Wu ZY, Lin ZQ. A nomogram to predict prognosis in Ewing sarcoma of bone. J Bone Oncol. 2019;15:100223. doi: 10.1016/j.jbo.2019.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosh M, Antar S, Nazzal A, et al. Uterine sarcoma analysis of 13,089 cases based on surveillance, epidemiology, and end results database. Int J Gynecol Cancer. 2016;26(6):1098–104. doi: 10.1097/IGC.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 22.Amant F, Dreyer L, Makin J, et al. Uterine sarcomas in South African black women: A clinicopathologic study with ethnic considerations. Eur J Gynaecol Oncol. 2001;22(3):194–200. [PubMed] [Google Scholar]
- 23.Li Y, Ren HT, Wang JM. Outcome of adjuvant radiotherapy after total hysterectomy in patients with uterine leiomyosarcoma or carcinosarcoma: A SEER-based study. BMC Cancer. 2019;19:697. doi: 10.1186/s12885-019-5879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livi L, Paiar F, Shah N, et al. Uterine sarcoma: Twenty-seven years of experience. Int J Radiat Oncol. 2003;57(5):1366–73. doi: 10.1016/s0360-3016(03)00750-8. [DOI] [PubMed] [Google Scholar]
- 25.Garg G, Shah JP, Liu JR, et al. Validation of tumor size as staging variable in the revised International Federation of Gynecology and Obstetrics stage i leiomyosarcoma a population-based study. Int J Gynecol Cancer. 2010;20(7):1201–6. doi: 10.1111/IGC.0b013e3181e9d0ba. [DOI] [PubMed] [Google Scholar]
- 26.Gadducci A, Landoni F, Sartori E, et al. Uterine leiomyosarcoma: Analysis of treatment failures and survival. Gynecol Oncol. 1996;62(1):25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]
- 27.Sagae S, Yamashita K, Ishioka S, et al. Preoperative diagnosis and treatment results in 106 patients with uterine sarcoma in Hokkaido, Japan. Oncology. 2004;67(1):33–39. doi: 10.1159/000080283. [DOI] [PubMed] [Google Scholar]
- 28.Levenback CF, Tortolero-Luna G, Pandey DK, et al. Uterine sarcoma. Obstet Gynecol Clin North Am. 1996;23(2):457–73. [PubMed] [Google Scholar]
- 29.Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44(6):808–18. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Chae SH, Shim SH, Chang M, et al. Effect of adjuvant therapy on the risk of recurrence in early-stage leiomyosarcoma: A meta-analysis. Gynecol Oncol. 2019;154(3):638–50. doi: 10.1016/j.ygyno.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Kushner DM, Webster KD, Belinson JL, et al. Safety and efficacy of adjuvant single-agent ifosfamide in uterine sarcoma. Gynecol Oncol. 2000;78(2):221–27. doi: 10.1006/gyno.2000.5875. [DOI] [PubMed] [Google Scholar]
- 32.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hensley ML, Blessing JA, DeGeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecologic Oncology. 2008;109(3):323–28. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hensley ML, Wathen JK, Maki RG, et al. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma Results of a Phase 2 Trial (SARC 005) Cancer. 2013;119(8):1555–61. doi: 10.1002/cncr.27942. [DOI] [PubMed] [Google Scholar]
- 35.Littell RD, Tucker LY, Raine-Bennett T, et al. Adjuvant gemcitabine-docetaxel chemotherapy for stage I uterine leiomyosarcoma: Trends and survival outcomes. Gynecol Oncol. 2017;147(1):11–17. doi: 10.1016/j.ygyno.2017.07.122. [DOI] [PubMed] [Google Scholar]
- 36.Hensley ML, Ishill N, Soslow R, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I–IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol Oncol. 2009;112(3):563–67. doi: 10.1016/j.ygyno.2008.11.027. [DOI] [PubMed] [Google Scholar]