Abstract
Background:
Multiple psychopathologies feature impaired clinical insight. Emerging evidence suggests that insight problems may similarly characterize addiction, perhaps due to aberrant functioning of self-referential brain circuitry, including the rostral anterior cingulate and ventromedial prefrontal cortices (rACC/vmPFC). We developed a new fMRI task to probe whether rACC/vmPFC abnormalities in cocaine use disorder (CUD) constitute neural correlates of readiness to change, one facet of insight.
Methods:
Eighteen individuals with current CUD and 15 healthy controls responded about their own need to change their drug use and eating behavior (control condition) and the need for a named acquaintance to do the same (two additional control conditions). Measures of simulated drug-choice behavior, addiction severity, and neuropsychological function were collected outside the scanner.
Results:
CUD participants perceived a greater need for behavior change than controls (as expected, given their diagnosis), but fell short of “agreeing” to a need for change; in CUD, lower perceived need correlated with higher simulated drug-choice behavior, a proxy measure of drug-seeking. During drug-related insight judgments, CUD participants had higher activation than controls in an anatomically-defined region of interest (ROI) in the medial orbitofrontal cortex, part of the rACC/vmPFC. Although not showing group differences, activation in an anatomically-defined ACC ROI correlated with insight-related task behavior (in all participants) and memory performance (in CUD).
Conclusions:
As a group, individuals with current CUD appear to show mild insight problems and rACC/vmPFC abnormalities vis-à-vis readiness to change behavior. With replication and extension of these results, insight-related circuitry may emerge as a novel therapeutic target.
Keywords: addiction, insight, self-awareness, behavior change, fMRI, orbitofrontal cortex
1. INTRODUCTION
Individuals with drug use disorders often take drugs despite adverse consequences and well-intentioned plans to abstain, and they routinely forego treatment. This behavior may reflect a core, underappreciated symptom of drug addiction involving dysfunction of insight and self-awareness (Goldstein et al., 2009), rather than deficiencies in willpower, character flaws, or intractable denial (Fine and Juni, 2001). For example, individuals with drug addiction rate their cognitive and emotional functioning as less impaired than do close informants (Verdejo-Garcia and Perez-Garcia, 2008), show impaired self-monitoring of task-related errors (Hester et al., 2007), and exhibit discordance between self-reports and objectively-measured behavior or brain function (Goldstein et al., 2007; Moeller et al., 2012; Parvaz et al., 2016).
We have suggested that impairments in behavioral insight and self-awareness can be plausibly mapped onto aberrant functioning of cortical midline regions subserving self-referential processing (Moeller and Goldstein, 2014). In this framework, we place special emphasis on the rostral (also referred to as perigenual) anterior cingulate cortex (ACC) (rACC) [Brodmann Areas (BAs) 24, 32] extending into the ventromedial prefrontal cortex (vmPFC) (BAs 10, 11, 25) (together: rACC/vmPFC). During self-referential processing, the rACC/vmPFC is thought to ascribe personal relevance or value to stimuli relevant to the self [meta-analyses: (Hu et al., 2016; Martinelli et al., 2013; Murray et al., 2012; Qin and Northoff, 2011; van der Meer et al., 2010)]. In support, individuals with cocaine use disorder (CUD) exhibit impaired self-monitoring of task behavior in correlation with smaller rACC/vmPFC gray matter volume (Moeller et al., 2016; Moeller et al., 2014). Studies from other laboratories have similarly shown correlations between behavioral self-awareness paradigms with rACC/vmPFC brain structure and function (Dean et al., 2015; Hester et al., 2009; Le Berre et al., 2013; Moreno-Lopez et al., 2014). Notably, the rACC/vmPFC also forms part of a larger, interconnected cortical network subserving self-referential processing comprised of: the entire ACC, dorsomedial PFC (dmPFC), posterior cingulate cortex (PCC), precuneus, insula, and temporoparietal junction [for reviews and meta-analyses, see (Cavanna and Trimble, 2006; Craig, 2009; D’Argembeau and Salmon, 2012; Denny et al., 2012; Martinelli et al., 2013; Murray et al., 2012; Qin and Northoff, 2011; Schmitz and Johnson, 2007; van der Meer et al., 2010; Wagner et al., 2012)]. Many of these regions also show functional abnormalities in drug addiction (Jasinska et al., 2014), including in studies assessing constructs related to self-awareness and treatment motivation (DeWitt et al., 2015; Moreno-Lopez et al., 2017; Prisciandaro et al., 2014). However, prior studies of addiction have assessed constructs less directly related to clinical insight, or they have examined neuroimaging associations with measures of insight collected outside the scanner, thereby constraining interpretations.
Here, we developed a new fMRI task to examine the rACC/vmPFC as underlying self-awareness of the need to change behavior, one component of clinical insight (David et al., 2012), among participants with active CUD. During the task, participants responded to statements about whether they needed to modify their drug use and seek treatment; fMRI was acquired while they made these judgments. We tested three hypotheses: (A) individuals with CUD will display a low perceived need to change their drug use behavior; (B) these self-judgments will be linked with aberrant activation in the rACC/vmPFC (i.e., compared with healthy controls); and (C) lower perceived readiness to change behavior and aberrant rACC/vmPFC activation will correlate with greater addiction severity. Hypothesis A was directly tested using task behavior. To test Hypothesis B, we used a priori, anatomically-defined regions of interest (ROIs); on a more exploratory basis, whole-brain analyses were also conducted to examine the broader spectrum of self-referential circuitry. To test Hypothesis C, we examined relationships between select task variables with simulated drug-seeking behavior (choice to view drug-related versus other salient images), indices of addiction severity, and cognitive functioning (Cambridge Neuropsychological Test Automated Battery: CANTAB). Supporting the latter, prior studies of addiction have revealed associations between neuropsychological deficits, especially of memory, and problems with insight, self-awareness, and motivation to change (Blume et al., 2005; Dean et al., 2015; Le Berre et al., 2012).
2. METHODS
2.1. Participants
Eighteen individuals meeting criteria for current CUD and 15 healthy controls participated. All CUD participants were abstinent from cocaine for ≥1 day, not intoxicated during the scan as determined by trained research staff. The controls, for whom the task is not directly relevant (because they have no problems with drugs), were a convenience sample to provide normative data in this first report. The groups were matched on gender, race, educational attainment, and IQ, but differed in age and self-report of depressive symptoms (CUD>control) (Table 1); the latter two factors were covaried in the analyses. Smoking history also differed between the groups, but could not be covaried due to the almost parallel distribution of cigarette smoking and CUD (Miller and Chapman, 2001). Participants were recruited through advertisements, word-of-mouth, and referrals. All provided written informed consent in accordance with the local Institutional Review Board. Participants underwent a comprehensive diagnostic interview, which in the CUD group confirmed their DSM-5 diagnosis, lack of treatment-seeking, and past-month use of cocaine. Current comorbidities within the CUD group included alcohol use disorder (N=2), post-traumatic stress disorder (N=2), and antisocial personality disorder (N=1). Past comorbidities (in remission) included alcohol use disorder (N=3), cannabis use disorder (N=3), and unipolar depression (N=1). The Supplement contains additional information regarding participant characterization.
Table 1.
Demographics and cocaine use of the study participants.
| Cocaine (N=18) | Control (N=15) | Test Statistic | |
|---|---|---|---|
| Gender: Male / Female | 15/3 | 9/6 | χ2=2.25 |
| Race: African-American / Caucasian / Asian / Other | 17/1/0/0 | 10/2/2/1 | χ2=4.92 |
| Age (years) | 51.0 ± 2.9 | 41.1 ± 10.4 | t=3.58* |
| Educational attainment (some high school / high school graduate / some college / college graduate) | 1/4/10/3 | 0/1/5/9 | χ2=7.25 |
| Verbal IQ: Wide Range Achievement Test (grade equivalent) | 12.6 ± 0.5 | 12.8 ± 0.5 | t=1.07 |
| Nonverbal IQ: WASI - Matrix Reasoning Scale | 9.9 ± 2.2 | 11.2 ± 3.2 | t=1.32 |
| Depression: Beck Depression Inventory II | 4.2 ± 3.6 | 1.7 ± 4.4 | U=55.5* |
| Smoking status (smoker / nonsmoker) | 17/1 | 2/13 | χ2=22.04* |
| Cigarettes per day (among current smokers) | 7.9 ± 5.5 | 4.3 ± 0.4 | t=0.89 |
| Body Mass Index (BMI) | 26.4 ± 3.9 | 28.6 ± 5.5 | t=1.17 |
| History of Comorbidity (No/Yes) | 7/11 | -- | -- |
| Cocaine urine status: positive / negative | 12/6 | -- | -- |
| Cocaine age of onset (years) | 23.5 ± 7.7 | -- | -- |
| Cocaine duration of use (years) | 21.1 ± 7.9 | -- | -- |
| Cocaine past month use: days/week | 2.2 ± 1.7 | -- | -- |
| Cocaine past month use: $/use | 120.8 ± 76.6 | -- | -- |
| Cocaine current abstinence: days | 6.9 ± 7.3 | -- | -- |
| Withdrawal symptoms: CSSA (0–126) | 17.1 ± 9.4 | -- | -- |
| Severity of Dependence Scale (0–15) | 5.1 ± 3.7 | -- | -- |
| Cocaine Craving Questionnaire (0–45) | 23.4 ± 11.5 | -- | -- |
Note. Values are frequencies or means ± standard deviation;
p<0.05.
Exclusion criteria were: (A) history of head trauma or loss of consciousness (> 30 min) or other neurological disease of central origin (including seizures); (B) abnormal vital signs; (C) history of major medical conditions, encompassing cardiovascular (including hypertension), endocrinological, oncological, or autoimmune diseases; (D) history of major psychiatric disorder, including disordered eating (given the task) (for CUD, exceptions were other substance use disorders and/or highly prevalent comorbidities, such as post-traumatic stress disorder; and for controls, the only exceptions were nicotine or caffeine dependence); (E) psychotropic or cardiovascular medication use within six months; (F) pregnancy; (G) contraindications to MRI; (H) except for cocaine in CUD participants, positive urine toxicology; and (I) evidence of current intoxication.
2.2. fMRI Task
2.2.1. Task Design
Task items were adapted statements from the University of Rhode Island Change Assessment (URICA) (McConnaughy, 1989), a questionnaire based on the Transtheoretical Model (Prochaska et al., 1992) (see Supplement for further description of the Model). During fMRI, participants viewed and responded to the eight items comprising the scale’s Contemplation subscale (e.g., “I think I might be ready for some self-improvement [regarding my drug use]”) intermixed with the eight items comprising the scale’s Precontemplation subscale (reverse coded) (e.g., “As far as I’m concerned, I don’t have any [drug use] problems that need changing”). A Likert-style scale was used for responding (1=strongly disagree, 2=disagree, 3=undecided, 4=agree, 5=strongly agree), with higher numbers coded to reflect greater perceived need for behavior change. Participants responded about the need to change their own drug use and eating behavior (control condition), and the need for a named acquaintance to change his/her drug use and eating behavior (two additional control conditions). Participants named an acquaintance with whom they previously used cocaine (for CUD) or had an alcoholic drink (for controls), but not a family member or significant other [due to potential overlap with the self (Murray et al., 2012)]. CUD acquaintances were estimated to have more drug use than healthy control acquaintances, but not a higher body mass index (Supplement). Together, the task conditions crossed the factors “Substance” (drug versus food) and “Person” (self versus other) in a 2×2 design (Figure 1). Control conditions about the participant’s acquaintance accounted for effects of general social cognition. Control conditions about eating helped to account for effects of general reward and reinforcement.
Figure 1.
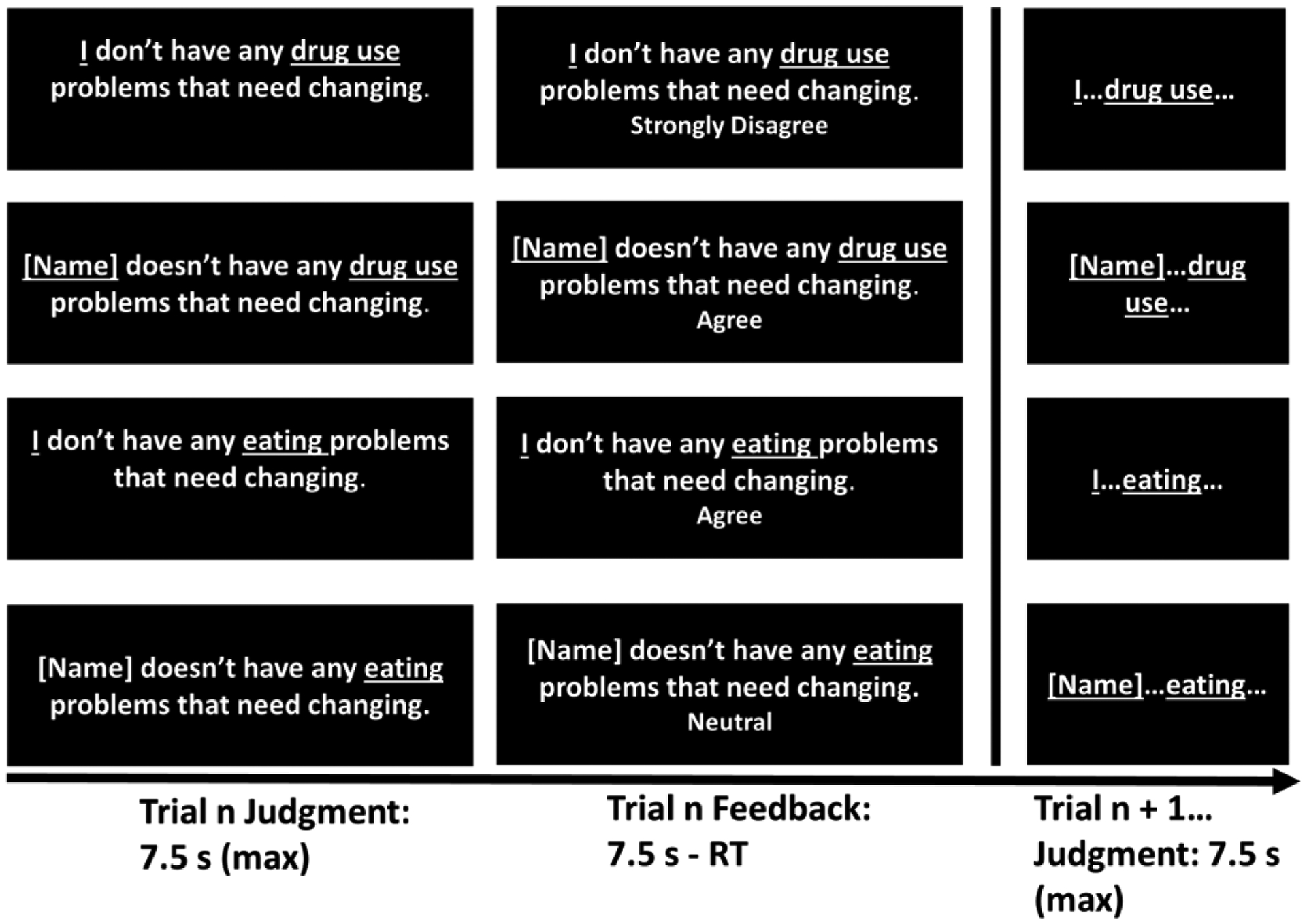
Schematic of the fMRI insight task. The task crossed the factors “Person” (self, other) and “Substance” (drug, food) in a 2 × 2 design.
To ensure adequate power, participants responded to each task item twice; reliability between the two responses was high (Supplement). Each trial lasted 7.5 seconds, followed immediately by the next 7.5-s trial. Once participants registered their response on a given trial, feedback was displayed until the 7.5 s trial time had elapsed. Responses could not be changed once entered, to avoid differential button pressing between trials and conditions. There were two 10-min task runs. Each run contained 4 experimental blocks, and each block contained 4 mini-blocks plus a 10-s fixation period at its beginning. The presentation of mini-blocks within each block was randomized. Each mini-block contained 4 trials of the same condition plus a 5-s instruction cue at its beginning, so that participants could anticipate the next 4 items and respond in time. Together, the task structure was 4 trials × 4 mini-blocks × 4 main blocks × 2 runs = 128 trials.
2.2.2. MRI Acquisition
Scanning was conducted on a Siemens 3T MAGNETOM Skyra (Siemens, Erlangen, Germany), using a 32-channel head coil. The BOLD-fMRI responses were measured as a function of time using a multiecho T2*-weighted, multiband-accelerated, single-shot EPI sequence [in-plane acceleration factor (GRAPPA) factor 2, multi-band factor 2, 3.6 mm isotropic voxel size, whole-brain coverage, TR=1500 ms, volumes=420, TEs=10.8, 28.68, 46.56 ms]. Absolute displacement was less than 3 mm, below the isotropic voxel size, except for 1 run in 1 CUD participant (which was removed from the analyses). T1-weighted MPRAGE was acquired with 0.9 mm isotropic resolution and whole-brain coverage.
2.2.3. MRI Data Processing
Functional MRI data were preprocessed using fMRIPrep software in combination with freely-available scripts derived from the multiecho independent components analysis (ME-ICA) pipeline (Kundu et al., 2013; Kundu et al., 2012). This ICA approach separates BOLD and non-BOLD signals based on quantitative T2* decay measures, in contrast to arbitrary preprocessing, and it mitigates signal dropout due to through-plane dephasing by acquiring an early echo and optimal combination of echoes. Multiecho imaging therefore increases signal-to-noise and improves statistical power compared with traditional denoising procedures. In the vmPFC, for example, simulations demonstrated that a significant effect (80% power) during a social cognition task required fewer than 15 participants (versus >20 with standard preprocessing) (Lombardo et al., 2016). Functional data were coregistered to anatomical images, then normalized to MNI standard space. No smoothing was applied, which is the default (and optimal) option for multiecho imaging (Lombardo et al., 2016).
2.3. Task Validation Measures
2.3.1. Simulated Drug-Choice
These well-validated drug-choice tasks instruct participants to select salient drug- and non-drug-related images for viewing, according to contingencies that are fully known (explicit task) and not fully known (probabilistic task) (Moeller et al., 2009). Three of the image categories were selected from the International Affective Image System (IAPS) (Lang et al., 2008): pleasant (e.g., smiling babies), unpleasant (e.g., mutilation), and neutral (e.g., household items) images. Another image category depicted cocaine-related images, matched on size and ratio of human to non-human content to IAPS. As done previously, we analyzed cocaine selections and cocaine minus pleasant (cocaine>pleasant) selections, separately for the two tasks. The tasks approximate drug-seeking behavior (drug versus pleasant choice), but without ethical concerns of real drug administration (Moeller and Stoops, 2015).
2.3.2. Addiction Severity
We analyzed in-house assessments for frequency and severity of drug use, and well-validated questionnaires including the Cocaine Selective Severity Assessment Scale (Kampman et al., 1998), Severity of Dependence Scale (Gossop et al., 1992), and Cocaine Craving Questionnaire (Tiffany et al., 1993).
2.3.3. Neuropsychological Functioning
To test multiple neuropsychological functions while minimizing the number of correlations, we created two CANTAB composite scores: (A) immediate recall memory (based on prior literature) and (B) executive function (core to addiction) (Goldstein and Volkow, 2011). The recall memory score was calculated from the following tasks (variables): Verbal Recognition Memory (free recall errors: insertions), Spatial Span measuring working memory capacity (total span), and Delayed Matching to Sample measuring spatial memory (total percent correct). The executive function score was calculated from the following tasks (variables): Intra-Extra Dimensional Set Shift measuring reversal learning (total errors), Information Sampling Task measuring impulsivity (total correct), One Touch Stockings of Cambridge measuring planning (number of problems solved on first choice), and the Stop Signal Task measuring response inhibition (stop signal reaction time). We centered and scaled each of the constituent test scores based on the mean and standard deviation of the healthy controls, and then averaged these standardized scores (Dean et al., 2012; Goldstein et al., 2004). Tests on which lower scores indicated better performance were multiplied by −1 to keep directionality consistent. An exploratory principal components analysis (PCA), with varimax rotation and two components specified, indicated that the various tasks indeed sorted into a “memory” component and an “executive function” component; one executive function task (One Touch Stockings of Cambridge) was removed and not considered further due to high cross-loadings.
2.4. Statistical Analyses
2.4.1. Behavior
2.4.1.1. One-Sample Tests.
We could not predict how healthy controls would interpret a task about the need to change (non-problematic) drug use. Therefore, to begin the analyses probing insight, we performed one-sample t-tests on the ‘Drug-Self’ condition mean against test values corresponding to key task response anchors (coded 1–5), in CUD participants only. First, we used a one-sample t-test against “3” to examine whether CUD participants’ Drug-Self condition score (i.e., judgments about the need to change their own drug use) differed from “Undecided.” Then (based on these results), we tested whether the Drug-Self mean differed from “Agree” (i.e., t-test against “4”).
2.4.1.2. Between-Group Tests.
We next performed a full 2 (Substance: drug, food) × 2 (Person: self, other) × 2 (Diagnosis: CUD, control) mixed ANCOVA, which controlled for age and depressive (BDI) symptoms (Beck, 1996), on the behavioral ratings. In addition to examining the ‘Drug-Self’ condition directly, we also computed and analyzed a targeted, a priori contrast of the Drug-Self Condition minus the mean of the remaining 3 conditions (‘Drug-Self > Remaining’). This contrast reflects perceived need specifically to change one’s own drug use, while accounting for the other three task conditions. It ensures that group differences (and correlations with severity, below) reflect a unique contribution of the Drug-Self Condition, rather than a byproduct of higher scores on all task conditions.
Together, these analyses tested Hypothesis A. Significance was p<0.05.
2.4.2. Neuroimaging
The fMRI BOLD data were modeled with a general linear model (GLM). The design matrix consisted of separate onset vectors for each task mini-block (8 mini-blocks for each of the 4 experimental conditions), convolved with a hemodynamic response function. The 6 motion parameters (3° rotation, and 3 mm translation) were included as regressors of no interest.
For the betas of the 1st-level regressors, we estimated a 2nd-level 2 (Substance: drug, food) × 2 (Person: self, other) × 2 (Diagnosis: CUD, control) flexible factorial model using Statistical Parametric Mapping (SPM12). Using this 2×2×2 model, we extracted (via MARSBAR toolbox) the fMRI-BOLD signal from anatomically-defined ROIs placed in the ACC and medial orbitofrontal cortex (OFC) (with the latter approximating the vmPFC, which has no real anatomical distinction) (Figure 3A). We then analyzed these extracted BOLD signals in SPSS using a corresponding 2×2×2 ANCOVA (controlling for age and BDI). Similar to behavior, we also computed a targeted ‘Drug-Self > Remaining’ conditions contrast. Significance was p<0.05. The Supplement contains information on all omnibus task activations and deactivations, and whole-brain results of factorial and regression SPM models. Together, these analyses tested Hypothesis B.
Figure 3.
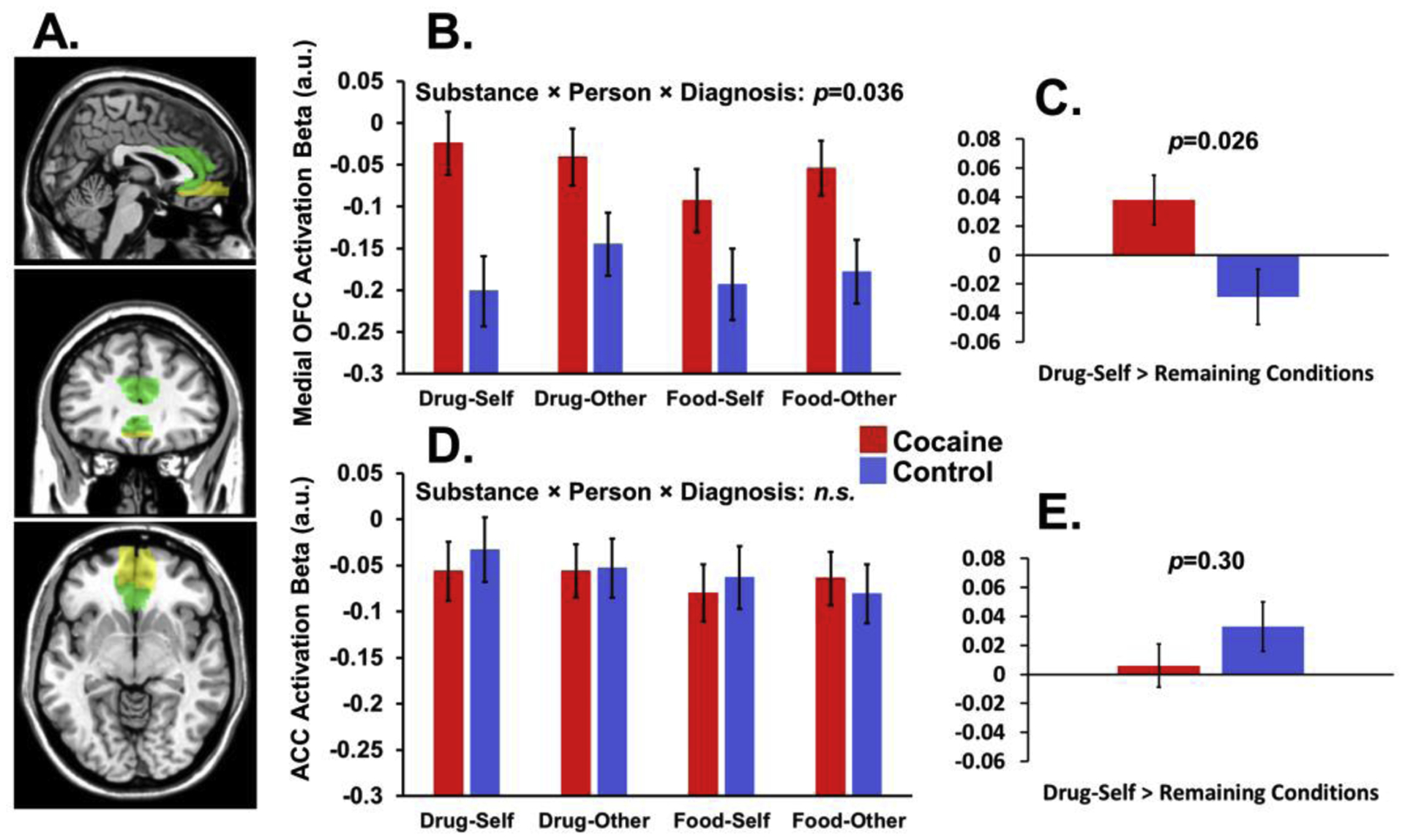
Neuroimaging ROI analyses. (A) Anatomical regions of interest (ROIs) in the medial orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC). (B) In addition to a Diagnosis main effect, there was a significant 3-way interaction between Substance, Person, and Diagnosis, driven in part by group differences in the Drug-Self condition. (C) Similarly to behavior, robustness of the Drug-Self between-group difference is further highlighted by a significant group difference for a targeted contrast of Drug-Self minus the mean of the other 3 conditions (Drug-Self > Remaining conditions). (D-E) In contrast, there were no significant effects in the ACC. All numbers are estimated marginal means after covarying age and dysphoric symptoms.
2.4.3. Correlation Analyses
2.4.3.1. Brain-Behavior Intercorrelations.
We tested for correlations between task behavior and ROI activations. For both brain and behavior, we examined (A) the Drug-Self condition and (B) the Drug-Self > Remaining conditions contrast. Correlations were tested across all participants and within CUD, resulting in 16 associations tested (see Table 2) (p<0.003 Bonferroni-corrected significance).
Table 2.
Associations between brain and behavior on the fMRI task.
| b (SE) | b (SE) | |
|---|---|---|
| All Participants | ||
| Behavior: Drug-Self Condition | Behavior: Drug-Self > Remaining Conditions | |
| Medial OFC: Drug-Self Condition | 0.11 (0.64) | −0.42 (0.54) |
| Medial OFC: Drug-Self > Remaining Conditions | 1.38 (1.51) | 0.54 (1.16) |
| ACC: Drug-Self Condition | −1.76 (0.77)+ | −1.98 (0.62)* |
| ACC: Drug-Self > Remaining Conditions | −3.88 (1.54)+ | −2.29 (1.18) |
| Cocaine Group Only | ||
| Behavior: Drug-Self Condition | Behavior: All Conditions | |
| Medial OFC: Drug-Self Condition | −2.40 (1.64) | −1.64 (1.53) |
| Medial OFC: Drug-Self > Remaining Conditions | −0.19 (1.58) | 0.13 (1.60) |
| ACC: Drug-Self Condition | −1.74 (2.35) | −1.48 (2.67) |
| ACC: Drug-Self > Remaining Conditions | −0.87 (1.75) | −0.06 (1.73) |
Note. Corrected significance was set p<0.003, due to 16 correlations tested. Generalized linear models with robust standard errors were used, due to heteroscedasticity of some of the measures. The “Drug-Self” condition (our primary condition of interest) refers to perceptions about the need to change one’s own drug use. “Drug-Self > Remaining conditions” refers to the Drug-Self condition minus the mean of the other 3 conditions. The models are defined such that brain activation is used to predict behavior. The asterisk (*) denotes the significant brain-behavior relationship across all participants; the plus sign (+) denotes trends at p<0.05 (uncorrected). Abbreviations: OFC=orbitofrontal cortex, ACC=anterior cingulate cortex.
2.4.3.2. Associations with Functioning.
Within CUD, we tested correlations between task variables and the following three classes of variables related to functioning: (A) drug-choice behavior (variables: explicit and probabilistic cocaine choice, and explicit and probabilistic cocaine>pleasant choice), (B) indices of drug use and severity (variables: days per week cocaine use in last 30 days, amount spent per use of cocaine in last 30 days, Cocaine Selective Severity Assessment Scale total score, Cocaine Craving Questionnaire total score, and Severity of Dependence Scale total score), and (C) the two CANTAB composite scores. As predictors, we used the same 6 task variables as tested for the brain-behavior correlations above. Significance reflected Bonferroni correction: p<0.002 for drug-choice behavior (6 task variables × 4 drug-choice metrics), p<0.002 for drug use severity (6 task variables × 5 drug use variables), and p<0.004 for CANTAB composite scores (6 task variables × 2 composite measures) (see Table 3). Due to heteroscedasticity with some of the measures, all correlation analyses used generalized linear models to enable the specification of robust standard errors. Together, these analyses tested Hypothesis C.
Table 3.
Associations of fMRI task variables with clinical severity.
| Behavior: Drug-Self Condition | Behavior: Drug-Self > Remaining Conditions | Medial OFC: Drug-Self Condition | Medial OFC: Drug-Self > Remaining Conditions | ACC: Drug-Self Condition | ACC: Drug-Self > Remaining Conditions | |
|---|---|---|---|---|---|---|
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| Drug-Choice Tasks | ||||||
| Probabilistic Cocaine Choice | −2.14 (3.64) | −0.31 (3.76) | −16.42 (25.71) | 12.03 (37.10) | −18.04 (25.13) | −8.96 (32.09) |
| Probabilistic Cocaine>Pleasant Choice | −8.53 (5.53) | −3.13 (5.80) | −31.51 (48.43) | 66.70 (47.84) | −27.06 (38.65) | 37.86 (39.44) |
| Explicit Cocaine Choice | −87.50 (21.30)* | −74.68 (20.60)* | 458.55 (256.47) | 687.87 (297.89)+ | 499.26 (298.30) | 764.30 (285.27)+ |
| Explicit Cocaine>Pleasant Choice | −23.40 (14.16) | −12.16 (21.35) | 22.68 (212.68) | 1142.43 (684.45) | 258.83 (442.05) | 842.16 (586.50) |
| Drug Use Variables | ||||||
| Cocaine Frequency (Days/Week, Last Month) | −1.09 (0.62) | −0.69 (0.82) | −5.39 (5.57) | 5.11 (6.91) | −1.38 (5.41) | 5.03 (5.65) |
| Cocaine Severity ($ Per Use, Last Month) | −5.34 (24.38) | 6.37 (26.87) | −385.57 (239.39) | 10.60 (371.17) | −388.25 (209.65) | −265.26 (269.15) |
| CSSA Questionnaire (Withdrawal Symptoms) | 2.89 (3.76) | −0.91 (2.41) | 14.49 (21.25) | 11.43 (27.84) | 51.97 (20.62)+ | −9.66 (32.61) |
| CQ Questionnaire (Craving) | −4.48 (2.87) | −1.38 (4.30) | 12.07 (37.51) | 57.15 (57.76) | 26.96 (36.07) | 40.07 (47.48) |
| SDS Questionnaire (Severity of Dependence) | 0.60 (1.04) | 1.64 (1.00) | −3.45 (12.29) | −24.27 (22.41) | −6.47 (15.88) | −19.13 (18.84) |
| Neuropsychological Functioning | ||||||
| Memory Composite | 0.22 (0.30) | 0.42 (0.27) | −2.17 (1.41) | −1.31 (1.46) | −4.33 (1.38)* | −3.17 (1.62) |
| Executive Function Composite | −0.16 (0.24) | −0.46 (0.29) | 2.09 (2.85) | −0.85 (3.08) | −0.60 (2.32) | −0.43 (1.96) |
Note. Analyses were generalized linear models, and were conducted in the cocaine use disorder (CUD) group only. Significance was based on the number correlations tested for a given class of variables: p<0.002 for drug-choice behavior and drug use severity, and p<0.004 for CANTAB composite scores. The models are defined such that the respective task variable is used to predict the respective severity measure. Asterisks denote (corrected) significant relationships; the plus sign denotes trends at p<0.05 (uncorrected). Abbreviations: OFC=orbitofrontal cortex, ACC=anterior cingulate cortex, CSSA=Cocaine Selective Severity Assessment, CQ=Cocaine Craving Questionnaire, SDS=Severity of Dependence Scale.
3. RESULTS
3.1. Behavior
3.1.1. One-Sample Tests
In CUD, the Drug-Self condition mean was higher than “undecided” [t(17)=4.04, p=0.001], but lower than “agree” [t(17)=2.28, p=0.036], consistent with mild insight problems.
3.1.2. Between-Group Tests (Figure 2)
Figure 2.
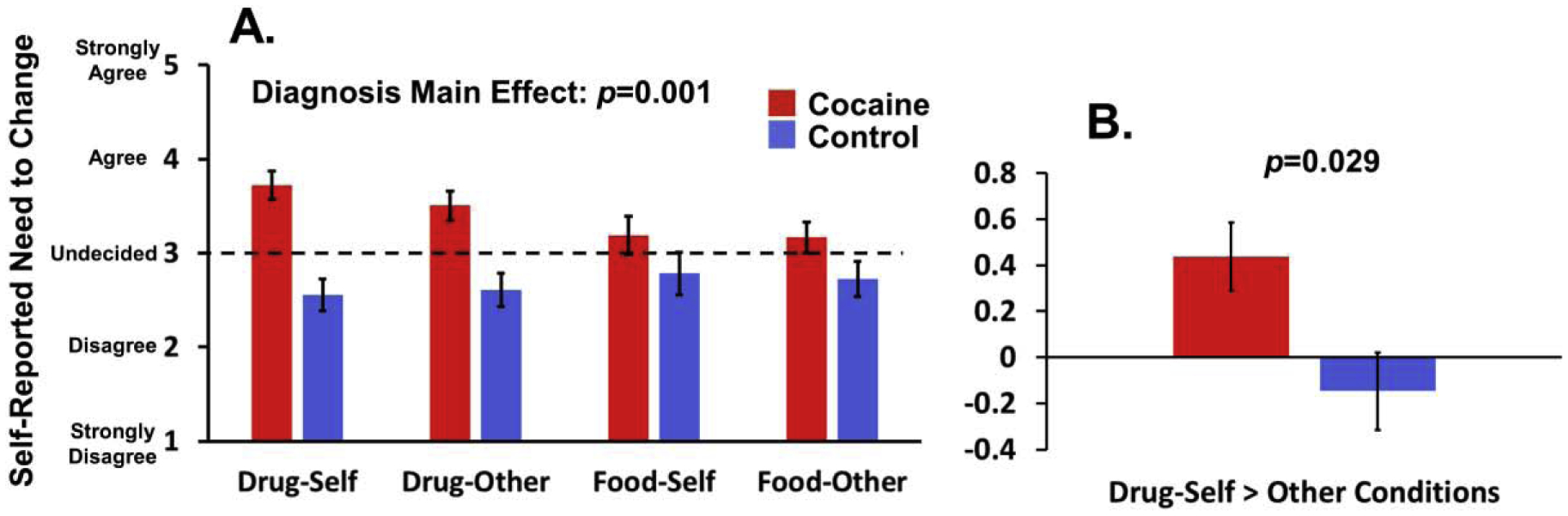
Behavioral results. (A) There was a Diagnosis main effect on the task, such that individuals with cocaine use disorder (CUD) had higher ratings than controls across all task conditions. The dashed line signifies “Undecided.” Despite a nonsignificant 3-way interaction between Substance, Person, and Diagnosis, the Drug-Self condition (i.e., our main task condition of interest) showed differences between the groups. (B) Robustness of the Drug-Self between-group difference is further highlighted by a significant group difference for a targeted contrast of Drug-Self minus the mean of the other 3 conditions (Drug-Self > Remaining conditions). All numbers are estimated marginal means after covarying age and dysphoric symptoms.
The full 2 (Substance: drug, food) × 2 (Person: self, other) × 2 (Diagnosis: CUD, control) ANCOVA (controlling for age and BDI) revealed only a main effect of Diagnosis, such that CUD reported a greater need than controls for behavior change across all task conditions [F(1,29)=12.69, p=0.001, partial eta-squared (ηp2)=0.30]. A trend Substance × Diagnosis interaction [F(1,29)=3.17, p=0.085, ηp2=0.10], which was significant without covariates (p=0.017, ηp2=0.17), suggested that the omnibus Diagnosis effect was partly driven by group differences in the two drug conditions [CUD>control: F(1,29)=17.23, p<0.001] versus the two food conditions (p=0.145). Despite a nonsignificant 3-way interaction [F(1,29)=0.85, p=0.364], the robustness and uniqueness of the Drug-Self Condition was evident, with significant group differences (CUD>control) observed for the Drug-Self condition [F(1,29)=20.32, p<0.001] and the Drug-Self > Remaining conditions contrast [F(1,29)=5.29, p=0.029].
3.2. Neuroimaging ROI Analyses (Figure 3)
The 2×2×2 ANCOVA on the medial OFC revealed a main effect of Diagnosis [F(1,29)=4.89, p=0.035, ηp2=0.14] and a Substance × Person interaction [F(1,29)=9.84, p=0.004, ηp2=0.25]. Both effects were qualified by a significant Substance × Person × Diagnosis interaction [F(1,29)=4.84, p=0.036, ηp2=0.14]: medial OFC activation was higher in CUD than controls during the Drug-Self condition [F(1,29)=7.53, p=0.010] and (somewhat surprisingly) the Food-Other condition [F(1,29)=4.76, p=0.037], but not in the Drug-Other or Food-Self conditions (p>0.084). Further supporting robust effects of Drug-Self condition, the targeted Drug-Self > Remaining conditions contrast differed between the groups (CUD>control) [F(1,29)=5.48, p=0.026]. The 2×2×2 ANCOVA on the ACC revealed no significant main effects or interactions (all p>0.22).
3.3. Correlation Analyses
3.3.1. Brain-Behavior Intercorrelations (Table 2)
The more participants activated the ACC (Drug-Self condition), the lower was their perceived need to change drug use behavior (Drug-Self > Remaining conditions) (Figure 4A). This correlation supports brain-behavior correspondence on the task, although it was not driven by CUD as expected. This correlation across participants was confirmed with whole-brain regression (Supplement). Other correlations did not reach significance.
Figure 4.
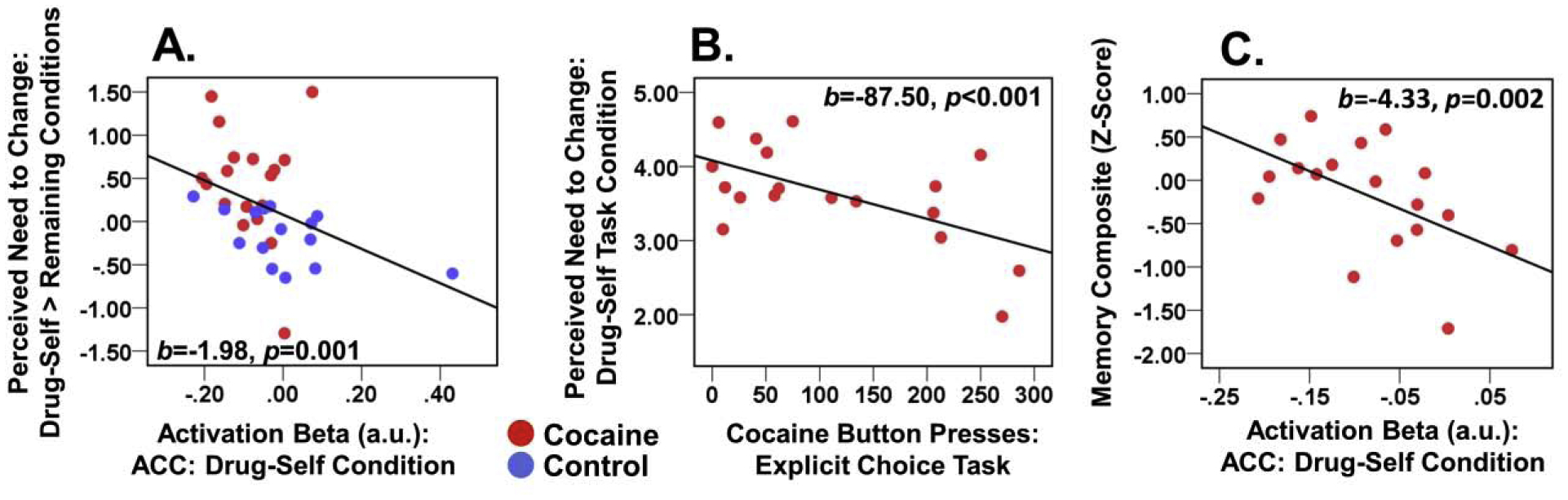
Brain-behavior correlations. Scatterplots show the associations between (A) task behavior and brain activation in the anterior cingulate cortex (ACC), (B) task behavior and drug-choice behavior (explicit task), and (C) ACC activation and memory performance. The correlation in (A) effect was across all participants and did not emerge for either diagnostic group tested alone. The correlations in (B) and (C) were conducted in CUD participants only.
3.3.2. Associations with Functioning (Table 3)
Within CUD, a higher self-reported need to change one’s drug use (Drug-Self condition with a similarly significant effect for Drug-Self > Remaining conditions) correlated with fewer cocaine image presses on the explicit drug-choice task (Figure 4B). Higher ACC activation during the Drug-Self condition correlated with worse memory performance (Figure 4C) though it was not confirmed whole-brain (Supplement). No significant relationships emerged with drug use severity, although several trends (p<0.05 uncorrected) largely corroborated directionality of the significant correlations.
4. DISCUSSION
We developed a new fMRI task assessing perceptions of the need to change, a feature of insight impairment, and underlying circuitry in individuals with current CUD. As expected, CUD participants reported a greater need to change their behavior (including drug use) than healthy controls (who had no drug use problems). Yet, despite symptoms severe enough to warrant a current DSM-5 CUD diagnosis, CUD individuals fell short of “agreeing” that they needed to alter their drug use, partially supporting Hypothesis A. This was true even among participants with higher CUD symptom counts (Supplement). These findings support prior reports of self-awareness and self-monitoring deficits in individuals with drug addiction (Hester et al., 2009; Hester et al., 2007; Moeller et al., 2014; Moeller et al., 2010; Parvaz et al., 2016; Payer et al., 2011; Verdejo-Garcia and Perez-Garcia, 2008). Findings of non-trivial perceptions of the need to change behavior were unexpected in the healthy controls. As the controls consumed alcohol recreationally, we speculate that the task may have led them to question the extent of their drinking (i.e., despite not meeting criteria for Alcohol Use Disorder), potentially due to priming effects of the task (Custers and Aarts, 2010).
Consistent with Hypothesis B, CUD participants activated the (anatomically-defined) medial OFC more than healthy controls, especially during the Drug-Self condition as hypothesized. We did not find similar group differences in an anatomical ACC ROI, although activation in this region was negatively correlated across all participants with perceived need to modify drug use. One potential mechanism for this somewhat unexpected direction of association is that confronting the potential severity of one’s substance use could have elicited enhanced conflict/ambivalence [a core ACC function (Chen et al., 2018; Etkin et al., 2011) implicated in addiction (Zilverstand et al., 2018)]. Participants may have resolved such conflict by reporting a lower need to change. Another potential explanation is optimism bias. Optimism bias has been previously linked to poor outcomes in substance use (Dillard et al., 2009; Jun and Nan, 2018; McKenna et al., 1993; Prokhorov et al., 2003), and some evidence in healthy individuals indicates that optimism bias involves the rACC/vmPFC (Sharot et al., 2007). In our study, optimism bias could have led CUD toward (potentially unfounded) beliefs that their drug use problems will improve without intervention or have not progressed enough to require intervention. In this way, and analogously to findings in patients with Alzheimer’s disease (Mograbi et al., 2009), CUD individuals may have activated an outdated (“petrified”) self-representation that omitted recent information about severity. Future studies should replicate these effects while also including tasks that tap unrealistic optimism and/or treatment ambivalence, to disentangle the underlying mechanisms. Despite this need for more research, our collective results – in conjunction with work from other laboratories (Le Berre et al., 2013; Moreno-Lopez et al., 2014) – support the view that drug-addicted individuals may have impaired drug-related insight, potentially rooted in aberrant functioning of self-referential brain regions (Goldstein et al., 2009; Moeller and Goldstein, 2014).
Consistent with Hypothesis C, lower perceived need for change correlated with higher cocaine-related choice on our explicit drug-choice task. This correlation agrees with prior research showing relationships between lower readiness to change and neurocognitive impairment in addiction (Dean et al., 2015). Enhanced drug-choice, as a proxy for actual drug-seeking, may indicate a vulnerability that culminates in future, real-world drug-seeking (Moeller et al., 2013; Moeller et al., 2019). In contrast, higher activation of self-referential circuitry during the task, especially in the ACC during the Drug-Self condition, was associated with a worse clinical phenotype: significantly worse memory performance, and a trend toward significantly more cocaine withdrawal symptoms. The differing directions of association between brain and behavior are not unexpected given the overall negative correlation between brain and behavior on the task (above). Still, such correlations should be replicated with larger samples.
This study has several limitations. First, because the overall sample size was relatively small, current results require replication. Second, results cannot generalize to treatment-seekers, who may report greater need to change (Le Berre et al., 2012). It would be interesting to evaluate whether perceived treatment need and its circuitry predicts treatment engagement and clinical progress. Third, our task did not capture all the components of clinical insight (David et al., 2012). Future studies can design more comprehensive tasks. Fourth, the study groups differed on age, dysphoric symptoms, and cigarette smoking history. Future studies can recruit groups with more parallel distributions on these variables, particularly smoking history which could not be covaried due to high overlap with CUD diagnosis. Fifth, 7 CUD participants had comorbidities, which may have affected insight and/or rACC/vmPFC functioning. Future studies with larger samples can better account for these comorbidities.
In conclusion, to our knowledge, this was the first study to image drug-addicted individuals while they made judgments of their need to change their drug use behaviors. A compromised capacity to recognize the need to change problematic drug-use behavior as a measure of disease severity has broad clinical significance, potentially undermining treatment pursuit, engagement, and adherence (Kim et al., 2007). The circuitry underlying such a deficit, potentially including the rACC/vmPFC, may provide a new target for clinical intervention studies.
Supplementary Material
HIGHLIGHTS.
Drug-addicted individuals may have impaired insight into their disease severity
Here, active cocaine users answered insight-relevant questions during fMRI
Participants did not agree they had drug use problems that needed changing
The task engaged the medial OFC, a core node of the self-referential network
Problems with insight and associated circuitry correlated with addiction severity
Acknowledgements
We thank Ms. Amanda Fisher and Dr. Mercedes Perez-Rodriguez for assistance with identifying and screening healthy controls for the study. We also thank Dr. Sameera Abeykoon for help with data preprocessing.
Role of Funding Source
This work was supported by grants from the National Institute on Drug Abuse (K01DA037452 and R21DA40046 to SJM; K23DA045928 to KB; K01DA043615 to MAP; and R01DA041528 and R21DA034954 to RZG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
References
- Beck AT, 1996. The Beck Depression Inventory (BDI-II). The Psychological Corporation. [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA, 2005. Memory, executive cognitive function, and readiness to change drinking behavior. Addict. Behav 30(2), 301–314. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR, 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(Pt 3), 564–583. [DOI] [PubMed] [Google Scholar]
- Chen T, Becker B, Camilleri J, Wang L, Yu S, Eickhoff SB, Feng C, 2018. A domain-general brain network underlying emotional and cognitive interference processing: evidence from coordinate-based and functional connectivity meta-analyses. Brain. Struct. Funct 223(8), 3813–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Custers R, Aarts H, 2010. The unconscious will: how the pursuit of goals operates outside of conscious awareness. Science 329(5987), 47–50. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Salmon E, 2012. The neural basis of semantic and episodic forms of self-knowledge: insights from functional neuroimaging. Adv. Exp. Med Biol 739, 276–290. [DOI] [PubMed] [Google Scholar]
- David AS, Bedford N, Wiffen B, Gilleen J, 2012. Failures of metacognition and lack of insight in neuropsychiatric disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci 367(1594), 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Hellemann G, Sugar CA, London ED, 2012. Educational attainment is not a good proxy for cognitive function in methamphetamine dependence. Drug Alcohol Depend. 123(1–3), 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED, 2015. Denial in methamphetamine users: Associations with cognition and functional connectivity in brain. Drug Alcohol Depend. 151, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN, 2012. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J. Cogn. Neurosci 24(8), 1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM, 2015. The hyper-sentient addict: an exteroception model of addiction. Am. J. Drug. Alcohol Abuse 41(5), 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard AJ, Midboe AM, Klein WM, 2009. The dark side of optimism: unrealistic optimism about problems with alcohol predicts subsequent negative event experiences. Pers. Soc. Psychol. Bull 35(11), 1540–1550. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J, Juni S, 2001. Ego atrophy in substance abuse: addiction from a socio-cultural perspective. Am. J. Psychoanal 61(3), 293–304. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND, 2007. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J. Psychiatry 164(1), 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND, 2009. The neurocircuitry of impaired insight in drug addiction. Trends Cogn. Sci 13(9), 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND, 2004. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia 42(11), 1447–1458. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12(11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J, 1992. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br. J. Addict 87(11), 1527–1536. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H, 2009. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 34, 2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simões-Franklin C, Garavan H, 2007. Post-error behavior in active cocaine users: Poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology 32(9), 1974–1984. [DOI] [PubMed] [Google Scholar]
- Hu C, Di X, Eickhoff SB, Zhang M, Peng K, Guo H, Sui J, 2016. Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neurosci. Biobehav. Rev 61, 197–207. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y, 2014. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J, Nan X, 2018. Comparative risk assessment and cessation information seeking among smokeless tobacco users. Addict. Behav 80, 14–21. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE, 1998. Reliability and validity of the Cocaine Selective Severity Assessment. Addict. Behav 23(4), 449–461. [DOI] [PubMed] [Google Scholar]
- Kim JS, Park BK, Kim GJ, Kim SS, Jung JG, Oh MK, Oh JK, 2007. The role of alcoholics’ insight in abstinence from alcohol in male Korean alcohol dependents. J. Korean Med. Sci 22(1), 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vertes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET, 2013. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl. Acad. Sci. USA 110(40), 16187–16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA, 2012. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60(3), 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. University of Florida, Gainsville, FL. [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Segobin S, Mezenge F, Boudehent C, Vabret F, Viader F, Eustache F, Pitel AL, Beaunieux H, 2013. Readiness to change and brain damage in patients with chronic alcoholism. Psychiatry Res. 213(3), 202–209. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Vabret F, Cauvin C, Pinon K, Allain P, Pitel AL, Eustache F, Beaunieux H, 2012. Cognitive barriers to readiness to change in alcohol-dependent patients. Alcohol Clin. Exp. Res 36(9), 1542–1549. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Auyeung B, Holt RJ, Waldman J, Ruigrok AN, Mooney N, Bullmore ET, Baron-Cohen S, Kundu P, 2016. Improving effect size estimation and statistical power with multi-echo fMRI and its impact on understanding the neural systems supporting mentalizing. Neuroimage 142, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P, 2013. Neural substrates of the self-memory system: new insights from a meta-analysis. Hum. Brain Mapp 34(7), 1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnaughy EA, DiClemente CC, Prochaska JO, Velicer WF, 1989. Stages of change in psychotherapy: Measurement and sample profiles. Psychother. Theor. Res. Prac 20, 368–375. [Google Scholar]
- McKenna FP, Warburton DM, Winwood M, 1993. Exploring the limits of optimism: the case of smokers’ decision making Br. J. Psychol (London, England: : 1953) 84 (Pt 3), 389–394. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP, 2001. Misunderstanding analysis of covariance. J. Abnorm. Psychol 110(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Beebe-Wang N, Woicik PA, Konova AB, Maloney T, Goldstein RZ, 2013. Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend. 130(1–3), 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Fleming SM, Gan G, Zilverstand A, Malaker P, dOleire Uquillas F, Schneider KE, Preston-Campbell RN, Parvaz MA, Maloney T, Alia-Klein N, Goldstein RZ, 2016. Metacognitive impairment in active cocaine use disorder is associated with individual differences in brain structure. Eur. Neuropsychopharmacology 26(4), 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ, 2014. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn. Sci 18(12), 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Hajcak G, Parvaz MA, Dunning JP, Volkow ND, Goldstein RZ, 2012. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain 135(Pt 11), 3481–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Hanley AW, Garland EL, 2019. Behavioral preference for viewing drug v. pleasant images predicts current and future opioid misuse among chronic pain patients. Psychol. Med, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, Goldstein RZ, 2014. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry 71(1), 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ, 2010. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain 133(Pt 5), 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ, 2009. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol. Psychiatry 66(2), 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Stoops WW, 2015. Cocaine choice procedures in animals, humans, and treatment-seekers: Can we bridge the divide? Pharmacol. Biochem. Behav 138, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mograbi DC, Brown RG, Morris RG, 2009. Anosognosia in Alzheimer’s disease--the petrified self. Conscious. Cogn 18(4), 989–1003. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez L, Albein-Urios N, Martinez-Gonzalez JM, Soriano-Mas C, Verdejo-Garcia A, 2014. Prefrontal Gray Matter and Motivation for Treatment in Cocaine-Dependent Individuals with and without Personality Disorders. Front. Psychiatry 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Albein-Urios N, Martinez-Gonzalez JM, Soriano-Mas C, Verdejo-Garcia A, 2017. Neural correlates of impaired self-awareness of apathy, disinhibition and dysexecutive deficits in cocaine-dependent individuals. Addict. Biol 22(5), 1438–1448. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbane M, 2012. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci. Biobehav. Rev 36(3), 1043–1059. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ, 2016. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry 73(11), 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED, 2011. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch. Gen. Psychiatry 68(3), 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT, 2014. Brain activation to cocaine cues and motivation/treatment status. Addict Biol 19(2), 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC, 1992. In search of how people change. Applications to addictive behaviors. Am. Psychol 47(9), 1102–1114. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Warneke C, de Moor C, Emmons KM, Mullin Jones M, Rosenblum C, Hudmon KS, Gritz ER, 2003. Self-reported health status, health vulnerability, and smoking behavior in college students: implications for intervention. Nicotine Tob. Res 5(4), 545–552. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G, 2011. How is our self related to midline regions and the default-mode network? Neuroimage 57(3), 1221–1233. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC, 2007. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci. Biobehav. Rev 31(4), 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA, 2007. Neural mechanisms mediating optimism bias. Nature 450(7166), 102–105. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE, 1993. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 34(1), 19–28. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS, 2010. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev 34(6), 935–946. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M, 2008. Substance abusers’ self-awareness of the neurobehavioral consequences of addiction. Psychiatry Res. 158(2), 172–180. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF, 2012. The Representation of Self and Person Knowledge in the Medial Prefrontal Cortex. Wiley Interdiscip. Rev. Cogn. Sci 3(4), 451–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ, 2018. Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron 98(5), 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


