Abstract
Peptide-based cancer vaccines have failed to provide sufficient clinical benefits in order to be approved in clinical trials since the 1990s. To understand the mechanisms underlying this failure, the present study investigated biomarkers associated with the lower overall survival (OS) among 2,588 patients receiving personalized peptide vaccination (PPV). Survival data were obtained from a database of 2,588 cancer patients including 399 patients with lung, 354 with prostate and 344 with colon cancer. They entered into phase II clinical trials of PPV in which 2 to 4 of 31 warehouse peptides were selected for vaccination on an individual patient basis based on human leukocyte antigen (HLA) class IA-types and pre-existing peptide-specific IgG levels. Higher pre-vaccination neutrophil, monocyte and platelet counts, and lower pre-vaccination lymphocyte and red blood cell counts were inversely associated with OS, with higher sensitivities in the proportions of neutrophils and lymphocytes, respectively. The most potent unfavorable and favorable factors for OS were the median percentage of neutrophils (>64.8%) or percentage of lymphocytes (>25.1%) with correlation coefficients (R2) of 0.98 and 0.92, respectively. Higher pre-vaccination levels of c-reactive protein and other inflammatory soluble factors were inversely associated with OS. Pre-vaccination peptide-specific immunity levels had no effect on OS, although lower immune boosting levels were inversely associated with OS. None of the 31 peptides was inversely associated with OS, although a few peptides were positively associated with it. On the whole, the findings of the present study suggested that pre-vaccination inflammatory signatures, but not those of post-vaccination immune induction, were associated with lower clinical benefits of PPV.
Keywords: biomarker, peptide vaccine, inflammatory responses, neutrophil proportion, lymphocyte proportion
Introduction
Cancer immunotherapy with anti-PD-1 or PDL-1 antibody has resulted in remarkable progress being made in the treatment of a number of different types of advanced cancers (1,2). By contrast, active specific immunotherapy using either tumor-associated antigens or their peptides capable of inducing cytotoxic T lymphocytes (CTLs) against tumor cells has been failing to provide sufficient clinical benefits in order to be approved for use, despite the large numbers of clinical trials beginning in the 1990s, and the mechanisms underlying this failure have not yet been clarified (3-5).
The authors have developed a novel approach to immunotherapy termed personalized peptide vaccination (PPV), in which peptides are selected for individual patients based on their secondary immune responses (6-8). Randomized phase II PPV trials achieved a longer overall survival (OS) for patients with certain types of cancer (9,10). However, no significant difference in OS was found between patients receiving PPV and those receiving the placebo in either of two randomized, double-blind, placebo-controlled phase III trials (11,12). A biomarker study revealed that both higher neutrophil and lower lymphocyte proportions prior to entry were unfavorable predictive factors for OS of only patients administered PPV with advanced prostate cancer (11). In another trial enrolling patients with recurrent glioblastoma, either very low or very high MCP-1 levels prior to study entry were identified as another biomarker discriminating between patients administered PPV exhibiting a significantly shorter OS and those exhibiting a significantly longer OS (12).
In the present study, these biomarker analyses of the 2,588 patients enrolled in the phase II PPV studies for the treatment of various types of cancer were extended, in order to elucidate the mechanisms involved in the inhibition of clinical benefits.
Patients and methods
Patients
From November, 2008 to March, 2017, phase II clinical trials of PPV were conducted at the Kurume University Cancer Vaccine Center, Kurume University Hospital, Sendai Kosei Hospital or Naito Hospital in Japan. Patients receiving no vaccination were excluded from the analysis, and thus a total of 2,588 patients were enrolled. Some of the results for certain cancer types have been previously reported (9,10,13-16). Eligibility criteria were the pathologically confirmed diagnosis of cancer, positive pre-vaccination plasma IgG responses for at least 2 of the 31 warehouse peptides (Table SI), positive status for HLA-A2, -A24, -A3supertypes (HLA-A3, -A11, -A31, or -A33: HLA-A3 sup), or -A26, age >20 years, Eastern Cooperative Oncology Group performance status (PS) of 0-2 and neurological 3 for only brain tumor patients, a life expectancy of at least 12 weeks, and adequate bone marrow function, hepatic function and renal function. Exclusion criteria were acute infection, a history of severe allergic reactions, or other systemic diseases. All patients provided written informed consent for the study participation and data collection. All patient sample collections, patient consent and recruitment followed protocols approved by the institutional review board of Kurume University. The study was conducted according to the principles of the Declaration of Helsinki.
Peptides and clinical analysis
Patients were vaccinated with 2 to 4 peptides based on their human leukocyte antigen (HLA) type and pre-existing immunity by measuring peptide-specific IgG levels. Each of the selected peptides was mixed with incomplete Freund's adjuvant (Montanide ISA-51VG; Product Code 36508H; Seppic) and injected subcutaneously into the inguinal, abdominal, or other sites. There were 3 different protocols with regards to the vaccination intervals. The details are presented as supplementary material (Data S1). The cancer patients who became resistant to the standard systemic therapies received 6 injections of PPV at 1-week intervals (the first cycle) followed by 6 injections at 2-week intervals (the second cycle); this protocol was termed PRT1 (for further details please see Data S1). The cancer patients at any stages, including those at the early stages of the disease, received 4 injections of PPV at 1-week intervals and then 4 injections at 2-week intervals (the first cycle), followed by 4 injections at 2-week intervals and then 4 injections at 4-week intervals (the second cycle) (PRT2: Data S1). Alternatively, the patients received 4 injections at 4-week intervals (the first cycle) followed by the same schedule for the second cycle (PRT3: Data S1). Detailed protocols are presented online only (https://upload.umin.ac.jp/cgi-open-bin/ctr/index.cgi). The detailed information of the protocols is presented in Data S1. All the trials were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. This is a retrospective study, i.e., all the patient data was already available and ethical approval provided when the original studies/trials were performed.
It was decided that only 2-4 peptides would be injected among the 31 candidates, mainly due to the following 3 reasons: Each peptide was independently injected to avoid a possible bias for CTL induction by different avidities of peptides when they are mixed together, and a maximum of 4 peptides were then independently injected every 7-14 days, which in turn was associated with a substantial burden for the patients. Therefore, >5 peptides did seem tolerable for certain patients. Secondly, the OS of the patients who received only one peptide, as only 1 of the 31 peptides was suitable for PPV due to lower baseline IgG levels, was shorter than that of patients who received at least 2 peptides (17). Thirdly, these antigens coding the 31 peptides were highly expressed on the majority of the histologically different cancer cells (6-17), a maximum of 4 peptides based on pre-existing immunity were expected to be sufficient to induce potent anti-tumor immunity.
Immune responses and biomarker analyses
T cell responses or IgG titers specific to the antigen peptides in peripheral blood mononuclear cells (PBMCs) or plasma were evaluated by interferon (IFN)y ELISPOT (Immunocyte IFNy ELISPOT kit; code no. 8223; Medical & Biological Laboratories Co., Ltd.) or bead-based multiplexed Luminex assay (Luminex; REF. LHC6003M; Invitrogen; Thermo Fisher Scientific) as previously described (8,9). For the measurement of the 35 different cytokines and proteins, bead-based multiplex assays or an enzyme-linked-immunosorbent assay (ELISA) were used as described previously (12). The vaccinated peptides, immunological responses, OS during PPV treatment and follow-up study were extracted from the database of the clinical trials for 2,588 patients. For the biomarker studies, the factors listed in the baseline characteristics and laboratory data at the screening time (14 days prior to the first vaccination) was provided.
Estimation of clinical efficacy of individual peptides and statistical analysis
The Kaplan-Meier method, log-rank test, univariate and multivariate proportional hazard regression models, Spearman's rank correlation coefficient test, Student's t-test, Chi-square test and Fisher's exact test were used for the statistical analyses. OS was calculated as the time in months from the date of study enrollment to death or to the date of last contact. The clinical efficacy of individual peptides for prolonging OS was evaluated by univariate and multivariate analyses with the Cox proportional hazards regression model, and the HR and 95% CI values were calculated. All reported P-values were two-sided, and P-values <0.05 were considered to indicate statistically significant differences. JMP version 12 or SAS version 9.4 software (SAS Institute Inc.) was used to perform all analyses.
Results
Baseline characteristics
The baseline characteristics of the 2,588 cancer patients and their median OS are presented in Table I. There were 399 patients with lung, 354 with prostate, 344 with colon, 290 with pancreatic, 200 with gastric and 183 with breast cancers. The remaining 818 cancer patients consisted of 139 patients with urothelial, 126 with biliary tract cancer, 118 with ovarian cancer, 79 with uterine cancer, 83 with hepatocellular carcinoma, 50 with head and neck cancer, 49 with sarcoma, 39 with esophageal cancer, 35 with brain cancer, 30 with renal and 70 with miscellaneous cancers (data not shown). There were 1,520 male and 1,068 female subjects with a median age of 63 years (Table I). The median OS among male and female patients was 10.5 and 13.7 months, respectively, and the OS of the female patients with lung or colon cancers was longer than that of male patients. Patients with a better PS or with early stages exhibited a trend for a longer OS in all types of cancer tested, respectively. No significant differences in the median OS were found with regard to different HLA-class I types. Table I also presents the number of patients receiving systemic therapies prior to the PPV vaccination and the number of patients receiving systemic therapies combined with the PPV vaccination. The median vaccination time was 11 months, ranging from 1 to 76 months, while the median study period was 5.4 months in all 2,588 cases. The median follow-up time was 11.3 months.
Table I.
Patient characteristics.
| All patients (mOS) | Lung cancer (mOS) | Prostate cancer (mOS) | Colon cancer (mOS) | Pancreatic cancer (mOS) | Gastric cancer (mOS) | Breast cancer (mOS) | Other types of cancer (mOS) | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 2588 | 399 | 354 | 344 | 290 | 200 | 183 | 818 |
| Age (years) | 63 | 64 | 69 | 61 | 64 | 64 | 56 | 62 |
| Sex | ||||||||
| Male | 1,520 (10.5) | 252 (9.5) | 354 (18.7) | 188 (11.0) | 159 (5.6) | 121 (9.7) | 0 | 446 (9.8) |
| Female | 1,068 (13.7) | 147 (18.0)a | 0 | 156 (16.2)a | 131 (6.2) | 79 (8.6) | 183 (21.2) | 372 (14.8) |
| Performance status | ||||||||
| 0 | 2,072 (13.7) | 279 (17.7) | 291 (22.3) | 308 (14.8) | 230 (6.4) | 157 (10.3) | 150 (26.5) | 657 (13.1) |
| 1 | 491 (5.7) | 117 (5.9) | 61 (9.8) | 34 (5.2) | 58 (3.7) | 42 (4.1) | 33 (10.9) | 146 (5.2) |
| 2 | 22 (3.3) | 3 (2.4) | 2 (3.8) | 2 (2.0) | 2 (1.7) | 1 (3.3) | 0 | 12 (4.6) |
| 3 | 3 (8.6) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (8.6) |
| Numbers of vaccinations Stage | 11 (1-76) | 10 (1-56) | 17 (1-76) | 11 (1-45) | 9 (1-60) | 9 (1-66) | 14 (1-49) | 11 (1-57) |
| I | 88 (58.9) | 30 (38.1) | 3 (41.7) | 3 (11.5) | 3 (-) | 6 (24.7) | 12 (-) | 31 (58.9) |
| II | 104 (29.5) | 19 (18.5) | 15 (71.3) | 6 (16.6) | 1 (-) | 8 (6.8) | 23 (32.3) | 32 (32) |
| III | 311 (15.4) | 95 (13.5) | 21 (26.1) | 53 (16.8) | 4 (53.5) | 41 (10.5) | 13 (21.9) | 84 (15.3) |
| IV | 1,753 (9.9) | 246 (10.0) | 315 (17.6) | 259 (12.3) | 246 (5.6) | 132 (7.7) | 78 (24.3) | 477 (8.3) |
| Recurrence | 332 (13.3) | 9 (22.9) | 0 | 23 (14.3) | 36 (6.1) | 13 (20.0) | 57 (12.2) | 194 (15.9) |
| Type of cancer | ||||||||
| Lung cancer | - | 399 (11.9) | - | - | - | - | - | - |
| Prostate cancer | - | - | 354 (18.7) | - | - | - | - | - |
| Colon cancer | - | - | 344 (13.1) | - | - | - | - | |
| Pancreatic cancer | - | - | - | - | 290 (5.7) | - | - | - |
| Gastric cancer | - | - | - | - | - | 200 (9.1) | - | - |
| Breast cancer | - | - | - | - | - | - | 183 (21.2) | - |
| Other | - | - | - | - | - | - | - | 818 (11.3) |
| HLA status | ||||||||
| A24 | 1,550 (12.0) | 233 (12.1) | 225 (20.2) | 209 (13.6) | 167 (5.7) | 119 (8.6) | 119 (23.5) | 478 (11.3) |
| A2 | 1,050 (11.5) | 164 (11.7) | 142 (18.3) | 133 (14.4) | 123 (5.6) | 89 (8.9) | 61 (18.8) | 338 (11.7) |
| A3 | 1,214 (11.1) | 164 (12.1) | 165 (18.2) | 168 (11.5) | 153 (5.8) | 90 (9.1) | 94 (26.5) | 380 (10.8) |
| A26 | 552 (12.4) | 107 (12.6) | 72 (22.4) | 71 (13.8) | 56 (4.7) | 36 (7.2) | 32 (23.7) | 178 (12.3) |
| Prior systemic therapy | ||||||||
| Operation | 1,574 | 155 | 86 | 317 | 126 | 127 | 160 | 603 |
| Chemotherapy | 2,185 | 362 | 303 | 308 | 241 | 167 | 180 | 624 |
| Radiation | 771 | 185 | 119 | 45 | 49 | 9 | 102 | 262 |
| Combination therapy | ||||||||
| Operation | 74 | 3 | 3 | 17 | 11 | 1 | 12 | 27 |
| Chemotherapy | 1,787 | 256 | 321 | 228 | 249 | 149 | 164 | 420 |
| Radiation | 272 | 76 | 29 | 25 | 20 | 4 | 33 | 85 |
| Local therapy | 99 | 1 | 5 | 8 | 4 | 2 | 7 | 72 |
| Study periods | 5.4 | 5.8 | 8.9 | 5.7 | 3.8 | 4.8 | 8.3 | 4.9 |
| mOS (95% CI) | 11.6 (11.0-12.3) | 11.9 (10.0-13.5) | 18.7 (16.1-22.3) | 13.1 (11.3-15.5) | 5.7 (5.1-6.7) | 9.1 (7.6-10.4) | 21.2 (15.6-28.4) | 11.3 (9.9-12.5) |
mOS, median overall survival (months).
P<0.05.
Circulating blood cells and OS
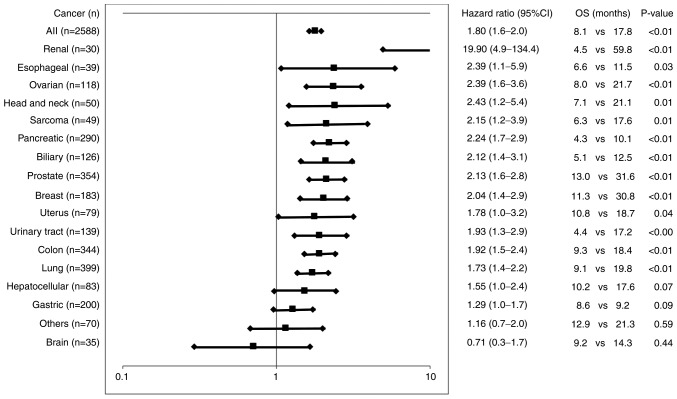
The association between the number or proportion of pre-vaccination circulating blood cells and OS was examined (Table II). The median OS of the patients administered PPV with greater than the median numbers of neutrophils, monocytes, or platelets was significantly shorter than that of the patients administered PPV with lower numbers, whereas the opposite was true for lymphocytes or red blood cells. Similar trends were obtained in the cellular proportions with more sensitive HRs as compared to those of the cellular numbers. The most potent unfavorable and favorable factors for OS were the median percentage of neutrophils >64.8% (HR, 1.70, Table II) or percentage lymphocytes >25.1% (HR, 0.53, Table II) with correlation coefficients (R2) of 0.98 and 0.92 (Fig. S1A and B), respectively. The median OS for 1,522 of the 2,588 cancer patients who met either or both the cut-offs of neutrophils >64.8% and lymphocytes <25.1% was significantly shorter than that of the remaining 1,066 patients who exhibited both <64.8% neutrophils and >25.1% lymphocytes together (8.1 months; vs. 17.8 months; HR, 1.8, 95% CI, 1.6-2.0; P<0.01) (Fig. 1). Significantly shorter OS times were also obtained in patients with all different cancer types tested, apart from gastric cancer, brain cancer, or other miscellaneous cancer types (Fig. 1). The median OS of the patients administered PPV with a greater than or equal to median neutrophil-lymphocyte-ratio (NLR) was also significantly shorter than that of the patients administered PPV with a less than median NLR (7.6 vs. 17.2 months; HR, 1.85; P<0.01) (Table II) with an (R2) value of 0.57 (Fig. S1C).
Table II.
Circulating blood cells and immune responses.
| Factors (no. of patients) | Median value | OS: Median < vs. median > value | HR (95% CI) | P-value |
|---|---|---|---|---|
| Pre-vaccination cell counts | ||||
| White blood cells (2,588) | 5,600 | 15.3/8.9 | 1.47 (1.35-1.60) | <0.01 |
| Red blood cells (2,588) | 384 | 8.7/16.4 | 0.61 (0.56-0.66) | <0.01 |
| Platelets (2,588) | 21.4 | 13.7/10.1 | 1.29 (1.19-1.40) | <0.01 |
| Neutrophils (2,587) | 3,519 | 16.1/8.3 | 1.62 (1.49-1.76) | <0.01 |
| % Neutrophils (2,588) | 64.8 | 16.5/8.2 | 1.70 (1.56-1.85) | <0.01 |
| Lymphocytes (2,588) | 1,346 | 10.2/13.6 | 0.78 (0.72-0.85) | <0.01 |
| % Lymphocyte (2,588) | 25.1 | 7.6/17.5 | 0.53 (0.48-0.57) | <0.01 |
| Basophiles (2,555) | 20.6 | 11.8/11.7 | 0.97 (0.89-1.05) | 0.43 |
| % Basophil (2,555) | 0.4 | 10.3/12.8 | 0.82 (0.75-0.89) | <0.01 |
| Eosinophils (2,562) | 101 | 11.6/11.7 | 0.98 (0.90-1.07) | 0.71 |
| % Eosinophils (2,562) | 1.9 | 10.8/12.4 | 0.90 (0.83-0.98) | 0.01 |
| Monocytes (2,575) | 352 | 16.5/8.5 | 1.67 (1.54-1.82) | <0.01 |
| % Monocyte (2,575) | 6.2 | 13.7/10.1 | 1.28 (1.18-1.40) | <0.01 |
| % Neutrophil-lymphocyte ratio (2,588) | 2.6 | 17.2/7.6 | 1.85 (1.70-2.02) | <0.01 |
| Pre-vaccination IgG (FIU) | ||||
| To 31 peptides (2,588) | 2,251 | 12.0/11.1 | 1.07 (0.98-1.16) | 0.12 |
| To vaccinated peptides (2,588) | 561 | 12.1/11.1 | 1.09 (1.01-1.19) | 0.04 |
| Post-vaccination IgG (FIU) | ||||
| To 31 peptides (2,116) | 27,266 | 9.9/22.3 | 0.48 (0.44-0.53) | <0.01 |
| To vaccinated peptides (2,116) | 22,716 | 9.8/22.3 | 0.48 (0.43-0.52) | <0.01 |
| Increased IgG levels (FIU) | ||||
| To 31 peptides (2,116) | 20,949 | 9.6/22.5 | 0.46 (0.42-0.51) | <0.01 |
| To vaccinated peptides (2,116) | 19,309 | 9.5/22.6 | 0.45 (0.41-0.50) | <0.01 |
| Pre-vaccination CTL to vaccinated peptides (IFNγ spots) (525) | 22 | 16.0/16.8 | 0.98 (0.82-1.19) | 0.86 |
| Post-vaccination CTL to vaccinated peptides (IFNγ spots) (525) | 68 | 11.4/19.5 | 0.68 (0.56-0.82) | <0.01 |
| Increased CTL levels to vaccinated peptides (IFNγ spots) (525) | 33 | 11.3/19.5 | 0.72 (0.60-0.87) | <0.01 |
FIU, fluorescence intensity unit; OS, overall survival; HR, hazard ratio; CI, confidence interval; CTL, cytotoxic T lymphocyte; IFNγ, interferon γ.
Figure 1.
Circulating blood cells and OS. The median OS for 1,522 of the 2,588 cancer patients (and those of each cancer type) who met either or both the cut-offs of neutrophils >64.8% and lymphocytes <25.1% was compared to that of the remaining 1,066 patients (and those of each cancer type) who exhibited both <64.8% neutrophils and >25.1% lymphocytes together. The hazard ratio, 95% confidence index, and P-value were also given. OS, overall survival (months).
Soluble factors and OS
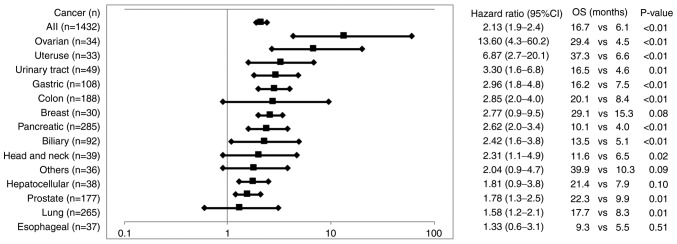
A total of 35 cytokine or protein levels were provided for the analyses of the association between the pre-vaccination levels and the OS of patients administered PPV from whom samples were available for the biomarker analysis. The total numbers tested were 1,432 for C-reactive protein (CRP) and 250 to 504 for the others. A cancer type having <20 tested samples was excluded from the analysis to avoid any biases. Higher levels of CRP (n=1,432), a typical protein involved in inflammation (18), were inversely associated with the OS of patients for all the cancer types tested (Fig. 2 and Table SII). Similar results were obtained for interleukin (IL)-6, B-cell activating factor (BAFF), haptoglobin (Hp), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), IL2-R and MIG levels, soluble factors involved in inflammatory responses (19), although the levels did not differ significantly in the majority of cancer types. Higher levels of IL-8, macrophage inflammatory protein (MIP)-1ß, interferon-inducible protein 10 (IP-10), or granulocyte-colony stimulating factor (G-CSF) were inversely associated with the OS of patients with only urinary tract, lung, or breast cancers, respectively (Table SII).
Figure 2.
Association of pre-vaccination CRP levels to OS. The HR (and 95% CI) of OS between the patients with pre-vaccination CRP levels of < median value and those with > median value in all cancer patients tested (and in each of the cancers tested) are shown in the figure. The median OS (months, M) of the patients with < median CRP value vs. that with > median value along with the P-value are shown. CRP, C-reactive protein; OS, overall survival; PPV, personalized peptide vaccination.
Discrepant results were obtained for several cytokines. Higher levels of IL-2, a cytokine for T cell activation (18), were positively associated with the OS of patients with certain types of cancer (urinary tract, breast and colon) (indicated by HR values <0.63), but were inversely associated with the OS of patients with prostate cancer (indicated by HR value of 2.15) (Table SII). Similar results were obtained for IL-1β or GM-CSF (cytokines involved in immune activation) or IL-10 (an anti-inflammatory cytokine) (18) (indicated by HR values of <0.63 for a positive association or HR values of >2.25 for an inverse association). Namely higher levels of IL-1β were positively associated with the OS of patients with urinary tract and breast cancers, but were inversely associated with the OS of patients with prostate cancer. Similarly, higher levels of GM-CSF or IL-10 were positively associated with the OS of patients with urinary tract or gastric cancer respectively, but were inversely associated with the OS of patients with lung or biliary cancer respectively. Higher levels of IFNa IFNy, IL-17a, or eotaxin were positively associated with the OS of patients with only certain types of cancer (indicated by HR values of <0.60). Pre-vaccination levels of the remaining 15 soluble factors tested [transforming growth factor (TGF), IL-21, fibroblast growth factor (FGF)-basic, IL-13, IL-12, IL-1RA, tumor necrosis factor (TNF)a, IL-7, IL-4, RANTES, MIP-1a, MCP-1, IL-15, epidermal growth factor (EGF), IL-5] had no significant effect on the OS of patients (Table SII).
Immune responses and OS
The median peptide-specific IgG levels in pre-vaccination plasma for all 31 of the peptides or for only the peptides selected for vaccination were 2,251 or 561 fluorescence intensity units (FIU) with no significant association between IgG levels to all the peptides and OS (Table II). The median IgG levels in post-vaccination plasma for all 31 of the peptides and for only the peptides selected for vaccination were 27,266 and 22,716 FIU, and lower IgG levels were inversely associated with OS in both the cases. Similar results were obtained with regard to the increase in IgG levels following vaccination. The median peptide-specific CTL activity in pre-vaccination PBMCs in response to the vaccinated peptides was 22 IFNy-spots with no association between higher levels and OS (Table II). The median CTL activity in post-vaccination PBMCs in response to the vaccinated peptides was 68 IFNy-spots with a positive association between the higher levels and OS. Similar results were obtained with regard to the increase in CTL levels following vaccination.
Immune responses to the 31 peptides
The pre-vaccination positive rates of the patients exhibiting >10 FIU (n=2,588) were largely different among the 31 peptides (12 to 87%) (Table III). The magnitude of IgG titers of the 31 peptides among the patients exhibiting detectable levels also differed between the peptides. Post-vaccination lgG titers were measured at the end of both the first cycle and second cycle, and the numbers of patients exhibiting a >2-fold increase in the IgG titer against a peptide at either the end of the first or second cycle as compared to the pre-vaccination IgG titer are listed in Table III as the number of positive patients. A portion of the patients (472 of 2,588 patients, 18.3%) were dropped from the clinical trials prior to the end of the first cycle due to a rapid disease progressive. The rates of patients exhibiting positive IgG responses among the 2,116 patients who completed at least the first cycle were largely different among the 31 peptides (Table III). The magnitudes of IgG titers of the 31 peptides among the patients exhibiting positive responses were also largely different from each other. Despite these large diversities, it was commonly observed that the patients exhibiting immune boosting to each of the vaccinated peptides had a longer survival than those without such immune boosting.
Table III.
Immune responses and association between 31 warehouse peptides and the OS of 2,588 patients.
| Peptides | Pre vaccination IgG
|
Post vaccination IgG
|
Association between immune responses and OS
|
Association between vaccination and OS
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive/negative | Median of positive patients (FIU) | Number of vaccinated patients (No. of positive patients) | Median (FIU) | ||||||||
| Positive (mOS)/Negative (mOS) | P-value | Vaccinate cases (mOS) | Non vaccinate cases (mOS) | HR | HR (95% CI) | HR (P-value) | |||||
| SART2-93 | 2,248/340 | 66 | 1,137 (362) | 3,435 | 362 (21.7)/568 (12.3) | <0.01 | 1,137 (12.3) | 413 (11.5) | 0.9 | 0.8 1.1 | 0.31 |
| Lck-486 | 2,184/404 | 42 | 879 (573) | 15,147 | 573 (19.4)/186 (7.2) | <0.01 | 879 (12.3) | 671 (11.3) | 0.9 | 0.8 1.0 | 0.01 |
| Lck-488 | 2,147/441 | 60 | 1,103 (635) | 11,594 | 635 (20.6)/277 (7.8) | <0.01 | 1,103 (12.0) | 447 (12.0) | 0.9 | 0.8 1.0 | 0.17 |
| Lck-90 | 2,087/501 | 44 | 502 (187) | 3,350 | 187 (20.9)/222 (10.3) | <0.01 | 502 (11.3) | 712 (11.0) | 1.0 | 0.9 1.1 | 0.92 |
| SART3-734 | 2,041/547 | 111 | 625 (225) | 4,816 | 225 (22.3)/280 (11.0) | <0.01 | 625 (10.9) | 589 (11.3) | 1.0 | 0.9 1.2 | 0.52 |
| PSA-248 | 2,035/553 | 48 | 283 (200) | 13,165 | 200 (20.2)/40 (7.0) | <0.01 | 283 (13.8) | 1,267 (11.6) | 0.9 | 0.8 1.1 | 0.24 |
| SART3-511 | 1,967/621 | 41 | 439 (145) | 921 | 145 (22.6)/213 (10.8) | <0.01 | 439 (11.0) | 775 (11.2) | 1.0 | 0.9 1.1 | 0.93 |
| SART3-309 | 1,921/667 | 35 | 328 (158) | 3,070 | 158 (21.4)/124 (10.5) | <0.01 | 328 (12.1) | 722 (11.1) | 0.9 | 0.8 1.1 | 0.25 |
| WHSC2-141 | 1,908/680 | 39 | 325 (173) | 13,078 | 173 (19.0)/94 (7.7) | <0.01 | 325 (12.1) | 725 (11.1) | 1.0 | 0.9 1.2 | 0.85 |
| CypB-129 | 1,888/700 | 31 | 483 (222) | 2,483 | 222 (23.8)/192 (9.0) | <0.01 | 483 (12.3) | 1,458 (11.2) | 0.9 | 0.8 1.1 | 0.24 |
| Lck-246 | 1,815/773 | 49 | 366 (169) | 10,806 | 169 (19.3)/128 (8.3) | <0.01 | 366 (11.4) | 684 (11.6) | 1.0 | 0.9 1.2 | 0.68 |
| WHSC2-103 | 1,673/915 | 43 | 729 (222) | 827 | 222 (19.6)/374 (12.1) | <0.01 | 729 (11.5) | 1,465 (11.6) | 1.0 | 0.9 1.1 | 0.52 |
| SART3-302 | 1,640/948 | 84 | 408 (271) | 14,958 | 271 (18.2)/78 (9.1) | <0.01 | 408 (12.7) | 642 (10.5) | 0.9 | 0.8 1.0 | 0.10 |
| MRP3-1293 | 1,558/1,030 | 27 | 324 (157) | 6,003 | 157 (20.6)/113 (8.6) | <0.01 | 324 (11.9) | 1,226 (12.1) | 1.1 | 0.9 1.2 | 0.31 |
| EGF-R-800 | 1,542/1,046 | 30 | 390 (156) | 715 | 156 (24.3)/188 (12.4) | <0.01 | 390 (14.3) | 1,160 (11.1) | 0.8 | 0.7 1.0 | 0.01 |
| PAP-213 | 1,422/1,166 | 33 | 279 (196) | 14,912 | 196 (21.7)/52 (7.0) | <0.01 | 279 (16.6) | 1,271 (11.4) | 0.9 | 0.8 1.0 | 0.06 |
| Lck-449 | 1,406/1,182 | 23 | 127 (67) | 18,919 | 67 (19.5)/44 (6.2) | <0.01 | 127 (13.7) | 1,087 (10.8) | 0.9 | 0.8 1.1 | 0.55 |
| ppMAPkkk-432 | 1,308/1,280 | 51 | 375 (97) | 757 | 97 (23.8)/203 (12.0) | <0.01 | 375 (11.1) | 1,087 (11.9) | 1.0 | 0.9 1.2 | 0.51 |
| HNRPL-140 | 1,295/1,293 | 39 | 200 (111) | 8,576 | 111 (23.9)/62 (9.8) | <0.01 | 200 (12.9) | 850 (11.1) | 0.8 | 0.7 0.9 | 0.01 |
| PAP-248 | 1,286/1,302 | 37 | 147 (62) | 2,136 | 62 (21.1)/63 (9.9) | 0.01 | 147 (11.4) | 1,067 (11.1) | 1.0 | 0.8 1.2 | 0.76 |
| UBE2V-43 | 1,238/1,350 | 34 | 230 (159) | 25,121 | 159 (18.5)/40 (7.0) | <0.01 | 230 (11.7) | 820 (11.5) | 0.9 | 0.8 1.0 | 0.15 |
| SART3-109 | 1,206/1,382 | 31 | 474 (223) | 7,875 | 223 (19.0)/191 (10.3) | <0.01 | 474 (12.3) | 1,948 (11.5) | 1.0 | 0.9 1.1 | 0.88 |
| HNRPL-501 | 1,189/1,399 | 45 | 355 (223) | 10,405 | 223 (19.3)/74 (6.6) | <0.01 | 355 (12.1) | 1,107 (11.6) | 1.0 | 0.9 1.1 | 0.75 |
| SART2-161 | 947/1,641 | 29 | 195 (61) | 312 | 61 (20.6)/109 (12.6) | 0.05 | 195 (12.3) | 13,552 (11.9) | 1.0 | 0.8 1.1 | 0.68 |
| PSMA-624 | 950/1,638 | 24 | 101 (50) | 7,772 | 50 (21.7)/37 (5.5) | <0.01 | 101 (11.7) | 1,449 (12.0) | 1.0 | 0.8 1.3 | 0.74 |
| PTHrP-102 | 886/1,702 | 27 | 204 (99) | 1,423 | 98 (20.0)/81 (11.4) | 0.01 | 204 (13.3) | 1,346 (11.9) | 0.9 | 0.8 1.1 | 0.22 |
| Lck-208 | 560/2,028 | 30 | 134 (64) | 4,597 | 64 (30.5)/62 (16.0) | 0.01 | 134 (19.7) | 1,416 (11.4) | 0.9 | 0.8 1.0 | 0.06 |
| EZH2-735 | 558/2,030 | 23 | 69 (51) | 17,691 | 51 (24.7)/11 (5.3) | <0.01 | 69 (13.3) | 1,481 (11.9) | 0.9 | 0.7 1.1 | 0.24 |
| MRP3-503 | 467/2,121 | 37 | 133 (85) | 6,401 | 85 (20.6)/36 (9.3) | 0.08 | 133 (14.1) | 1,417 (11.9) | 0.9 | 0.8 1.1 | 0.55 |
| UBE2V-85 | 362/2,226 | 28 | 51 (26) | 3,941 | 26 (16.8)/18 (8.4) | 0.09 | 51 (9.6) | 999 (11.6) | 1.2 | 0.9 1.5 | 0.32 |
| Lck-422 | 316/2,272 | 33 | 92 (20) | 436 | 20 (18.5)/60 (11.7) | 0.10 | 92 (12.0) | 1,849 (11.4) | 1.2 | 0.9 1.4 | 0.22 |
FIU, fluorescence intensity unit; mOS, median overall survival (months); HR, hazard ratio; CI, confidence interval. Values in bold font indicate statistically significant differences.
The association between each of the 31 peptides and OS was evaluated by the Cox proportional hazards regression model. This analysis revealed that the patients administered PPV that included each of the 3 peptides (Lch-486, EGFR-800 or HNRPL-140 peptide) exhibited a significantly longer survival than the patients whose PPV did not include each of these peptides with HR values of 0.9, 0.8 or 0.8, respectively (Table III). The HR values of the 25 peptides were between 0.8 to 1.0, and those of the remaining 3 peptides were 1.1 to 1.2; however, none of these were statistically significant.
Discussion
The results of the present study demonstrated that both higher neutrophil and lower lymphocyte proportions prior to entry were unfavorable biomarkers for the OS of patients administered PPV. When the most relevant cut-off levels in terms of inhibiting the clinical benefits of PPV were set as neutrophils >64% and lymphocytes <26%, the median OS of more than half of the cancer patients who met either or both the cut-offs of neutrophils >64% and lymphocytes <26% was significantly shorter than that of the remaining patients who exhibited both <64% neutrophils and >26% lymphocyte together. These results are consistent with those of the randomized, placebo-controlled, phase III trial (11). It has been previously reported that both circulating myeloid-derived suppressor cells (MDSCs) and CD4+CD45RA- activated T cells prior to study entry suppress or promote the OS of patients administered PPV in the other phase III study of PPV (12). The abnormal circulating granulocytes at the gene expression level, as well as the increase in granulocytic MDSCs following PPV treatment have been shown to negatively contribute to the OS of certain patients receiving PPV (20,21). These findings suggest that certain types of circulating neutrophils or lymphocytes suppress or promote the PPV-induced clinical benefits, respectively. However, the underlying immunological mechanisms are unclear. Cellular subset analyses of the PBMCs, regional lymph nodes and tumor sites of cancer patients are required to obtain a better understanding of the mechanisms.
The neutrophil-to-lymphocyte ratio (NLR) has been reported to be a risk factor for the OS of advanced cancers (22), which was consistent with the results of the present study. However, the co-efficiency (R2) between HR of the OS and NLR (0.57) was much lower than that between HR and the neutrophil (0.97) or lymphocyte proportion (0.92). NLR was less sensitive as compared to the proportion of neutrophils or lymphocytes as a biomarker to predict the efficacy of PPV when the OS was compared between the PPV and placebo patients in the randomized phase III trial (11). The results suggest that the pre-vaccination neutrophil or lymphocyte proportion is more suitable than pre-vaccination NLR as a biomarker for the clinical efficacy of PPV.
The present study indicated that higher pre-vaccination levels of CRP along with IL-6, BAFF, Hp, HGF, VEGF, IL2-R and MIG were inversely associated with the OS of cancer patients receiving PPV regardless of the cancer types tested. However, the higher levels of IL-2, IL-1β, GM-CSF and IL-10 were well associated with the OS of patients with certain cancer types, but were inversely associated with those with other cancer types. The mechanisms involved in this discrepancy are also presently unknown, and will need to be confirmed in a relatively large number of cancer patients in the future. Furthermore, all these results with the exception of CRP need to be confirmed with a larger number of samples.
Discrepant results with regard to the cytokine biomarkers are one of the unsolved hot points in cancer immunotherapy, and the more in depth underlying mechanisms reason cannot be explained at present. The hypothesis is that the discrepancy is due in part to the ambiguity of cytokines with regard to the promotion and inhibition of immune responses. For example, IL-2 and IFN-γ are key cytokines for both cytotoxic and helper T cell activation, which play a role in tumor elimination, but they also activate other types of immune cells (suppressor macrophages and T regulatory cells) that have roles in immune suppression in certain types of cancers that depend on the doses of IL-2 or IFN-γ. Similarly, IL-10 can suppress T regulatory cells, but can also inhibit the TNFa production that is needed for the infiltration of T cells into cancers IL-10 dose-dependently. This hypothesis is based in part on a recent randomized double-blind, phase III trial of personalized peptide vaccination for recurrent glioblastoma (12). It was found that the median OS of the patients with very low or high CCL2 levels (CCL2low/high) or IL-6 (IL-6low/high) was shorter than that of the patients with an intermediate level of CCL2 (CCL2im) or IL-6 (IL-6im). By contrast, the median level of CCL2 or IL-6 failed to discriminate one from the other. Instead, CCL4 was an indicator to discriminate one from the other, with higher levels favorable to longer OS. Further studies need to be conducted to clarify this issue.
Higher CCL2 levels were unfavorable for the OS of patients (n=338; HR, 1.5, 95% CI, 0.7-3.5; P=0.06), but not for the OS of each cancer type tested, including brain tumor (n=35) (data not shown). These results were also consistent with the results of the randomized PIII study of recurrent glioblastoma (12).
The results shown in Table I were also evaluated with the multivariate proportional hazard regression model. The 10 factors consisting of 5 higher HRs and 5 lower HRs in the univariate analysis (Table I) were provided for the multivariate analysis, and the results are presented in Table SIII. The HR values of red blood cell numbers (0.56; 95% CI, 0.46-0.68; P<0.01) or monocyte numbers (1.40; 95% CI, 1.13-1.74; P<0.01) were statistically significant, respectively (Table SIIIA). The red blood cell number was a prognostic risk factor for the OS of both the PPV and placebo groups in the double-blind placebo control phase III study for advanced prostate cancer, whereas the monocyte number was a risk factor for the OS of only the placebo group (11). By contrast, the lymphocyte ratio or neutrophil ratios were predictive a favorable or unfavorable factor for the OS of only the PPV group (11). This analysis also revealed that the lymphocyte (P=0.09) or neutrophil ratio (P=0.16) was a favorable or unfavorable factor for the OS of the PPV group, although not statistically significant. Both the post-vaccination increment of peptide-specific IgG levels (P<0.01) and CTL activities (P=0.04) were favorable factors for OS, as expected from previous studies (6,7). The results of the soluble factors shown in Table SII were then evaluated with the multivariate proportional hazard regression models. The 10 factors consisting of 5 higher HRs and 5 lower HRs in the univariate analysis using >300 test cases (Table SII) were provided for the multivariate analysis, and the results are presented in Table SIIIB of the revised manuscript. The levels of only CRP, and not those of the other cytokines (IL-6, VEGF, MIG, IL-2, IL1β, IP-10, GM-CSF, IFNa and IFNy) were significantly (P<0.05) associated with OS. It has previously been reported that CRP is an unfavorable factor for the OS of patients administered PPV (6,7). Collectively, the results with the multivariate analyses revealed that the well-known pre-vaccination unfavorable factors (red blood cell numbers, monocyte numbers and CRP levels) and well-known post-vaccination favorable markers as the statistically significant biomarkers for the OS of the patients administered PPV. Further studies are required to identify novel biomarkers influencing the OS of cancer patients administered PPV in order to better understand the mechanisms involved the long-lasting failure of the peptide-based cancer vaccine with regard to the clinical efficacy.
Inflammatory responses associated with tumors are considered to be one of the major events involved in cancer development (22-26), which in turn may be responsible for the hindering of the clinical benefits of PPV in patients when pre-vaccination levels of inflammatory soluble factors are higher than the median levels, as shown in the present study. However, the immunological mechanisms responsible for this soluble factor-mediated inhibition of clinical benefits are presently unknown. One might think that higher levels of inflammatory soluble factors cause the higher neutrophil or lymphocyte proportion, which in turn may be responsible for the inhibition of clinical efficacy. However, the results of the present study suggested that this was not the case, since the R2 values by Spearman's rank correlation coefficient test between the CRP level and the proportion of neutrophils and lymphocytes in the 1,432 patients tested were as low as 0.007 and 0.005, respectively (data not shown). Cellular subset analyses of the PBMCs, regional lymph nodes, and tumor sites of cancer patients and the correlation of each of these parameters and the soluble factor levels are required to better understand the immunological mechanisms.
Both the antibody positivity rate and the magnitude of IgG titers in pre-vaccination samples were largely different among the 31 peptides. These divergence among the peptides was also observed in the post-vaccination samples (the positive IgG responses and magnitude of IgG titers). However, it was commonly observed that the patients showing immune boosting to each of the vaccinated peptides had a longer survival than those without it, suggesting that all 31 of the peptides used in this study maintained their ability to prolong clinical benefits through immune boosting. Furthermore, the patients administered PPV that included certain peptides exhibited a significantly longer survival than the patients administered PPV without these peptides. Therefore, the divergence among the peptides used for PPV may not be a risk factor hampering the clinical benefits of PPV.
The results revealed that, among the vaccinated peptides, only three peptides (Lck-486, EGFR-800 and HNRPL-140) were significantly associated with OS. Although the underlying mechanisms remain unclear, it was reported that a monoclonal antibody reacting to the Lck-486 peptide exhibited an antitumor activity in a murine model with suppression of T regulatory cells at tumor sites (27). Lck (a member of the Src family of non-receptor protein tyrosine kinases expressed in both activated T lymphocytes and metastatic cancers cells with oncogenic properties in human cancers), is pivotal for T regulatory cells (Tregs) and program death-1-positive T-cell activities (26). The anti-Lck-486 antibody augmented by the vaccination may have promoted the antitumor activity in the present study on PPV. Monoclonal antibodies to the other peptides, including EGFR-800 and HNRPL-140 have not yet been created.
The results revealed that the baseline neutrophil and lymphocyte ratios affected the clinical benefits. Although the reasons why the baseline neutrophil and lymphocyte ratios most strongly affected the clinical benefits cannot be explained, the following hypothesis could be considered. A tumor mass causes inflammation to escape from immune attack (28) and promotes the production of inflammatory cytokines (IL-6, VEGF, CRP and haptoglobin, etc.), which in turn results in the circulation of a few lymphocytes along with many neutrophils. Subsequently, fewer peptide-specific T cells may enter the vaccinated lymph nodes for binding to HLA-peptide complex on dendritic cells, which in turn results in the induction of tolerogenic dendritic cells, suppressive macrophages and T regulatory cells (29). The normal range of the baseline lymphocyte ratio in cancer patients may thus be one of the keys for successful immune induction (28-30).
The present study demonstrated that pre-vaccination inflammatory signatures, but not those of post-vaccination immune induction, were associated with lower clinical benefits of PPV. The major limitation of the present study is the exploratory nature of the retrospective analyses on 2,588 patients under PPV treatment, which means that our results are not clinically applicable. Thus, the present results are purely informative, but may facilitate the better understanding of one of the mechanisms involved in the long-lasting failure of peptide-based cancer vaccines to provide clinical benefits enough to be approved in clinical trials since the 1990s.
Supplementary Data
Acknowledgements
Not applicable.
Abbreviations
- OS
overall survival
- PPV
personalized peptide vaccination
- HLA
human leukocyte antigen
- CTLs
cytotoxic T lymphocytes
- PS
performance status
- MHC
major histocompatibility complex
- FIU
fluorescence intensity units
- NLR
neutrophil to lymphocyte ratio
- MDSCs
myeloid derived suppressor cells
- (R2)
correlation coefficients
Funding
The present study did not receive specific funding, but was performed as part of the Kurume University, Kurume Fukuoka, Japan, and in part by Sendai Kousei Hospital, Sendai, Japan.
Availability of data and materials
The data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
SSuekane, SShichijo, SY, MNo and KI were involved in the conception and design of the study. SSuekane, SY, SShichijo, AY, SM, KI, NK and MNo were involved in the collection and assembly of the data. SSuekane, SShichijo, MNo and KI were involved in data analysis and interpretation, and were involved in the writing of the manuscript. SSuekane, SY, TS, ST, UT, KK, KY, SSakamoto, SSugawara, TY, MNa, MT, TM, KI and MNo were involved in the clinical studies by treating cancer patients under personalized peptide vaccination. All these 19 authors were involved in the provision of study materials or patients. All have read and approved the final manuscript.
Ethics approval and consent to participate
All patient sample collections, patient consent and recruitment followed protocols approved by the institutional review board of Kurume University. The study was conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent for the study participation and data collection.
Patient consent for publication
Not applicable.
Competing interests
MN has served as an advisory board consultant for BrightPath Biotherapeutics Co. Ltd. TS received a grant from BrightPath Biotherapeutics Co. AY is a part-time executive of BrightPath Biotherapeutics Co. KI received research funding from Taiho Pharmaceutical Company. KI and SShichijo gained income by selling stock of BrightPath Biotherapeutics Co., Ltd. The other authors have no competing interests to declare.
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Eng J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezu L, Kepp O, Cerrato G, Pol J, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Peptide-based vaccines in anticancer therapy. OncoImmunol. 2018;7:e1511506. doi: 10.1080/2162402X.2018.1511506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi M, Sasada T, Itoh K. Personalized peptide vaccination: A new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol Immunother. 2013;62:919–929. doi: 10.1007/s00262-012-1379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem. 2014;21:2332–2345. doi: 10.2174/0929867321666140205132936. [DOI] [PubMed] [Google Scholar]
- 8.Kibe S, Yutani S, Motoyama S, Nomura T, Tanaka N, Kawahara A, Yamaguchi T, Matsueda S, Komatsu N, Miura M, et al. Phase II study of personalized peptide vaccination for previously treated advanced colorectal cancer. Cancer Immunol Res. 2014;2:1154–1162. doi: 10.1158/2326-6066.CIR-14-0035. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M, Matsumoto K, Uemura H, Arai G, Eto M, Naito S, Ohyama C, Nasu Y, Tanaka M, Moriya F, et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res. 2016;22:54–60. doi: 10.1158/1078-0432.CCR-15-1265. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura K, Minami T, Nozawa M, Kimura T, Egawa S, Fujimoto H, Yamada A, Itoh K, Uemura H. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur Urol. 2016;70:35–41. doi: 10.1016/j.eururo.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Fujimoto K, Arai G, Uemura H, Hashine K, Matsumoto H, Fukasawa S, Nakatsu H, Takenaka A, Fujisawa M, et al. Personalized peptide vaccination for castration-resistant prostate cancer progressing after docetaxel: A randomized, double-blind, placebo-controlled, phase III trial. J Clin Oncol. 2019;37(Suppl 15):S5033. doi: 10.1200/JCO.2019.37.15_suppl.5033. [DOI] [Google Scholar]
- 12.Narita Y, Arakawa Y, Yamasaki F, Nishikawa R, Aoki T, Kanamori M, Nagane M, Kumabe T, Hirose Y, Ichikawa T, et al. A randomized, double-blind, phase III trial of personalized peptide vaccination for recurrent glioblastoma. Neuro Oncol. 2019;21:348–359. doi: 10.1093/neuonc/noy200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirahama T, Muroya D, Matsueda S, Yamada A, Shichijo S, Naito M, Yamashita T, Sakamoto S, Okuda K, Itoh K, et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer. Cancer Sci. 2017;108:838–845. doi: 10.1111/cas.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suekane S, Ueda K, Nishihara K, Sasada T, Yamashita T, Koga N, Yutani S, Shichijo S, Itoh K, Igawa T, Noguchi M. Personalized peptide vaccination as second-line treatment for metastatic upper tract urothelial carcinoma. Cancer Sci. 2017;108:2430–2437. doi: 10.1111/cas.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama K, Sugawara S, Saijo Y, Maemondo M, Sato A, Takamori S, Harada T, Sasada T, Kakuma T, Kishimoto J, et al. Randomized phase II study of docetaxel plus personalized peptide vaccination versus docetaxel plus placebo for patients with previously treated advanced wild type EGFR non-small-cell lung cancer. J Immunol Res. 2016;2016:1745108. doi: 10.1155/2016/1745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi M, Koga N, Moriya F, Suekane S, Yutani S, Yamada A, Shichijo S, Kakuma T, Itoh K. Survival analysis of multiple peptide vaccination for the selection of correlated peptides in urological cancers. Cancer Sci. 2018;109:2660–2669. doi: 10.1111/cas.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mine T, Sato Y, Noguchi M, Sasatomi T, Gohara R, Tsuda N, Tanaka S, Shomura H, Katagiri K, Rikimaru T, et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing, peptide-specific cellular responses. Clin Cancer Res. 2004;10:929–937. doi: 10.1158/1078-0432.CCR-1117-3. [DOI] [PubMed] [Google Scholar]
- 18.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy K, Weaver C. Immunobiology Zayets E (eds) Chapter 9, Garland New York and London. Science Taylor and Francis Group; 2017. pp. 345–398. [Google Scholar]
- 20.Komatsu N, Matsueda S, Tashiro K, Ioji T, Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al. Gene expression profiles in peripheral blood as a biomarker in cancer patients receiving peptide vaccination. Cancer. 2012;118:3208–3221. doi: 10.1002/cncr.26636. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi M, Moriya F, Koga N, Matsueda S, Sasada T, Yamada A, Kakuma T, Itoh K. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol Immunother. 2016;65:151–160. doi: 10.1007/s00262-015-1781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolan RD, Laird BJA, Horgan PG, McMillan DC. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: A systematic review. Crit Rev Oncol Hematol. 2018;132:130–137. doi: 10.1016/j.critrevonc.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: Neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 25.Shaul ME, Fridlender ZG. Cancer-related circulating and tumor-associated neutrophils-subtypes, sources and function. FEBS J. 2018;285:4316–4342. doi: 10.1111/febs.14524. [DOI] [PubMed] [Google Scholar]
- 26.Ritter B, Greten FR. Modulating inflammation for cancer therapy. J Exp Med. 2019;216:1234–1243. doi: 10.1084/jem.20181739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsueda S, Itoh K, Shichijo S. Antitumor activity of antibody against cytotoxic T lymphocyte epitope peptide of lymphocyte-specific protein tyrosine kinase. Cancer Sci. 2018;109:611–617. doi: 10.1111/cas.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: Wounds that do not heal-redux. Cancer Immunol Res. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinman RM, Howiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 30.Basile D, Garattini SK, Bonotto M, Ongaro E, Casagrande M, Cattaneo M, Fanotto V, De Carlo E, Loupakis F, Urbano F, et al. Immunotherapy for colorectal cancer: Where are we heading? Expert Opin Biol Ther. 2017;17:709–721. doi: 10.1080/14712598.2017.1315405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.