Abstract
Despite enormous scientific advancements in cancer treatment, there is a need for research to combat cancer, particularly bladder cancer. Drugs once proved to be effective in treating bladder cancer have shown reduced efficacy; hence, the cancer recurrence rate is increasing. To overcome this situation, several strategies have been considered, including the development of novel active drugs or modification of existing therapeutic regimens by combining two or more existing drugs. In recent years, atypical protein kinase Cs (PKCs), phospholipid-dependent serine/threonine kinases, have been considered as a central regulator of various cancer-associated signaling pathways, and they control cell cycle progression, tumorigenesis and metastasis. Additionally, the biologically crucial mTOR signaling pathway is altered in numerous types of cancer, including bladder cancer. Furthermore, despite independent activation, atypical PKC signaling can be triggered by mTOR. The present study examined whether the concurrent inhibition of atypical PKCs and mTOR using a combination of novel atypical PKC inhibitors (ICA-I, an inhibitor of PKC-ι; or ζ-Stat, an inhibitor of PKC-ζ) and rapamycin blocks bladder cancer progression. In the present study, healthy bladder MC-SV-HUCT2 and bladder cancer TCCSUP cells were tested and subjected to a WST1 assay, western blot analysis, immunoprecipitation, a scratch wound healing assay, flow cytometry and immunofluorescence analyses. The results revealed that the combination therapy induced a reduction in human bladder cancer cell viability compared with control and individual atypical PKC inhibitor and rapamycin treatment. Additionally, the concurrent inhibition of atypical PKCs and mTOR retards the migration of bladder cancer cells. These findings indicated that the administration of atypical PKC inhibitors together with rapamycin could be a useful therapeutic option in treating bladder cancer.
Keywords: bladder cancer, atypical protein kinase C, mTOR, rapamycin, ICA-I, ζ-Stat, combination treatment
Introduction
Bladder cancer is the fourth most common type of cancer, with ~25,000 estimated cases of mortality in the United States in 2019 (1). Carcinoma in the urinary bladder occurs in multiple forms, such as low-grade superficial or high-grade muscle-invasive tumor (2). Urothelial cell carcinoma is the most common form of bladder cancer that accounts >90% of all cases (2). The superficial tumor can be removed surgically even though the recurrence rate is higher, and the disease can progress to high-grade stage eventually. Metastatic muscle-invasive bladder cancer, on the other hand, is extreme and accounts for fatality in 50% of the patients (3). The most reliable prognostic evaluation for recurrence includes tumor stage and grade (4). Transurethral resection combined with immunotherapy for superficial tumor, and cystectomy and chemo/radiation therapy for invasive carcinoma are the most common treatment strategies (5,6). Nevertheless, identification of novel targets or molecules may provide insights into prognosis and therapeutic options for bladder cancer, since the available prognosis and treatment strategies fail to meet expectations.
Protein kinase C (PKC) is a family of serine/threonine enzymes consisting of three subclasses of enzymes, including conventional, novel and atypical. All PKCs function by phosphorylating and activating different downstream proteins and are involved in various transmembrane cross-talks and signal transduction pathways (7). Activation of conventional PKCs (α, β1, β2 and γ) depends on diacylglycerol and Ca2+, whereas novel PKCs (θ, δ, η and ε) are activated by diacylglycerol. However, atypical PKCs (ι and ζ) require neither diacylglycerol, nor calcium, instead they require protein-protein interaction (8). PKCs are involved in various cell signaling events that stimulate cell growth, proliferation, apoptosis, metastasis and regulation of gene expression (9).
mTOR is considered to be an essential downstream molecule of the PI3K/AKT1 signaling pathway, which triggers various signaling cascades that mediate cellular growth, survival, metastasis, metabolism and angiogenesis, and is often hyperactivated in different types of cancer (10-12).
mTOR is a serine/threonine kinase which can exist in two different complexes, namely mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (13). Raptor and Rictor are two critical components of mTORC1 and mTORC2, respectively. Furthermore, previous studies have demonstrated that mTORC2 phosphorylates turn motif (TF) and hydrophobic motif (HM) of conventional PKCs and AKT1, which ultimately leads to the maturation and stabilization of these kinases (14-16).
Protein kinases, specifically atypical PKCs, have been implicated in numerous types of cancer, such as breast cancer, colon cancer, melanoma, ovarian cancer and glioblastoma (8,9,17-20). Additionally, unlike other PKCs, atypical PKC has a glutamic acid at the HM region, which makes the TM of the atypical PKC sensitive to mTOR-mediated phosphorylation and stabilization of PKC-ι and PKC-ζ (14). Therefore, in the present study, bladder cancer TCCSUP cells were treated with a combination of atypical PKC inhibitors (either ICA-I or ζ-Stat) and rapamycin (mTOR inhibitor; Fig. 1) to examine the effect of mTOR and atypical PKC, as well as the mTOR complex, on atypical PKCs associated with bladder cancer cell survival. The healthy bladder epithelial MC-SV-HUCT2 cell line, was used in the present study as a healthy bladder cell line. The data revealed that the combination of atypical PKC inhibitor and rapamycin reduced metastatic bladder cancer cell progression by inducing cell cycle arrest and eventual senescence.
Figure 1.
Structures of inhibitors. (A) ICA-I (nucleoside analog, PKC-ι inhibitor), (B) ζ-Stat (PKC-ζ inhibitor), (C) Rapamycin (mTOR inhibitor). PKC, protein kinase C.
Materials and methods
Antibodies and reagents
ICA-I [5-amino-1-(2, 3-dihydroxy-4- hydroxymethyl) cyclopentyl)-1H-imidazole-4-carboxamide)] and ζ-Stat (8-hydroxynaphthalene-1, 3, 6-trisulfonic acid) were obtained from the National Cancer Institute, and rapamycin (cat. no. BP29631) was procured from Thermo Fisher Scientific, Inc. Anti-phospho PKC-ι (T555; cat. no. ab5813) and anti-phospho PKC-ζ (T560; cat. no. ab59412) were purchased from Abcam, Inc.. Anti-PKC-ι (cat. no. 610176) and anti-E-cadherin (cat. no. 610181) antibodies were purchased from BD Biosciences. The antibodies, anti-PKC-ζ (cat. no. 9372), anti-phospho Rictor (T1135; cat. no. 3806), anti-Rictor (cat. no. 9476), anti-β-galactosidase (cat. no. 2372), anti-β-catenin (cat. no. 25362), anti-P70S6K (cat. no. 2708), anti-phospho P70S6K (T421/S424; cat. no. 9204), anti-AKT1 (cat. no. 4691), anti-phospho AKT1 (S473; cat. no. 4058), anti-phospho AKT1 (T308; cat. no. 4056), anti-phospho Src homology 2 domain cotaining transforming protein (SHC) (S239/240) (cat. no. 2434) and anti-SHC (cat. no. 2432), and the Senescence β-Galactosidase Staining kit (cat. no. 9860) were procured from Cell Signaling Technology, Inc. The antibodies purchased from Santa Cruz Biotechnology, Inc. were: Anti-cyclin D1 (cat. no. sc70899), anti-CDK4 (cat. no. sc53636), anti-p27 (cat. no. sc527), anti-phospho p53 (S315; sc101763), anti-p53 (cat. no. sc126), anti-Lamin B1 (cat. no. sc374015), anti-p21 (cat. no. sc817), anti-phospho mouse double minute 2 homolog (MDM2; S166; cat. no. sc53368) and anti-MDM2 (cat. no. sc965). Anti-β-actin (cat. no. A3854) was obtained from Sigma-Aldrich; Merck KGaA. The Annexin-V APC apoptosis assay kit (cat. no. 601411) was procured from Cayman Chemical Company. Super Signal West Pico
Chemiluminescent substrate (cat. no. 34580) was purchased from Pierce; Thermo Fisher Scientific, Inc. Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (H+L) (cat. no. 1706516) and goat anti-rabbit IgG (H+L) (cat. no. 1706515) secondary antibodies were purchased from Bio-Rad Laboratories, Inc., and water-soluble tetrazolium salts (WST-1; cat. no. 11644807001) reagent was purchased from Sigma-Aldrich; Merck KGaA. Eagle's minimum essential medium (EMEM, cat. no ATCC® 30-2003) was procured from American Type Culture Collection. F12K was obtained from Corning Inc. (cat. no. 10025CV), and Trypsin-EDTA solution (cat. no. 25200056) was obtained from Thermo Fisher Scientific, Inc.
Cell lines and subculture
The metastatic bladder cancer TCCSUP (ATCC® HTB-5) and healthy bladder epithelial MC-SV-HUCT2 (ATCC® CRL-9519) cell lines were obtained from American Type Culture Collection. The TCCSUP and MC-SV-HUCT2 cells were sub-cultured and maintained in T75 flasks containing EMEM and F12K media, respectively. Both flasks were supplemented with 10% FBS (R&D Systems, Inc.) and 1% antibiotics (penicillin, 10 U/ml; streptomycin, 10 mg/ml). Cells were incubated at 37°C with 5% CO2. Cells were used for the experiments a few days after subculture when cells were 70-80% confluent.
Viability of metastatic TCCSUP cells and normal bladder MC-SV-HUCT2 cells following treatment with ICA-I, ζ-Stat and rapamycin
Metastatic TCCSUP cells (3.0×103/well) and healthy MC-SV-HUCT2 cells (3.0×103/well) were subcultured in a 96-well plate. Following incubation for 24 h at 37°C with 5% CO2, the cells were treated with either ICA-I (7.5 μM), ζ-Stat (7.5 μM) or rapamycin (100 nM) separately, or combination of ICA-I and rapamycin or ζ-Stat for 72 h at 37°C with 5% CO2. Subsequently, cells were washed with 200 μl 1X DPBS buffer and incubated with suitable media (either EMEM or F12K) and WST-1 reagent (final dilution, 1:10) at 37°C with 5% CO2 for 3 h according to the manufacturer's protocol. Finally, the cells were analyzed after 3 h at 450 nm using a microplate reader (BioTek Instruments, Inc.) (21).
Flow cytometric analysis of apoptosis of combination- treated bladder cancer cells
Annexin-V/APC and DAPI-based flow cytometry was used to distinguish the apoptotic population from a healthy population. Cells were plated in 100-mm cell culture plates, treated for 5 consecutive days as aforementioned for the cell viability test, lifted, harvested and analyzed according to the manufacturer's protocol. Briefly, the treated and untreated cells were then resuspended in 100 μl Annexin-V/APC and DAPI solution. Subsequently, the cells were incubated in the dark for 10 min at room temperature. The samples were run at 633 nm excitation and 700 nm emission wavelengths for APC, and 350 nm excitation and 450 nm emission wavelengths for DAPI using the BD FacsCanto II instrument (Becton, Dickinson and Company), and analyzed using FacsDiva 6.3.1 software (BD Biosciences).
Cell lysate preparation and immunoblot analysis
Cells (1.5×105) were cultured in 100-mm cell culture flasks. After incubation for 24 h at 37°C with 5% CO2 (to obtain ≥50% confluency), new medium was supplied, and each experimental flask was treated with 7.5 μM ICA-I or ζ-Stat, 100 nM rapamycin or a combination of ICA-I/ζ-Stat and rapamycin for 72 h at 37°C with 5% CO2. An equal volume of DMSO (vehicle control), as the volume of the drug, was added to the control. After continuing the treatment for 3 consecutive days, cells were placed on ice, washed with 1X DPBS, scraped into a 1.5-ml centrifuge tube and resuspended in 500 μl cell 1× Cell Lysis buffer (cat. no. 9803; Cell Signaling Technology, Inc.). The re-suspended cells were subsequently sonicated and centrifuged at 16,128 x g at 4°C for 30 min, followed by the determination of protein concentration using a Bradford assay (22). An equal amount (30 μg/lane) of protein was loaded and separated by 10% SDS-PAGE, followed by electroblotting onto a nitrocellulose membrane (0.45-μm). The proteins were then blocked for 1 h in 5% fat-free milk in TBS with 0.05% Tween-20 (TBST) at room temperature. Subsequently, the membranes were incubated with primary antibody solution in 5% BSA (cat. no. BP1600-100; Thermo Fisher Scientific, Inc.) in TBST (dilution, 1:1,000) overnight at 4°C, followed by incubation with secondary antibody of either goat anti-mouse IgG HRP conjugate or goat anti-rabbit IgG HRP conjugate in 5% fat-free milk in TBST (dilution, 1:3,000) for 1.5 h at room temperature. The immune reactive bands were finally visual-ized using Supersignal West Pico Chemiluminescent substrate under Amersham Imager 600 (GE Healthcare Life Sciences), as previously described (23).
β-galactosidase staining of bladder cancer cells for senescence
TCCSUP metastatic bladder cells (1×103 cells/well) were plated in 6-well plates, followed by treatment with the combination of atypical PKC inhibitors and rapamycin for 7 consecutive days. Cells were then fixed and stained according to the manufacturer's protocol using the senescence β-Gal staining kit. Briefly, TCCSUP cells were fixed with 1× β-Gal fixative for 20 min at room temperature and stained with complete 1× β-Gal stain solution in a dry incubator overnight at 37°C. Images were captured using Jenoptik Gryphax® software (Jenoptik AG) with differential interference contrast brightfield microscopy (Motic AE31E; magnification, x20).
Scratch wound healing assay
TCCSUP malignant cells were plated into 6-well plates. At ~90% confluency, old media were discarded, a line was scratched across the cell monolayer using a 100-μl sterile pipette tip and the cells were washed with 1X DPBS to remove floating cells and debris. Subsequently, fresh complete media (supplemented with 10% FBS) was supplied and the cells were treated using ICA-I (7.5 μm), ζ-Stat (7.5 μm), rapamycin (100 nM), combination of ICA-I and rapamycin, or combination of ζ-Stat and rapamycin for 72 h and incubated at 37°C with 5% CO2. Finally, the cells that moved into the interspace of the wound were observed after 72 h using a phase contrast microscope (Motic Incorporation, Ltd.).
Immunoprecipitation (IP)
Protein (3 μg) was immuno-precipitated from 300 μg protein containing cell lysate suspension using primary antibody of interest and separated by SDS-PAGE and finally analyzed using the western blotting technique to determine the associated proteins with the IP protein as described in our previous study (8).
Densitometry
The intensity of each band was quantified using 1D analysis software, Alpha View (v3.4.0.0; ProteinSimple). The background intensity was subtracted from each band to quantify the correct intensity.
Statistical analysis
To determine the statistical significance of the data, the results were presented as the mean ± SEM of at least three independent experiments. The data were compared by one-way ANOVA (Dunnett's multiple comparison tests) or two-way ANOVA (Tukey's multiple comparison test) using GraphPad Prism 8 software (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Atypical PKC inhibitors and rapamycin reduce the survival of bladder cancer cells without affecting healthy bladder cells
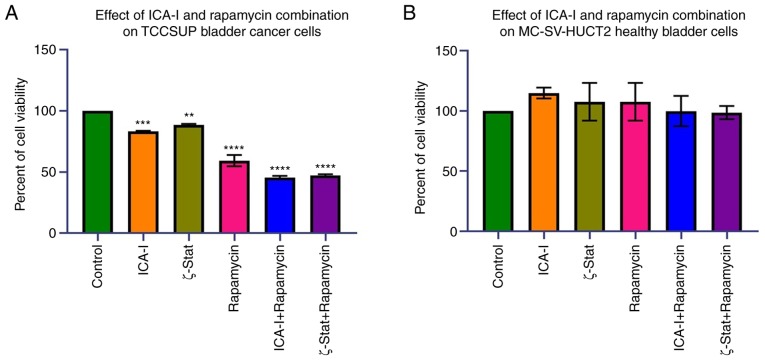
To examine the effects of the combination of atypical PKC inhibitors and rapamycin on bladder cancer TCCSUP cells, the present study first examined the viability of bladder cancer following the combination treatment. The data indicated that the combination of ICA-I with rapamycin and the combination of ζ-Stat with rapamycin decreased the viability of bladder cancer TCCSUP cells by >50% (P<0.0001) compared with the control (Fig. 2A). Additionally, the reduction in the viability of the cells treated with rapamycin or atypical PKC inhibitors alone was less compared with combination-treated cells (Fig. 2A). By contrast, the use of either combination or single drug therapy did not produce any significant effect in healthy bladder MC-SV-HUCT2 cells (Fig. 2B). These discoveries suggested that the concomitant administration of mTOR and atypical PKC inhibitors can be used to treat bladder cancer cells without affecting healthy bladder cells.
Figure 2.
Effect of atypical PKC inhibitors and rapamycin on normal and malignant bladder cells. (A) TCCSUP and (B) MC-SV-HUCT2 cells (3×103 cells/well) were plated in 96-well plates and treated with 7.5 μM ICA-I or ζ-Stat, or 100 nM rapamycin, or combination of atypical PKC inhibitor and rapamycin for 72 h. Following incubation with a WST-1 reagent for 3 h, absorbance was determined at 450 nm using a microplate reader. All the experiments were performed for at least three times. Data are presented as the mean ± SEM. **P<0.02, ***P<0.01 and ****P<0.0001 vs. untreated control cells. PKC, protein kinase C.
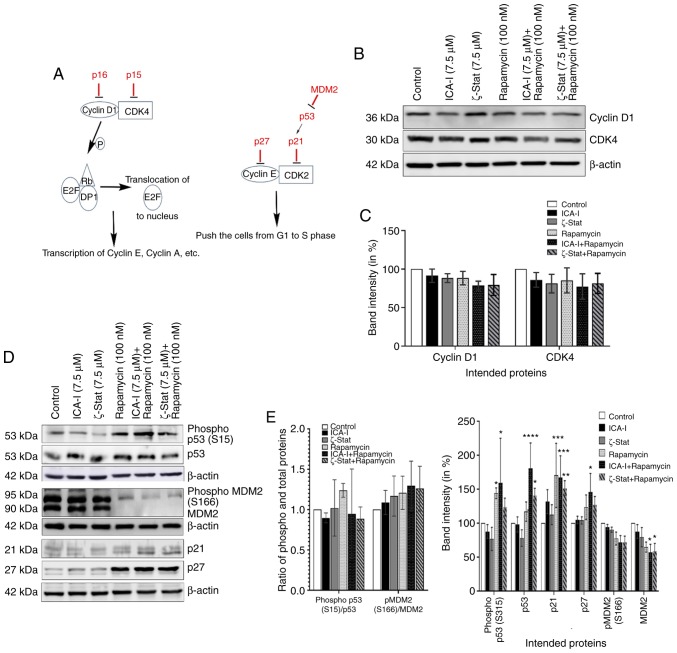
Combination of atypical PKC inhibitors and rapamycin induces cell cycle arrest by activating tumor suppressors in bladder cancer cells
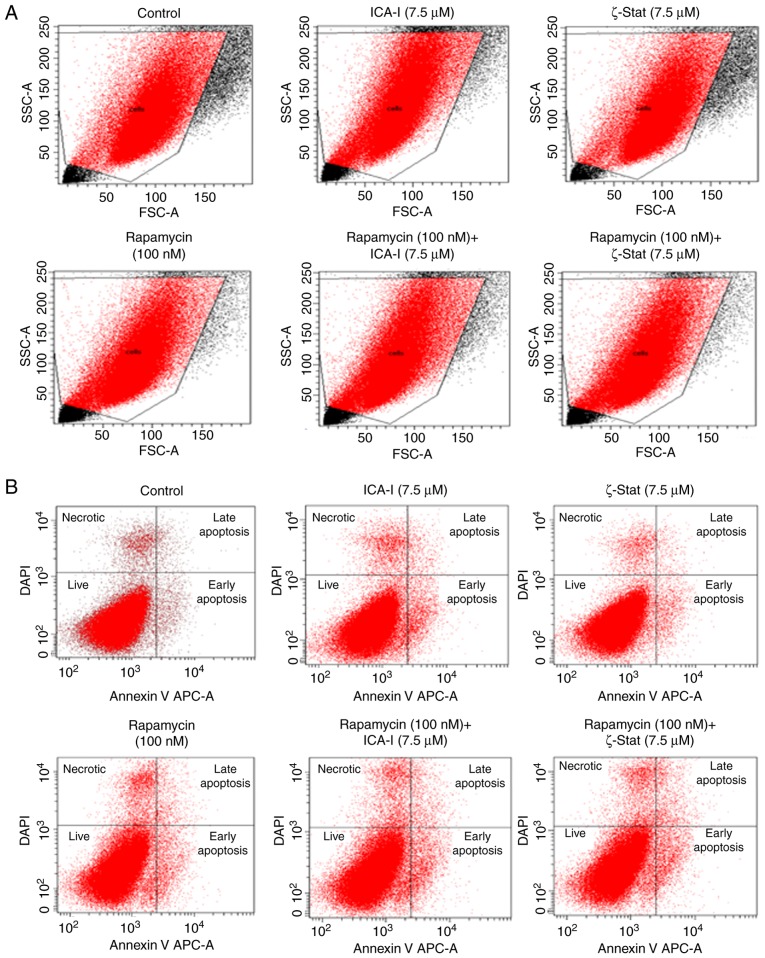
Since reduced viability of TCCSUP bladder cancer cells was observed for the combination therapy of rapamycin and atypical PKC inhibitor, the present study investigated the reason for the reduced bladder cancer cell viability. The flow cytometric analysis of apoptosis revealed that there was no substantial change in the early and late apoptotic population of TCCSUP cells treated with the combination even after 5 days (Fig. 3). Subsequently, the present study investigated the status of cell cycle progression. During cell cycle progression, cyclin E can work in conjunction with CDK2 to transit the cells from G1 to S phase (24). The CDK interacting protein/kinase inhibitory protein family members, such as p21 and p27, and inhibitor of kinase 4/alternative reading frame family members, such as p16, serve a critical role in inhibiting cell cycle progression by binding and inactivating cyclin-CDK complexes (Fig. 4A) (25). The findings indicated that there was no significant change in the expression of cyclin D1 and CDK4 (Fig. 4B and C). However, the data demonstrated that the simultaneous inhibition of mTOR and atypical PKC by the combination of ICA-I and rapamycin significantly increased the expression levels of p53 (P<0.0001), p21(P<0.01), p27 (P<0.05; Fig. 4D and E) in treated cancerous urinary tract cells compared with control cells.
Figure 3.
Effect of atypical PKC inhibitors and rapamycin on malignant bladder cell apoptosis. TCCSUP cells (1×106) were plated in 100-mm plates and treated with 7.5 μM ICA-I or ζ-Stat, or 100 nM rapamycin or combination of atypical PKC inhibitor and rapamycin for 72 h. Following treatment, the cells were harvested and labeled with an APC-bound Annexin V and DAPI to detect different stages of apoptosis using flow cytometry. (A) Targeted cells used for gating during flow cytometric analysis to detect necrotic and apoptotic populations. (B) Percentage of different phases of apoptotic and necrotic population using flow cytometer. The experiment was repeated for N=3 independent times. PKC, protein kinase C; SSC, side scattered light; FSC, forward scattered light.
Figure 4.
Combination of atypical PKC inhibitors and rapamycin decreases bladder cancer cell progression. (A) Proteins involved in cell cycle progression. Cells were grown in 100-mm cell culture plates, followed by treatment with either ICA-I (7.5 μM), ζ-Stat (7.5 μM), rapamycin (100 nM) or a combination. The treated lysates were then subjected to western blot analysis to observe the expression of cell cycle regulatory proteins. (B) Western blotting bands of cyclin D1 and CDK4 expression. (C) Expression levels of cyclin D1 and CDK4 presented as corresponding band intensity. (D) Western blotting bands of phospho and total p53 and MDM2, as well as p21 and p27 expression in TCCSUP cells following 3 days of treatment of ICA-I, ζ-Stat and rapamycin alone or in combination. (E) Densitometric analysis of the phospho and total p53, and MDM2, p21 and p27. on β-actin was used as a loading control (different β-actin loading controls represent the protein bands from different blots). All experiments were performed for at least three times. Data are presented as the mean ± SEM. *P<0.05, **P<0.02, ***P<0.01 and ****P<0.0001 vs. untreated control cells. MDM2, mouse double min 2 homolog; p, phosphorylated; PKC, protein kinase C.
Furthermore, in bladder cancer, p53 is known as the guardian of the cell cycle and is the most important tumor suppressor that serves a crucial role in regulating the cell cycle via p21 (26). However, the phosphorylation of p53, in turn, depends on the status of tumor promoting MDM2 (26). The results demonstrated that there was a notable change in the expression of total MDM2 (P<0.05) in TCCSUP cells following treatment with atypical PKC inhibitor and rapamycin combination (Fig. 4D and E). These findings suggested that the combination of rapamycin with ICA-I or ζ-Stat reduced malignant bladder cell growth by retarding cell cycle progression.
TCCSUP cells undergo senescence following prolonged treatment with atypical PKC inhibitor and rapamycin
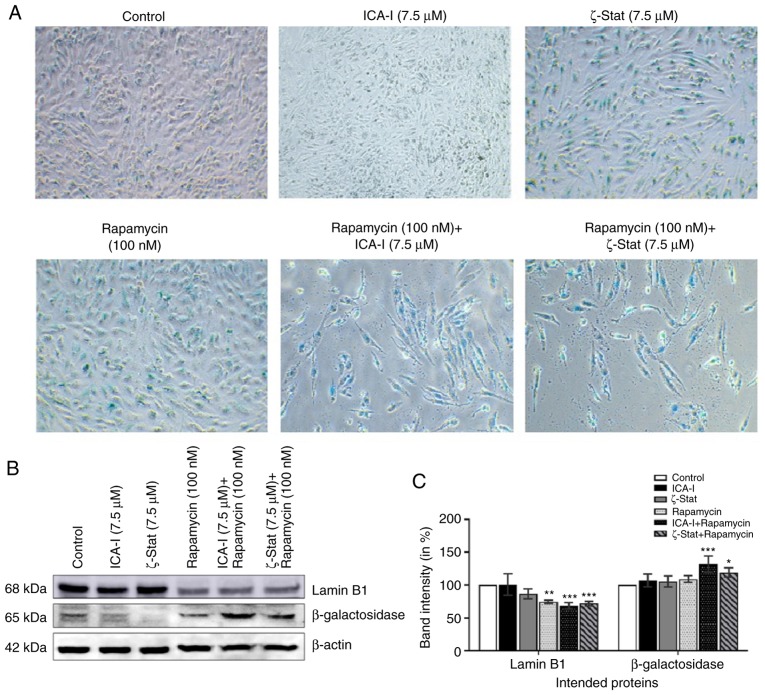
Cellular aging or senescence, gradual degradation of functional characteristics, can be identified by the upregulation of senescence associated β-Galactosidase (SA β-Gal) activity and downregulation of a nuclear membrane protein, Lamin B1 (27,28). To investigate whether SA β-Gal activity and Lamin B1 could be used to quantify senescence in bladder cancer cells, metastatic cancer cells were treated with atypical PKC inhibitors, rapamycin or the combination for 7 days. The immunofluorescence analysis demonstrated that with the combination treatment of rapamycin with either ICA-I or ζ-Stat there was an increased SA β-Gal activity (Fig. 5A), as well as, significant overexpression of β-Galactosidase by >20% (P<0.05) in TCCSUP cells (Fig. 5B and C). Additionally, the combination of rapamycin and atypical PKC inhibitor (either ICA-I or ζ-Stat) reduced the expression levels of Lamin B1 by >30% (P<0.01; Fig. 5B and C). These results demonstrated that the prolonged simultaneous inhibition of atypical PKC and mTOR induced senescence in TCCSUP cells.
Figure 5.
Prolonged exposure of atypical PKC inhibitors and rapamycin combination induces senescence in bladder cancer cells. Bladder cancer cells were treated with ICA-I, ζ-Stat, and rapamycin alone or in combination for 7 consecutive days. (A) Cells were stained with β-Galactosidase (blue) for the evaluation of senescence. Magnification, x10. (B) Expression levels of senescence markers, such as Lamin B1 and β-Galactosidase, were also observed. (C) Bar charts representing the corresponding band intensities. β-actin was used as a loading control. All the experiments were performed for at least three times. Data are presented as the mean ± SEM. All the data were compared with untreated control cells where *P<0.05, **P<0.02 and ***P<0.01 vs. untreated control cells. PKC, protein kinase C.
Concurrent inhibition of atypical PKCs and mTOR reduces the migration of bladder cancer cells
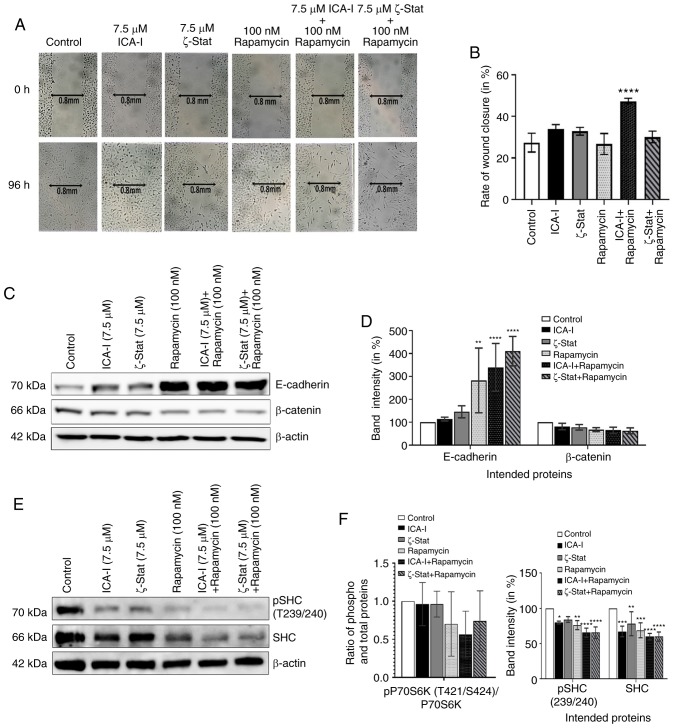
To detect the anti-metastatic potential of the combination, a preliminary scratch wound healing assay was performed. The data depicted that the combination of ICA-I and rapamycin treatment significantly prolonged the wound closure time comapred with the control in TCCSUP bladder cancer cells (Fig. 6A and B). During metastasis, the alterations of two critical proteins, namely E-cadherin and β-catenin, in cells contribute to the acquisition of the motile phenotype. E-cadherin, a cell adhesion protein, works in a reverse relationship with β-catenin, a key molecule of the WNT/β-catenin signaling pathway, to form an adherent junction which ultimately gives the rigid structure of healthy cells (29,30). The results revealed that the atypical PKC inhibitor and rapamycin combination significantly increased the expression of E-cadherin (P<0.0001; Fig. 6C and D). Additionally, the combination of atypical PKC inhibitor and rapamycin decreased the β-catenin levels in treated metastatic bladder cancer cells; however, this was not statistically significant (Fig. 6C and D). Furthermore, SHC is a substrate for receptor tyrosine kinase that has previously been identified to be involved in the dynamic regulation of cell adhesion and motility of cancer cells (31,32). To determine whether the atypical PKC and mTOR serve any role in regulating the functionality of SHC protein in metastatic bladder cancer cells, the level of SHC was examined. The data demonstrated that the combination can also impeded the migration of bladder cancer cells by reducing both phosphorylated and the total level of SHC by >30% (P<0.0001) in treated cancerous cells compared with control cells; however, the change in the ratio of phosphorylated to total SHC levels was not significant (Fig. 6E and F). These results suggested that the atypical PKC and mTOR mediated expression of E-cadherin, β-catenin and SHC control the migration of TCCSUP cells.
Figure 6.
Combination of atypical PKC inhibitors and rapamycin blocks migration of bladder cancer cells. (A) Microscopic images of metastatic TCCSUP cells which were grown to ~90% confluency and subjected to a wound healing assay by making a scratch using a sterile 100-μl pipette tip, followed by concurrent inhibition of atypical PKC and mTOR. Original magnification, x10. (B) Bar charts presenting the rate of wound closure after treating the cells for 3 consecutive days. In addition, TCCSUP cells were grown in 100-mm cell culture plates followed by treatment with either ICA-I (7.5 μM) or ζ-Stat (7.5 μM) or rapamycin (100 nM) or combination for 3 days. (C) Western blot analysis for the effect of ICA-I, ζ-Stat and rapamycin alone or in combination on E-cadherin and β-catenin expression. (D) Densitometric analysis of E-cadherin and β-catenin levels. In addition, the levels of phospho and total SHC in TCCSUP cells were assessed. (E) Bands and (F) their intensities (in percent). β-actin was used as a loading control. All experiments were performed at least three times. All data are presented as the mean ± SEM. *P<0.05, **P<0.02, ***P<0.01 and ****P<0.0001 vs. untreated control cells. p, phosphorylated; PKC, protein kinase C; SHC, Src homology 2 domain cotaining transforming protein.
Combination treatment of ICA-I and rapamycin or ζ-Stat and rapamycin inhibits mTOR and atypical PKC signaling pathways in bladder cancer cells
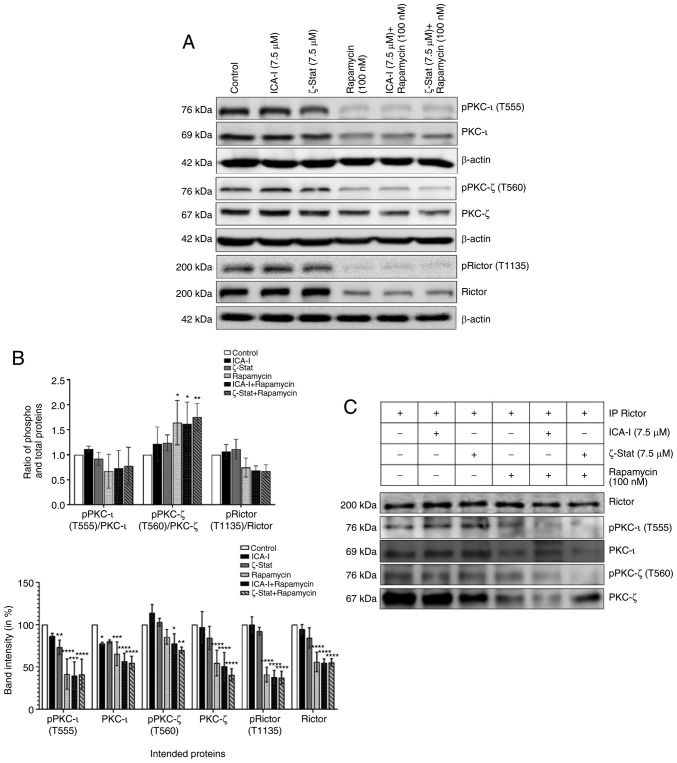
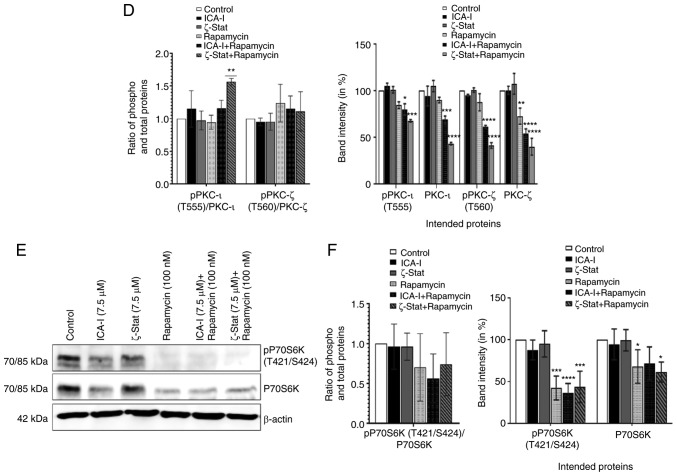
To examine whether the concurrent administration of rapamycin and ICA-I or ζ-Stat could block the mTOR and atypical PKC signaling pathways in bladder cancer cells, the present study tested the changes in the functionality and expression of major components of mTOR and atypical PKC signaling pathways in treated TCCSUP cells. Our observed data indicated that the simultaneous inhibition of mTOR and atypical PKC reduced the level of both phosphorylated PKC-ι at T555 (by >50%; P<0.01) and phosphorylated PKC-ζ at T560 (by >30%; P<0.02; Fig. 7A and B). The ratio of phosphorylated to total PKC-ι was decreased, whereas, the ratio of phosphorylated to total PKC-ζ was increased (Fig. 7A and B). Additionally, the phosphorylation of the critical component of mTORC2, Rictor at T1135 was also decreased by >50% (P<0.0001) in treated TCCSUP cells. Furthermore, the total expression levels of PKC-ι, PKC-ζ and Rictor (P<0.0001) were decreased following combination treatment compared with the control (Fig. 7A and B). The ratio of phosphorylated and total PKC-ι and Rictor decreased (Fig. 7A and B); however, this was not statistically significant. Consistent with the aforementioned findings, the present study examined whether atypical PKC and mTOR signaling pathways occur downstream and upstream of each other by immunoprecipitating Rictor and checking for PKC-ι and PKC-ζ. It was observed that Rictor was associated with atypical PKC and the combination therapy of rapamycin and atypical PKC inhibitors minimized the association of Rictor with both PKC-ι by >30% (P<0.05) and PKC-ζ by >50% (P<0.0001) in atypical PKC inhibitors and rapamycin combination-treated cells compared with the control (Fig. 7C and D). Finally, the expression of both total and phosphorylated P70S6K, a downstream molecule of mTOR responsible for triggering cell cycle progression (33), also declined as a function of combination treatment compared with control. Contrarily, the reduction in the ratio of phospho P70S6K and P70S6K was found to be statistically insignificant (Fig. 7E and F). These findings suggest that the concomitant inhibition of atypical PKC and mTOR can be used effectively to target both atypical PKC and mTOR signaling to treat bladder cancer cells when atypical PKC and mTOR are overexpressed.
Figure 7.
Combination of atypical PKC inhibitors and rapamycin reduces the functionality of mTOR complexes and atypical PKC in bladder cancer cells. (A) Western blot analysis for the effect of ICA-I, ζ-Stat and rapamycin alone or in combination on phospho and total PKC-ι, PKC-ζ and Rictor in TCCSUP cells following 3 days of treatment. β-actin was used as a loading control (different β-actin loading controls represent the protein bands from different blots). (B) Densitometric analysis of phospho and total PKC-ι, PKC-ζ and Rictor bands. (C) Rictor, key component of mTORC2, was used in IP (3 μg) separately from whole cell extracts (300 μg) of both treated (72 h) and untreated metastatic TCCSUP cells using Rictor antibody, and evaluated for atypical PKC molecules. The IP samples were separated by SDS-PAGE and subjected to western blot analysis. Data are presented as the mean ± SEM of three independent experiment. *P<0.05, **P<0.02, ***P<0.01 and ****P<0.0001 vs. untreated control cells. Combination of atypical PKC inhibitors and rapamycin reduces the functionality of mTOR complexes and atypical PKC in bladder cancer cells. (D) Effect of atypical PKC inhibitors on total and phospho PKC-ι and PKC-ζ were quantified using densitometry. (E) Effect of the combination on a mTORC1 downstream protein, P70S6K and its phosphorylated form. (F) Densitometric analysis of phospho and total P70S6K. Data are presented as the mean ± SEM of three independent experiment. *P<0.05, **P<0.02, ***P<0.01 and ****P<0.0001 vs. untreated control cells. IP, immunoprecipitation; p, phosphorylated; PKC, protein kinase C.
Combination of atypical PKC inhibitors and rapamycin reduces the expression of AKT1 in metastatic TCCSUP cells
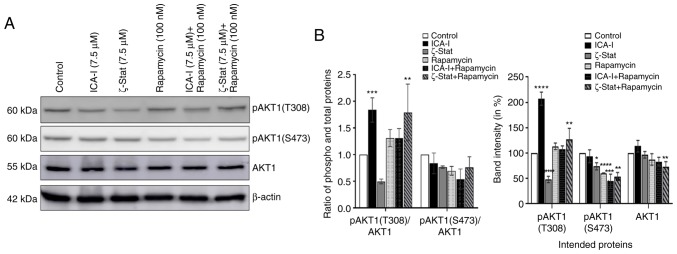
AKT1 serves an interesting role in mediating mTOR complexes driven pathways; it acts as an upstream regulator of mTORC1 and downstream molecule of mTORC2 (34). The present study examined the expression levels of AKT1 to observe whether the inhibition of atypical PKCs and mTOR affects its functionality. The results demonstrated that compared with the control, the combination therapy significantly reduced the phosphorylation of AKT1 at S473 position by >45% (P<0.0001; Fig. 8) which is a phosphorylation site for mTORC2. However, there was no significant change in the ratio of phosphorylated to total AKT1 (Fig. 8). These findings suggested that the combination of atypical PKC inhibitor and rapamycin affects the activation of AKT1 in bladder cancer cells.
Figure 8.
Combination of atypical PKC inhibitors and rapamycin affects the activation of AKT1 in bladder cancer cells. (A) Expression of phospho (both at S473 and T308 positions) and total AKT1 following 3 days of treatment with atypical PKC inhibitor and rapamycin combinations. (B) Bar chart indicating the corresponding band intensities. β-actin was used as a loading control. All experiments were performed at least three times. Data are presented as the mean ± SEM. *P<0.05, **P<0.02, ***P<0.01 and ****P<0.0001 vs. untreated control cells. p, phosphorylated; PKC, protein kinase C.
Discussion
Recent advances in life sciences have shifted the therapeutic approach of bladder cancer and are currently focusing on segregating bladder cancer based on the genetic profile. The activation of the PI3K/mTOR signaling pathway is crucial in bladder cancer and is associated with resistance to chemotherapeutic agents (35). The genetic profile of a patient with bladder cancer seems to be promising in designing targeted therapy, since the genomic analysis of patients with bladder cancer who responded well to everolimus (rapamycin-containing drug) indicated that they have high expression levels of tuberous sclerosis 1, TSC1 (36). One of the main challenges in using mTOR inhibitor is that it can cause reactivation of oncogenic AKT1 signaling resulting from the attenuation of the negative regulatory loop from mTOR which can ultimately lead to disease recurrence (36). Therefore, the present study aimed to establish a novel therapeutic regimen that can cause dual inhibition of cancer-causing pathways, namely atypical PKCs and mTOR signaling in bladder cancer.
In our previous studies, it was observed that atypical PKCs are overexpressed in prostate cancer, colon cancer, melanoma, breast cancer, glioma and neuroblastoma cells (8,19,23,37,38). Similarly, hyperactivation of mTOR is associated with different types of cancer, such as bladder and breast cancer (11,39). Notably, combination regimen, such as rapamycin and resveratrol, have been identified to be effective in treating breast and bladder cancer by inducing autophagy and preventing reactivation of AKT1 in vitro and in a mouse xenograft model (40,41). Keeping the hypothesis in mind that both atypical PKC and mTOR serve crucial carcinogenic roles in bladder cancer cells, the present study aimed to inhibit both atypical PKC and mTOR in bladder cancer cells. Another reason for trying this combination is that in a recent study, a combination of atypical PKC inhibitor and a widely used clinical agent, known as 5-flouorouracil, was trialed in CRC cells, and it was observed that the combination can reduce the growth and proliferation of CRC cells by blocking the DNA repair mechanism of the cancer cells (42). First, the present study investigated the effi-cacy of the inhibitors in bladder cancer cells compared with healthy bladder cells. The cell viability investigation revealed that the simultaneous inhibition of atypical PKC and mTOR using the combination of either ICA-I or Stat and rapamycin for 3 days reduced the viability of TCCSUP bladder cancer cells markedly (>50%; P<0.0001) compared with control untreated bladder cancer cells. However, the combination therapy did not induce any significant changes in the MC-SV-HUCT2 healthy bladder cell viability. It is interesting to note that the flow cytometry based apoptosis assay did not detect any significant apoptotic population even after treating the cells for 5 days. The subsequent western blot analysis of cell cycle proteins following treatment of TCCSUP cells with atypical PKC and mTOR inhibitors revealed that there was an upregulation of p27 and p21, which are two important tumor suppressors that work by inhibiting cyclin E and CDK2, respectively, of the cyclin E-CDK2 cell cycle regulatory complex (25,43). The activation of p21 depends on another critical tumor suppressor protein known as p53, which in turn, is negatively regulated by MDM2 (43). The further investigation revealed that the combination of atypical PKC inhibitor and rapamycin increased the functionality of tumor suppressing p53 while retarding MDM2 expression. However, the combination treatment did not induce any significant changes in other upstream cell cycle regulatory molecules, such as cyclin D1and CDK4.
Interestingly, treatment was continued for 7 consecutive days to examine the fate of cells following cell cycle arrest, and it was observed that prolonged treatment made the cells undergo irreversible growth arrest or senescence. Two of the crucial factors that are indicative of cellular senescence are: i) Downregulation of Lamin B1, a nuclear membrane component important in maintaining normal cellular function; and ii) increased SA β-Gal activity (27). Based on this observation, it was speculated that the prolonged inhibition of atypical PKC and mTOR induced senescence as evident by reduced Lamin B1 expression and increased SA β-Gal activity. Considering the fact that mTOR and atypical PKCs may stimulate bladder cancer cell progression, the present study also examined the metastatic profile of bladder cancer cells as a function of combination treatment. Similar to our previous study (20), combined inhibition of atypical PKC and mTOR using ICA-I and rapamycin prolonged the rate of wound closure in TCCSUP cells, as demonstrated by the scratch wound healing assay. Although serum has a significant impact on the proliferation of cells, the scratch wound healing assay was performed using media containing 10% FBS to maintain consistency across all experimental protocols, since changes in serum concentration could affect the phosphorylation of proteins that have a critical role in the cell signaling pathway (44). One of the first metastatic events that takes place inside the cell is the loss of E-cadherin, a cell adhesion molecule. Loss of E-cadherin causes loose junction between cells and induces the cells to acquire a motile phenotype (29). Additionally, increased activity of WNT/β-catenin signaling is another hallmark of cancer cell metastasis (30). The present study revealed that the combination treatment induced an increased expression of E-cadherin and decreased expression of β-catenin. Furthermore, the expression of SHC, another dynamic regulator of adhesion and migration has been implicated in metastasis of cancerous cells (31,32). Interestingly, the combination of atypical PKC inhibitors and rapamycin notably reduced the expression of SHC in bladder cancer cells.
One of the purposes of the present study was to establish a link between atypical PKCs and mTOR complexes in bladder cancer cells. It was considered that atypical PKCs may serve a role as a downstream molecule of the mTOR signaling pathway, since PKCs, in general, are found as a downstream protein of mTORC2 that facilitates cancer cell migration and invasion by cytoskeleton remodeling (11). The results demonstrated that the combination of atypical PKC inhibitor and rapamycin induced a pronounced inhibition in the expression of phos-phorylated atypical PKC which may occur because of the role of mTOR in mediating the phosphorylation of protein kinases at their turn and hydrophobic region (14-16). Rapamycin is the most commonly used extremely selective drug to target mTOR, which is already in clinical use. It is well established that mTOR complexes, specifically mTORC1, are sensitive towards rapamycin but mTORC2 is somewhat insensitive to rapamycin (45); hence, the goal of the present study was to examine the effect of rapamycin and atypical PKC inhibitor combination on mTORC2. It was demonstrated that the combination induced an inactivation and downregulation of the most crucial mTORC2 component, Rictor. Additionally, atypical PKCs were associated with Rictor and the combination treatment reduced the association in the treated bladder cancer cells. Furthermore, there was a marked decrease in the expression of phosphorylated P70S6K, a mTORC1 downstream protein responsible for the cell cycle progression, following treatment with the combination (33). This finding was consistent with the cell cycle protein analysis, which could result from the direct inhibition of both mTORC1 and atypical PKC, or there may be some links between these two proteins. Furthermore, AKT1 serves a role in mTOR pathways; it acts as an upstream molecule of mTORC1 where AKT1 acts by inhibiting tuberous sclerosis 2 (TSC2) required for mTORC1 activation and is a downstream protein of mTORC2, whereas mTORC2 phosphorylates AKT1 at Serine 473 (34). The results demonstrated that the combination of both ICA-I and rapamycin, and ζ-Stat and rapamycin therapy reduced the phosphorylation of AKT1 at Serine 473; which may be due to the inactivation of upstream mTORC2 or indirect inhibition of mTORC2 due to decreased activation of atypical PKCs. However, more investigations need to be performed to validate this statement.
The present study has some limitations; only in vitro study is not adequate to justify the efficacy of the proposed combination treatment in bladder cancer; hence, future in vivo studies would increase the credibility of the proposed statement. Furthermore, in this investigation, even though a significant change in the phosphorylated and total levels of intended proteins was observed following treatment with the combination of atypical PKC inhibitors and rapamycin compared with the control, the change in the ratio between the phosphorylated and total protein levels appeared to be statistically insignificant in most cases. One possible reason for not achieving statistical significance is that in some cases the combination treatment reduced the phosphorylated form of the protein without affecting total protein levels, or there was fluctuating change between the reduction of phosphorylated and total levels of protein, or in some cases the phosphorylated and total proteins changed at the same rate. Therefore, further studies need to be performed to understand the change in effect between phosphorylated and total protein and their ratios. Furthermore, the present study used only one aggressive bladder cancer cell line that represents grade IV transitional cell carcinoma; thus, the proposed combination should be evaluated in another bladder cancer cell line.
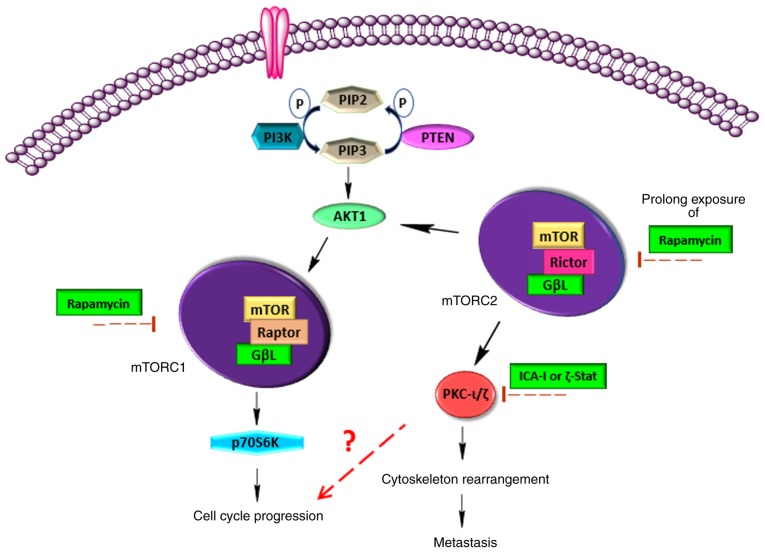
In conclusion, atypical PKC and mTOR serve a crucial role in the carcinogenesis of bladder cancer cells (Fig. 9). Additionally, mTOR can enhance the activity of atypical PKC. Hence, the combination of atypical PKC inhibitors and rapamycin can be used as a novel therapeutic regimen for individualized therapy.
Figure 9.
mTOR and atypical PKC triggers bladder cancer cell progression. mTOR can exist in two distinct complexes, mTORC1 and mTORC2. The main structural difference between these two complexes is their key components, namely Rictor (mTORC1) and Raptor (mTORC2). mTORC1 regulates cell cycle progression via p70S6K, whereas mTORC2 facilitates the metastatic phenotype of cancer cells via atypical PKC. Additionally, atypical PKC may serve a vital role in regulating the cell cycle. Hence, the combination of rapamycin, an mTOR complex inhibitor, and atypical PKC inhibitor may reduce bladder cancer cell progression. PKC, protein kinase C.
Acknowledgments
The authors would like to thank Mr. Gene Prenzo, Trustee of the Leo and Anne Albert Charitable Trust for his generous financial support of this work. The abstract of the present study was presented at the AACR Annual Meeting 2019; March 29-April 3, 2019; Atlanta, GA, USA, and was published as Abstract no. 4290.
Funding
The present study was supported by the Leo and Anne Albert Charitable Trust, Frederick H (grant no. 42-0142). Leonhardt Foundation (grant no. 42-0044), Kyrias Foundation (grant no. 42-0044), Jin and Joo Lee Family Foundation (grant no. 42-0044), and Miami Foundation for Cancer Research, Inc. (grant no. 42-0044).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RP and SMAI designed the experiment. SMAI wrote the draft of the manuscript. RP performed most of the experiments and analyzed the data. TS and RRB assisted RP in western blotting and immunoprecipitation analyses. MAD was involved in the conception, design and mentoring, as well as critical interpretation and evaluation of the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Montironi R, Cheng L, Scarpelli M, Lopez-Beltran A. Pathology and genetics: Tumours of the urinary system and male genital system: Clinical implications of the 4th edition of the WHO classification and beyond. Eur Urol. 2016;70:120–123. doi: 10.1016/j.eururo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed SI. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–360. doi: 10.1158/1078-0432.CCR-05-1505. [DOI] [PubMed] [Google Scholar]
- 4.Reuter VE. Pathology of bladder cancer: Assessment of prognostic variables and response to therapy. Semin Oncol. 1990;17:524–532. [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 6.Gschwend JE, Dahm P, Fair WR. Disease specific survival as endpoint of outcome for bladder cancer patients following radical cystectomy. Eur Urol. 2002;41:440–448. doi: 10.1016/S0302-2838(02)00060-X. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DL, Cox MM. Lehninger principles of biochemistry. 5th ed. Book; 2008. pp. 1–1294. [Google Scholar]
- 8.Islam SMA, Patel R, Acevedo-Duncan M. Protein Kinase C-ζ stimulates colorectal cancer cell carcinogenesis via PKC-ζ/Rac1/Pak1/β-Catenin signaling cascade. Biochim Biophys Acta Mol cell Res. 2018;1865:650–664. doi: 10.1016/j.bbamcr.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Islam SMA, Patel R, Bommareddy RR, Khalid KM, Acevedo-Duncan M. The modulation of actin dynamics via atypical Protein Kinase-C activated Cofilin regulates metastasis of colorectal cancer cells. Cell Adh Migr. 2019;13:106–120. doi: 10.1080/19336918.2018.1546513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phos-phoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 17.Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, Kango-Singh M, Lu KH, Warneke CL, Atkinson EN, et al. Atypical PKC contributes to poor prognosis through loss of apical-basal polarity and Cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo H, Ma Y, Zhang B, Sun B, Niu R, Ying G, Zhang N. Pivotal Advance: PKC is required for migration of macrophages. J Leukoc Biol. 2009;85:911–918. doi: 10.1189/jlb.0708429. [DOI] [PubMed] [Google Scholar]
- 19.Smalley T, Islam SMA, Apostolatos C, Apostolatos A, Acevedo-Duncan M. Analysis of PKC-ζ protein levels in normal and malignant breast tissue subtypes. Oncol Lett. 2019;17:1537–1546. doi: 10.3892/ol.2018.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam SMA, Dey A, Acevedo-Duncan M. Abstract 719: Combination of atypical protein kinase-C inhibitor and 5-fluo-rouracil retards the proliferation of colorectal cancer cells. Mol Cell Biol. 2019;719 [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Patel R, Win H, Desai S, Patel K, Matthews JA, Acevedo-Duncan M. Involvement of PKC-ι in glioma proliferation. Cell Prolif. 2008;41:122–135. doi: 10.1111/j.1365-2184.2007.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 25.Hardie DG, Hanks S. The protein kinase factsbook. Academic Press; 1995. [Google Scholar]
- 26.Kriegmair MC, Balk M, Wirtz R, Steidler A, Weis CA, Breyer J, Hartmann A, Bolenz C, Erben P. Expression of the p53 inhibitors mdm2 and mdm4 as outcome predictor in muscle-invasive bladder cancer. Anticancer Res. 2016;36:5205–5213. doi: 10.21873/anticanres.11091. [DOI] [PubMed] [Google Scholar]
- 27.Wang AS, Ong PF, Chojn owski A, Clavel C, Dreesen O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci Rep. 2017;7:15678. doi: 10.1038/s41598-017-15901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein S. Replicative senescence: The human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 29.Wheelock MJ, Johnson KR. Cadherins as Modulators of Cellular Phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 30.De P, Carlson JH, Wu H, Marcus A, Leyland-Jones B, Dey N. Wnt-beta-catenin pathway signals metastasis-associated tumor cell phenotypes in triple negative breast cancers. Oncotarget. 2016;7:43124–43149. doi: 10.18632/oncotarget.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauro L, Sisci D, Bartucci M, Salerno M, Kim J, Tam T, Guvakova MA, Ando S, Surmacz E. SHC-α5β1 integrin interactions regulate breast cancer cell adhesion and motility. Exp Cell Res. 1999;252:439–448. doi: 10.1006/excr.1999.4639. [DOI] [PubMed] [Google Scholar]
- 32.Jackson JG, Yoneda T, Clark GM, Yee D. Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clin Cancer Res. 2000;6:1135–1139. [PubMed] [Google Scholar]
- 33.Xiao L, Wang YC, Li WS, Du Y. The role of mTOR and phospho-p70S6K in pathogenesis and progression of gastric carcinomas: An immunohistochemical study on tissue micro-array. J Exp Clin Cancer Res. 2009;28:152. doi: 10.1186/1756-9966-28-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malley CO, Pidgeon GP. The mTOR pathway in obesity driven gastrointestinal cancers: Potential targets and clinical trials. BBA Clin. 2016;5:29–40. doi: 10.1016/j.bbacli.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: The PI3K/Akt/mTOR pathway. Lab Invest. 2010;90:1406–1414. doi: 10.1038/labinvest.2010.133. [DOI] [PubMed] [Google Scholar]
- 36.Carneiro BA, Meeks JJ, Kuzel TM, Scaranti M, Abdulkadir SA, Giles FJ. Emerging therapeutic targets in bladder cancer. Cancer Treat Rev. 2015;41:170–178. doi: 10.1016/j.ctrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Apostolatos AH, Ratnayake WS, Win-Piazza H, Apostolatos CA, Smalley T, Kang L, Salup R, Hill R, Acevedo-Duncan M. Inhibition of atypical protein kinase C-ι effectively reduces the malignancy of prostate cancer cells by downregulating the NF-κB signaling cascade. Int J Oncol. 2018;53:1836–1846. doi: 10.3892/ijo.2018.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratnayake WS, Apostolatos AH, Ostrov DA, Acevedo-Duncan M. Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis. Int J Oncol. 2017;51:1370–1382. doi: 10.3892/ijo.2017.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto-Leite R, Arantes-Rodrigues R, Sousa N, Oliveira PA, Santos L. mTOR inhibitors in urinary bladder cancer. Tumor Biol. 2016;37:11541–11551. doi: 10.1007/s13277-016-5083-1. [DOI] [PubMed] [Google Scholar]
- 40.Alayev A, Salamon RS, Schwartz NS, Berman AY, Wiener SL, Holz MK. Combination of rapamycin and resveratrol for treatment of bladder cancer. J Cell Physiol. 2017;232:436–446. doi: 10.1002/jcp.25443. [DOI] [PubMed] [Google Scholar]
- 41.Alayev A, Berger SM, Kramer MY, Schwartz NS, Holz MK. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J Cell Biochem. 2015;116:450–547. doi: 10.1002/jcb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islam SMA, Dey A, Patel R, Smalley T, Acevedo-Duncan M. Atypical Protein Kinase-C inhibitors exhibit a synergistic effect in facilitating DNA damaging effect of 5-fluorouracil in colorectal cancer cells. Biomed Pharmacother. 2020;120:109665. doi: 10.1016/j.biopha.2019.109665. [DOI] [PubMed] [Google Scholar]
- 43.Orlando DA, Lin CY, Bernard A, Wang JY, Socolar JE, Iversen ES, Hartemink AJ, Haase SB. Global control of cell-cycle transcription by coupled CDK and network oscillators. Nature. 2008;453:944–947. doi: 10.1038/nature06955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin VA, Panchabhai SC, Shen L, Kornblau SM, Qiu Y, Baggerly KA. Different changes in protein and phosphoprotein levels result from serum starvation of high-grade glioma and adenocarcinoma cell lines. J Proteome Res. 2010;9:179–191. doi: 10.1021/pr900392b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. J Chem Biol. 2008;1:27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.