ABSTRACT
Background
Blueberries are dietary sources of polyphenols, specifically anthocyanins. Anthocyanins have been identified as having a strong association with type 2 diabetes risk reduction; however, to date few human clinical trials have evaluated the potential beneficial health effects of blueberries in populations with type 2 diabetes.
Objectives
We investigated the effects of blueberry consumption for 8 wk on cardiometabolic parameters in men with type 2 diabetes.
Methods
In a double-blind, parallel-arm, randomized controlled trial, 52 men who are US veterans [mean baseline characteristics: age, 67 y (range: 51–75 y); weight, 102 kg (range: 80–130 kg); BMI (in kg/m2), 34 (range: 26–45)] were randomly assigned to 1 of 2 intervention groups. The interventions were either 22 g freeze-dried blueberries or 22 g placebo. The study participants were asked to consume 11 g freeze-dried blueberries or placebo with each of their morning and evening meals along with their typical diet.
Results
Mean ± SE hemoglobin A1c (7.1% ± 0.1% compared with 7.5% ± 0.2%; P = 0.03), fructosamine (275.5 ± 4.1 compared with 292.4 ± 7.9 µmol/L; P = 0.04), triglycerides (179.6 ± 10.1 compared with 199.6 ± 19.9 mg/dL; P = 0.03), aspartate transaminase (23.2 ± 1.4 compared with 30.5 ± 2.7 units/L; P = 0.02), and alanine transaminase (35.6 ± 1.5 compared with 48.3 ± 2.9 units/L; P = 0.0003) were significantly lower for those consuming blueberries for 8 wk than for those consuming the placebo. Fasting plasma glucose concentrations; serum insulin, total cholesterol, LDL-cholesterol, HDL-cholesterol, and C-reactive protein concentrations; blood pressure; and body weight were not significantly different after 8 wk consumption of blueberries compared with the placebo.
Conclusions
Consumption of 22 g freeze-dried blueberries for 8 wk may beneficially affect cardiometabolic health parameters in men with type 2 diabetes.
This trial was registered at clinicaltrials.gov as NCT02972996.
Keywords: blueberries, polyphenols, flavonoids, anthocyanins, type 2 diabetes
Introduction
Type 2 diabetes develops owing to a continuous loss of β-cell insulin secretion frequently accompanied by insulin resistance (1). In the United States, >30.3 million individuals, ∼9.4% of the population, have diabetes; type 2 diabetes accounts for 90%–95% of all diabetes cases. Prevalence of type 2 diabetes is increasing owing to aging, physical inactivity, overweight [BMI (in kg/m2) > 25], and obesity (BMI > 30) status, all of which are serious risk factors (2). Hypertension and dyslipidemia are typical comorbidities presenting with type 2 diabetes and are risk factors for atherosclerotic cardiovascular disease (ASCVD). ASCVD is defined as coronary artery disease, cerebrovascular disease, or peripheral artery disease, and is the major cause of morbidity and mortality for those with type 2 diabetes (3).
The American Diabetes Association Standards of Medical Care in Diabetes presents guidance for evidence-based disease management (4). An important component of type 2 diabetes management is the provision of diabetes self-management education and support services. The goals of interventions for type 2 diabetes are to delay microvascular and macrovascular complications, along with maintaining quality of life. This requires control of glycemia and cardiovascular disease risk factor management (5). Robust evidence supports the efficacy and cost effectiveness of medical nutrition therapy (MNT) as a component of quality care for type 2 diabetes (6, 7). MNT that maintains or improves glycemic targets, weight management, and cardiovascular disease risk factors within individualized treatment goals is recommended for adults with type 2 diabetes. In addition, an important goal of MNT for type 2 diabetes is to support healthful eating patterns, which emphasizes a variety of nutrient-dense foods in appropriate portion sizes (8). Currently, the amount of carbohydrate required for ideal human health is debated, although the quality of carbohydrate foods chosen in the management of type 2 diabetes should be addressed as part of an individualized eating plan for optimum nutrition. High-quality carbohydrate foods should be high in dietary fiber, vitamins, and minerals, and low in added sugars, fats, and sodium. Fruits and vegetables are considered high-quality carbohydrate foods (9). Various components of fruits and vegetables contribute to their beneficial health effects, but there has been keen interest in the possibility that plant polyphenols may have a role (10).
Blueberries are dietary sources of polyphenols, specifically anthocyanins. Anthocyanins may comprise ≤60% of total phenolic compounds in blueberries, followed by hydroxycinnamic acid derivatives (especially chlorogenic acid), flavonols, and flavanols (11). Anthocyanins have been identified as having a strong association with type 2 diabetes risk reduction (12–15); however, to date there are limited human clinical trials evaluating the beneficial health effects of blueberries in populations with type 2 diabetes. Several randomized, parallel-design human studies have reported that consumption of blueberries may beneficially affect early biomarkers of cardiovascular disease and diabetes, such as blood pressure, lipids, oxidative stress, and vascular function (16–20). However, the effects of blueberry consumption on glycemic control have presented inconsistent results in these studies (16, 17, 19, 20).
Here we conducted a randomized, double-blind, human intervention trial with 22 g freeze-dried blueberries (equivalent to ∼1 cup fresh per day), implementing a parallel-arm design with a placebo control for 8 wk. We hypothesized that the intake of 11 g freeze-dried blueberries (equivalent to 1/2 cup fresh), consumed with morning and evening meals, would have promising effects on cardiometabolic parameters including glycemic control, lipids, blood pressure, and inflammation in men with type 2 diabetes.
Methods
Study population
Men aged 45–75 y were recruited from the Stratton Veterans Administration (VA) Medical Center in Albany, NY, USA from January 2017 to March 2018. The gender and age range were chosen because the potential study participants meeting these criteria have a greater frequency of type 2 diabetes at the Medical Center. Inclusion in the study was based on having a medical diagnosis of type 2 diabetes for ≥6 mo; glycated hemoglobin (HbA1c) > 6.5 and <9; and BMI > 25. Exclusion criteria were for those who used insulin; had chronic kidney disease (glomerular filtration rate ≤ 45 mL/min), liver cirrhosis, gastrointestinal disease, pancreatic disease, or malabsorption syndromes; had lost 10% of their body weight within the past 12 mo or were planning to initiate weight loss; were routinely participating in a heavy exercise program (≥5 h physical exercise such as running or cycling per week) or initiated an exercise program during the study; were heavy smokers (>20 cigarettes/d); were unable or unwilling to give informed consent or communicate with study staff; had other medical, psychiatric, or behavioral factors that in the judgment of the principal investigators may have interfered with study participation or the ability to follow the intervention protocol; and/or had a known (self-reported) allergy or adverse reaction to blueberries or blueberry products. Potential participants underwent a medical evaluation by a physician or nurse practitioner. The study endocrinologist or nurse practitioner and research investigators reviewed the participant's medical history, medication use, and previous clinical laboratory tests (including a profile of hematological and biochemical parameters including HbA1c), and used the clinical screening and evaluation results to identify individuals who qualified for the study. Potential participants were required to attend an individual information session where the study coordinator detailed the requirements, risks, and benefits of participating in the study. After the session, those interested were required to complete and sign a consent form and complete a study application. Participants gave their informed consent to take part in the study, and the institutional review board approved the intervention protocol, which was followed in accordance with the ethical standards of the Albany Stratton VA Institutional Review Board. The participants received monetary compensation for their travel and meal expenses for their participation in the study.
Study design
The study (NCT02972996) was a double-blind, placebo-controlled, parallel-arm study with 2 intervention groups for a total study duration of 8 wk. The randomization plan was generated by the study biostatistician before the start of the study. Participants were assigned to the randomization plan in order of recruitment. The study was conducted at the Stratton VA Medical Center in Albany, NY, USA. Parti-cipants were randomly assigned to 1 of 2 intervention groups: either 1) 22 g freeze-dried blueberries along with their free-living diet per day or 2) 22 g placebo powder (matched in energy and carbohydrate content to the freeze-dried blueberries) along with their free-living diet per day. One cup of fresh blueberries is equivalent to ∼22 g freeze-dried blueberries. This amount was chosen because it is a reasonable amount of fruit for subjects with type 2 diabetes to consume. Participants were asked to consume 11 g freeze-dried blueberries or placebo, reconstituted with 240 mL water, with their morning and evening meals for 8 wk. Participants were counseled by a registered dietitian/nutritionist (RDN) regarding how the intervention was to be consumed, and adherence was monitored by a daily questionnaire. In addition, the number of used and unused intervention packets were tracked during each study visit. Study adherence was defined as missing ≤2 doses/wk. There was a total of 3 study visits: at baseline, 4 wk, and 8 wk. Participants received a 4-wk supply of the study interventions during study visits 1 (baseline) and 2 (4 wk) at the Stratton VA Medical Center. The interventions were supplied in packets by the US Highbush Blueberry Council and were differentiated by labels marked either A or B. The interventions were blinded to the study investigators and participants. The US Highbush Blueberry Council held the code to the study interventions. All analyses were completed before the blinding code was revealed.
Study interventions
The blueberry intervention consisted of freeze-dried highbush blueberries [50/50 blend of tifblue (Vaccinium virgatum) and rubel (Vaccinium corymbosum)] from the same harvest. The blueberry intervention was produced and packed in September 2016 by Mercer Foods. The placebo intervention consisted of maltodextrin, fructose, artificial and natural blueberry flavoring, colorants (red and blue), citric acid, and silica dioxide. The placebo intervention was produced and packed in July 2016 by Covance Labs. The study interventions, both blueberries and placebo, were stored at 10°C in the pharmacy at the Stratton VA Medical Center until use. Samples of the interventions were collected, then stored at 10°C for independent nutrient and polyphenol analyses at the end of the study. Proximate sugar profile, total phenolics, and anthocyanins were determined by International Chemistry Testing. Phenolics were analyzed using the Folin–Ciocalteu reagent and the method of Slinkard and Singleton (21). Anthocyanins were analyzed by a pH differential method measuring the monomeric anthocyanin pigments (22). Table 1 shows the nutrient composition of the interventions.
TABLE 1.
Nutrient composition of freeze-dried blueberries and placebo compared with fresh blueberries1
| Nutrient | Freeze-dried blueberries (22 g)2 | Placebo (22 g)2 | Fresh blueberries (per 1 cup)3 |
|---|---|---|---|
| Energy, kcal | 85 | 84 | 84 |
| Carbohydrates, g | 20.2 | 20.9 | 21.5 |
| Protein, g | 0.6 | ND | 1.1 |
| Fat, g | 0.2 | ND | 0.5 |
| Fiber, g | 5.2 | 0.3 | 3.6 |
| Vitamin C, mg | 1.7 | ND | 14.4 |
| Potassium, mg | 104.7 | 3.2 | 114 |
| Anthocyanins, mg | 261.8 | 2.2 | 241.6 |
| Phenolics, mg | 765.6 | 48.8 | Unknown |
ND, nondetectable.
Analyzed by International Chemistry Testing.
USDA, Agricultural Research Service, FoodData Central, FDC ID: 171711, USDA Database for Flavonoid Content of Selected Foods, release 3.2 (2015), NDB no. 09050.
Dietary intake assessment
Study participants were responsible for their own meals and continued to follow their typical patterns of food intake. Dietary intake data were collected and analyzed by 8 weekly 3-d (2 weekdays and 1 weekend day) food records throughout the study duration. Three-day mean macronutrient and micronutrient intakes were analyzed using Nutritionist Pro version 7.4 (2018; Axxya System). At the first study visit, participants received instruction, using appropriate food models and measuring utensils, about completing food records from an RDN. At the second and third study visits, the RDN reviewed the 3-d food records for accuracy with the participant. The days of data collection, 2 weekdays and 1 weekend day, ensured proportional representation of food intake for weekdays and weekend days. All food intake data entry was performed by an RDN and the data entry was confirmed by a second RDN for quality control.
Participants completed a daily questionnaire regarding their general health; any consumption of prescription and over-the-counter medications; factors related to intervention compliance; and physical activity performed. The questionnaire also gave participants the opportunity to write any questions they had regarding the study intervention. Participants were encouraged to maintain their habitual diet and normal physical activity throughout the study. Participants were asked to maintain their same medication usage, dose, and timing without any change throughout the study.
Anthropometrics and blood pressure
Height was measured with participants positioned in the Frankfort frontal plane. Each participant's height without shoes was measured to the nearest 0.1 cm with a wall-mounted stadiometer. Weight was assessed using a digital scale (DETECTO) to the nearest 0.1 kg. Waist circumferences were measured above the right ilium on the midaxillary line using a fiberglass tape measure by 2 trained individuals following a written protocol, with almost 90% of the measures performed by 1 of the 2 individuals. Blood pressure and heart rate were collected according to a standard protocol. Participants were taken to a quiet room with subdued lighting and rested quietly for ∼10 min. A cuff of appropriate size was attached to the upper right arm according to the manufacturer's instructions. Blood pressure and heart rate were measured in duplicate using an automatic monitor (Welch Allyn Spot Vital Signs), which was programmed to take an initial reading and then a reading after 5 min per a standardized protocol. Participants were instructed to refrain from consuming coffee for ≥30 min before the measurement. With the exception of height, the measurements of body weight, waist circumference, and blood pressure were obtained at baseline, at 4 wk, then after 8 wk of the study.
Biological sample collection and analysis
Twice during the study, at baseline and after the 8-wk study period, blood was collected from the antecubital veins of participants after a 12-h fast. Serum was collected in serum separator vacutainer tubes, then allowed to clot at room temperature for a minimum of 20 min, then spun at 3000 rpm for 5 min at room temperature at the Stratton VA Medical Center Clinical Laboratory, Albany, NY, USA. Serum concentrations of total, HDL, and LDL cholesterol, triglycerides, and high-sensitivity C-reactive protein (CRP), a complete metabolic panel, and plasma glucose concentrations were determined by spectrophotometric procedures using a Siemens Vista 500 Analyzer. HbA1c, collected using potassium EDTA–coated tubes, was determined by a Tosoh G8 Analyzer using HPLC methodology. These analyses were also performed at the Stratton VA Medical Center Clinical Laboratory. Serum insulin concentrations were measured by immunoassay with chemiluminescent detection on a Siemens Immulite 2000 XPi Immunoassay System, and serum fructosamine was measured by using spectrophotometry at Quest Diagnostics. Twice during the study, at baseline and after the 8-wk study period, participants provided a urine sample of ≥10 mL after a 12-h fast. Urine was collected in plastic containers and was stored on ice during collection and until samples were analyzed at the Stratton VA Medical Center Clinical Laboratory. Urine was analyzed for the microalbumin-to-creatinine ratio (MA:CR). Microalbumin was measured by nephelometry and creatinine was measured by enzymatic testing using a Siemens Vista 500 Analyzer. MA:CR was then calculated to determine the level of nephropathy and expressed as mg/g. MA:CR of 30–300 mg/g = evidence of early nephropathy and >300 mg/g = nephropathy.
Statistical analysis
To determine the effect of the blueberry intervention, statistical analyses were performed in SAS version 9.4 (SAS Institute, Inc.). For blood pressure, weight, BMI, and waist circumference, a repeated-measures linear mixed-model ANOVA with covariates of age, baseline (preintervention values), BMI, and weight was used in all models. For laboratory biomarkers, a linear mixed-model ANOVA with covariates of age, baseline (preintervention values), BMI, and weight was used in all models. For the lipid data, subgroup analysis was performed in those participants prescribed lipid-lowering medication. Data were tested for normality with the Shapiro–Wilk statistic and by inspection of stem-leaf plots and normal probability plots of residuals. In all statistical comparisons, differences with P < 0.05 were considered significant. Results are presented as means ± SEs unless otherwise stated.
The sample size for this study was based on an SD from a previous study conducted by Johnson et al. (18), which showed that 22 g freeze-dried blueberries lowered systolic and diastolic blood pressure. A sample size calculation was estimated for a parallel-arm study with >80% power to detect a significant difference at P = 0.05. Twenty-four parti-cipants per group were needed, and we determined to recruit 30/group based on a 20% dropout rate. No power calculation was determined for HbA1c and fructosamine because of the limited number of published studies evaluating the health effects of blueberries in type 2 diabetes.
Results
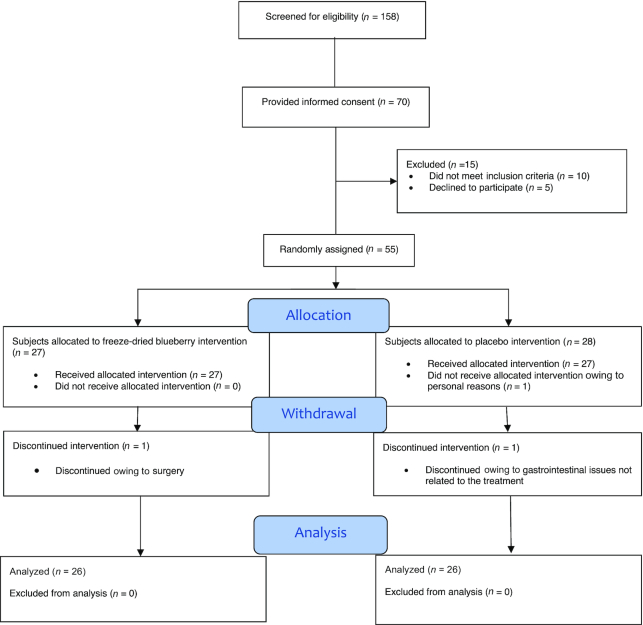
Seventy potential study participants provided informed consent, 55 of whom met the inclusion criteria and were selected for study participation. Three participants withdrew from the study. One participant did not start the study protocol owing to personal reasons, 1 discontinued because of gastrointestinal issues not related to the intervention administration, and 1 discontinued because of surgery. A total of 52 participants successfully completed the study protocol. Twenty-six participants completed the freeze-dried blueberry arm and 26 participants completed the placebo arm (Figure 1).
FIGURE 1.
CONSORT diagram for the study trial.
Table 2 presents the baseline characteristics of the 52 participants included in the final data. Participants were men, with an age range of 51–75 y and BMI range of 26–45. Body weight (102.4 ± 0.4 kg compared with 102.4 ± 0.4 kg; P = 0.19) and BMI (34.0 ± 0.1 compared with 33.7 ± 0.1; P = 0.13) were not significantly different after 8 wk of consumption of freeze-dried blueberries compared with placebo, respectively. Noninsulin diabetes medications were prescribed to 100% of the participants with 81% biguanides (metformin), 15% sulfonyl-ureas (glimepiride, glyburide, and glipizide), 2% thiazolidinediones (pioglitazone), and 2% dipeptidyl peptidase-4 inhibitors (sitagliptin and saxagliptin). The freeze-dried blueberry group participants at baseline showed n = 21 had treated hypertension with blood pressure values within the normal range; n = 3 had treated hypertension with stage 1 hypertension (systolic blood pressure 130–139 mm Hg or diastolic blood pressure 80–89 mm Hg); and n = 2 untreated had blood pressure values within the normal range. In the placebo group at baseline, n = 20 had treated hypertension with blood pressure values within the normal range; n = 1 had treated hypertension with stage 1 hypertension (systolic blood pressure 130–139 mm Hg or diastolic blood pressure 80–89 mm Hg); and n = 5 untreated had blood pressure values within the normal range. No medication changes were made throughout the 8 wk of the study, with the exception of 1 participant who changed their noninsulin diabetes medication after 4 wk of the study. Statistical analysis was run with and without this 1 participant's data with no changes in study results; therefore, it was determined to use the participant's data. Participants’ dietary and medical history showed that 83% had received MNT for type 2 diabetes. At baseline, participants in both intervention groups had equal glycemic control as assessed by HbA1c. Approximately 4% of participants used tobacco products (1 participant smoked ∼10 cigarettes/d and 1 participant chewed tobacco twice daily).
TABLE 2.
Characteristics of study participants at baseline1
| Blueberry (n = 26) | Placebo (n = 26) | |||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 4 wk | 8 wk | Baseline | 4 wk | 8 wk |
| Age, y | 67.1 ± 1.1 | – | – | 66.7 ± 1.1 | – | – |
| Height, cm | 173.6 ± 1.3 | – | – | 174.3 ± 1.3 | – | – |
| Weight, kg | 102.7 ± 1.1 | 102.5 ± 0.4 | 102.4 ± 0.4 | 102.4 ± 1.4 | 102.1 ± 0.4 | 102.4 ± 0.4 |
| BMI, kg/m2 | 34.2 ± 0.7 | 34.1 ± 0.1 | 34.0 ± 0.1 | 34.0 ± 0.9 | 33.8 ± 0.1 | 33.7 ± 0.1 |
| Waist circumference, cm | 116.4 ± 1.0 | 117.4 ± 0.5 | 116.8 ± 0.5 | 116.4 ± 1.0 | 116.1 ± 0.5 | 116.4 ± 0.5 |
| Race | ||||||
| White, alone | 25 (96) | 24 (92) | ||||
| Black or African American, alone | 1 (4) | 2 (8) | ||||
| Comorbidities | ||||||
| Hypertension | 24 (92) | 21 (81) | ||||
| Coronary heart disease | 19 (73) | 20 (77) | ||||
| Dyslipidemia | 17 (65) | 23 (88) | ||||
| Noninsulin diabetes medication users | 26 (100) | 26 (100) | ||||
| Biguanides | 20 (77) | 21 (80) | ||||
| Sulfonylureas | 5 (19) | 3 (12) | ||||
| Thiazolidinediones | 0 (0) | 1 (4) | ||||
| Dipeptidyl peptidase-4 inhibitors | 1 (4) | 1 (4) | ||||
| Antihypertensive medication users | 24 (92) | 21 (81) | ||||
| Angiotensin1 receptor blockers | 4 (16) | 5 (23) | ||||
| β-receptor blockers | 3 (13) | 1 (5) | ||||
| Angiotensin-converting enzyme inhibitors | 10 (42) | 9 (43) | ||||
| Calcium-channel blockers | 1 (4) | 1 (5) | ||||
| Diuretics | 6 (25) | 5 (24) | ||||
| Lipid-lowering medication users | 17 (65) | 23 (88) | ||||
| HMG-CoA reductase inhibitors | 17 (65) | 23 (88) | ||||
| Three-day food record dietary intake at baseline | ||||||
| Energy, kcal/d | 1824 ± 99 | 1840 ± 183 | ||||
| Carbohydrate, g | 186 ± 10 | 191 ± 18 | ||||
| Protein, g | 81 ± 5 | 80 ± 6 | ||||
| Fat, g | 84 ± 6 | 84 ± 11 | ||||
| Fiber, g | 15 ± 1 | 15 ± 2 | ||||
All values are means ± SEs or n (%). Statistical comparisons were made by ANOVA. No differences between groups were observed at baseline. HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA.
Mean daily energy intake was 1824 ± 99 kcal (41% energy from carbohydrate, 18% energy from protein, and 41% energy from fat) and 1840 ± 183 kcal (42% energy from carbohydrate, 17% energy from protein, and 41% energy from fat) for the freeze-dried blueberry and placebo groups, respectively, and did not differ significantly between the 2 intervention groups. There were no significant differences in either of the intervention groups on changes in carbohydrate, protein, fat, cholesterol, or fiber intakes, as determined by the 3-d food records during the course of the intervention; 92% of participants completed ≥8 sets of 3-d food records. In addition, review of the 3-d food records showed that participants did not consume high-anthocyanin foods throughout the study period, although they were not asked to change their habitual diets. Participants from each group consumed on average 1 serving of fruit and 2 servings of vegetables per day; this did not include the freeze-dried blueberry intervention, which may be considered 2 servings of fruit. Anthocyanins and other nutrients in the intervention products were found to be stable at the end of the study when compared with analyses at the beginning of the study (data not shown).
Participants were asked to self-monitor their adherence to the consumption of the interventions (freeze-dried blueberry and placebo packets) on a daily questionnaire. Furthermore, participants verbally reported the amounts of intervention packets they consumed to researchers. Researchers also counted the number of returned intervention packets at the study visits. The daily questionnaires, subject self-reports, and numbers of intervention packets showed 98% adherence for the freeze-dried blueberry and placebo groups.
Fasting plasma glucose and serum insulin were not significantly different after 8 wk of consumption of freeze-dried blueberries compared with placebo, although HbA1c (7.1% ± 0.1% compared with 7.5% ± 0.2%; P = 0.03) and fructosamine (275.5 ± 4.1 compared with 292.4 ± 7.9 µmol/L; P = 0.04) were significantly lower for those consuming freeze-dried blueberries for 8 wk than for those consuming the placebo (Table 3).
TABLE 3.
Fasting biomarkers of glycemic control and complete metabolic panel in men with type 2 diabetes at baseline and after 8 wk consuming freeze-dried blueberries or placebo1
| Blueberry (n = 26) | Placebo (n = 26) | ||||
|---|---|---|---|---|---|
| Variable | Baseline2 | 8 wk3 | Baseline2 | 8 wk3 | P 4 |
| Glucose, mg/dL | 148.4 ± 4.8 | 146.3 ± 5.4 | 150.8 ± 4.7 | 153.3 ± 10.2 | 0.54 |
| Insulin, µIU/mL | 12.8 ± 1.4 | 16.8 ± 2.3 | 13.4 ± 2.1 | 13.2 ± 4.4 | 0.48 |
| Glycated hemoglobin, % | 7.2 ± 0.2 | 7.1 ± 0.1 | 7.4 ± 0.2 | 7.5 ± 0.2 | 0.03 |
| Fructosamine, µmol/L | 290.5 ± 8.7 | 275.5 ± 4.1 | 289.5 ± 9.2 | 292.4 ± 7.9 | 0.04 |
| Sodium, mEq/L | 140.1 ± 0.4 | 139.8 ± 0.4 | 139.7 ± 0.5 | 139.1 ± 0.7 | 0.41 |
| Potassium, mEq/L | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.4 ± 0.1 | 4.3 ± 0.1 | 0.89 |
| Chloride, mEq/L | 104.1 ± 0.6 | 103.6 ± 0.5 | 103.7 ± 0.5 | 104.0 ± 0.9 | 0.69 |
| Carbon dioxide, mEq/L | 26.9 ± 0.6 | 27.1 ± 0.5 | 27.2 ± 0.3 | 26.4 ± 0.9 | 0.47 |
| Urea nitrogen, mg/dL | 19.9 ± 1.2 | 17.2 ± 0.9 | 16.6 ± 0.8 | 19.1 ± 1.6 | 0.32 |
| Creatinine, mg/dL | 1.0 ± 0.04 | 1.0 ± 0.02 | 0.9 ± 0.03 | 1.0 ± 0.04 | 0.94 |
| Calcium, mg/dL | 8.8 ± 0.1 | 8.8 ± 0.1 | 8.9 ± 0.1 | 8.9 ± 0.1 | 0.35 |
| eGFR, mL/min | 76.7 ± 3.5 | 80.9 ± 2.0 | 85.8 ± 3.7 | 79.7 ± 3.7 | 0.77 |
| Protein, total, g/dL | 7.3 ± 0.1 | 7.2 ± 0.1 | 7.1 ± 0.1 | 7.2 ± 0.1 | 0.66 |
| Albumin, g/dL | 3.9 ± 0.04 | 3.9 ± 0.03 | 3.9 ± 0.04 | 3.9 ± 0.06 | 0.93 |
| Bilirubin, total, mg/dL | 0.6 ± 0.04 | 0.6 ± 0.03 | 0.7 ± 0.08 | 0.7 ± 0.06 | 0.38 |
| Alkaline phosphatase, units/L | 77.8 ± 4.6 | 74.7 ± 1.6 | 76.5 ± 5.1 | 78.5 ± 2.9 | 0.27 |
| AST, units/L | 23.5 ± 1.9 | 23.2 ± 1.4 | 24.9 ± 2.9 | 30.5 ± 2.7 | 0.02 |
| ALT, units/L | 36.5 ± 2.7 | 35.6 ± 1.5 | 39.9 ± 3.8 | 48.3 ± 2.9 | 0.0003 |
| Urine MA:CR, mg/g | 124.5 ± 90.1 | 123.8 ± 77.9 | 180.1 ± 80.4 | 178.9 ± 78.9 | 0.74 |
ALT, alanine transaminase; AST, aspartate transaminase; eGFR, estimated glomerular filtration rate; MA:CR, microalbumin-to-creatinine ratio.
Values are arithmetic means ± SEs from samples collected before the dietary intervention (preintervention values).
Values are least-squares means ± SEs (adjusted for the baseline values) from a linear mixed-model ANOVA that included covariates of age, baseline (preintervention values), BMI, and weight.
Values are for the blueberry and placebo comparison at 8 wk.
Serum total cholesterol, LDL cholesterol, and HDL cholesterol were not significantly different after 8 wk of consumption of freeze-dried blueberries compared with the placebo. Serum triglycerides were significantly lower for those participants consuming freeze-dried blueberries for 8 wk than for those consuming the placebo (179.6 ± 10.1 and 199.6 ± 19.9 mg/dL, respectively; P = 0.03). In addition, serum total cholesterol (148.8 ± 4.9 compared with 163.6 ± 3.4 mg/dL; P = 0.03), LDL cholesterol (77.2 ± 6.5 compared with 84.1 ± 5.4 mg/dL; P = 0.04), and triglycerides (160.6 ± 13.1 compared with 183.9 ± 10.7 mg/dL; P = 0.03) were significantly lower for those consuming freeze-dried blueberries (n = 17) than for those consuming the placebo (n = 23) in those using lipid-lowering medication. High-sensitivity CRP was not significantly different after 8 wk of consumption of freeze-dried blueberries compared with the placebo (Table 4). Systolic blood pressure, diastolic blood pressure, and heart rate were not significantly different after 8 wk of consumption of freeze-dried blueberries compared with the placebo (Table 5).
TABLE 4.
Fasting serum lipids, lipoproteins, and CRP in men with type 2 diabetes at baseline and after 8 wk consuming freeze-dried blueberries or placebo1
| Blueberry (n = 26) | Placebo (n = 26) | ||||
|---|---|---|---|---|---|
| Variable | Baseline2 | 8 wk3 | Baseline2 | 8 wk3 | P 4 |
| Total cholesterol, mg/dL | 161.4 ± 9.6 | 155.8 ± 3.8 | 166.4 ± 9.6 | 161.2 ± 7.3 | 0.51 |
| LDL cholesterol, mg/dL | 82.8 ± 7.8 | 84.2 ± 5.0 | 87.2 ± 8.4 | 83.2 ± 9.6 | 0.93 |
| HDL cholesterol, mg/dL | 44.7 ± 1.9 | 47.1 ± 1.2 | 48.6 ± 2.6 | 44.1 ± 2.2 | 0.25 |
| Triglycerides, mg/dL | 186.1 ± 24.6 | 179.6 ± 10.1 | 176.4 ± 15.3 | 199.6 ± 19.9 | 0.03 |
| CRP, mg/dL | 0.57 ± 0.1 | 0.50 ± 0.1 | 0.51 ± 0.1 | 0.50 ± 0.1 | 0.99 |
| Lipid-lowering medication users5 | |||||
| Total cholesterol, mg/dL | 164.2 ± 6.9 | 148.8 ± 4.9 | 164.3 ± 10.6 | 163.6 ± 3.4 | 0.03 |
| LDL cholesterol, mg/dL | 102.9 ± 19.7 | 77.2 ± 6.5 | 95.7 ± 40.7 | 84.1 ± 5.4 | 0.04 |
| HDL cholesterol, mg/dL | 46.4 ± 2.6 | 44.9 ± 1.5 | 50.3 ± 2.8 | 45.6 ± 1.3 | 0.74 |
| Triglycerides, mg/dL | 166.9 ± 24.7 | 160.6 ± 13.1 | 164.5 ± 12.1 | 183.9 ± 10.7 | 0.03 |
| CRP, mg/dL | 0.51 ± 0.1 | 0.56 ± 0.1 | 0.52 ± 0.2 | 0.54 ± 0.1 | 0.86 |
CRP, C-reactive protein.
Values are arithmetic means ± SEs from samples collected before the dietary intervention (preintervention values).
Values are least-squares means ± SEs (adjusted for the baseline values) from a linear mixed-model ANOVA that included covariates of age, baseline (preintervention values), BMI, and weight.
Values are for the blueberry and placebo comparison at 8 wk.
n = 17, blueberry lipid-lowering medication users; n = 23, placebo lipid-lowering medication users.
TABLE 5.
Blood pressure and heart rate in men with type 2 diabetes at baseline and after 8 wk consuming freeze-dried blueberries or placebo
| Blueberry (n = 26) | Placebo (n = 26) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Baseline1 | 4 wk2 | 8 wk2 | Baseline1 | 4 wk2 | 8 wk2 | P 3 |
| Systolic blood pressure, mm Hg | 130 ± 3 | 129 ± 3 | 126 ± 3 | 126 ± 3 | 126 ± 3 | 126 ± 3 | 0.56 |
| Diastolic blood pressure, mm Hg | 73 ± 1 | 75 ± 1 | 75 ± 1 | 76 ± 2 | 76 ± 1 | 75 ± 1 | 0.48 |
| Heart rate, beats/min | 70 ± 2 | 72 ± 2 | 70 ± 2 | 71 ± 2 | 69 ± 2 | 71 ± 2 | 0.72 |
Values are arithmetic means ± SEs from samples collected before the dietary intervention (preintervention values).
Values are least-squares means ± SEs (adjusted for the baseline values) from a repeated-measures linear mixed-model ANOVA that included covariates of age, baseline (preintervention values), BMI, and weight.
Values are for the blueberry and placebo comparison at 8 wk.
Serum liver enzymes [aspartate transaminase (AST): 23.2 ± 1.4 compared with 30.5 ± 2.7 units/L; P = 0.02, and alanine transaminase (ALT): 35.6 ± 1.5 compared with 48.3 ± 2.9 units/L; P = 0.0003] were significantly lower for those consuming freeze-dried blueberries for 8 wk than for those consuming the placebo. Serum concentrations of blood urea nitrogen, of creatinine, and all other metabolic variables including urinary MA:CR were not significantly different after 8 wk of consumption of freeze-dried blueberries compared with the placebo (Table 3).
Discussion
This randomized, double-blind, placebo-controlled, parallel-design human intervention study was the first to our knowledge to assess the health effects of consuming 22 g freeze-dried blueberries (∼1 cup fresh blueberries) daily for 8 wk in men with type 2 diabetes. Results showed that HbA1c and fructosamine, biomarkers of glycemic control, were significantly lower for those consuming freeze-dried blueberries for 8 wk than for those consuming the placebo. Total cholesterol, LDL cholesterol, HDL cholesterol, and CRP concentrations, blood pressure, and body weight were not significantly different after 8 wk of consumption of freeze-dried blueberries compared with the placebo. However, triglyceride concentrations were significantly lower for those consuming freeze-dried blueberries for 8 wk than for those consuming the placebo. Further subgroup analyses demonstrated that in lipid-lowering medication users only, total cholesterol, LDL-cholesterol, and triglyceride concentrations were significantly lower for those consuming freeze-dried blueberries than for those consuming the placebo; the subgroup not taking lipid-lowering medication showed no statistically significant effect of freeze-dried blueberries on total cholesterol, LDL-cholesterol, and triglyceride concentrations. The liver enzymes AST and ALT were significantly lower for those consuming freeze-dried blueberries for 8 wk than for those consuming the placebo.
In the present study, participants consuming freeze-dried blueberries had lower HbA1c and fructosamine after 8 wk than those consuming the placebo, although fasting glucose and insulin concentrations were not significantly different. Participants also maintained their body weight throughout the study. To our knowledge, the effect of freeze-dried blueberry consumption for 8 wk on glycemic control in participants with type 2 diabetes has not been previously investigated. Human clinical trials with freeze-dried blueberries and glycemic control have presented inconsistent results. Some showed that consuming freeze-dried blueberries (ranging from 13 to 50 g) had no significant effect on glycemic control and surrogate markers of insulin sensitivity in adults with metabolic syndrome and risk factors for cardiovascular disease (16, 17, 19). However, another study showed daily consumption of 22.5 g freeze-dried blueberries for 6 wk improved insulin resistance in obese adults with insulin resistance (20). In addition, supplementation with 320 mg purified anthocyanins daily for 6 mo lowered fasting glucose along with a trend toward lowering HbA1c (P = 0.06) in adults with type 2 diabetes (23). HbA1c and fructosamine were not evaluated in several of the freeze-dried blueberry studies owing to shorter-term study durations and/or study participants having metabolic complications other than type 2 diabetes. Our participants were instructed throughout the study to consume the freeze-dried blueberries with their morning and evening meals, because a proposed mechanism of the polyphenol-rich blueberries is interference with carbohydrate digestion and glucose absorption through the inhibition of α-amylase and/or α-glucosidase activity in the intestinal epithelium (24, 25). Also, other mechanisms have shown that anthocyanins may delay intestinal absorption of glucose through inhibition of sodium glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2) (26). Further, anthocyanins may modify GLUT4 via AMP-activated protein kinase (AMPK) activation reducing glucose production in the liver (27). In addition, consuming freeze-dried blueberries with meals may have improved glycemic control in our participants by delaying carbohydrate breakdown and glucose absorption over the 8-wk study period. The results of a meta-analysis showed that polyphenols are associated with a significant reduction in HbA1c with the effect more pronounced in those with type 2 diabetes; when the analysis was restricted to longer clinical trials, i.e., >6 wk, a reduction of 0.4% in HbA1c was found (28). This result is similar to what was found in the present 8-wk study. HbA1c is a surrogate marker of glycemic control over the preceding 2–3 mo, and current clinical practice recognizes an individualized approach in using HbA1c in type 2 diabetes management (29). Fructosamine is strongly correlated with HbA1c and fasting glucose. It is a short-term glycemic marker of approximately a 2- to 4-wk duration and is useful in evaluating an earlier glycemic response. An advantage of the use of several measures of glycemic control is that they each provide distinctive aspects of type 2 diabetes physiology (30–32). We evaluated fructosamine at baseline and after 8 wk of our study. It would have been beneficial to measure fructosamine after 4 wk of the study to determine if the freeze-dried blueberries affected short-term glycemic control. Further research is warranted to evaluate blueberry consumption in those with type 2 diabetes using continuous glucose monitoring, along with short-term and long-term biomarkers of glycemic control in human clinical trials.
Individuals with type 2 diabetes have altered lipid and lipoprotein metabolism, causing an increased prevalence of dyslipidemia contributing to ASCVD (3). We found triglyceride concentrations were lowered in our participants with type 2 diabetes consuming freeze-dried blueberries, although our study participants’ baseline lipid concentrations were well controlled. In addition, in statistical analysis restricted to those participants using lipid-lowering medication, total cholesterol, LDL-cholesterol, and triglyceride concentrations were lower for those consuming freeze-dried blueberries than for those consuming the placebo. The synergistic effect of the combination of consuming freeze-dried blueberries along with lipid-lowering therapy may be a novel approach for decreasing risk of ASCVD in type 2 diabetes. Whereas there have been positive effects of blueberries and anthocyanins shown in studies in vitro and in animals with diabetic dyslipidemia, human research provides inconsistent results (33, 34). Previous research showed supplementation with 320 mg purified anthocyanins daily decreased triglycerides and LDL cholesterol, and increased HDL cholesterol, in participants with type 2 diabetes (n = 58; 58% male; ages 56–67 y; mean ± SD BMI: 25.2 ± 2.6) after 6 mo (23). However, some human clinical trials utilizing freeze-dried blueberries (ranging from 22.5 to 50 g) had no significant effect on triglycerides, LDL cholesterol, and HDL cholesterol in adults with metabolic syndrome and risk factors for cardiovascular disease after 6–8 wk (16, 19, 20). A recent clinical trial reported participants with metabolic syndrome consuming 26 g freeze-dried blueberries showed a significant increase in HDL-cholesterol concentrations with a trend toward a dose-related increase (17). More human research is required to differentiate between the effects of the blueberry fruit and those of the anthocyanin supplements on lipid and lipoprotein metabolism in those with well-controlled lipids and dyslipidemia in type 2 diabetes.
Although participants in this study had liver enzyme values within the normal range, those consuming freeze-dried blueberries had lower AST and ALT concentrations at the end of the intervention than those consuming the placebo. There are few studies in humans evaluating liver enzymes and blueberry consumption. Blueberries (equivalent to 50 g fresh) added to a weight-reducing diet for 6 wk lowered AST and ALT concentrations in overweight and obese women and men (35). Typically, AST and ALT concentrations are used as biomarkers for liver diseases or liver damage. Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent chronic diseases, affecting up to one-third of individuals in high-income countries. NAFLD is characterized by steatosis—liver fat deposition (36). Those with type 2 diabetes have an increased prevalence of NAFLD ≤70%–75%, and may be disposed to more severe forms of NAFLD along with increased progression to hepatocellular carcinoma (37). In the current study, there was insufficient evidence on baseline prevalence of NAFLD in our participants. Lifestyle intervention, such as decreasing body weight and increasing physical activity, is the standard of care for those with NAFLD, although anthocyanins may have a beneficial effect (38). In vitro studies show that anthocyanins, such as cyanidin 3-glucoside, may inhibit lipogenesis, promote lipolysis, and decrease steatosis by activating the AMPK pathway (39). Also, blueberry consumption stimulates liver peroxisome proliferator–activated receptor-α activity and decreases hepatic lipid accumulation in obese Zucker rats (40). In the current study, participants who consumed freeze-dried blueberries compared with those who consumed placebo had lower triglyceride concentrations and better glycemic control. These outcomes may have contributed to the lowering of liver enzymes which ameliorated hepatotoxicity. Future research directions should include human clinical trials evaluating effects of blueberry consumption in those with NAFLD.
The strengths and limitations of our study design warrant consideration. To our knowledge, this is one of the first human studies to evaluate the effects of consuming freeze-dried blueberries compared with placebo for 8 wk in participants with type 2 diabetes, with a double-blind, randomized, placebo-controlled clinical trial design. There were, however, several limitations to the study. The sample size was limiting, and the study may not have been adequately powered to detect existing differences in some biomarkers. Also, the current study did not measure the bioavailability of the freeze-dried blueberries. Although our participants had good glycemic control, they had evidence of early nephropathy; type 2 diabetes and its complications alter absorption and metabolism, which may affect the bioavailability and effectiveness of phenolic compounds (41). It is also important to note that our study did not control for fiber in the interventions. The freeze-dried blueberry intervention contained 5.2 g fiber and the placebo contained 0.3 g fiber. Fiber is known to influence glycemic response and the Diabetes Prevention Program multicenter, randomized clinical trial reported that an intensive lifestyle intervention group had a 1-y weight loss of 1.45 kg per 5 g fiber intake, independent of changes in energy intake (9, 42). Dietary intake data show our participants consumed ∼15 g fiber/d. Those in the freeze-dried blueberry intervention group, therefore, would have increased their fiber intake to 20.2 g/d. Individuals with type 2 diabetes should consume the amount of fiber recommended by the Dietary Guidelines for Americans (minimum 14 g fiber/1000 kcal) (9, 43). Participants in the freeze-dried blueberry and placebo groups met 77% and 58% of their fiber requirements, respectively. Future studies are needed to clarify whether effects on glycemic control are dependent on the phenolic profile or fiber content of blueberries. The self-reported questionnaires (food records and daily questionnaires) used were another limitation of this study; response bias could occur. In addition, our findings may lack generalizability to the non-VA population, because our study population included US veterans who were mostly older white men with optimal type 2 diabetes control. Approximately 25% of US veterans have type 2 diabetes, which is more than double the prevalence of the general US population; therefore, the Veterans Health Administration provides high access to comprehensive primary care (including in-person and telemedicine), MNT, and prescription coverage for those with type 2 diabetes (44).
In conclusion, the daily consumption of 22 g freeze-dried blueberries (equivalent to 1 cup fresh blueberries) may beneficially affect cardiometabolic health parameters in men with type 2 diabetes. Additional research is needed to determine whether polyphenol-rich foods reduce ASCVD by modifying glycemic control and dyslipidemia in those with type 2 diabetes without or with NAFLD.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—KSS, MMW, and ARG: designed the research; KSS, MMW, DH, KT, JMR, and ARG: conducted the research; KSS and MMW: analyzed the data with the study biostatistician, wrote the initial draft of the paper, and had primary responsibility for the final content; DH, KT, JMR, MIS, KTG-P, and ARG: reviewed the draft and provided critical feedback; and all authors: read and approved the final manuscript.
Notes
Supported by the US Highbush Blueberry Council (to KSS, MMW, and ARG) and by resources and the use of facilities at the Stratton VA Medical Center, Albany, NY, USA.
Author disclosures: KSS, MMW, and ARG received intervention products from the US Highbush Blueberry Council. All other authors report no conflicts of interest.
The US Highbush Blueberry Council supplied the funds to conduct the study but was not involved in the design, implementation, analysis, or interpretation of data.
Abbreviations used: ALT, alanine transaminase; AMPK, AMP-activated protein kinase; ASCVD, atherosclerotic cardiovascular disease; AST, aspartate transaminase; CRP, C-reactive protein; GLUT, glucose transporter; HbA1c, glycated hemoglobin; MA:CR, microalbumin-to-creatinine ratio; MNT, medical nutrition therapy; NAFLD, nonalcoholic fatty liver disease; RDN, registered dietitian/nutritionist; VA, Veterans Administration.
References
- 1. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S13–28. [DOI] [PubMed] [Google Scholar]
- 2. CDC. National diabetes statistics report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. [Google Scholar]
- 3. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103–S23. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S7–S12. [DOI] [PubMed] [Google Scholar]
- 5. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–98. [DOI] [PubMed] [Google Scholar]
- 6. Briggs Early K, Stanley K. Position of the Academy of Nutrition and Dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet. 2018;118(2):343–53. [DOI] [PubMed] [Google Scholar]
- 7. Marincic PZ, Salazar MV, Hardin A, Scott S, Fan SX, Gaillard PR, Wyatt C, Watson L, Green P, Glover P et al.. Diabetes self-management education and medical nutrition therapy: a multisite study documenting the efficacy of registered dietitian nutritionist interventions in the management of glycemic control and diabetic dyslipidemia through retrospective chart review. J Acad Nutr Diet. 2019;119(3):449–63. [DOI] [PubMed] [Google Scholar]
- 8. MacLeod J, Franz MJ, Handu D, Gradwell E, Brown C, Evert A, Reppert A, Robinson M. Academy of Nutrition and Dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: nutrition intervention evidence reviews and recommendations. J Acad Nutr Diet. 2017;117(10):1637–58. [DOI] [PubMed] [Google Scholar]
- 9. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, Mitri J, Pereira RF, Rawlings K, Robinson S et al.. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calvano A, Izuora K, Oh EC, Ebersole JL, Lyons TJ, Basu A. Dietary berries, insulin resistance and type 2 diabetes: an overview of human feeding trials. Food Funct. 2019;10(10):6227–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borges G, Degeneve A, Mullen W, Crozier A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J Agric Food Chem. 2010;58(7):3901–9. [DOI] [PubMed] [Google Scholar]
- 12. Grosso G, Stepaniak U, Micek A, Kozela M, Stefler D, Bobak M, Pajak A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br J Nutr. 2017;118(1):60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo X, Yang B, Tan J, Jiang J, Li D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70(12):1360–7. [DOI] [PubMed] [Google Scholar]
- 14. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95(4):925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140(9):1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curtis PJ, van der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, Evans M, Fernandez BO, Meiss MS, Minnion M et al.. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr. 2019;109(6):1535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson SA, Figueroa A, Navaei N, Wong A, Kalfon R, Ormsbee LT, Feresin RG, Elam ML, Hooshmand S, Payton ME et al.. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: a randomized, double-blind, placebo-controlled clinical trial. J Acad Nutr Diet. 2015;115(3):369–77. [DOI] [PubMed] [Google Scholar]
- 19. Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, Johnson WD, Cefalu WT. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2015;7(6):4107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28(1):49–55. [Google Scholar]
- 22. Cheng GW, Breen PJ. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hortic Sci. 1991;116(5):865–9. [Google Scholar]
- 23. Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145(4):742–8. [DOI] [PubMed] [Google Scholar]
- 24. Xiao J, Kai G, Yamamoto K, Chen X. Advance in dietary polyphenols as α-glucosidases inhibitors: a review on structure-activity relationship aspect. Crit Rev Food Sci Nutr. 2013;53(8):818–36. [DOI] [PubMed] [Google Scholar]
- 25. Xiao J, Ni X, Kai G, Chen X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit Rev Food Sci Nutr. 2013;53(5):497–506. [DOI] [PubMed] [Google Scholar]
- 26. Baron G, Altomare A, Regazzoni L, Redaelli V, Grandi S, Riva A, Morazzoni P, Mazzolari A, Carini M, Vistoli G et al.. Pharmacokinetic profile of bilberry anthocyanins in rats and the role of glucose transporters: LC-MS/MS and computational studies. J Pharm Biomed Anal. 2017;144:112–21. [DOI] [PubMed] [Google Scholar]
- 27. Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140(3):527–33. [DOI] [PubMed] [Google Scholar]
- 28. Palma-Duran SA, Vlassopoulos A, Lean M, Govan L, Combet E. Nutritional intervention and impact of polyphenol on glycohemoglobin (HbA1c) in non-diabetic and type 2 diabetic subjects: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2017;57(5):975–86. [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S61–70. [DOI] [PubMed] [Google Scholar]
- 30. Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9(2):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malmström H, Walldius G, Grill V, Jungner I, Gudbjörnsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies – cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9(10):e111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14(11):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reis JF, Monteiro VV, de Souza Gomes R, do Carmo MM, da Costa GV, Ribera PC, Monteiro MC. Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med. 2016;14(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu X, Wang TTY, Prior RL, Pehrsson PR. Prevention of atherosclerosis by berries: the case of blueberries. J Agric Food Chem. 2018;66(35):9172–88. [DOI] [PubMed] [Google Scholar]
- 35. Nilgun Istek OG. Investigation of the impact of blueberries on metabolic factors influencing health. J Funct Foods. 2017;38:298–307. [Google Scholar]
- 36. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65(8):1096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41(2):372–82. [DOI] [PubMed] [Google Scholar]
- 38. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–30. [DOI] [PubMed] [Google Scholar]
- 39. Guo H, Liu G, Zhong R, Wang Y, Wang D, Xia M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vendrame S, Daugherty A, Kristo AS, Klimis-Zacas D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br J Nutr. 2014;111(2):194–200. [DOI] [PubMed] [Google Scholar]
- 41. Redan BW, Buhman KK, Novotny JA, Ferruzzi MG. Altered transport and metabolism of phenolic compounds in obesity and diabetes: implications for functional food development and assessment. Adv Nutr. 2016;7(6):1090–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. US Department of Health and Human Services and the US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. [Internet] 8th ed Washington (DC): US Deparment of Health and Human Services and US Department of Agriculture; 2015; [accessed 29 September, 2019] Available from:https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 44. Davis PJ, Liu M, Sherman S, Natarajan S, Alemi F, Jensen A, Avramovic S, Schwartz MD, Hayes RB. HbA1c, lipid profiles and risk of incident type 2 diabetes in United States veterans. PLoS One. 2018;13(9):e0203484. [DOI] [PMC free article] [PubMed] [Google Scholar]