ABSTRACT
Background
A number of investigations have highlighted the importance of vitamin C in maintaining brain health. Biologically, vitamin C has exhibited roles in neuromodulation, neurodevelopment, vascular support, and neuroprotection. Vitamin C's contribution to cognitive function in both cognitively intact and impaired cohorts has previously been assessed, with little focus on gender variability.
Objective
The present study explored the interaction between gender and plasma vitamin C on cognitive performance, and the effect of different amounts of plasma vitamin C (adequate/inadequate) on various cognitive tasks by gender.
Methods
This retrospective analysis was conducted in healthy adults (n = 80, female = 52, male = 28, 24–96 y) with a range of blood plasma vitamin C concentrations. Cognitive assessments included the Swinburne University Computerized Cognitive Assessment Battery (SUCCAB) and 2 pen-and-paper tests, the Symbol Digits Modalities Test (SDMT) and the Hopkins Verbal Learning Test–Revised (HVLT-R). Food-frequency questionnaires were used to elucidate dietary consumption.
Results
After adjusting for a number of potential covariates such as age, number of prescribed medications and dose of vitamin C supplementation, results indicated a significant interaction (P < 0.001) between plasma vitamin C and gender on cognitive function, on both the computerized and pen-and-paper assessments. A novel finding was that the performance of males with inadequate plasma vitamin C was poorer on tasks involving components of memory (short/delayed), inhibition, and visual perception, whereas females presenting with inadequate vitamin C were more compromised on tasks involving psychomotor performance/motor speed. Additionally, females with adequate vitamin C concentrations exhibited higher performance than males on tasks involving recall, recognition, attention, and focus.
Conclusions
Further larger-scale investigations are required to establish a cause-and-effect relation and to elucidate whether differences in cognitive function between genders may be attributed to plasma vitamin C status.
This trial was registered at https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369440&isReview=true as ACTRN12615001140549.
Keywords: vitamin C, ascorbic acid, cognition, central nervous system, gender
Biologically, vitamin C has exhibited roles in neuromodulation, neurodevelopment, vascular support and neuroprotection. Vitamin C's contribution to cognitive function in both cognitively intact and impaired cohorts has previously been assessed, with little focus on gender variability. The present pilot study is the first to assess the interaction between gender and plasma vitamin C on cognitive performance, and the effect of different levels of plasma vitamin C (adequate/inadequate) on various cognitive tasks by gender. Results indicated a significant interaction (p < 0.001) between plasma vitamin C and gender on cognitive function, on both the computerised and pen and paper assessments. A novel finding was that the performance of males with inadequate plasma vitamin C was poorer on tasks involving components of memory (short/delayed), inhibition and visual perception, whereas females presenting with inadequate vitamin C were more compromised on tasks involving psychomotor performance/motor speed. Additionally, females with adequate vitamin C levels exhibited higher performance than males on tasks involving recall, recognition, attention and focus. These preliminary findings are hypothesis generating, with larger scale studies being required for more conclusive results and mechanisms of action to be identified.
Introduction
There has been increasing research aimed at uncovering the roles of vitamin C on central nervous system (CNS) function and its importance in maintaining brain health (1). The reduced form of vitamin C, ascorbic acid, is a potent water-soluble antioxidant, not capable of being synthesized by any human tissue, including the brain. Studies have demonstrated a key neuromodulatory role of vitamin C within the brain (2). This function is exerted through vitamin C's involvement as a cofactor for the synthesis of catecholamines such as norepinephrine (3) and serotonin (3), alongside synaptic acetylcholine release (4) and re-uptake of glutamate (5).
Additional roles of vitamin C on the CNS include neurodevelopment (6), stimulation of brain-derived neurotrophic factor (7), cardiovascular support through collagen production, angiogenesis, reduced NO metabolism, and the biosynthesis of carnitine (8). Vitamin C's neuroprotective roles include prevention of excitotoxicity (9), regeneration of other antioxidants (10), reduction in lipid peroxidation (11), and reduction in proinflammatory cytokines (12) and scavenger of brain reactive oxygen species (ROS) (13).
Human autopsy studies have indicated that the most saturated vitamin C brain regions include the cerebral cortex, hippocampus, and amygdala (14, 15). Ascorbic acid is maintained at concentrations 100 times higher in neurons than those present in the circulating plasma (15) and 2.5–4 times higher concentrations in cerebrospinal fluid than in plasma (16). A number of factors, including aging, have demonstrated an association with depletions of vitamin C in brain tissue, especially within the cerebral cortex, pituitary gland, and hippocampus (17, 18).
A meta-analysis consisting of multiple prospective cohort studies showed that the relative risk for dementia was significantly decreased with higher intakes of vitamin C (19), while another meta-analysis showed significantly lower values of blood vitamin C in Alzheimer's patients than those in healthy controls (20, 21).
Based on 50 studies, our recent systematic review revealed higher mean plasma vitamin C concentrations in the cognitively intact groups of participants compared with cognitively impaired groups (1). In our recent cross-sectional study, cognitively intact participants presenting with adequate plasma vitamin C concentrations, as opposed to those with inadequate concentrations, demonstrated significantly better performance on sensitive cognitive assessments involving immediate and delayed recall, attention, focus, and recognition (22). In line with these results, a recent randomized controlled trial using middle-aged adults (23) revealed significant improvements on tasks relating to verbal and visual memory, visual motor performance, and processing speed following 200 mg vitamin C supplementation for 8 wk.
Epidemiological studies have frequently shown higher vitamin C serum/plasma concentrations in women than in men (24–26) and significantly higher rates of deficiency in males (27). Furthermore, at equal vitamin C intake amounts, women achieve higher serum/plasma concentrations than men (26, 28).
Overall, no conclusive gender-related mechanistic differences in the pharmacokinetics of vitamin C have been observed in humans (29–31), despite women reaching the plasma ascorbate concentration plateau (70 µmol/L) at lower vitamin C intake amounts than men (32). Recent research has suggested that body weight is considered to be an essential factor for these gender-related differences (33), while another study (34) indicated that a higher absolute fat-free mass (muscle mass) in males, and thus a higher distribution volume of vitamin C, may contribute. Various lifestyle differences between gender groups may also play a role, such as higher levels of physical activity, alcohol consumption, and smoking among males and higher intakes of vitamin C–containing foods and supplements among females (34, 35). These differences point to increased ROS and possible vitamin utilization in males. As a consequence, studies have recommended higher daily intakes of vitamin C for males (33).
Few studies have addressed gender differences in brain tissue vitamin C concentrations. One rat model demonstrated that male rats had greater cerebral vitamin C loss in response to stress and ROS generated during ischemia than females (36). A number of additional factors may influence gender variations in brain vitamin C distribution, including variations in hydrogen peroxide, ROS (36), excitotoxicity (37), proinflammatory mediators such as TNF-α, IL-1β, IL-6, and inducible NO synthase inducible nitric oxide synthase (iNOS), and hormonal factors such as mineralocorticoids (38), 17β-estradiol (E2), and progesterone (39). A handful of studies have assessed potential differential associations between vitamin C and cognition by gender. A study conducted in a large cohort of elderly Korean participants reported a significant relation between vitamin C consumption and Mini-Mental State Examination (MMSE) scores in men but not in women (40). Another study revealed that men who reported low MMSE scores consumed less vitamin C than females with low MMSE scores; however, little variability in vitamin C consumption was observed in those who demonstrated high MMSE scores (41).
One study (42) has assessed the interaction between gender and plasma vitamin C concentrations on cognitive function using a cognitively intact sample. The study revealed that higher plasma concentrations were associated nonsignificantly with higher MMSE scores for men but not for women. Older age was associated with lower MMSE scores in men but not in women. However, the range of plasma vitamin C concentrations and MMSE scores was similar between the gender groups. Symbol Digits Modalities Test (SDMT) scores increased with increasing plasma concentrations for both gender groups. The authors concluded that more sensitive cognitive assessments were required to determine true associations in cognitively intact cohorts. Previous investigations which assessed vitamin C intake or plasma concentrations controlled for gender as a potential confounding factor rather than considering gender as a potential vitamin C effect modifier (43, 44).
Although efforts have been made to establish the links between plasma vitamin C concentrations and cognitive function in healthy participants and to distinguish gender differences in the vitamin's plasma and structural concentrations (using animal models), a paucity of research has explored the interaction between gender and plasma vitamin C concentrations on cognitive function. A major limitation of the research to date has been the use of a cognitive measure not suitable for distinguishing subtle differences in cognitively intact samples and the failure to consider gender as a potential vitamin C effect modifier when assessing cognition.
To our knowledge, this is the first pilot study to advance the findings from previous research by examining how plasma vitamin C concentrations interact with cognitive functions between cognitively intact males and females with the use of suitable cognitive instruments. Using a sensitive battery of computerized and pen-and-paper assessments, the present pilot study explored the interaction between gender, plasma vitamin C, and cognitive performance, assessing how gender groups with different concentrations of plasma vitamin C (adequate/inadequate) perform on various cognitive tasks.
Methods
This article is a retrospective analysis of a recently published study (22), in which we investigated the association between plasma vitamin C concentrations and cognitive function. The methodology of cognitive and vitamin C assessments, briefly outlined below, is described in more detail in the related publication (22). Here we focus only on statistical analyses relevant to the present study investigating the interaction between cognition and plasma vitamin C by gender.
This study was approved by the Human Research Ethics Committees at the National Institute of Integrative Medicine and Swinburne University of Technology. The trial was registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12615001140549). Participants provided informed written consent.
Cognitively intact adults (≥18 y) were sought to participate in this cross-sectional study. Asymptomatic participants were primarily recruited from the National Institute of Integrative Medicine. We included participants with no major neurodegenerative condition (i.e., dementia; 3MS score >79), and included those likely to be displaying a range of plasma vitamin C concentrations (i.e., varying diets, supplementation, age groups). Participants were excluded if they were pregnant or lactating, color blind, or taking antidepressants, antipsychotics, anxiolytics, illicit drugs, or any cognition-enhancing drugs. Participants were also excluded if they were not able to give informed consent.
Cognitive assessments
A number of cognitive assessments were performed. These included the Hopkins Verbal Learning Test–Revised (HVLT-R) (45), SDMT (46), and the Swinburne University Computerized Cognitive Assessment Battery (SUCCAB) (47).
HVLT-R/SDMT (paper-and-pen tests)
The HVLT-R is a validated paper-and-pen test designed to examine verbal learning, immediate and delayed recall, and delayed recognition. Participants were required to recognize and recall a list of 12 words immediately (across 3 trials) and after 40 min (fourth trial). The SDMT (Sheridan, Fitzgerald et al. 2006) (46) required participants to pair numbers (1–9) with geometric figures within 90 s. This primarily assessed divided attention, tracking, and visual screening. For more information, see reference 22.
SUCCAB
The SUCCAB is a validated cognitive test battery consisting of 8 computer-based cognitive tasks assessing various aspects of cognitive performance (47). Participants were asked to respond as quickly and accurately as possible in each task. A 4-button response box was used to complete the tasks, with buttons corresponding to task-specific response dimensions: color (red, blue, green, or yellow), “yes” or “no,” or the spatial location of objects on the screen (top, bottom, left, or right). The 8 tasks that comprised the SUCCAB battery included simple reaction time (SRT), choice reaction time (CRT), incongruent Stroop (ISTR), congruent Stroop (CSTR), immediate recognition memory (IREC), spatial working memory (SWM), contextual memory (CMEM), and delayed recognition memory (DREC). Ratio performance was calculated by dividing percentage of correct responses by response times (seconds) for each task. (For more information see reference 22.)
Questionnaires
A questionnaire assessed intake of prescribed medications, dietary supplementation (dose/frequency), smoking status, highest level of education, exercise (duration/type), family history of neurodegenerative disease, and any history of an incident possibly contributing to cognitive dysfunction.
A long-term (1 y) dietary intake of a number of additional nutrients was assessed using the computerized version of the Dietary Questionnaire for Epidemiological Studies, version 2 (DQES v2) (48). The Cancer Council DQES v2 covered 5 types of dietary intake based on the previous 12 mo, incorporating 80 items. Estimated mean daily intake of a number of vitamins and minerals was derived from the consumption of these foods. Second, an “in house”–developed short-term food-frequency questionnaire (FFQ) was administered. This FFQ (based on the Cancer Council FFQ) assessed specifically what the consumption of vitamin C– and vitamin B-12–rich foods was within 2 wk prior to the testing session. The consumption of other nutrients such as zinc, magnesium, etc, was assessed due to their potential link with cognitive function (49). Serum vitamin B-12 was assessed, in particular, due to the vitamin's prominent link with cognition (50, 51).
Plasma vitamin C assessment
Fasting (8–12 h) blood tests were performed by the researcher/phlebotomist immediately after the completion of the SDMT. Blood was collected in two 6-mL lithium heparin Vacutainer blood tubes. The blood tubes were immediately wrapped in foil, kept away from light, and transported on dry ice. Each blood tube was spun in a centrifuge at 3600 rpm for 10 min at room temperature. Plasma from the heparin tubes was aliquoted into separate 3-mL tubes. These were also wrapped in foil and kept away from light. Two biochemical techniques were used to assess plasma concentrations, these included an HPLC analysis that was conducted by an external laboratory and a colorimetric analysis (52) conducted within our laboratory. For more information regarding the biochemical analysis, see reference 22.
Statistical analyses
Analyses were performed using SPSS IBM statistics version 23 package (IBM SPSS). Statistical significance was set at P < 0.05. Values that were 2 SDs away from their means for the cognitive assessments were excluded from analysis. Tests of normality were conducted to ensure a normal distribution of cognitive scores before any subsequent analyses were undertaken.
Primary analyses were conducted to compare cognitive performance between gender groups displaying adequate plasma vitamin C with those in the inadequate group. Average plasma vitamin C concentrations were subgrouped into established reference ranges representing adequate (≥28 μmol/L) and inadequate (also defined as depleted, <28 μmol/L) concentrations (25, 53, 54), based on expert committee recommendation (55). Cognitive performance was compared using a 2 (gender) by 2 (plasma vitamin C category) ANOVA for continuous variables.
This was followed by a 2-factor multivariate ANCOVA (MANCOVA) on cognitive performance measures that examined the interaction between gender and vitamin C group after adjusting for potential confounders. Potential confounding variables were assessed using the correlation regression analyses generated in the MANCOVA, with covariates included in the model only if they had a significant effect (P < 0.05) on the cognitive performance variables. A Bonferroni correction adjustment was used in the MANCOVA analyses to avoid a type 1 error.
For the SUCCAB, mean response time and percentage accuracy were calculated for each task. The accuracy percentage was divided by the mean response time (seconds) to provide the overall performance ratio score. This ratio also assisted in accounting for the issue of speed versus accuracy trade-off that exists with increasing age (56).
A number of cognitive variables were derived from the HVLT-R assessment. Trials 1–3, delayed recall (trial 4), total recall, and recognition index were scored based on the number of correct responses. HVLT-R is the sum of correct responses in trials 1–4. SDMT performance was scored out of 110 points.
Sample size calculation
Our initial study indicated that a sample size calculation of 80 participants was sufficient (with a 10% attrition rate) to detect a significant mean difference on the spatial working memory task on the SUCCAB, with 80% power and 95% confidence (22). This was based on a study that established aged-related mean SUCCAB values for each task using cognitively intact adults (47).
The literature suggests that a pilot study sample should consist of 10% of the projected sample for the larger study (n = 80) (57, 58). The sample size for the present pilot study comprised 13 participants in the inadequate group and 47 in the adequate group.
In order to confirm whether this pilot study was sufficiently powered, we ran nonparametric analyses (Mann-Whitney test), which took medians into account. This demonstrated a number of significant trends that were consistent with our MANCOVA presented below. The Mann-Whitney subgroup analysis revealed that even with a small sample size in those with inadequate vitamin C concentrations, we had sufficient power to detect differences in cognitive function between gender and vitamin C groups. A parametric MANCOVA was conducted in the present study due to this analysis being a more powerful analysis than a nonparametric analysis assessing for an interaction while also controlling for a number of key covariates, such as age.
Results
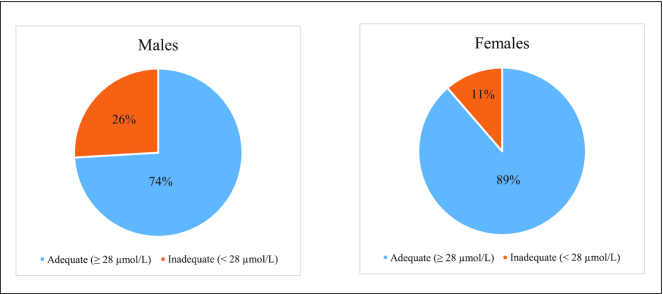
ANOVA of all participants revealed a marginal, nonsignificant difference in mean plasma vitamin C concentrations between genders [males (mean, plus/minus SD): 44.46 ± 4.43, 8–111 µmol/L; females: 48.45 ± 3.13, range: 8–116 µmol/L; P = 0.46]. The percentage of males presenting with inadequate plasma vitamin C concentrations (26%) was higher than the percentage of female participants (11%) (Figure 1). The inadequate group included 3 participants who displayed deficient vitamin C concentrations (<11 µmol/L). Table 1 shows the descriptive statistics comparing the adequate and inadequate plasma vitamin C categories by gender. An ANOVA was conducted between gender groups for each vitamin C group separately and a 2 × 2 factorial ANOVA (gender by vitamin C group) was conducted and is shown in Table 1.
FIGURE 1.
Proportion of males and females presenting with adequate (≥28 µmol/L) and inadequate/depleted (<28 µmol/L) plasma vitamin C concentrations. The inadequate group included 3 participants (2 males, 1 female) who displayed deficient vitamin C concentrations (<11 µmol/L).
TABLE 1.
Descriptive characteristics between adequate and inadequate plasma vitamin C categories for gender groups1
| Variable | Adequate plasma vitamin C (≥28 µmol/L) | Inadequate plasma vitamin C (<28 µmol/L) | P (2 × 2 factorial ANOVA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | P (M vs. F adequate) | Males | Females | P (M vs. F inadequate) | ||||||
| n | Values | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||||
| Age, y | 20 | 65 ± 3 | 47 | 57 ± 2 | 0.08 | 7 | 73 ± 6 | 6 | 63 ± 22 | NS | 0.25 |
| Gender, % | 20 | 30 | 47 | 70 |
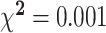
|
7 | 53.8 | 6 | 46.2 | NS |
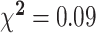
|
| BMI, kg/m2 | 20 | 27 ± 1 | 47 | 25 ± 0.4 | 0.024 | 7 | 27 ± 1 | 6 | 25 ± 1 | NS | NS |
| Smoker, % | 1 | 5 | 0 | 0 | NS | 2 | 28.6 | 1 | 16.7 | NS |
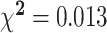
|
| Prescribed medications | 20 | 2 ± 1 | 47 | 1 ± 0.2 | 0.08 | 7 | 2 ± 1 | 6 | 1 ± 0.2 | NS | NS |
| Education, y | 20 | 13 ± 0.3 | 47 | 13 ± 0.2 | NS | 7 | 13 ± 1 | 6 | 13 ± 1 | NS | NS |
| Exercise, min/wk | |||||||||||
| Moderate intensity | 8 | 146 ± 40 | 25 | 138 ± 23 | NS | 2 | 145 ± 81 | 2 | 120 ± 81 | NS | NS |
| Vigorous intensity | 4 | 143 ± 35 | 8 | 250 ± 25 | 0.03 | 1 | 90 ± 71 | 1 | 180 ± 71 | NA | NS |
| Nutrient intake | |||||||||||
| Dose of vitamin C supplementation, mg/d | 7 | 800 ± 233 | 13 | 892 ± 168 | NS | 0 | 0 | 0 | 0 | NA | NA |
| Vitamin C dietary intake, mg/d | 20 | 86 ± 5 | 47 | 85 ± 3 | NS | 7 | 76 ± 8 | 6 | 72 ± 8 | NS | NS |
| Plasma vitamin C, μmol/L | 20 | 54 ± 4 | 47 | 53 ± 3 | NS | 7 | 18 ± 7 | 6 | 15 ± 8 | NS | NS |
| Vitamin B-12 supplementation, µg/d | 4 | 210 ± 124 | 17 | 211 ± 60 | NS | 0 | 0 | 0 | 0 | NA | NA |
| Vitamin B-12 dietary, µg/d | 20 | 3 ± 0.2 | 47 | 3 ± 0.1 | NS | 7 | 3 ± 0.4 | 5 | 3 ± 0.4 | NS | NS |
| Serum vitamin B-12, pmol/L | 20 | 439 ± 71 | 47 | 648 ± 119 | 0.02 | 7 | 269 ± 129 | 6 | 429 ± 60 | 0.07 | NS |
| Vitamin D supplementation, IU/d | 6 | 1417 ± 188 | 18 | 1556 ± 108 | NS | 1 | 1000 ± 460 | 0 | 0 | NA | NA |
| Vitamin D dietary, μg/d | 10 | 4 ± 1 | 28 | 3 ± 0.3 | NS | 4 | 3 ± 1 | 5 | 4 ± 1 | NS | NS |
| Magnesium supplementation, mg/d | 7 | 621 ± 74 | 13 | 685 ± 54 | NS | 1 | 500 ± 194 | 0 | 0 | NA | NA |
| Magnesium dietary, mg/d | 10 | 485 ± 68 | 28 | 517 ± 40 | NS | 4 | 593 ± 107 | 5 | 452 ± 96 | NS | NS |
| Fish oil/krill oil supplementation, mg/d | 4 | 1250 ± 144 | 8 | 1313 ± 92 | NS | 3 | 1167 ± 167 | 0 | 0 | NA | NA |
| Dietary omega-3, mg/d | 10 | 386 ± 94 | 28 | 594 ± 56 | 0.07 | 4 | 466 ± 149 | 5 | 101 ± 133 | NS | NS |
| Vitamin E, mg/d | 10 | 16 ± 3 | 28 | 14 ± 1 | NS | 4 | 12 ± 4 | 5 | 13 ± 2 | NS | NS |
| Vitamin B-1, mg/d | 10 | 2 ± 0.3 | 28 | 2 ± 0.2 | NS | 4 | 2 ± 1 | 5 | 2 ± 0.3 | NS | NS |
| Vitamin B-2, mg/d | 10 | 2 ± 0.3 | 28 | 2 ± 0.2 | NS | 4 | 2 ± 1 | 5 | 2 ± 1 | NS | NS |
| Vitamin B-3, mg/d | 10 | 23 ± 3 | 28 | 23 ± 2 | NS | 4 | 28 ± 5 | 5 | 25 ± 4 | NS | NS |
| Vitamin B-5, mg/d | 10 | 4 ± 0.4 | 28 | 3 ± 0.2 | NS | 4 | 3 ± 1 | 5 | 4 ± 1 | NS | NS |
| Vitamin B-6, mg/d | 10 | 1 ± 0.2 | 28 | 1 ± 0.1 | NS | 4 | 1 ± 0.3 | 5 | 1 ± 0.3 | NS | NS |
| Vitamin B-7, mg/d | 10 | 36 ± 5 | 28 | 33 ± 3 | NS | 4 | 32 ± 7 | 5 | 40 ± 6 | NS | NS |
| Iron, mg/d | 10 | 12 ± 2 | 28 | 12 ± 1 | NS | 4 | 13 ± 2 | 5 | 13 ± 2 | NS | NS |
| Zinc, mg/d | 10 | 11 ± 1 | 28 | 10 ± 1 | NS | 4 | 8 ± 2 | 5 | 12 ± 2 | 0.06 | NS |
| Iodine, µg/d | 10 | 135 ± 15 | 28 | 116 ± 9 | NS | 4 | 111 ± 24 | 5 | 125 ± 21 | NS | NS |
| Alcohol, g/d | 10 | 15 ± 3 | 28 | 3 ± 2 | <0.001 | 4 | 1 ± 4 | 5 | 1 ± 4 | NS | 0.07 |
| Caffeine, mg/d | 10 | 488 ± 114 | 28 | 557 ± 68 | NS | 4 | 742 ± 180 | 5 | 322 ± 161 | NS | 0.08 |
Values are means ± SEs unless otherwise indicated. Moderate exercise = 50–60% of maximum heart rate; vigorous exercise = 70–85% of maximum heart rate. NS: P > 0.05. NA, not applicable.
A 1-factor ANOVA revealed no significant differences on any of the variables in Table 1 between males and females exhibiting inadequate plasma vitamin C concentrations. Females in the inadequate plasma vitamin C group displayed a borderline significant higher mean zinc supplementation dose (P = 0.06) and mean serum vitamin B-12 concentrations (P = 0.07) than males. In those with adequate plasma vitamin C concentrations, females undertook significantly more vigorous intensity exercise, on average, per week than males (P = 0.03) and presented with significantly higher mean serum vitamin B-12 concentrations than males (P = 0.023). Males with adequate plasma vitamin C concentrations presented with significantly higher mean BMIs (P = 0.023) and a significantly higher average daily alcohol consumption than females (P < 0.001). Additionally, a chi-square likelihood ratio test using Fisher's exact test revealed a significant interaction between smoking status and plasma vitamin C category in both genders, with both male and female smokers less likely to display adequate vitamin C concentrations (P = 0.013).
A 2 × 2 factorial ANOVA revealed no significant associations between gender and vitamin C group among the analyzed descriptive variables. Trends revealed higher mean BMIs in both males with adequate and inadequate vitamin C, and more prescribed medications in the male group in both vitamin C groups. Higher mean dietary intake of vitamin C was evident in both males and females who displayed adequate vitamin C concentrations than in those with inadequate concentrations. Serum vitamin B-12 concentrations tended to be higher in males displaying adequate vitamin C concentrations than in those presenting with inadequate concentrations, and were higher in females with adequate vitamin C concentrations. The highest mean daily alcohol consumption was found in males presenting with adequate vitamin C concentrations (P = 0.07) and the highest average daily caffeine consumption in males with inadequate vitamin C concentrations (P = 0.08), with more than twice the daily caffeine consumption in this latter group.
Results of a MANCOVA examining the interaction of gender and plasma vitamin C group on SUCCAB ratio performance are shown in Table 2. Missing values were reported on a number of SUCCAB tasks due to a coding programming error during score generation. As a result, the MANCOVA model for SUCCAB performance was performed with data missing from 8 participants displaying adequate plasma vitamin C concentrations (n = 72). The correlation regression analyses generated in the MANCOVA examined whether the variables in Table 1 had a significant contribution to the MANCOVA model. Covariates were controlled for if they had a significant contribution to the model. Age had a significant, negative impact on CSTR ratio (P = 0.02), ISTR ratio (P = 0.031), and SWM ratio (P = 0.006). Number of medications had a significant, negative impact on IREC (P = 0.04) and on DREC (P = 0.02). After adjusting for the significant covariates, there was a statistically significant gender by plasma vitamin C group interaction [F (32, 219) = 7.38; P < 0.001, Wilks’ Lambda = 0.068] on the SUCCAB task ratios. The adjusted model displayed an R2 > 89% for each of the SUCCAB ratio tasks, indicating a large proportion of the variance on the SUCCAB ratios accounted for by the gender and vitamin C interaction effects in the MANCOVA model after covariate adjustment.
TABLE 2.
MANCOVA: associated univariate F tests for gender by group interaction for SUCCAB performance1
| Model | Task | F-test interaction P value | R 2 | Covariate name | β ± SE | Covariate P value |
|---|---|---|---|---|---|---|
| Gender by adequate/inadequate plasma group interaction term | Simple reaction time (SRT) | <0.001 | 0.97 | — | — | — |
| Choice reaction time (CRT) | <0.001 | 0.95 | — | — | — | |
| Immediate recognition memory (IREC) | <0.001 | 0.97 | No. of Meds | −3.06 ± 1.47 | 0.04 | |
| Congruent Stroop (CSTR) | <0.001 | 0.96 | Age | −0.47 ± 0.02 | 0.02 | |
| Incongruent Stroop (ISTR) | <0.001 | 0.92 | Age | −0.43 ± 0.19 | 0.03 | |
| Spatial working memory (SWM) | <0.001 | 0.89 | Age | −0.53 ± 0.19 | 0.006 | |
| Contextual memory (CMEM) | <0.001 | 0.96 | — | — | — | |
| Delayed recognition memory (DREC) | <0.001 | 0.96 | No. of Meds | −2.65 ± 1.11 | 0.02 |
MANCOVA, multivariate ANCOVA; SUCCAB, Swinburne University Computerized Cognitive Assessment Battery; β, β-slope coefficient related to the covariates accounted for in the MANCOVA.
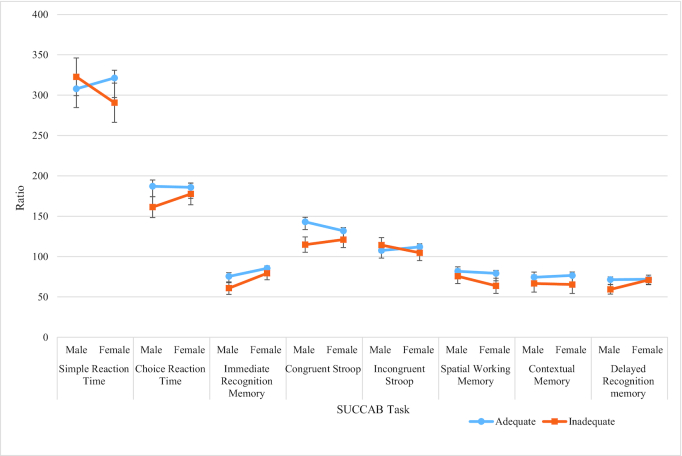
Overall, both male and female participants in the adequate vitamin C group performed better with higher performance ratios than those in the inadequate group. A gender by vitamin C interaction was observed in all individual SUCCAB task ratios (Table 3 and Figure 2).
TABLE 3.
Means, SEs, and 95% CIs for gender by group interaction for SUCCAB ratios across both plasma vitamin C groups and gender categories1
| Cognitive measure | Plasma category (adequate/inadequate) | Gender | Sample size, n | Mean | SE | 95% CI |
|---|---|---|---|---|---|---|
| Simple reaction time (SRT) | Adequate | Male | 18 | 307.95↓ | 14.1 | 279, 336 |
| Female | 41 | 321.35 | 9.60 | 303, 340 | ||
| Inadequate | Male | 7 | 322.73 | 23.3 | 276, 369 | |
| Female | 6 | 290.69↓ | 24.29 | 242, 339 | ||
| Choice reaction time (CRT) | Adequate | Male | 18 | 187.08 | 7.88 | 171, 203 |
| Female | 41 | 185.78↓ | 5.31 | 175, 196 | ||
| Inadequate | Male | 7 | 161.22↓ | 12.97 | 135, 187 | |
| Female | 6 | 177.63 | 13.5 | 150, 205 | ||
| Immediate recognition memory (IREC) | Adequate | Male | 18 | 75.39↓ | 4.67 | 66, 85 |
| Female | 41 | 85.44 | 3.14 | 79, 92 | ||
| Inadequate | Male | 7 | 60.91↓ | 7.70 | 46, 76 | |
| Female | 6 | 79.32 | 7.98 | 63, 95 | ||
| Congruent Stroop (CSTR) | Adequate | Male | 18 | 143.00 | 5.80 | 131, 154 |
| Female | 41 | 131.95↓ | 3.91 | 124, 140 | ||
| Inadequate | Male | 7 | 114.81↓ | 9.54 | 96, 133 | |
| Female | 6 | 121.01 | 9.94 | 101, 146 | ||
| Incongruent Stroop (ISTR) | Adequate | Male | 18 | 107.47↓ | 5.69 | 96, 119 |
| Female | 41 | 112.01 | 3.84 | 104, 120 | ||
| Inadequate | Male | 7 | 114.81 | 9.37 | 95, 133 | |
| Female | 6 | 104.72↓ | 9.78 | 85, 124 | ||
| Spatial working memory (SWM) | Adequate | Male | 18 | 81.76 | 5.49 | 71, 93 |
| Female | 41 | 79.30↓ | 3.71 | 72, 87 | ||
| Inadequate | Male | 7 | 75.63 | 9.05 | 58, 94 | |
| Female | 6 | 63.84↓ | 9.41 | 45, 83 | ||
| Contextual memory (CMEM) | Adequate | Male | 18 | 74.41↓ | 6.42 | 62, 87 |
| Female | 41 | 76.64 | 4.33 | 68, 85 | ||
| Inadequate | Male | 7 | 66.70 | 10.56 | 46, 88 | |
| Female | 6 | 65.33↓ | 11.00 | 43, 87 | ||
| Delayed recognition memory (DREC) | Adequate | Male | 18 | 71.33↓ | 3.51 | 64, 78 |
| Female | 41 | 71.93 | 2.37 | 67, 77 | ||
| Inadequate | Male | 7 | 59.31↓ | 5.76 | 48, 71 | |
| Female | 6 | 71.13 | 6.01 | 59, 83 |
MANCOVA, multivariate ANCOVA; SUCCAB, Swinburne University Computerized Cognitive Assessment Battery; ↓, lower mean value between genders (male/female) for vitamin C subgroup (adequate/inadequate).
FIGURE 2.
Adequate versus inadequate vitamin C groups for SUCCAB ratios. Largest interaction (largest difference between gender groups) is represented by nonparallel and/or crossing lines: ratio = accuracy ÷ response time. Adequate concentrations: ≥28 µmol/L; inadequate: <28 µmol/L. The inadequate/depleted group included 3 participants who displayed deficient vitamin C concentrations (<11µmol/L). SUCCAB, Swinburne University Computerized Cognitive Assessment Battery.
Graphically, the nonparallel lines and/or lines that crossed demonstrated greater differences between males and females and were indicative of a prominent gender by vitamin C interaction on the task. This was demonstrated on the CRT (males = 161.22 vs. 177.63), IREC (males = 60.91 vs. 79.32), CSTR (males = 114.81 vs. 121.01), and DREC (males = 59.31 vs. 71.13) tasks (Figure 2 and Table 3). For each specific task, there was a marginal disparity in cognitive ratios between the inadequate and adequate vitamin C groups in females. However, males in the inadequate vitamin C group exhibited lower ratio performance than females in the inadequate group. Besides the CSTR task, females in the adequate vitamin C group displayed higher performance ratios than males in the adequate group. For SWM and CMEM, the parallel lines indicated similar rates of reduced performance ratio in the inadequate vitamin C group for both genders (Figure 2 and Table 3).
Conversely, there was a disparity in SRT ratio performance (322.73 vs. 307.95) in males between the vitamin C groups. However, disparity between females displaying inadequate and adequate vitamin C concentrations (321.35 vs. 290.69) was clearer than in males on the SRT ratio performance (307.95 vs. 322.73). Overall, these results suggest a lower performance in males with inadequate vitamin C than females with inadequate concentrations, specifically on CRT, IREC, CSTR, and DREC. Females with inadequate vitamin C were predominantly lower in SRT performance than males with inadequate concentrations.
Additionally, a MANCOVA examined the interaction of gender and plasma vitamin C group on HVLT-R scores and the SDMT (Table 4). Covariates were only considered if they had a significant contribution to the model. Vitamin C supplementation dose had a significant positive impact on trial 2 (P = 0.03), trial 3 (P = 0.006), and delayed recall (P = 0.01). Additionally, age had a significant impact on delayed recall (P = 0.002), recognition index (P = 0.04), and SDMT (P < 0.001). After adjusting for the significant covariates, there was a statistically significant MANOVA [F (28, 246) = 11.74; P < 0.001; Wilks’ Lambda = 0.047] for a gender by plasma vitamin C category interaction on the HVLT-R tasks and SDMT. The adjusted model displayed R2 values >95% for each of the SUCCAB ratio tasks, indicating a large proportion of the variance on the HVLT-R and SDMT tasks was accounted for by the gender and vitamin C interaction MANCOVA model after adjusting for covariates.
TABLE 4.
MANCOVA: associated univariate F tests for gender by group interaction for HVLT-R and SDMT task performance1
| Model | Task | F-test interaction P value | R 2 | Covariate | β ± SE | Covariate P value |
|---|---|---|---|---|---|---|
| Gender by adequate/inadequate plasma group interaction term | Trial 1 | <0.001 | 0.95 | — | — | — |
| Trial 2 | <0.001 | 0.97 | Vit C dose | 0.001 ± 0.001 | 0.03 | |
| Trial 3 | <0.001 | 0.98 | Vit C dose | 0.001 ± 0.001 | 0.006 | |
| Total recall | <0.001 | 0.98 | Vit C dose | 0.002 ± 0.001 | 0.02 | |
| Delayed recall | <0.001 | 0.97 | Age | −0.04 ± 0.013 | 0.003 | |
| Vit C dose | 0.001 ± 0.001 | 0.01 | ||||
| Recognition index | <0.001 | 0.99 | Age | −0.019 ± 0.009 | 0.04 | |
| SDMT | <0.001 | 0.97 | Age | −0.36 ± 0.06 | <0.001 |
HVLT-R, Hopkins Verbal Learning Test–Revised; MANCOVA, multivariate ANCOVA; SDMT, Symbol Digits Modalities Test; Vit, vitamin; β, β-slope coefficient related to the covariates accounted for in the MANCOVA.
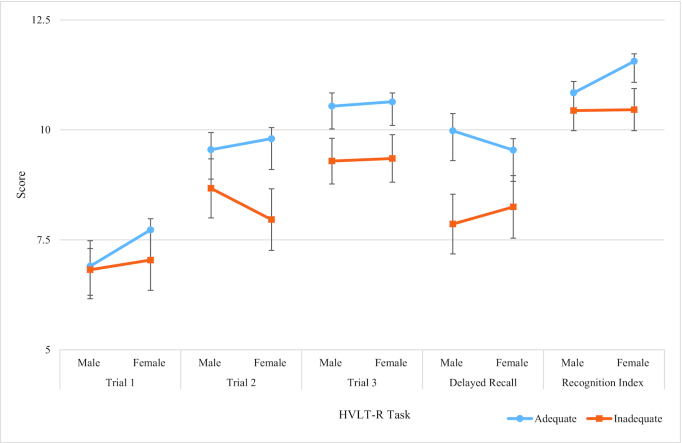
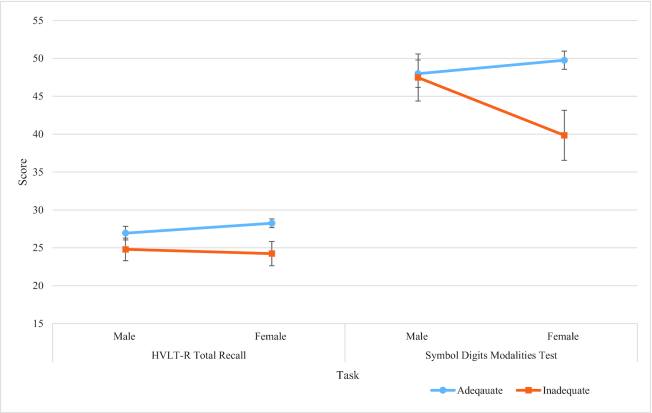
Similarly to the SUCCAB tasks, participants in the adequate vitamin C group displayed higher performance on all the HVLT-R and SDMT tasks than those in the inadequate group in both males and females. A gender by vitamin C interaction was observed on all individual HVLT-R tasks and on the SDMT (Table 5, Figures 3 and 4).
TABLE 5.
Means, SEs, and 95% CIs for gender by group interaction for HVLT-R and SDMT scores across both plasma vitamin C groups and gender categories1
| Cognitive measure | Plasma category (adequate/inadequate) | Gender | Sample size, n | Mean | SE | 95% CI |
|---|---|---|---|---|---|---|
| Trial 1 | Adequate | Male | 27 | 6.89↓ | 0.38 | 6.15, 7.65 |
| Female | 53 | 7.73 | 0.25 | 7.32, 8.23 | ||
| Inadequate | Male | 7 | 6.82↓ | 0.66 | 5.61, 8.03 | |
| Female | 6 | 6.99 | 0.69 | 5.51, 8.57 | ||
| Trial 2 | Adequate | Male | 27 | 9.56↓ | 0.39 | 8.78, 10.32 |
| Female | 53 | 9.80 | 0.26 | 9.30, 10.31 | ||
| Inadequate | Male | 27 | 8.67 | 0.62 | 7.44, 9.90 | |
| Female | 53 | 7.96↓ | 0.78 | 6.40, 9.53 | ||
| Trial 3 | Adequate | Male | 27 | 10.55↓ | 0.30 | 9.96, 11.13 |
| Female | 53 | 10.64 | 0.20 | 10.25, 11.03 | ||
| Inadequate | Male | 7 | 9.29↓ | 0.47 | 8.36, 10.23 | |
| Female | 6 | 9.35 | 0.60 | 8.16, 10.15 | ||
| Total recall | Adequate | Male | 27 | 26.95↓ | 0.88 | 25.20, 28.70 |
| Female | 53 | 28.26 | 0.58 | 27.10, 29.41 | ||
| Inadequate | Male | 7 | 24.70 | 1.41 | 21.90, 27.51 | |
| Female | 6 | 24.26↓ | 1.78 | 20.70, 27.81 | ||
| Delayed recall | Adequate | Male | 27 | 9.98 | 0.39 | 9.21, 10.76 |
| Female | 53 | 9.54↓ | 0.26 | 9.02, 10.05 | ||
| Inadequate | Male | 7 | 7.72↓ | 0.68 | 6.36, 9.08 | |
| Female | 6 | 8.33 | 0.71 | 6.90, 9.75 | ||
| Recognition index | Adequate | Male | 27 | 10.84↓ | 0.26 | 10.32, 11.36 |
| Female | 53 | 11.56 | 0.17 | 11.22, 11.90 | ||
| Inadequate | Male | 7 | 10.59 | 0.46 | 9.68, 11.50 | |
| Female | 6 | 10.29↓ | 0.48 | 9.34, 11.24 | ||
| SDMT | Adequate | Male | 27 | 47.98↓ | 1.79 | 44.42, 52.14 |
| Female | 53 | 49.76 | 1.18 | 47.41, 52.11 | ||
| Inadequate | Male | 7 | 47.46 | 3.13 | 41.25, 53.71 | |
| Female | 6 | 40.87↓ | 3.67 | 33.34, 46.36 |
HVLT-R, Hopkins Verbal Learning Test–Revised; SDMT, Symbol Digits Modalities Test; ↓, lower mean value between genders (male/female) for vitamin C subgroup (adequate/inadequate).
FIGURE 3.
Adequate versus inadequate vitamin C groups for the HVLT-R. Largest interaction (largest difference between gender groups) is represented by nonparallel and/or crossing lines. Adequate concentrations: ≥28 µmol/L; inadequate/depleted: <28 µmol/L. The inadequate/depleted group included 3 participants who displayed deficient vitamin C concentrations (<11 µmol/L). HVLT-R, Hopkins Verbal Learning Test–Revised.
FIGURE 4.
Adequate versus inadequate vitamin C groups for HVLT-R total recall and Symbol Digits Modalities Test. Largest interaction (largest difference between gender groups) is represented by nonparallel and/or crossing lines. Adequate concentrations: ≥28 µmol/L; inadequate/depleted: <28 µmol/L. The inadequate/depleted group included 3 participants who displayed deficient vitamin C concentrations (<11 µmol/L). HVLT-R, Hopkins Verbal Learning Test–Revised.
Nonparallel lines related to significant interaction/gender differences were observed on trial 1, trial 2, recognition index, total recall, and the SDMT (Figures 3 and 4, Table 5). Females with adequate vitamin C displayed higher SDMT scores and HVLT-R scores than males in the adequate group on all measures besides the delayed recall, on which males in the adequate group demonstrated higher scores.
For trial 1 (male = 6.89 vs. 6.82, female = 7.73 vs. 6.99) and recognition index (male = 10.84 vs. 10.59, female = 11.56 vs. 10.29) a smaller discrepancy was demonstrated between the adequate and inadequate vitamin C groups in males than in females (Table 5). For trial 2 (7.96 vs. 9.80) and the SDMT (40.87 vs. 49.76), females in the inadequate vitamin C group scored lower than females in the adequate group, whereas there was less disparity on these tasks in males between the adequate and inadequate vitamin C groups (trial 2: 9.56 vs. 8.67; SDMT: 47.98 vs. 47.46). Furthermore, males displayed a larger disparity on delayed recall between the adequate and inadequate vitamin C groups (9.98 vs. 7.72) than females (9.54 vs. 8.33). Taken together, these results suggest higher performance in males presenting with inadequate plasma vitamin C on the SDMT than females with an inadequate plasma vitamin C. Results further revealed lower performance on delayed recall in males presenting with inadequate plasma vitamin C concentrations than females, and females presenting with adequate vitamin C concentrations had higher performance on the recognition index than males in the adequate group.
Discussion
In summary, our pilot study found that females with adequate plasma vitamin C performed better than males on tasks involving recall, recognition, attention and focus, SDMT scores (assessing psychomotor performance), and HVLT-R scores (assessing verbal learning, immediate and delayed recall, and recognition). Females with inadequate plasma vitamin C concentrations performed better than males on IREC/DREC, CSTR, and CRT, whereas males with inadequate vitamin C performed better on tasks involving psychomotor performance/motor speed (SDMT task/SRT). Given that our results are derived from a small, retrospective analysis, our findings are hypothesis generating, with larger-scale studies being required for more conclusive results and mechanisms of action to be identified.
Based on previous research, it has been observed that those with adequate plasma vitamin C concentrations performed better on a series of cognitive tasks than those displaying inadequate and deficient concentrations (22). To our knowledge, this pilot study is the first to extend these previous investigations and demonstrate that gender may play a role on how vitamin C concentrations affect cognitive performance.
The observation of females performing better than males on tasks involving memory (short/delayed), inhibition, and visual perception may be explained by possible variations in the distribution of brain vitamin C between genders. Due to a larger abundance of cell bodies, ascorbate concentrations are higher in gray matter than in white matter (15). Differences in gray matter volume are thought to be due to the combination of the neuroprotective effect of estrogen (which is more prominent in females) tending to decrease the loss of gray matter (59), particularly within the hippocampus (60).
Our findings of females with inadequate vitamin C concentrations demonstrating superior performance (higher performance ratios and scores) than males on CRT, IREC, CSTR, and DREC tasks may also be explained by nutritional and health differences between the gender groups. Females in the inadequate plasma vitamin C group displayed a borderline significant higher mean zinc supplementation dose and mean serum vitamin B-12 concentrations than males. Based on our assessment of potential covariates, females with adequate vitamin C concentrations undertook significantly more vigorous intensity exercise, on average, per week than males, and presented with significantly higher mean serum vitamin B-12 concentrations. Although these variables did not have a significant impact on the analyses as covariates, this is suggestive of a healthier lifestyle in the female sample, and could explain the greater performance in females.
In this study, male participants may have had higher oxidative stress, more prescribed medications, less vitamin C supplementation, and higher alcohol consumption than females. Although these variables did not have a significant impact on the analyses as covariates, they reveal a trend of a possibly lower overall health status in the male sample and elevated oxidative stress (61, 62). Studies have indicated a link between alcohol consumption and elevated oxidative stress, which is associated with gray matter volume reduction in specific regions, such as the frontal lobe (63), with these reductions in gray matter volume observed only in men.
Recent research has indicated that the hippocampus in guinea pigs requires more dietary vitamin C to become saturated than other brain regions (64). Due to the possibility of increased cerebral oxidative stress, males would require greater dietary vitamin C intake and plasma vitamin C to reach hippocampal saturation, making males more vulnerable to cognitive decline in scenarios of depleted concentrations of vitamin C.
Female performance on IREC/DREC, CSTR, and CRT with inadequate vitamin C concentrations was superior to that of males with inadequate concentrations. Although studies (65) have indicated a possible plateau in ascorbic cerebrospinal fluid once concentrations reach 45 µmol/L, the concentration of this plateau may be lower for females. Male mice have been shown to experience more dramatic age-associated decline in reduced glutathione (GSH) content in the brain (66), possibly affecting the brain's ability to recycle ascorbate. This may imply that the female brain might require less vitamin C to function sufficiently. The observation that females depleted of vitamin C perform similarly on these tests to those females displaying adequate concentrations is consistent with the retention of vitamin C in brain tissue.
There may be a number of explanations accounting for females presenting with inadequate vitamin C to exhibit lower performance on tasks involving psychomotor performance/motor speed (SDMT task/SRT). Recent research has demonstrated that the cerebellum of female guinea pigs, a structure that is involved in the timing and accuracy of movements, becomes saturated at relatively low intakes of vitamin C (64). Although not previously examined, this saturation threshold may be lower for males, enabling this brain region to efficiently utilize ascorbate at lower plasma concentrations.
Additionally, one animal model (using genetically modified mice unable to synthesize vitamin C like humans) revealed that during states of restricted ascorbate intake and depleted intracellular cerebellum ascorbate, sodium-dependent vitamin C transporter 2 (SVCT2) protein expression was increased within the cerebellum (67), possibly to upregulate the absorption of ascorbate. Despite the study not taking potential gender differences into account, males may be capable of expressing more cerebellar SVCT2 proteins during states of depletion, consequently absorbing and utilizing more ascorbate during depleted states.
In a cohort of 1004 adults, males showed significantly larger volumes for globus pallidus and putamen of the basal ganglia while controlling for total gray matter volume and age of the participants (68). These regions have been associated with higher order movement, providing a connection between motor centers and cortical areas for higher brain function (69).
Additional neuroimaging investigations have highlighted larger cerebellar hemispheres in men (70). Given these larger volumes, males may have larger ascorbate demands in these regions and be better at maintaining ascorbate concentrations in these regions. Vitamin C brain distribution and the observed cognitive performance in our male sample may further be accounted for by differences in regional gray matter (which contains the largest amount of vitamin C) distribution.
A large cross-sectional MRI study that explored how gender interacts with gray matter structure revealed that the cerebellar gray matter volume in males was significantly larger in the anterior and middle posterior lobes (59). A recent study also detected more gray matter volume within subcortical temporal structures in men, which included the putamen, anterior cerebellum, and premotor cortex (71). The premotor cortex is involved in movement planning and the execution of higher-order motor skills (72).
Males with inadequate vitamin C concentrations, on the other hand, may preserve their vitamin C more efficiently for psychomotor tasks involving neuromodulatory functions such as synaptic acetylcholine release (4) and sympathetic nerve activity (which is stimulated during tasks involving movement) (73). Elevated sympathetic activation in men (74) has been linked to increases in the secretion of the antidiuretic peptide hormone vasopressin (75), which is more present in men (76) and requires the involvement of ascorbate for the synthesis of the peptide (77).
There are a number of limitations to the present pilot study. It is difficult to ascertain a causative relation based on the cross-sectional design. Furthermore, due to the study design, it is difficult to predict the duration of inadequate/adequate plasma vitamin C concentrations. Inadequate plasma concentrations can be maintained from a diet consisting of <45 mg vitamin C/d. Multiple assessments of participants over time of vitamin C and cognitive assessments would help to determine the length of depletion and the effects of this length on cognitive performance.
To elucidate the effects of inadequate concentrations of vitamin C on the brain, increased knowledge about the distribution of vitamin C to the brain and within different brain regions during varying plasma vitamin C concentrations between gender groups would be advantageous for interpretation of findings. Assessing levels of oxidative stress through superoxide dismutase, glutathione peroxidase, and malondialdehyde would give a clearer indication on the variability between the gender groups and whether this could account for variances in the way vitamin C is being utilized.
Given that our subsample with inadequate plasma vitamin C concentrations consisted of only 7 males and 6 females, our results need to be interpreted with caution, in particular those relating to participants with inadequate plasma vitamin C. There was a discrepancy in the number of males and females included in the study, particularly in those presenting with adequate concentrations. Future research should assess larger numbers of adults with inadequate plasma vitamin C concentrations and more equal numbers of males and females with adequate concentrations for more generalizable results.
Additionally, physiological differences between gender groups may have played a role. These include cardiovascular, immune, and hormonal biomarkers, alongside oxidative stress, all of which may have an impact on both cognitive performance and vitamin C concentrations and should be considered in future research. While our study measured plasma vitamin C and vitamin B-12 concentrations biochemically, additional nutrients important for cognitive health, such as a range of B vitamins, vitamin D, and homocysteine, should also be assessed in future studies (78). Measuring these through blood tests will assess nutritional absorption as well as avoid any potential recall and response bias associated with FFQs (79).
In conclusion, the results from this pilot study extend the findings from our previous study and provide an avenue of exploration for future studies examining the link between plasma vitamin C and cognition. This pilot study demonstrated differences in the effects of vitamin C on cognition between males and females after controlling for potential covariates such as age, numbers of prescribed medications, and vitamin C supplementation dose. One novel finding was that females with inadequate plasma vitamin C performed better than males on IREC/DREC, CSTR, and CRT, whereas males with inadequate vitamin C performed better on tasks involving psychomotor performance/motor speed. The results further suggest that, in the group with adequate plasma vitamin C concentrations, females perform better than males on tasks involving recall, recognition, attention, and focus. In adults with inadequate vitamin C concentrations, females performed better on tasks involving components of memory (short/delayed), inhibition, and visual perception, whereas males performed better on tasks involving psychomotor performance/motor speed (SDMT task/SRT). The findings from this study warrant future cohort or longitudinal, randomized controlled trials to determine a cause-and-effect relation more conclusively and the way in which cognition is affected by varying plasma vitamin C concentrations in both males and females.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—A Sali and NT: conceptualized the study in discussion with KR and AP; IH: provided software and statistical expertise; NT: undertook data analysis and interpreted findings in discussion with KR and prepared the manuscript with contributions from all co-authors (KR, AP, A Sali, IH, and A Scholey); and all authors: read and approved the final manuscript.
Notes
No funding was received for the study. Study expenses were subsidized by the National Institute of Integrative Medicine.
Author disclosures: A Scholey and AP have received research funding, consultancy, travel support, and speaking fees from the nutrition and supplement industry. The other authors report no conflicts of interest.
Abbreviations used: CMEM, contextual memory; CNS, central nervous system; CRT, choice reaction time; CSTR, congruent Stroop; DQES v2, Dietary Questionnaire for Epidemiological Studies, version 2; DREC, delayed recognition memory; FFQ, food-frequency questionnaire; HVLT-R, Hopkins Verbal Learning Test–Revised; IREC, immediate recognition memory; ISTR, incongruent Stroop; MANCOVA, multivariate ANCOVA; MMSE, Mini-Mental State Examination; ROS, reactive oxygen species; SDMT, Symbol Digits Modalities Test; SRT, simple reaction time; SUCCAB, Swinburne University Computerized Cognitive Assessment Battery; SVCT2, sodium-dependent vitamin C transporter 2; SWM, spatial working memory.
References
- 1. Travica N, Ried K, Sali A, Scholey A, Hudson I, Pipingas A. Vitamin C status and cognitive function: a systematic review. Nutrients. 2017;9(9):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ballaz SJ, Rebec GV. Neurobiology of vitamin C: expanding the focus from antioxidant to endogenous neuromodulator. Pharmacol Res. 2019;146:104321. [DOI] [PubMed] [Google Scholar]
- 3. Gupta P, Tiwari S, Haria J. Relationship between depression and vitamin C status: a study on rural patients from western Uttar Pradesh in India. Int J Sci Study. 2014;1(4):37–9. [Google Scholar]
- 4. Kuo C-H, Hata F, Yoshida H, Yamatodani A, Wada H. Effect of ascorbic acid on release of acetylcholine from synaptic vesicles prepared from different species of animals and release of noradrenaline from synaptic vesicles of rat brain. Life Sci. 1979;24(10):911–5. [DOI] [PubMed] [Google Scholar]
- 5. Majewska MD, Bell JA. Ascorbic acid protects neurons from injury induced by glutamate and NMDA. Neuroreport. 1990;1(3):194–6. [DOI] [PubMed] [Google Scholar]
- 6. Hansen SN, Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency affect cognitive development and function?. Nutrients. 2014;6(9):3818–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grant MM, Barber VS, Griffiths HR. The presence of ascorbate induces expression of brain derived neurotrophic factor in SH‐SY5Y neuroblastoma cells after peroxide insult, which is associated with increased survival. Proteomics. 2005;5(2):534–40. [DOI] [PubMed] [Google Scholar]
- 8. Johnston CS, Corte C, Swan PD. Marginal vitamin C status is associated with reduced fat oxidation during submaximal exercise in young adults. Nutr Metab. 2006;3(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kocot J, Luchowska-Kocot D, Kiełczykowska M, Musik I, Kurzepa J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders?. Nutrients. 2017;9(7):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lykkesfeldt J, Trueba GP, Poulsen HE, Christen S. Vitamin C deficiency in weanling guinea pigs: differential expression of oxidative stress and DNA repair in liver and brain. Br J Nutr. 2007;98(6):1116–9. [DOI] [PubMed] [Google Scholar]
- 11. May JM, Qu Z-C. Ascorbic acid prevents increased endothelial permeability caused by oxidized low density lipoprotein. Free Radic Res. 2010;44(11):1359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowie AG, O'Neill LA. Vitamin C inhibits NF-κB activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165(12):7180–8. [DOI] [PubMed] [Google Scholar]
- 13. Duarte TL, Lunec J. When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39(7):671–86. [DOI] [PubMed] [Google Scholar]
- 14. Oke AF, May L, Adams RN. Ascorbic acid distribution patterns in human brain. Ann NY Acad Sci. 1987;498(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15. Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–16. [DOI] [PubMed] [Google Scholar]
- 16. Bowman GL, Dodge H, Frei B, Calabrese C, Oken BS, Kaye JA, Quinn JF. Ascorbic acid and rates of cognitive decline in Alzheimer's disease. Journal of Alzheimer's Disease. 2009;16(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schaus R. The ascorbic acid content of human pituitary, cerebral cortex, heart, and skeletal muscle and its relation to age. Am J Clin Nutr. 1957;5(1):39–41. [DOI] [PubMed] [Google Scholar]
- 18. Siqueira IR, Elsner VR, Leite MC, Vanzella C, dos Santos Moysés F, Spindler C, Godinho G, Battú C, Wofchuk S, Souza DO et al.. Ascorbate uptake is decreased in the hippocampus of ageing rats. Neurochem Int. 2011;58(4):527–32. [DOI] [PubMed] [Google Scholar]
- 19. Cao L, Tan L, Wang H-F, Jiang T, Zhu X-C, Lu H, Tan M-S, Yu J-T. Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53(9):6144–54. [DOI] [PubMed] [Google Scholar]
- 20. da Silva SL, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, Sijben J, Groenendijk J, Stijnen T. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimer Dementia. 2014;10(4):485–502. [DOI] [PubMed] [Google Scholar]
- 21. Fenech M. Vitamins associated with brain aging, mild cognitive impairment, and Alzheimer disease: biomarkers, epidemiological and experimental evidence, plausible mechanisms, and knowledge gaps. Adv Nutr. 2017;8(6):958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Travica N, Ried K, Sali A, Hudson IL, Scholey A, Pipingas A. Plasma vitamin C concentrations and cognitive function: a cross-sectional study. Front Aging Neurosci. 2019;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denniss RJ, Barker LA, Day CJ. Improvement in cognition following double-blind randomised micronutrient interventions in the general population. Front Behav Neurosci. 2019;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birlouez-Aragon I, Delcourt C, Tessier F, Papoz L. Associations of age, smoking habits and diabetes with plasma vitamin C of elderly of the POLA study. Int J Vitam Nutr Res. 2001;71(1):53–9. [DOI] [PubMed] [Google Scholar]
- 25. Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health. 2004;94(5):870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252–63. [DOI] [PubMed] [Google Scholar]
- 27. Pearson JF, Pullar JM, Wilson R, Spittlehouse JK, Vissers M, Skidmore PM, Willis J, Cameron VA, Carr AC. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: findings of the CHALICE cohort study. Nutrients. 2017;9(8):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci. 2001;98(17):9842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanchard J. Effects of gender on vitamin C pharmacokinetics in man. J Am Coll Nutr. 1991;10(5):453–9. [DOI] [PubMed] [Google Scholar]
- 30. Bachar M, Raimann JG, Kotanko P. Impulsive mathematical modeling of ascorbic acid metabolism in healthy subjects. J Theor Biol. 2016;392:35–47. [DOI] [PubMed] [Google Scholar]
- 31. Garry PJ, Goodwin JS, Hunt WC, Gilbert BA. Nutritional status in a healthy elderly population: vitamin C. Am J Clin Nutr. 1982;36(2):332–9. [DOI] [PubMed] [Google Scholar]
- 32. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J et al.. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci. 1996;93(8):3704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. German Nutrition Society. New reference values for vitamin C intake. Ann Nutr Metab. 2015;67(1):13–20. [DOI] [PubMed] [Google Scholar]
- 34. Jungert A, Neuhäuser-Berthold M. The lower vitamin C plasma concentrations in elderly men compared with elderly women can partly be attributed to a volumetric dilution effect due to differences in fat-free mass. Br J Nutr. 2015;113(05):859–64. [DOI] [PubMed] [Google Scholar]
- 35. Galan P, Viteri F, Bertrais S, Czernichow S, Faure H, Arnaud J, Ruffieux D, Chenal S, Arnault N, Favier A et al.. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005;59(10):1181. [DOI] [PubMed] [Google Scholar]
- 36. Ferris D, Kume-Kick J, Russo-Menna I, Rice M. Gender differences in cerebral ascorbate levels and ascorbate loss in ischemia. Neuroreport. 1995;6(11):1485–9. [DOI] [PubMed] [Google Scholar]
- 37. Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26(5):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nathani MG, Nath M. Effect of corticosteroids on ascorbic acid metabolism in rats. Metabolism. 1972;21(8):779–86. [DOI] [PubMed] [Google Scholar]
- 39. Kume-Kick J, Ferris DC, Russo-Menna I, Rice ME. Enhanced oxidative stress in female rat brain after gonadectomy. Brain Res. 1996;738(1):8–14. [DOI] [PubMed] [Google Scholar]
- 40. Lee L, Kang SA, Lee HO, Lee BH, Park JS, Kim JH, Jung IK, Park YJ, Lee JE. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health. 2001;115(2):133–8. [DOI] [PubMed] [Google Scholar]
- 41. Ortega RM, Requejo AM, Andrés P, López-Sobaler AM, Quintas ME, Redondo MR, Navia B, Rivas T. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66(4):803–9. [DOI] [PubMed] [Google Scholar]
- 42. Sato R, Helzlsouer K, Comstock G, Hoffman S. A cross-sectional study of vitamin C and cognitive function in older adults: the differential effects of gender. J Nutr Health Aging. 2006;10(1):37. [PubMed] [Google Scholar]
- 43. Perrig WJ, Perrig P, Stähelin H. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45(6):718–24. [DOI] [PubMed] [Google Scholar]
- 44. Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996;312(7031):608–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clinical Neuropsychologist. 1991;5(2):125–42. [Google Scholar]
- 46. Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, Wong MM, Zucker RA. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch Clin Neuropsychol. 2006;21(1):23–8. [DOI] [PubMed] [Google Scholar]
- 47. Pipingas A, Harris E, Tournier E, King R, Kras M, Stough CK. Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr Topics Nutraceutical Res. 2010;8(2):79. [Google Scholar]
- 48. Ireland P, Jolley D, Giles G, O'Dea K, Powles J, Rutishauser I, Wahlqvist ML, Williams J. Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac J Clin Nutr. 1994;3(1):19–31. [PubMed] [Google Scholar]
- 49. Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. 2016;8(2):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith AD, Refsum H. Vitamin B-12 and cognition in the elderly. Am J Clin Nutr. 2008;89(2):707S–11S. [DOI] [PubMed] [Google Scholar]
- 52. Chung WY, Chung JKO, Szeto YT, Tomlinson B, Benzie IF. Plasma ascorbic acid: measurement, stability and clinical utility revisited. Clin Biochem. 2001;34(8):623–7. [DOI] [PubMed] [Google Scholar]
- 53. Ravindran RD, Vashist P, Gupta SK, Young IS, Maraini G, Camparini M, Jayanthi R, John N, Fitzpatrick EK, Chakravarthy U et al.. Prevalence and risk factors for vitamin C deficiency in north and south India: a two centre population based study in people aged 60 years and over. PLoS One. 2011;6(12):e28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lim DJ, Sharma Y, Thompson CH. Vitamin C and alcohol: a call to action. BMJ Nutr Prev Health. 2018:bmjnph–2018-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 56. Starns JJ, Ratcliff R. The effects of aging on the speed–accuracy compromise: boundary optimality in the diffusion model. Psychol Aging. 2010;25(2):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Connelly LM. Pilot studies. Medsurg Nursing. 2008;17(6):411–2. [PubMed] [Google Scholar]
- 58. Lackey NR, Wingate AL. The pilot study: one key to research success. Adv Design Nursing Res. 1998;2(1):375–87. [Google Scholar]
- 59. Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One. 2011;6(7):e22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Österlund MK, Overstreet DH, Hurd YL. The Flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17β-estradiol. Mol Brain Res. 1999;74(1–2):158–66. [DOI] [PubMed] [Google Scholar]
- 61. Tunc O, Bakos H, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43(2):121–8. [DOI] [PubMed] [Google Scholar]
- 62. Lecomte E, Herbeth B, Pirollet P, Chancerelle Y, Arnaud J, Musse N, Paille F, Siest G, Artur Y. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am J Clin Nutr. 1994;60(2):255–61. [DOI] [PubMed] [Google Scholar]
- 63. de Bruin EA, Pol HEH, Schnack HG, Janssen J, Bijl S, Evans AC, Kenemans JL, Kahn RS, Verbaten MN. Focal brain matter differences associated with lifetime alcohol intake and visual attention in male but not in female non-alcohol-dependent drinkers. Neuroimage. 2005;26(2):536–45. [DOI] [PubMed] [Google Scholar]
- 64. Hasselholt S, Tveden-Nyborg P, Lykkesfeldt J. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br J Nutr. 2015;113(10):1539–49. [DOI] [PubMed] [Google Scholar]
- 65. Paraskevas G, Kapaki E, Libitaki G, Zournas C, Segditsa I, Papageorgiou C. Ascorbate in healthy subjects, amyotrophic lateral sclerosis and Alzheimer's disease. Acta Neurol Scand. 1997;96(2):88–90. [DOI] [PubMed] [Google Scholar]
- 66. Wang H, Liu H, Liu R-M. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38(5):507–17. [DOI] [PubMed] [Google Scholar]
- 67. Meredith ME, Harrison FE, May JM. Differential regulation of the ascorbic acid transporter SVCT2 during development and in response to ascorbic acid depletion. Biochem Biophys Res Commun. 2011;414(4):737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rijpkema M, Everaerd D, van der Pol C, Franke B, Tendolkar I, Fernández G. Normal sexual dimorphism in the human basal ganglia. Hum Brain Mapp. 2012;33(5):1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moretti R, Caruso P, Crisman E, Gazzin S. Basal ganglia: their role in complex cognitive procedures in experimental models and in clinical practice. Neurol India. 2017;65(4):814–82. [DOI] [PubMed] [Google Scholar]
- 70. Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. Am J Neuroradiol. 2001;22(6):1161–7. [PMC free article] [PubMed] [Google Scholar]
- 71. Lotze M, Domin M, Gerlach FH, Gaser C, Lueders E, Schmidt CO, Neumann N. Novel findings from 2,838 adult brains on sex differences in gray matter brain volume. Sci Rep. 2019;9(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci. 2012;109(Suppl 2):17186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hart EC, Joyner MJ. The curse of the sympathetic nervous system: are men or women more unfortunate?. J Physiol. 2010;588(22):4345–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hinojosa‐Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26(2):122–6. [DOI] [PubMed] [Google Scholar]
- 75. Kvetňanský R, Ježová D, Opršalová Z, Földes O, Michajlovskij N, Dobrakovova M, Lichardus B, Makara GB. Regulation of the sympathetic nervous system by circulating vasopressin. Circulating Regulatory Factors and Neuroendocrine Function. Boston (MA): Springer; 1990. pp. 113–34. [DOI] [PubMed] [Google Scholar]
- 76. Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders?. Behav Brain Res. 2007;176(1):170–86. [DOI] [PubMed] [Google Scholar]
- 77. Carr AC, Shaw GM, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock?. Crit Care. 2015;19(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kennedy DO, Haskell CF. Vitamins and cognition. Drugs. 2011;71(15):1957–71. [DOI] [PubMed] [Google Scholar]
- 79. Naska A, Lagiou A, Lagiou P. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res. 2017;6:926. [DOI] [PMC free article] [PubMed] [Google Scholar]