Abstract
Background: Sarcoidosis goes into remission in two-thirds of patients with sarcoidosis, but about 20 % of patients develop pulmonary fibrosis. The mechanisms of pulmonary fibrosis in sarcoidosis and differences in pathogenesis between clinical stages are still unclear. Objectives: The aim of this study was investigating proteins associated with clinical stages by comparing bronchoalveolar lavage fluid (BALF) protein between stage I and stage IV using proteome analysis. Methods: Proteomic differences in BALF were compared between stage I and stage IV by examining BALF from 8 stage I patients and 5 stage IV patients by two-dimensional gel electrophoresis and mass spectrometry. Results: In individual comparisons of BALF samples, the levels of apolipoprotein (Apo) A-I fragment, fibrinogen γ chain, calcyphosine, complement C3, and surfactant protein A were significantly higher in stage I than in stage IV. In contrast, none of the proteins examined significantly higher in stage IV than in stage I. To confirm the results of Apo A-I in the BALF proteome, we performed enzyme-linked immunosorbent assay (ELISA) in a larger group. The concentration of BALF Apo A-I was significantly higher in stage I patients than in stage IV patients (0.70 [0.13-0.89] vs. 0.15 [0.08-0.21] ng/μg protein, p=0.003). Conclusion: The involvement of BALF Apo A-I in sarcoidosis may differ between stage I and stage IV. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 5-15)
Keywords: apolipoprotein A-I, sarcoidosis, interstitial pneumonia, proteomics, bronchoalveolar lavage fluid
Supplement Table 1. Characteristics of patients performed ELISA
| Stage I (n=19) | Stage IV (n=12) | p Value | |
|---|---|---|---|
| Gender | |||
| Male | 4 | 10 | |
| Female | 15 | 2 | <0.001* |
| Age (yr) | 61 (44-68) | 42 (35-61) | 0.074 |
| Pack-year | 0 (0-2) | 18 (6-25) | <0.001* |
| Extrathoracic lesion | eye 18, skin 1, kidney1 | eye 5, skin 2 | |
| Serum CRP (mg/dl) | 0.1 (0.0-0.2) | 0.1 (0.1-0.2) | 0.700 |
| Serum LDH (IU/l) | 200 (163-213) | 182 (168-209) | 0.776 |
| Serum KL-6 (U/ml) | 324 (228-475)§ | 796 (559-1478) | 0.001* |
| Serum SP-D (ng/ml) | 34 (25-42)¶ | 108 (58-123) | <0.001* |
| Serum ACE (U/l) | 15 (13-21) | 20 (16-34) | 0.045* |
| Serum Lysozyme (μg/ml) | 8 (6-12) | 16 (9-21) | 0.030* |
| %VC (%) | 108.8 (102.8-119.7) | 91.9 (74.9-104.6) | 0.005* |
| %FEV1.0 (%) | 98.5 (93.0-114.1) | 88.5 (72.7-95.2) | 0.009* |
| BALF profiles | |||
| Recovery rate (%) | 59.3 (52.0-66.7) | 64.0 (53.0-67.9) | 0.556 |
| Cell density (×105/ml) | 2.4 (1.4-3.7) | 2.5 (1.4-4.1) | 0.685 |
| Macrophages (%) | 64.7 (44.9-74.0) | 72.0 (50.2-84.1) | 0.320 |
| Lymphocytes (%) | 32.8 (25.4-53.5) | 24.4 (14.0-48.9) | 0.265 |
| Neutrophils (%) | 0.0 (0.0-1.5) | 0.8 (0.2-1.4) | 0.125 |
| Eosinophils (%) | 0.0 (0.0-0.9) | 0.7 (0.0-1.2) | 0.256 |
| CD4/CD8 | 5.3 (3.4-9.0) | 5.7 (2.5-9.1) | 0.984 |
* p<0.05
§: n=17
¶: n=15.
Values are given as the numbers or medians (25th and 75th percentiles).
ELISA, enzyme-linked immunosorbent assay; KL-6, Krebs von den Lungen 6; SP-D, surfactant protein D; ACE, angiotensin converting enzyme; %VC, percentage of predicted vital capacity; %FEV1.0, percentage of forced expiratory volume in one second
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown etiology characterized by non caseating epithelioid granulomas. The disease primarily affects the lungs, lymph nodes and eyes but any organ can be affected (1). The lungs are affected in more than 90 percent of patients with sarcoidosis. Two-thirds of sarcoidosis patients can expect remission, but about 20 % of those with pulmonary sarcoidosis develop pulmonary fibrosis (1). Four stages of intrathoracic findings have been identified on chest X-ray : stage I with bilateral hilar adenopathy, stage II with bilateral hilar adenopathy and diffuse reticulonodular opacities, stage III with reticulonodular pattern, and stage IV with pulmonary fibrosis. Though sarcoidosis with pulmonary fibrosis has a worse prognosis, the mechanisms of pulmonary fibrosis in sarcoidosis and the differences in pathogenesis between the chest X-ray stages remain unclear.
Apolipoprotein (Apo) A-I is a major component of high-density lipoprotein. Apo A-I plays an important role in reverse cholesterol transport (RCT) and possesses both anti-inflammatory and anti-oxidative properties. Apo A-I mimetics have also been shown to effectively attenuate atherosclerosis and several forms of inflammation in animal models (2, 3). Recent reports on Apo A-I in bronchoalveolar lavage fluid (BALF) from patients with idiopathic pulmonary fibrosis (IPF) and asthma have suggested that Apo A-I might confer an anti-inflammatory property in the lungs (4, 5).
Proteomic analysis of human body fluids has emerged as a very important tool for investigating biomarkers. The analysis of BALF proteome can contribute to the identification of protein profiles among various lung diseases. Previous studies in different lung diseases have shown alterations in the protein profiles of BALF (4, 6-12). Bronchoalveolar lavage (BAL) has been widely used to collect cells and other soluble components from epithelial lining fluids that cover the airway and the alveoli (13). BALF contains proteins secreted from various cell types such as epithelial and inflammatory cells, as well as a wide variety of proteins from the bloodstream. The analysis of BALF can therefore reveal important biological activities related to inflammation, oxidation-reduction, tissue matrix turnover, and immunity.
The aim of this study was investigating the proteins associated with the pathogenesis of the clinical stage by comparing differences of BALF protein expression between stage I and stage IV by the proteomic approach. We also focused on Apo A-I, which was observed at higher levels in stage I, and studied the relationship between Apo A-I and sarcoidosis.
Methods
Patients
The study conformed to the Declaration of Helsinki and was approved by the internal review boards of our institution (No. 1850). With an adherence to the Ethical Guidelines for Medical and Health Research Involving Human Subjects, we made public information concerning the implementation of this study to ensure the opportunities to withdraw such consent.
Patients with sarcoidosis diagnosed in our hospital between 2007 and 2014 were recruited for this retrospective study. The diagnosis of sarcoidosis was established on the basis of a combination of histological and clinical findings according to the ATS/ERS/WASOG statement on sarcoidosis (1). The patients were divided into two groups, stage I (with bilateral hilar adenopathy) and stage IV (with pulmonary fibrosis), based on chest X-ray findings. Fifty eight patients were diagnosed with stage I and stage IV. Patients who had medical histories such as bronchial asthma, connective tissue disease, and malignant neoplasm and received insufficient BAL (recovery rate of less than 35 %) were excluded. Patients whose samples had not been stored were also excluded. The remaining 31 patients (19 patients with stage I and 12 with stage IV) could be evaluated by enzyme-linked immunosorbent assay (ELISA) to confirm the result of two-dimensional gel electrophoresis (2-DE). Not all patients enrolled to this analysis were treated with systemic corticosteroids or immunosuppressive therapies because not all patients had any organ impairment of sarcoidosis at the time of diagnosis. Then, we excluded the patients who had not sufficient examination such as Krebs von den Lungen 6 (KL-6), and surfactant protein D (SP-D) and the remaining patients were matched age and gender. Finally, 13 patients (8 patients with stage I and 5 with stage IV) were assigned to screening group for investigation of the protein associated with the pathogenesis by 2-DE.
Pulmonary function data on the percentage predicted vital capacity percentage (%VC) and percentage predicted forced expiratory volume in one second (%FEV1.0) and laboratory data on serum levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), Krebs von den Lungen 6 (KL-6), surfactant protein D (SP-D), angiotensin converting enzyme (ACE), and lysozyme were collected.
Bronchoalveolar lavage (BAL)
BAL was performed using three 50-ml aliquots of sterile 0.9% saline at the time of initial sarcoidosis diagnosis, as previously described (14). The cellular profile of BALF was determined by counting 200 cells in a cytospin smear with Wright’s stain. The lymphocyte phenotypes were analyzed by flow cytometry using monoclonal antibodies for CD4 and CD8. BALF were stored at -80°C until use.
Two-dimensional gel electrophoresis
2-DE was performed as previously described (10). Supernatant of BALF was concentrated by acetone precipitation and diluted in lysis buffer (8 M urea, 4% CHAPS, 65 mM dithioerythritol, 0.1 M acetic acid, ampholytes pH 3-10, and a trace of bromophenol blue). Samples containing 108 μg of proteins were loaded on immobilized pH gradient (IPG) strips (pH 4 to 7, 18 cm; GE Healthcare, Uppsala, Sweden) in each analytical experiment. The samples were rehydrated in the strip holder of an IPG-IEF Cool-PhoreStar system (Anatech, Tokyo, Japan) at 20°C and isoelectrofocusing was terminated at 47 kV. The IPG strips were equilibrated in the urea/SDS/Tris buffer for 40 minutes. The second dimensional run was performed on 10% polyacrylamide linear gradient gels at a constant current of 20 to 30 mA/gel at 20°C until the dye front reached the bottom of the gels. The gels were stained with SYPRO Ruby Protein Gel Stain (Molecular Probes, Carlsbad, CA, USA).
Evaluation and identification of proteins
The gels were scanned using a FluoroPhoreStar 3000 image analysis system (Anatech, Tokyo, Japan) and analyzed with Progenesis PG220 Software (Nonlinear Dynamics Ltd, Newcastle upon Tyne, UK). The proteins were analyzed automatically using the spot detection feature of the software, with automatic warping and matching. Spot volumes were corrected for background and then normalized. The normalized spot volume was calculated as a percentage of volumes of each spot to the volumes of all spots in a gel and compared between the two clinical groups. Differences between the two groups were confirmed by analyzing the spot quantities of pooled samples from stage I and stage IV patients. Non-matching spots or spots more than 2-fold higher or lower in quantity were regarded as differentially expressed in stage I compared with stage IV. Next, BALF samples of individuals were compared between the two groups to verify the results. Proteins were identified by comparing BALF maps such as the SWISS-2D PAGE human plasma map or published BALF maps (15, 16), or by liquid chromatography nano electron spray ionization tandem mass spectrometry (LC-nESI-MS/MS) performed in the Laboratory of Cytometry and Proteome Research at Tokyo Medical and Dental University. LC separation was performed by the nano-UHPLC system (BrukerDaltonics, Bremen, Germany). Mass analysis was performed on a maXis-4G-CPR (BrukerDaltonics) mass spectrometer equipped with a nano-ESI source.
Enzyme-linked immunosorbent assay (ELISA)
BALF and serum Apo A-I by ELISA were measured in a larger group to confirm the results on Apo A-I in the BALF proteome. An ELISA kit (ALerCHEK, Inc., Portland, ME, USA) was used to measure the Apo A-I concentration in undiluted BALF in 31 patients (19 in stage I and 12 in stage IV) and in serum at a 1:10000 dilution in 22 patients (12 in stage I and 10 in stage IV). BALF Apo A-I was also measured in 13 patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) (8 with organizing pneumonia [OP] pattern and 5 with usual interstitial pneumonia [UIP] pattern based on the findings of high-resolution computed tomography), 8 patients with IPF, and 5 healthy volunteers (HV). The levels of Apo A-I detected by this assay ranged from 52 to 3330 μg/ml.
BALF Apo A-I in disease activity
We collected information on the contents of treatment from the time of diagnosis until October 2015 and divided each stage I and IV patients into two groups to investigate the disease activity markers. One group was composed of patients treated with systemic corticosteroid for any organ impairment of sarcoidosis and the other received no medication during the following periods. Pulmonary function data, BALF profiles, and five markers of disease activity at the time of diagnosis were compared between the two groups: normalized BALF Apo A-I, serum ACE, lysozome, CRP, and soluble interleukin-2 receptor (sIL-2R).
Statistical analysis
All statistical analyses were performed with GraphPad Prism version 5.2 (GraphPad Software Inc., U.S.A.). Data were presented as medians with 25th and 75th percentiles. The two groups were compared using the Mann-Whitney U test. The two gender groups were compared using Fisher’s test. Correlation coefficients were obtained using Spearman’s correlation coefficient test. All statistical comparisons were two-sided and p values of less than 0.05 were considered statistically significant.
Results
Patient characteristics
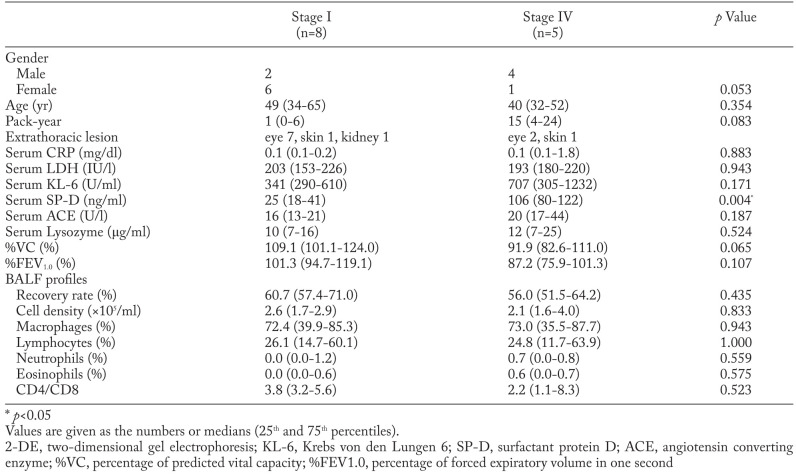
The patient characteristics and BALF findings analysed by 2-DE are shown in Table 1. The serum SP-D level was significantly higher in stage IV than in stage I. The stage IV patients tended to have a higher serum KL-6 concentration and a lower %VC. No difference in BALF profiles was observed between the two groups. No microbiological or clinical evidence of bacterial or fungal infection was found in any patient. Clinical characteristics of patients with sarcoidosis analysed by ELISA are provided in the online supplement (Supplement Table 1).
Table 1.
Characteristics of patients performed 2-DE
Two-dimensional gel electrophoresis
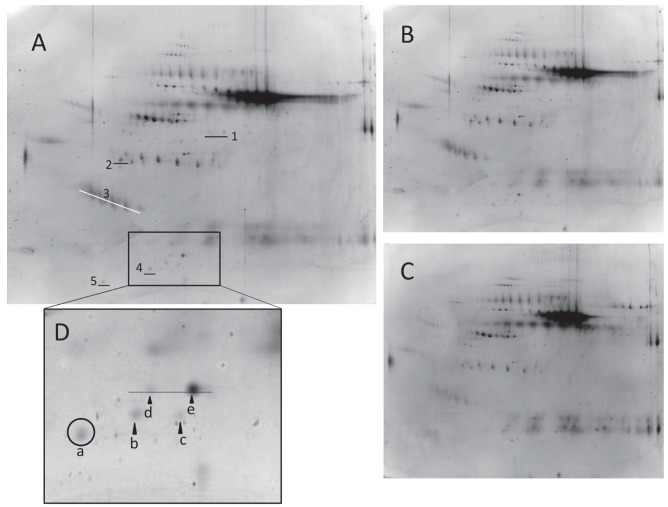
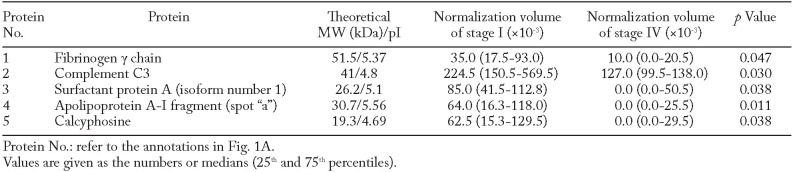
The BALF proteome in stage I and stage IV patients was analyzed by the 2-DE technique to evaluate the differences in protein expression between the two groups. The 2-DE patterns of BALF are shown in Fig. 1A. There was no significant difference in the total number of spots between stage I and stage IV (312 [300 - 390] vs. 281 [256 - 305] spots, p=0.143; Fig.1B, C). Differentially expressed protein spots were identified using LC-nESI-MS/MS and/or gel matching with the 2-DE database from Swiss-PROT. The levels of fibrinogen γ chain, complement C3, surfactant protein A, Apo A-I fragment, and calcyphosin were significantly higher in stage I than in stage IV. In contrast, no protein was significantly higher in stage IV than in stage I (Table 2). Apo A-I fragment (spots “a” - “c”) and Apo A-I (spots “d” - “e”) are shown collectively as five spots (Fig. 1D). The quantity of spot “a” was significantly higher in stage I than in stage IV. The quantities of spots “b”, “c”, “d” and “e” tended to be higher in stage I than in stage IV, but not significantly (normalization volumes [x10-3] of spots “b”, “c”, “d”, and “e” stage I vs. stage IV ; spot “b” 104.0 [0.0-156.5] vs. 0.0 [0.0-32.5] p=0.166, spot “c” 63 [0.0-116.8] vs. 0.0 [0.0-11.5] p=0.095, spot “d” 30.5 [0.0-97.8] vs. 0.0 [0.0-86.0] p=0.609, spot “e” 135.0 [35.0-337.8] vs. 79.0 [37.5-285.0] p=1.000).
Fig. 1.
Two-Dimensional Electrophoresis (2-DE) of BALF from sarcoidosis patient with stage I (A) stained by SYPRO-Ruby. Numbers indicate proteins, as listed in Table 2. Representative 2-DE patterns of BALF from stage I (B) and stage IV (C). The square area is magnified in Fig. 1D. Apo A-I fragments (spot“ a ” - “ c ”) and Apo A-I (spot“ d ” - “ e ”) were shown as five spots
Table 2.
Proteins with higher normalization volumes in stage I than in stage IV
Measurement of Apo A-I in BALF and serum
The concentrations of BALF Apo A-I in sarcoidosis (stages I and IV), RA-ILD (OP and UIP patterns), IPF, and HV were determined by ELISA (Fig. 2). The normalized concentration of Apo A-I with total protein was significantly higher in stage I than in stage IV (0.70 [0.13-0.89] vs. 0.15 [0.08-0.21] ng/μg protein, p=0.003). The concentration of BALF Apo A-I was significantly higher in stage I sarcoidosis than in RA-ILD OP (0.07 [0.07-0.19] ng/μg protein, p=0.001) and UIP pattern (0.06 [0.06-0.10] ng/μg protein, p=0.001), IPF (0.08 [0.05-0.12] ng/μg protein, p<0.001), and HV (0.20 [0.05-0.29] ng/μg protein, p=0.033). No significant difference in serum Apo A-I was found between the stage I and stage IV sarcoidosis patients (1456 [1220-1722] vs. 2248 [1361-6701] μg/ml, p=0.052).
Fig. 2.
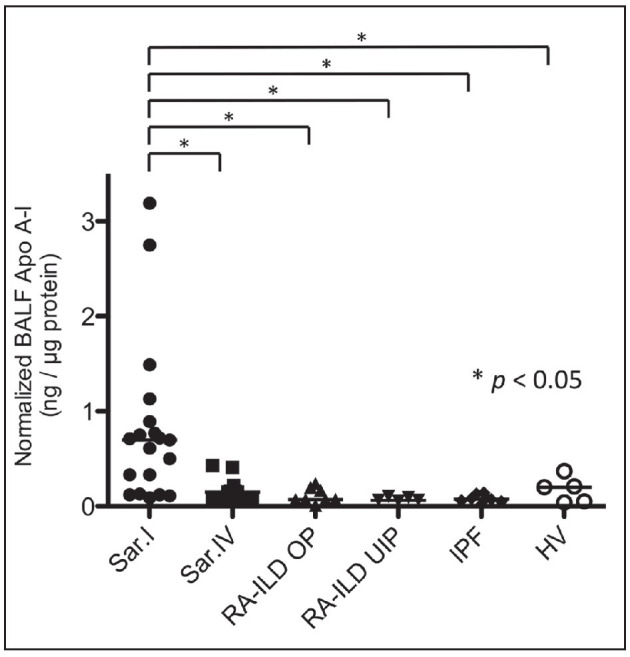
The normalized BALF Apo A-I concentration with total protein in sarcoidosis with stage I (Sar. I), sarcoidosis with stage IV (Sar. IV), rheumatoid arthritis-associated interstitial lung disease with organizing pneumonia pattern (RA-ILD OP), rheumatoid arthritis-associated interstitial lung disease with usual interstitial pneumonia pattern (RA-ILD UIP), idiopathic pulmonary fibrosis (IPF), and healthy volunteers (HV). * p<0.05
We analysed correlation between normalized concentration of BALF Apo A-I in sarcoidosis and clinical parameters (Table 3). We were interested to find a significant positive correlation between the concentration of BALF Apo A-I and the percentage of lymphocytes in BALF, especially in the stage I sarcoidosis (p=0.005, r=0.620; Fig. 3). In contrast, there was a significant negative correlation between the concentration of BALF Apo A-I in stage I and the percentage of macrophages in BALF. The concentration of BALF Apo A-I in stage IV was significantly correlated with serum ACE levels. No correlation was found between the serum level and normalized concentration of BALF Apo A-I in stage I and stage IV.
Table 3.
Correlation between normalized BALF Apo A-I and the clinical parameters

Fig. 3.

Correlation between normalized concentration of BALF Apo A-I and the percentage of lymphocytes in BALF in stage I sarcoidosis
Apo A-I in BALF in disease activity
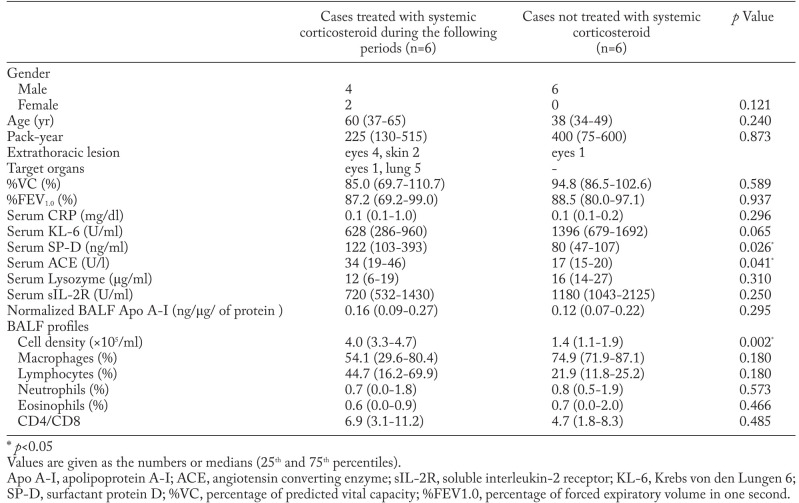
Five patients with stage I were treated with systemic corticosteroid during the following periods. Four of five patients were treated with systemic corticosteroid for eye involvements of sarcoidosis and the other patient was treated for eye and kidney involvements of sarcoidosis. Among the patients with stage I sarcoidosis, normalized BALF Apo A-I and serum ACE were significantly higher in those treated with systemic corticosteroid than in those receiving no treatment (Table 4).
Table 4.
Comparison of BALF and serum markers between cases treated with systemic corticosteroid and not treated in sarcoidosis with stage I
Six patients with stage IV were treated with systemic corticosteroid during the following periods. Five of six patients were treated with systemic corticosteroid for lung involvements of sarcoidosis and the other patient was treated for eye involvements of sarcoidosis. Among the patients with stage IV sarcoidosis, serum ACE, serum SP-D, and the cell density of BALF were significantly higher in those treated with systemic corticosteroid than in those receiving no treatment. On the other hand, normalized BALF Apo A-I was not significantly higher in those treated with systemic corticosteroid than in those receiving no treatment (Table 5).
Table 5.
Comparison of BALF and serum markers between cases treated with systemic corticosteroid and not treated in sarcoidosis with stage IV
Discussion
In this study we identified different patterns of protein expression in BALF between stage I and stage IV sarcoidosis analyzed by the proteomic approach. The expression levels of five proteins significantly differed between stage I and stage IV. We also visualized Apo A-I and Apo A-I fragments as five spots by 2-DE. One fragment (spot “a”) was found to be significantly increased in the stage I. The quantities of the other Apo A-I fragments tended to be higher in stage I than in stage IV, but not significantly. In our next experiment we tried to find any association between Apo A-I and pulmonary sarcoidosis. To confirm the results of Apo A-I in BALF proteome, we performed ELISA in a larger group. The concentration of BALF Apo A-I was significantly higher in stage I than in stage IV. Apo A-I may be associated with the pathogenesis of the clinical stage.
Apo A-I, the major component of high-density lipoprotein, originates from the liver and small intestine (17). It is secreted as a 249 amino acid proprotein containing a prosegment linked to the N-terminal of the mature 243-amino-acid Apo A-I. Proapoprotein A-I is converted to Apo A-I after its secretion (18, 19). Apo A-I plays an important role in RCT, one of the major anti-atherosclerotic mechanisms, by extracting cholesterol and phospholipids from macrophages via the ATP-binding cassette transporter A-I (ABCA-I) and transferring them to the liver (20). ABCA-I is located in the cell of the liver, small intestine, brain, kidney, and lung, including alveolar type II cells and pulmonary macrophages (21-23).
The concentration of BALF Apo A-I was significantly higher in stage I sarcoidosis than in stage IV sarcoidosis, RA-ILD, IPF, and HV. We also found a significant positive correlation between the concentration of BALF Apo A-I and the percentage of lymphocytes in BALF in stage I sarcoidosis patients. The significant negative correlation between the concentration of BALF Apo A-I and the percentage of macrophages in BALF in stage I sarcoidosis patients was seen as the result of increasing the percentages of lymphocytes in BALF in stage I. These results suggest that an increase of BALF Apo A-I may serve as a relatively supportive marker of disease activity. Previous reports have shown variation in BALF protein profiles between sarcoidosis and other interstitial lung diseases. Wattiez et al. found that BALF Apo A-I tended to have a higher relative intensity in stage I sarcoidosis than in IPF, hypersensitivity pneumonitis, and healthy adults (6). Magi et al. reported that the relative volume of BALF Apo A-I fragment was significantly higher in active sarcoidosis (chest X-ray stage unknown) than in IPF (7). Landi et al. proved that Apo A-I might play a key role in the pathogenesis of sarcoidosis in a proteomic analysis of BALF in patients with chronic active sarcoidosis (stage II and III), smokers, and no-smoker controls (9). All of these results are very similar to our own. The high levels of BALF Apo A-I might be a consequence of the defensive response against lung inflammation.
In sarcoidosis, stimulated and proliferated CD4+ T cells and macrophages produce inflammatory mediators considered pivotal for granuloma formation, such as IL-2, interferon gamma (IFN-γ), and tumor necrosis factor-α (TNF-α) (24). Apo A-I has known anti-inflammatory action, including removal of reactive oxygen species and an inhibition of secretions of TNF-α, IL-1β, IL-6, and IL-8 (25, 26). Kim et al. also reported that Apo A-I inhibited IL-12p70 and IFN-γ production by blocking the T-cell and natural killer cell stimulation of dendritic cells (27). Apo A-I in BALF might play an anti-inflammatory role in stage I of pulmonary sarcoidosis. Another possible explanation for the high levels of BALF Apo A-I in stage I might be inflammatory damage to the alveolar-capillary barrier permitting leakage of plasma proteins. Sarcoidosis is characterized by inflammatory damage to the pulmonary tissues and compromised function of the alveolar-capillary barrier (6). Higher concentrations of plasma proteins observed in previous BALF proteomic studies of sarcoidosis were similarly attributed to the leakage of plasma proteins through the damaged alveolar-capillary barrier (6, 7). Since Apo A-I is a plasma protein, the concentration of BALF Apo A-I might reflect the damaged alveolar-capillary barrier.
The concentration of BALF Apo A-I was lower in stage IV sarcoidosis than in stage I sarcoidosis. Pulmonary fibrosis in sarcoidosis is often described as a “burnt-out” disease reflecting a lack of granulomatous inflammation on histopathology (28). The lower concentration of Apo A-I in stage IV might reflect decreased granulomatous inflammation. While the exact causes of pulmonary fibrosis in sarcoidosis have yet to be demonstrated, the augmented activity of transforming growth factor (TGF)-β, the Th1 to Th2 transition, and macrophage phenotype switching have all been proposed as explanations (28, 29). In the study by Kim et al., BALF Apo A-I concentration was lower in IPF than in normal controls. Low levels of BALF Apo A-I might be associated with mechanisms of fibrosis. Further studies will be needed to clarify the role of Apo A-I in the development of pulmonary fibrosis in sarcoidosis.
Serum ACE levels had been reported reflecting the granulomatous lesion of the whole body and proposed as a marker for the activity of sarcoidosis (30). We also found significant positive correlation between the concentration of BALF Apo A-I in stage IV and serum ACE levels. This result might support BALF Apo A-I concentration reflect disease activity of sarcoidosis. However, there was no significant correlation serum ACE levels and BALF Apo A-I concentration in stage I. Westall et al. reported that serum ACE levels might reflect the granulomatous lesion of the whole body rather than the lung involvement (31). There was difference of distribution of extrathoracic lesion between stage I and stage IV in this study. The distribution of extrathoracic lesion might be contribute to the difference of correlation of ACE between stage I and stage IV.
We divided the stage I and IV patients into two groups to investigate disease activity markers in sarcoidosis. Among the patients with stage I sarcoidosis, the BALF Apo A-I concentration was significantly higher in patients treated with systemic corticosteroid than in non-treated patients. This result suggests that the BALF Apo A-I concentration may reflect systemic granulomatous inflammation as a marker of disease activity in stage I. Among the patients with stage IV sarcoidosis, the BALF Apo A-I concentration was not significantly higher in patients treated with systemic corticosteroid than in non-treated patients. Fibrosis in sarcoidosis extends from granuloma and therefore generally occurs in the same lymphatic distribution as active inflammation. Some sarcoidosis patients with pulmonary fibrosis coexisted with active inflammation (32). The patients treated with systemic corticosteroid in stage IV might be more heterogeneous than that in stage I. The heterogeneity of inflammation in stage IV might be contribute to the no difference of BALF Apo A-I concentration between patients treated with systemic corticosteroid and non-treated patients in stage IV. On the other hand, serum SP-D and ACE levels were significantly higher in patients treated with systemic corticosteroid than in non-treated patients. This result suggests that the serum SP-D and ACE levels might become disease activity markers in stage IV. As the number of patients with stage IV was small, we will need to further study to clarify the disease activity markers among the patients with stage IV.
There were several limitations in this study. First, we were unable to investigate hydrophobic proteins by the 2-DE technique. Second, the relatively small number of patients in this study limited our power to detect statistical differences between stage I and stage IV.
In conclusion, we identified several proteins that may be involved in the clinical differences between stage I and stage IV in sarcoidosis. Apo A-I in BALF may be associated with the pathogenesis of the clinical stage and serve as a marker of disease activity in stage I.
Abbreviations
- ABCA-I
ATP-binding cassette transporter A-I
- ACE
angiotensin converting enzyme
- Apo A-I
apolipoprotein A-I
- BALF
bronchoalveolar lavage fluid
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- HV
healthy volunteers
- IPF
idiopathic pulmonary fibrosis
- IPG
immobilized pH gradient
- KL-6
Krebs von den Lungen-6
- LC-nESI-MS/MS
liquid chromatography nano electron spray ionization tandem mass spectrometry
- LDH
lactate dehydrogenase
- OP
organizing pneumonia
- RA-ILD
rheumatoid arthritis-associated interstitial lung disease
- RCT
reverse cholesterol transport
- sIL-2R
soluble interleukin-2 receptor
- SP-D
surfactant protein D
- UIP
usual interstitial pneumonia
- 2-DE
two-dimensional gel electrophoresis
- %FEV1.0
percentage predicted forced expiratory volume in one second
- %VC
percentage predicted vital capacity.
Acknowledgments
The authors wish to thank Makiko Nawa from the Laboratory of Cytometry and Proteome Research at Tokyo Medical and Dental University for her help with the mass spectrum analysis, and we also thank Dr. Takumi Akashi from the Department of Pathology at Tokyo Medical and Dental University for discussions.
Statement of Authors’ Contributions:
Yoshihisa Nukui, Yasunari Miyazaki, Tsukasa Okamoto, Haruhiko Furusawa and Naohiko Inase designed the study and wrote the manuscript. Yoshihisa Nukui, Yasunari Miyazaki, Kozo Suhara and Tsukasa Okamoto performed statistical analysis and interpretation of the results. Kozo Suhara and Tsukasa Okamoto contributed to data collection. All authors read and approved the final manuscript.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. AJRCCM. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Van Lenten BJ, Navab M, Anantharamaiah GM, Buga GM, Reddy ST, Fogelman AM. Multiple indications for anti-inflammatory apolipoprotein mimetic peptides. Curr Opin Investig Drugs. 2008;9:1157–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Chyu KY, Faria Neto JR, et al. Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;110:1701–5. doi: 10.1161/01.CIR.0000142857.79401.69. [DOI] [PubMed] [Google Scholar]
- 4.Kim TH, Lee YH, Kim KH, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. AJRCCM. 2010;182:633–42. doi: 10.1164/rccm.200905-0659OC. [DOI] [PubMed] [Google Scholar]
- 5.Park SW, Lee EH, Lee EJ, et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin Exp Allergy. 2013;43:914–27. doi: 10.1111/cea.12143. [DOI] [PubMed] [Google Scholar]
- 6.Wattiez R, Hermans C, Cruyt C, Bernard A, Falmagne P. Human bronchoalveolar lavage fluid protein two-dimensional database: study of interstitial lung diseases. Electrophoresis. 2000;21:2703–12. doi: 10.1002/1522-2683(20000701)21:13<2703::AID-ELPS2703>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Magi B, Bini L, Perari MG, et al. Bronchoalveolar lavage fluid protein composition in patients with sarcoidosis and idiopathic pulmonary fibrosis: a two-dimensional electrophoretic study. Electrophoresis. 2002;23:3434–44. doi: 10.1002/1522-2683(200210)23:19<3434::AID-ELPS3434>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Rottoli P, Magi B, Cianti R, et al. Carbonylated proteins in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics. 2005;5:2612–8. doi: 10.1002/pmic.200401206. [DOI] [PubMed] [Google Scholar]
- 9.Landi C, Bargagli E, Carleo A, et al. A functional proteomics approach to the comprehension of sarcoidosis. J Proteomics. 2015;128:375–87. doi: 10.1016/j.jprot.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Shirahama R, Miyazaki Y, Okamoto T, Inase N, Yoshizawa Y. Proteome analysis of bronchoalveolar lavage fluid in lung fibrosis associated with systemic sclerosis. Allergol Int. 2010;59:409–15. doi: 10.2332/allergolint.10-OA-0176. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Miyazaki Y, Shirahama R, Tamaoka M, Inase N. Proteome analysis of bronchoalveolar lavage fluid in chronic hypersensitivity pneumonitis. Allergol Int. 2012;61:83–92. doi: 10.2332/allergolint.11-OA-0315. [DOI] [PubMed] [Google Scholar]
- 12.Suhara K, Miyazaki Y, Okamoto T, Ishizuka M, Tsuchiya K, Inase N. Fragmented gelsolins are increased in rheumatoid arthritis-associated interstitial lung disease with usual interstitial pneumonia pattern. Allergol Int. 2015;65:88–95. doi: 10.1016/j.alit.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis. 1987;135:250–63. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- 14.Kishi M, Miyazaki Y, Jinta T, et al. Pathogenesis of cBFL in common with IPF? Correlation of IP-10/TARC ratio with histological patterns. Thorax. 2008;63:810–6. doi: 10.1136/thx.2007.086074. [DOI] [PubMed] [Google Scholar]
- 15.Fietta A, Bardoni A, Salvini R, et al. Analysis of bronchoalveolar lavage fluid proteome from systemic sclerosis patients with or without functional, clinical and radiological signs of lung fibrosis. Arthritis Res Ther. 2006;8:R160. doi: 10.1186/ar2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wattiez R, Falmagne P. Proteomics of bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:169–78. doi: 10.1016/j.jchromb.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Bolanos-Garcia VM, Miguel RN. On the structure and function of apolipoproteins: more than a family of lipid-binding proteins. Prog Biophys Mol Biol. 2003;83:47–68. doi: 10.1016/s0079-6107(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 18.Chau P, Fielding PE, Fielding CJ. Bone morphogenetic protein-1 (BMP-1) cleaves human proapolipoprotein A1 and regulates its activation for lipid binding. Biochemistry. 2007;46:8445–50. doi: 10.1021/bi700028u. [DOI] [PubMed] [Google Scholar]
- 19.Edelstein C, Gordon JI, Toscas K, Sims HF, Strauss AW, Scanu AM. In vitro conversion of proapoprotein A-I to apoprotein A-I. Partial characterization of an extracellular enzyme activity. J Bio Chem. 1983;258:11430–3. [PubMed] [Google Scholar]
- 20.Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Bio Chem. 2001;276:23742–7. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- 21.Lawn RM, Wade DP, Couse TL, Wilcox JN. Localization of human ATP-binding cassette transporter 1 (ABC1) in normal and atherosclerotic tissues. Arterioscler Thromb Vasc Biol. 2001;21:378–85. doi: 10.1161/01.atv.21.3.378. [DOI] [PubMed] [Google Scholar]
- 22.Bortnick AE, Favari E, Tao JQ, et al. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L869–78. doi: 10.1152/ajplung.00077.2003. [DOI] [PubMed] [Google Scholar]
- 23.Bates SR, Tao JQ, Yu KJ, et al. Expression and biological activity of ABCA1 in alveolar epithelial cells. Am J Respir Cell Mol Bio. 2008;38:283–92. doi: 10.1165/rcmb.2007-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunewald J, Eklund A. Role of CD4+ T cells in sarcoidosis. Proc Am Thorac Soc. 2007;4:461–4. doi: 10.1513/pats.200606-130MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galbois A, Thabut D, Tazi KA, et al. Ex vivo effects of high-density lipoprotein exposure on the lipopolysaccharide-induced inflammatory response in patients with severe cirrhosis. Hepatology. 2009;49:175–84. doi: 10.1002/hep.22582. [DOI] [PubMed] [Google Scholar]
- 26.Hyka N, Dayer JM, Modoux C, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–9. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 27.Kim KD, Lim HY, Lee HG, et al. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun. 2005;338:1126–36. doi: 10.1016/j.bbrc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 28.Patterson KC, Hogarth K, Husain AN, Sperling AI, Niewold TB. The clinical and immunologic features of pulmonary fibrosis in sarcoidosis. Translational research. 2012;160:321–31. doi: 10.1016/j.trsl.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc. 2013;10:362–70. doi: 10.1513/AnnalsATS.201303-069FR. [DOI] [PubMed] [Google Scholar]
- 30.Selroons OB. Biochemical markers in sarcoidosis. Crit Rev Clin Lab Sci. 1986;24:185–216. doi: 10.3109/10408368609110273. [DOI] [PubMed] [Google Scholar]
- 31.Westall GP, Stirling RG, Cullinan P, et al. 4th ed. Hamilton, London, England: BC Decker; 2003. Interstital Lung Disease; pp. 332–386. [Google Scholar]
- 32.Patterson KC, Strek ME, et al. Pulmonary Fibrosis in Sarcoidosis. Ann Am Thorac Soc. 2013;10:362–70. doi: 10.1513/AnnalsATS.201303-069FR. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table 1. Characteristics of patients performed ELISA
| Stage I (n=19) | Stage IV (n=12) | p Value | |
|---|---|---|---|
| Gender | |||
| Male | 4 | 10 | |
| Female | 15 | 2 | <0.001* |
| Age (yr) | 61 (44-68) | 42 (35-61) | 0.074 |
| Pack-year | 0 (0-2) | 18 (6-25) | <0.001* |
| Extrathoracic lesion | eye 18, skin 1, kidney1 | eye 5, skin 2 | |
| Serum CRP (mg/dl) | 0.1 (0.0-0.2) | 0.1 (0.1-0.2) | 0.700 |
| Serum LDH (IU/l) | 200 (163-213) | 182 (168-209) | 0.776 |
| Serum KL-6 (U/ml) | 324 (228-475)§ | 796 (559-1478) | 0.001* |
| Serum SP-D (ng/ml) | 34 (25-42)¶ | 108 (58-123) | <0.001* |
| Serum ACE (U/l) | 15 (13-21) | 20 (16-34) | 0.045* |
| Serum Lysozyme (μg/ml) | 8 (6-12) | 16 (9-21) | 0.030* |
| %VC (%) | 108.8 (102.8-119.7) | 91.9 (74.9-104.6) | 0.005* |
| %FEV1.0 (%) | 98.5 (93.0-114.1) | 88.5 (72.7-95.2) | 0.009* |
| BALF profiles | |||
| Recovery rate (%) | 59.3 (52.0-66.7) | 64.0 (53.0-67.9) | 0.556 |
| Cell density (×105/ml) | 2.4 (1.4-3.7) | 2.5 (1.4-4.1) | 0.685 |
| Macrophages (%) | 64.7 (44.9-74.0) | 72.0 (50.2-84.1) | 0.320 |
| Lymphocytes (%) | 32.8 (25.4-53.5) | 24.4 (14.0-48.9) | 0.265 |
| Neutrophils (%) | 0.0 (0.0-1.5) | 0.8 (0.2-1.4) | 0.125 |
| Eosinophils (%) | 0.0 (0.0-0.9) | 0.7 (0.0-1.2) | 0.256 |
| CD4/CD8 | 5.3 (3.4-9.0) | 5.7 (2.5-9.1) | 0.984 |
* p<0.05
§: n=17
¶: n=15.
Values are given as the numbers or medians (25th and 75th percentiles).
ELISA, enzyme-linked immunosorbent assay; KL-6, Krebs von den Lungen 6; SP-D, surfactant protein D; ACE, angiotensin converting enzyme; %VC, percentage of predicted vital capacity; %FEV1.0, percentage of forced expiratory volume in one second