Abstract
There are no specific data on the management of pulmonary fibrosis post-H1N1 ARDS. We present the cases of three patients who responded positively to treatment with pirfenidone, azithromycin and prednisolone. Three males, aged 40, 45 and 59 years, had H1N1 ARDS requiring mechanical ventilation for two weeks or longer. After weaning off ventilation, they had persistent symptoms and hypoxemia at rest despite receiving prednisolone and home oxygen for at least three weeks following discharge. Computed tomography (CT) of the chest showed fibrosis and traction bronchiectasis. At presentation, they could not perform spirometry. Investigations ruled out infection. Pirfenidone (600 mg daily escalated to maximum tolerable dose of 2.4 gm daily) and azithromycin (500 mg thrice weekly) were added off-label to prednisolone. In one patient pirfenidone was discontinued after three months due to an adverse reaction and azithromycin was continued for nine months. At one year follow-up, all patients had symptomatic improvement, better effort tolerance, regression of opacities and no progression of fibrosis on CT, and improvement in spirometry and six minute walk tests. Pirfenidone and azithromycin added to prednisolone may have led to clinical and radiological improvement. The current experience suggests that this treatment approach to pulmonary fibrosis post-H1N1 ARDS be studied further. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 85-90)
Keywords: H1N1, ARDS, pulmonary fibrosis, pirfenidone, azithromycin, prednisolone
Introduction
Pulmonary fibrosis may follow acute respiratory distress syndrome (ARDS) caused by the 2009 H1N1 influenza A virus (1, 2). To our knowledge there are no data on the management of this condition, nor standardised pharmacotherapy targeting post-ARDS fibrosis (3).
Corticosteroids have anti-inflammatory action and are effective in the fibroproliferative phase of ARDS (4-6). They are still considered in the management of non-IPF usual interstitial pneumonia (UIP). Pirfenidone is recommended in the management algorithm of IPF (7). It regulates fibroblast proliferation, improving symptoms and slowing the progression of pulmonary fibrosis. Long-term macrolide therapy is used in the management of post-infection bronchiectasis (8). Macrolides also have immunomodulatory properties, and azithromycin has been shown to be anti-fibrotic in vivo in murine models (9, 10). There is an ongoing trial examining the role of azithromycin in IPF (University Hospital Inselspital Berne: Azithromycin in Idiopathic Pulmonary Fibrosis; ClinicalTrials.gov Identifier NCT02173145; https://clinicaltrials.gov/ct2/show/NCT02173145).
We report three cases of pulmonary fibrosis post-H1N1 ARDS who showed clinical and radiological improvement after pirfenidone and azithromycin were added to prednisolone.
Case Reports
All three patients were of Indian ethnicity and non-smokers. H1N1 influenza had been diagnosed by real-time polymerase chain reaction (RT-PCR) on throat swab samples. They had received oseltamivir during hospitalisation. All these patients had manifested ARDS, requiring ventilator support during ICU stay and received systemic corticosteroids during hospitalisation. They were discharged with oral prednisolone and home oxygen support. At presentation, all three patients had persistent cough, dyspnoea and hypoxemia at rest even after at least three weeks on oral prednisolone post discharge. The first patient had resting partial pressure of oxygen 53 mm Hg. The other two patients required 4 L/min of nasal oxygen to maintain SpO2 more than 90%. Blood counts, C-reactive protein, serum procalcitonin, liver and renal function tests were within normal limits.
Pirfenidone 600 mg daily, escalated over a month to a maximum tolerable dose of 2.4 gm daily, and azithromycin 500 mg thrice weekly were added off-label to prednisolone 10 mg daily on compassionate grounds with patients’ consents (7, 11-13).
Institutional approval and patients’ consents were obtained for this observational report.
Patient 1
A 40 year old male with no past or family history of pulmonary disease, or comorbidities had been hospitalised for three months and had received mechanical ventilation for five weeks.
At presentation, chest X-ray showed fibrotic opacities and patchy areas of consolidation in bilateral mid zones. High resolution computed tomography (HRCT) scan of the chest revealed bilateral fibrosis with interlobar and intralobular septal thickening, bronchiectatic changes involving right middle, right lower lobes and left lingula, and mild pleural thickening (Figure 1a). Arterial blood gas analysis on room air revealed pH 7.435, pO2 53.3 mm Hg, pCO2 38.7 mm Hg and bicarbonate (cHCO3) 25.4 mmol/L. He was unable to perform spirometry at presentation. Spirometry performed a month after beginning therapy revealed forced vital capacity (FVC) 1.75 L (42% of predicted) and forced expiratory volume in 1 s (FEV1) 1.56 L (46% of predicted). Six-minute walk test distance was 175 meters and lowest SpO2 was 74%.
Fig. 1.
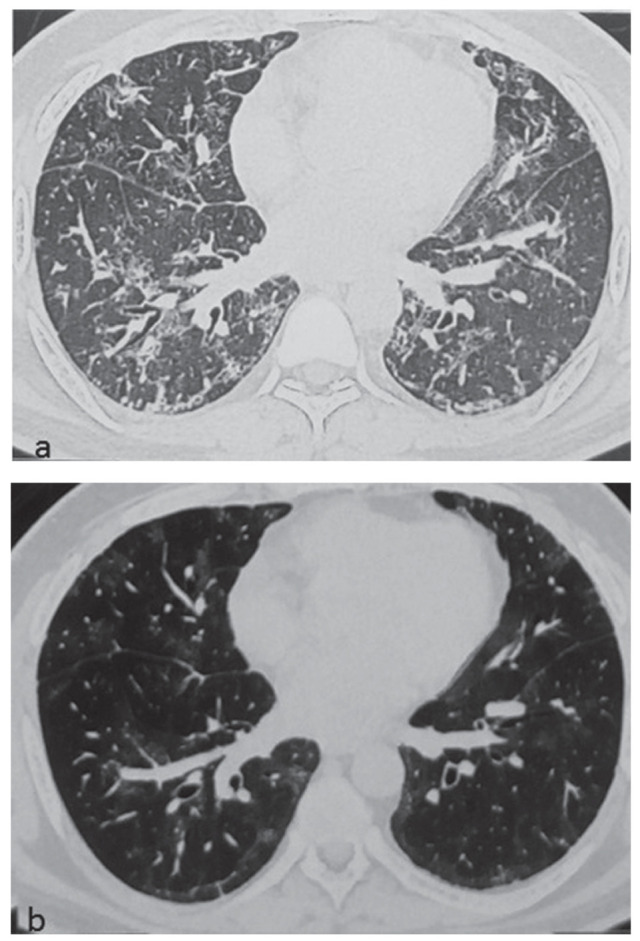
HRCT chest showing fibrotic, bronchiectatic and consolidative changes at presentation (a), and improvement after one year of therapy (b)
At three months, the patient had remarkable clinical improvement and home oxygen support could be discontinued. At this stage, azithromycin was also stopped. At one year follow up, he had only occasional cough and was able to walk two kilometres at moderate pace over level ground without rest and climb three flights of stairs. HRCT scan of the chest showed some regression of fibrotic and bronchiectatic changes (Figure 1b). Spirometry revealed FVC 2.12 L (51% of predicted) and FEV1 1.88 L (55% of predicted). Six-minute walk test distance was 450 meters and lowest SpO2 was 93%. Pirfenidone and prednisolone were stopped at one year. The patient resumed full-time work.
Patient 2
A 45 year old male with no past or family history of pulmonary disease, or comorbidities had been hospitalised for a month, requiring mechanical ventilation for two weeks.
At presentation, chest X-ray showed patchy opacities in both lower lobes. HRCT scan of the chest revealed patchy consolidations in basal segments of bilateral lower lobes, posterior segment of right lower lobe, left lingula and anterior segment of left upper lobe associated with some fibrotic bands and mild bronchiectatic changes. Patchy ground glass opacities were also observed in both lung fields (Figure 2a). A month after initiation of therapy, spirometry revealed FVC 1.33 L (39% of predicted), FEV1 1.14 L (40% of predicted), FEV1/FVC 85%. On six-minute walk test the minimum SpO2 was 90% and the distance was 325 m. Three months into therapy he developed a generalised erythematous macular pruritic rash that resolved on stopping pirfenidone. Thrice weekly azithromycin and daily prednisolone were continued.
Fig. 2.
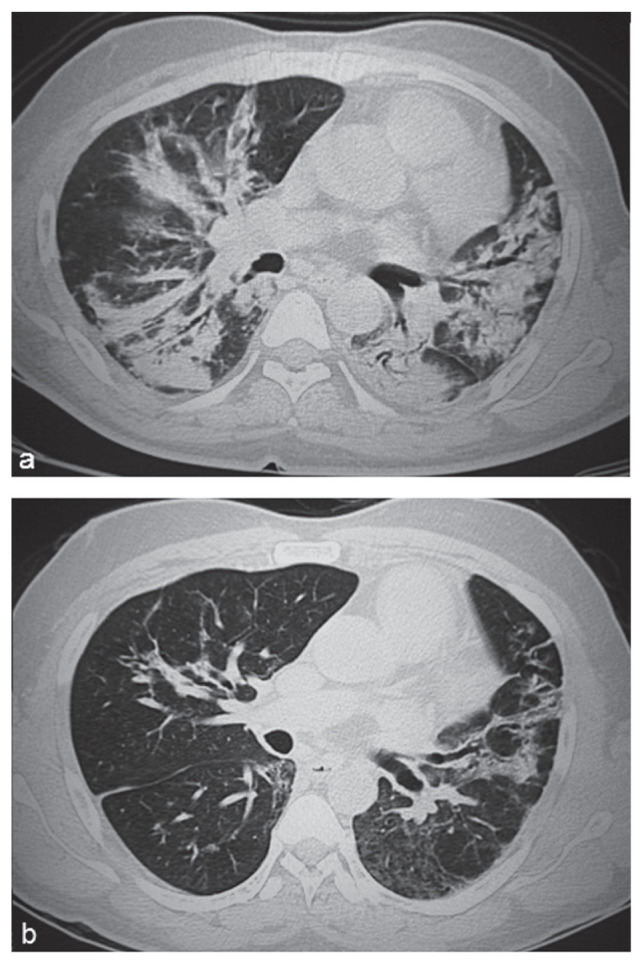
HRCT chest showing predominantly consolidative and some fibrotic changes with scattered ground glass opacities at presentation (a), and improvement after one year of therapy (b)
At one year follow up, the effort tolerance was ability to walk two kilometres at a moderate pace over level ground without rest and climb one flight of stairs. HRCT scan of the chest showed regression of consolidation and no progression of fibrotic and bronchiectatic changes (Figure 2b). Spirometry revealed FVC 1.79 L (50% of predicted) and FEV1 1.56 L (53% of predicted). Six-minute walk test revealed minimum SpO2 96% and distance 550 m. Azithromycin and prednisolone were stopped. The patient resumed full-time work.
Patient 3
A 59 year old male with a history of ischaemic heart disease, subsequent percutaneous transluminal coronary angioplasty twelve years ago, type 2 diabetes mellitus and dyslipidaemia was hospitalised for a month and required mechanical ventilation for two weeks.
Four weeks following discharge from hospital, arterial blood gas (ABG) analysis revealed pH 7.421, pO2 49.9 mm Hg, pCO2 38.9 mm Hg, HCO3 24.8 m and SpO2 92% on oxygen via nasal prongs at 4 L/min. HRCT scan of the chest revealed extensive bilateral ground glass opacities, intra and interlobular interstitial thickening, and traction bronchiectasis with areas of honeycombing (Figure 3a). He was unable to perform spirometry. Rheumatoid factor test was borderline positive (13.76 IU/ml), but anti-cyclic citrullinated peptide and antinuclear antibodies were negative. A two dimensional echocardiography with Doppler haemodynamic study was unremarkable (left ventricular ejection fraction 62% and no pulmonary hypertension). He was unable to perform pulmonary function testing.
Fig. 3.
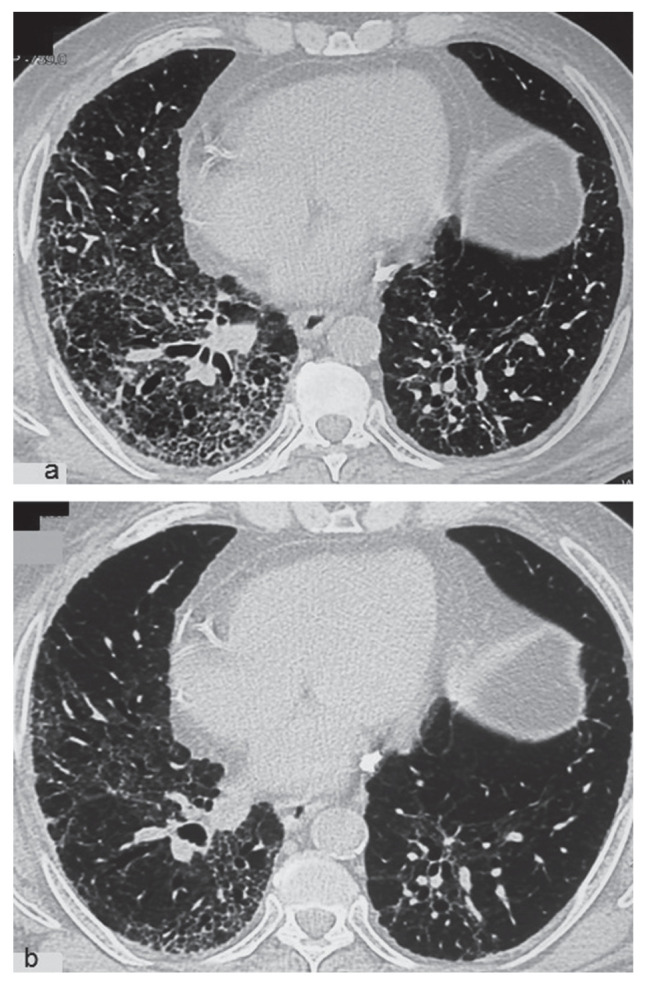
HRCT chest showing fibrosis, honeycombing, traction bronchiectasis and ground glass opacities at presentation (a), and improvement after one year of therapy (b)
Patient had remarkable clinical recovery and oxygen supplementation was stopped after five months. At one year follow up, the patient was able to walk 1.5 km at a moderate pace without rest and climb one flight of stairs without requiring oxygen support. SpO2 was 98% on room air. HRCT chest showed resolution of ground glass opacities and no progression of fibrotic and bronchiectatic changes (Figure 3b). Spirometry revealed FVC 1.95 L (68% of predicted), FEV1 1.75 L (76% of predicted) FEV1/FVC 89.9%. Steroids and azithromycin were stopped. Pirfenidone is being continued in view of honeycombing on CT scan chest.
Discussion
Pulmonary fibrosis may follow H1N1 ARDS as a result of increased fibroblast activity in the post-inflammatory repair pathways, with transforming growth factor-β (TGF-β) and procollagen I playing important roles (14-16). The development of pulmonary fibrosis following acute lung injury by the H1N1 virus due to inflammatory cytokine and pro-fibrotic cascades has been demonstrated in murine models (17). In general, fibrosis occurs after about three weeks of evolution of ARDS (18). A one-year outcome study of post-H1N1 ARDS patients showed that among patients treated and not treated with extracorporeal lung assist, 50% and 40% patients reported exertional dyspnoea respectively, 83% and 64% had returned to work, and all showed diminished exercise capacity and residual CT scan changes (19). However, this study did not report on the conditions of the patients at discharge from ICU in terms of chest CT scan findings and oxygen requirements. Our patient population is plausibly different from the report of Luyt et al (19). The patients in our report had high morbidity at presentation, were severely incapacitated, dependent on oxygen for 24 hours at home with CT scans of the chest showing ground glass opacities, interstitial septal thickening or lung fibrosis despite being on oral corticosteroids for at least three weeks following discharge from ICU. There is a lack of data regarding outcomes of such patients with severe morbidity post-H1N1 ARDS. Furthermore, there is no standardised pharmacotherapy targeting fibroproliferation post-ARDS (3). Thus we initiated add-on off-label therapy as described in the current report.
Corticosteroids reduce inflammation and fibroproliferation in the late fibrotic phase of ARDS (5). All three patients were treated with corticosteroids whilst in hospital. However, following discharge from hospital, all three had persistent symptoms and required continued home oxygen support. They received at least 10 mg of prednisolone daily for at least three weeks following discharge. Pirfenidone and azithromycin were added off-label and temporal association leads us to believe that they contributed to improvement in symptoms, regression of chest CT scan abnormalities and improved respiratory physiology leading to withdrawal of home oxygen therapy in at least the two patients who continued pirfenidone therapy for at least a year. The role of pirfenidone in patient 2 is a matter for speculation as the drug had to be stopped three months into therapy due to an adverse reaction.
TGF-β is overproduced in H1N1 pneumonia (14, 16). Pirfenidone is a broad-spectrum oral anti-fibrotic agent that has been shown to suppress bleomycin induced overexpression of TGF-β, procollagen I and III gene expressions, and lung basic-fibroblast growth factor in murine models (20-22). Pirfenidone reduces lung fibrosis significantly more than prednisolone in bleomycin-induced pulmonary fibrosis in mice (22). Successful treatment of bleomycin induced lung disease with pirfenidone and prednisolone has been reported in humans (23). In patients with IPF, decline in FVC is associated with increased mortality. Pirfenidone is recommended in IPF as it reduces this decline in pulmonary function (6, 7, 11, 12). In the current report, pirfenidone was additionally administered to patients already receiving prednisolone and still having significant respiratory morbidity.
Macrolide antibiotics have immunomodulatory properties. They assist in the regeneration of alveolar epithelium following injury and in the maintenance of alveolar surfactant homeostasis, besides inhibiting pro-fibrotic cytokines like IL-8, NF-κB, eotaxins and matrix metalloproteinases (10, 24). Long-term azithromycin is effective in the management of cystic fibrosis (CF) and diffuse panbronchiolitis, rationalising its use in non-CF bronchiectasis (8, 10, 13). Success has been reported in the use of long-term azithromycin therapy for post-infectious bronchiolitis obliterans in children (25). Azithromycin has also been shown to reduce bleomycin induced pulmonary fibrosis and restrictive lung function in murine models (9). There is an ongoing trial examining the use of macrolides in pulmonary fibrosis (University Hospital Inselspital Berne: Azithromycin in Idiopathic Pulmonary Fibrosis; ClinicalTrials.gov Identifier NCT02173145; https://clinicaltrials.gov/ct2/show/NCT02173145). Our patients were severely incapacitated at presentation and had not responded to at least three weeks’ oral corticosteroid and home oxygen therapy. Therefore we extrapolated the role of azithromycin to the present clinical situations, adding it to pirfenidone and prednisolone.
In summary, the patients recovering from the fibrotic sequelae of H1N1 ARDS in the current report had severe morbidity despite continued home oxygen support and oral corticosteroid therapy following discharge from the ICU. They experienced favourable treatment outcomes with the use of pirfenidone and azithromycin in addition to prednisolone (although the role of pirfenidone in one patient remains speculative due to cessation of the drug after three months due to an adverse reaction). At one year follow-up, all three patients had remarkable clinical improvement, enhanced effort tolerance and increased FEV1 and FVC. HRCT scans of the chest showed no progression of fibrosis and resolution of ground glass opacities. Two patients returned to work and the other maintains a good retired life and an active social life.
Based on this experience, we believe that there may be a potential for the use of pirfenidone, azithromycin and prednisolone in the management of pulmonary fibrosis post-H1N1 ARDS. Further studies on the treatment of this morbid condition would help establish their role.
References
- 1.Mineo G, Ciccarese F, Modolon C, Landini MP, Valentino M, Zompatori M. Post-ARDS pulmonary fibrosis in patients with H1N1 pneumonia: role of follow-up CT. Radiol Med. 2012;117:185–200. doi: 10.1007/s11547-011-0740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V, Sharma BB, Patel V. Pulmonary sequelae in a patient recovered from swine flu. Lung India. 2012;29:277–9. doi: 10.4103/0970-2113.99118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnham EL, Janssen WJ, Riches DWH, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43:276–85. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Thoracic Society (ATS), European Respiratory Society (ERS) Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 5.Biffl WL, Moore FA, Moore EE, Haenel JB, McIntyre RC, Jr, Burch JM. Are corticosteroids salvage therapy for refractory acute respiratory distress syndrome. Am J Surg. 1995;170:591–6. doi: 10.1016/s0002-9610(99)80022-1. [DOI] [PubMed] [Google Scholar]
- 6.Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem. 2008;15:1911–24. doi: 10.2174/092986708785132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 8.Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J. 2013;42:239–51. doi: 10.1183/09031936.00136712. [DOI] [PubMed] [Google Scholar]
- 9.Wuyts WA, Willems S, Vos R, et al. Azithromycin reduces pulmonary fibrosis in a bleomycin mouse model. Exp Lung Res. 2010;36:602–14. doi: 10.3109/01902148.2010.492895. [DOI] [PubMed] [Google Scholar]
- 10.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble PW, Albera C, Bradford WZ, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 12.King TEJ, Bradford WZ, Castro-Bernardini S, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 13.Amsden GW. Anti-inflammatory effects of macrolides - an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions. J Antimicrob Chemother. 2005;55:10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- 14.Wen Y, Deng BC, Zhou Y, et al. Immunological features in patients with pneumonitis due to influenza A H1N1 infection. J Investig Allergol Clin Immunol. 2011;21:44–50. [PubMed] [Google Scholar]
- 15.Liebler JM, Qu Z, Buckner B, Powers MR, Rosenbaum JT. Fibroproliferation and mast cells in the acute respiratory distress syndrome. Thorax. 1998;53:823–9. doi: 10.1136/thx.53.10.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Ishii K, Yamada M, Kunishima H, Kaku M. Lung fibrosis after acute lung injury caused by H1N1 influenza virus infection in mice. Am J Respir Crit Care Med. 2013;187:A4171. [Google Scholar]
- 18.Thille AW, Esteban A, Fernandez-Segoviano P, et al. Time to onset of pulmonary fibrosis in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;187:A2216. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 19.Luyt CE, Combes A, Becquemin MH, et al. REVA Study Group. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012;142:583–92. doi: 10.1378/chest.11-2196. [DOI] [PubMed] [Google Scholar]
- 20.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-β gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291:367–73. [PubMed] [Google Scholar]
- 21.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;289:211–8. [PubMed] [Google Scholar]
- 22.Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–8. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya PJ, Sandeepa HS, Singh T, et al. Combined prednisolone and pirfenidone in bleomycin-induced lung disease. J Cancer Res Ther. 2016;12:1198–202. doi: 10.4103/0973-1482.197530. [DOI] [PubMed] [Google Scholar]
- 24.Guillot L, Tabary O, Nathan N, Corvol H, Clement A. Macrolides: new therapeutic perspectives in lung diseases. Int J Biochem Cell Biol. 2011;43:1241–6. doi: 10.1016/j.biocel.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Liu C, Wang M, Zhang YI, Li H, Liu G. Clinical features of post-infectious bronchiolitis obliterans in children undergoing long-term azithromycin treatment. Exp Ther Med. 2015;9:2379–83. doi: 10.3892/etm.2015.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]


