Abstract
Background: While the efficacy of pulmonary rehabilitation (PR) in chronic obstructive pulmonary disease (COPD) has been well established, emerging evidence also suggests its benefit in idiopathic pulmonary fibrosis (IPF). However, the differences and similarities between how PR affects diseases with different physiologies remain unknown. Objective: This study aimed to compare the efficacy of PR in COPD and IPF patients by performing multifactorial evaluation with various exercise capacity measurements, and dyspnea and health-related quality of life (QoL) assessment. Methods: Twenty-two IPF patients (%vital capacity: 72%) and 27 COPD patients (%forced expiratory volume1: 43%) were recruited. Subjects who completed a 10-week outpatient PR program were analyzed. We assessed five exercise capacity indicators (6-minute walking distance, incremental shuttle walking distance, endurance time, peak work rate, and peak values for oxygen uptake [peak VO2]), dyspnea (Baseline Dyspnea Index: BDI), and health-related QoL (St. George’s Respiratory Questionnaire: SGRQ) at baseline and immediately following completion of the PR program. Results: After 10 weeks of PR, all exercise capacity measurements, except VO2, as well as BDI and SGRQ score improved significantly (p<0.05) in both disease groups. The magnitude of the observed changes in each outcome, assessed by the effect size, was comparable between IPF and COPD patients. This was also true for endurance time, the measurement most responsive to PR, with a large effect size. Conclusions: PR can result in comparable improvements in exercise capacity, including endurance time, and dyspnea and HRQoL in both IPF and COPD patients after 10 weeks of exercise training. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 283-289)
Keywords: endurance time, idiopathic pulmonary fibrosis, COPD, pulmonary rehabilitation, exercise capacity
Introduction
Despite the difference in pathophysiology and disease mechanisms, patients with chronic lung diseases, including chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), share the common manifestations of reduced exercise capacity and skeletal muscle function, along with exertional dyspnea and impaired health-related quality of life (HRQoL) (1-4). Multiple studies have shown that pulmonary rehabilitation (PR), including aerobic exercise training, improves exercise capacity, dyspnea, and HRQoL in COPD subjects (4, 5). The efficacy of PR in COPD patients has been repeatedly validated by means of various measurements of exercise capacity, including 6-minute walking distance (6MWD) and incremental shuttle walk distance (ISWD), providing strong evidence for the benefit of PR in COPD patients (4, 5). In addition, endurance time has been reported to be the most responsive exercise measurement during the evaluation of PR efficacy in both IPF and COPD patients (6, 7). PR was also shown to improve subjective measurements such as dyspnea and HRQoL in both IPF and COPD (4, 5).
Recent studies (1, 8-13) have suggested that PR has beneficial effects in IPF patients. One recent systematic review (10) emphasized the efficacy of PR in terms of improving 6MWD. However, robust evidence based on other exercise capacity measurements is unavailable to support the benefit of PR in IPF patients. It also remains largely unknown whether the effect of PR is different or similar in these two chronic lung diseases (COPD and IPF), which have different underlying pathophysiologies.
The current study aimed to compare the effects of PR in COPD and IPF subjects by assessing a variety of exercise capacities, including endurance time, along with dyspnea and HRQoL.
Methods
Study design
The study was a prospective observational study. Patients referred to our outpatient clinic between April 2008 and March 2012 were recruited into this study and classified into the IPF and COPD patient groups. IPF and COPD patients who had undergone evaluation at diagnosis, as is the general practice in Tosei General Hospital (Aichi, Japan), were assessed for inclusion in the study according to the eligibility criteria described below. Enrolled patients in both groups were assessed at baseline and immediately following the 10-week PR program. Patients in both groups underwent an identical exercise training program. Informed consent was obtained from all study participants. This study was approved by the ethics committee of Tosei General Hospital (approval number 213). The registration number for the trial registry was R000022887 (http://www.umin.ac.jp/).
Inclusion and exclusion criteria
The following inclusion criteria were used for IPF patients: (i) age less than 75 years; (ii) diagnosis of IPF; (iii) shortness of breath on effort; and (iv) stable clinical condition with no infection or exacerbation in the previous 3 months. Exclusion criteria were severe comorbid illnesses, collagen vascular diseases, and the need for long-term oxygen therapy. The diagnosis of IPF was made in accordance with the American Thoracic Society and European Respiratory Society statement (14).
Inclusion criteria for COPD patients were as follows: patient-reported exertional dyspnea and a constant medication regimen without any history of an acute exacerbation for at least 3 months prior to recruitment. The diagnosis of COPD was based on the following criteria: (i) a history of smoking of more than 20 pack-years, (ii) a forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) of less than 70%, (iii) no obvious abnormal shadows on chest X-ray, and (iv) no clinical diagnosis of asthma. The exclusion criteria were a history of lung surgery, the use of long-term oxygen therapy, or any comorbid conditions likely to reduce exercise capacity. At the time of the study, none of the subjects were current smokers.
Assessment
The following measurements were conducted at baseline and immediately following the 10-week PR program. All patients underwent body anthropometry, pulmonary function tests, arterial blood-gas tension measurements, exercise tests, muscle strength tests, and dyspnea and HRQoL assessments at baseline and 10-weeks. The primary outcome measures were scores on four exercise tests, including an incremental load ergometry test (ILET), constant load ergometry test (CLET), 6MWT, and incremental shuttle walk test (ISWT). Secondary outcome measures were dyspnea (Baseline Dyspnea Index; BDI), HRQoL (St. George’s Respiratory Questionnaire: SGRQ), and muscle strength.
Exercise tests
The ILET was performed on an electronically braked cycle ergometer in accordance with published guidelines (15) to evaluate maximal exercise capacity. Peak values for oxygen uptake (peak VO2) and work rate (peak WR) during exercise were recorded. The anaerobic threshold was determined using the V-slope technique. Endurance time was determined by performing the CLET using the same cycle ergometer as that used for the ILET (16). The patients continued cycling at a constant submaximal workload (80% of the peak WR). They were stopped according to the same criteria used in the ILET, and the endurance time was measured. Measurement of 6MWT was performed according to the American Thoracic Society statement (17). The total distance walked was recorded as the 6MWD. The ISWT was performed in a 10-m course identified by two cones placed 0.5 m from each endpoint (18). The total distance walked was recorded as the ISWD. All patients underwent both the 6MWT and the ISWT at least once prior to study entry. Transcutaneous oxygen saturation was monitored by pulse oximetry throughout all tests.
Pulmonary function tests
Spirometry was performed according to published recommendations (19). Single-breath diffusion capacity for carbon monoxide (DLCO) was also measured. All values are expressed as a percentage of the predicted values reported by the Japan Society of Respiratory Diseases (20).
Muscle strength tests
Quadriceps force was measured using a dynamometer. The peak torque (Newton-meters, Nm) was measured in both legs during a maximal isokinetic knee extension maneuver, with the hip in 90° flexion (21). The highest value from at least four maneuvers for each leg was recorded. Grip strength was measured with a hydraulic hand dynamometer. The highest value of at least three maneuvers was recorded for each hand. All subjects underwent respiratory muscle testing to determine the maximal inspiratory pressure and maximal expiratory pressure. The highest value from at least three maneuvers was recorded.
Pulmonary rehabilitation program
The program comprised twice-weekly supervised exercise training for a period of 10 weeks in Tosei General Hospital (6). The supervised sessions lasted 90 minutes and consisted of respiratory care, subject education, and endurance and strength training. Subjects performed supervised endurance training on a braked cycle ergometer, with a target of 20 minutes of continuous cycling. The target intensity was 80% of the peak WR obtained from the ILET. Peripheral muscle strength training included upper and lower limb resistance training with weight machines, hand weights, or elastic bands. The respiratory muscle training was performed using an inspiratory threshold device. If desaturation was under 80% during the CLET, subjects received oxygen therapy during exercise training. Supplemental oxygen was administered to maintain oxygen saturation above 80% during exercise training.
Statistical analysis
Within-group changes in the outcome measures following the PR program were compared using paired t-tests or Wilcoxon signed rank tests. Differences between the two groups at baseline and the differences in outcome measures were compared using unpaired t-tests. Differences between the two groups in the change in outcome measures following PR were compared using two-way analysis of variance (ANOVA). When a significant difference was found, post hoc analysis was performed with the Bonferroni adjustment method to identify the differences that were significant. The effect size represented the mean change in the score divided by the standard deviation of the baseline scores. Following Cohen, effect sizes of ≥0.2 to <0.5 were regarded as small, ≥0.5 to <0.8 as moderate, and ≥0.8 as large changes (22). A p value of less than 0.05 was considered significant. All data are given as mean ± standard deviation (SD). Analyses were performed using SPSS 22.0 for Windows.
Results
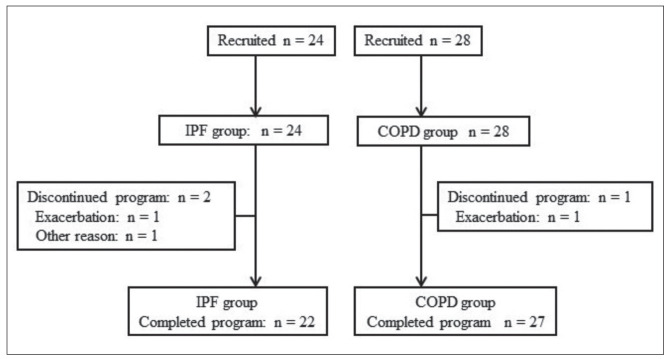
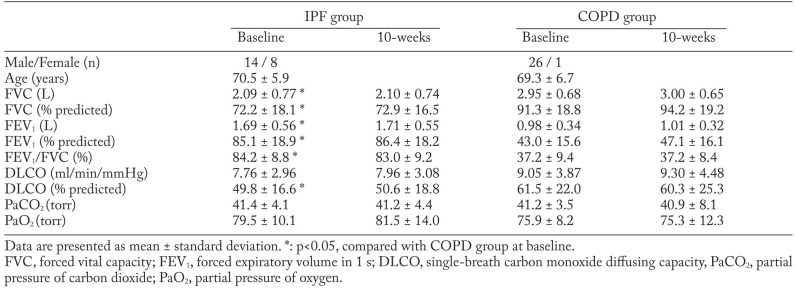
The flow of patients through the study is shown in Figure 1. Of the 52 patients recruited for the study, 24 patients were included in the IPF group and commenced the PR program; among them, 22 finished the program and completed the second evaluation. In the COPD group, 28 patients underwent baseline evaluation, and 27 of them completed the second evaluation. Patients were evaluated by Medical Research Council (MRC) grade at baseline. In IPF patients, 3 patients were grade 2, 18 patients were grade 3, and one patient was grade 4. In COPD patients, 8 patients were grade 2, 17 patients were grade 3, and two patients were grade 4. The average GAP score of the 22 IPF patients was 4.0 ± 1.3 points. When classified by GAP stage, 10 patients were GAP stage 1, 9 patients were GAP stage 2, and 3 patients were GAP stage 3. In terms of baseline measurement, %VC and %DLCO were lower, while FEV1 and FEV1.% were higher in the IPF group than in the COPD group. Arterial blood gas tension, BDI, SGRQ score, and exercise capacity at baseline were not significantly different between the two groups (Table 1, Table 2).
Fig. 1.
Participant flow diagram. IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease
Table 1.
Lung function data at baseline and immediately following 10 weeks of pulmonary rehabilitation
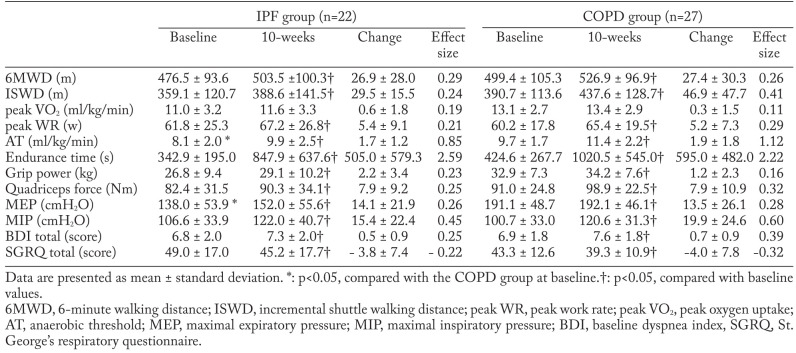
Table 2.
Muscle strength, exercise test data, dyspnea and health status at baseline and immediately following pulmonary rehabilitation
In both the IPF and COPD groups, endurance time, peak WR, anaerobic threshold, work efficiency, 6MWD, and ISWD improved significantly after 10 weeks of PR (p<0.05), whereas peak VO2 did not improve. The improvement in each exercise capacity outcome after PR showed no significant difference between the IPF and COPD groups. Further, we compared the magnitude of change in each measurement after the program using effect size. The effect size for each measurement was comparable between both groups for most measurements. A larger effect size for endurance time was observed in the IPF group (2.59) than in the COPD group (2.22).
After the 10-week PR program, BDI and SGRQ scores improved significantly in both the IPF and COPD groups (p<0.05). The improvement in BDI and SGRQ scores was not different between IPF and COPD groups. Lung function and arterial blood-gas tension did not change after 10 weeks of PR in either group (Table 1).
In terms of muscle strength, significant improvements were seen in grip strength, quadriceps force, maximal expiratory pressure, and maximal inspiratory pressure after the 10-week PR program in both the IPF and COPD groups (Table 2). The improvement in the four muscle strength outcomes was not significantly different between the IPF and COPD groups.
Discussion
In the present study, we first evaluated the effects of PR in IPF patients by using various exercise measurements, including endurance time, and compared their improvements with those in COPD patients. We found that the same indicators of exercise capacity, HRQoL, and dyspnea were improved by PR in both the IPF and COPD groups. Furthermore, the magnitudes of the improvements in major outcomes in IPF patients were comparable to those observed in COPD patients. Additionally, the PR-induced improvements, evaluated using effect size, were comparable between IPF and COPD patients. The effect size of endurance time following PR was observed to be larger than those of other exercise measurements in both diseases.
Among the several different measurements of exercise capacity, endurance time showed the largest effect size following the PR program in both patient groups. This result supports our previous finding (6, 7) that, among the exercise capacity measurements, endurance time was the most responsive to PR in IPF and COPD patients. In addition to this, PR was associated with a moderately significant increase in peak WR, anaerobic threshold, 6MWD, and ISWD in IPF and COPD patients. Anaerobic threshold, 6MWD, and ISWD reflect submaximal exercise capacity. We assumed that the effectiveness of PR on submaximal exercise capacity was similar in both IPF and COPD.
In this study, we assessed the magnitude of improvement in exercise capacity outcomes between IPF and COPD groups by effect size, and found that the observed changes in exercise capacity in IPF were similar to those in COPD. The present study and a previous study by Vainshelboim et al. (23) in IPF patients with relatively preserved exercise capacity showed large improvements in exercise capacity after PR. Conversely, Kozu et al. (11) and Jackson et al. (13) reported that PR was associated with poor improvement in exercise capacity in IPF patients with poor baseline exercise capacity. The subjects in the present study had average GAP scores of 4 points, with most patients in GAP stage 1 or 2. Because many IPF patients in this study had mild and early-stage disease, it might have been easier for them to adhere to the PR program, including high-intensity training. This indicates that PR should be considered in earlier disease stages before patients experience severe impairment in exercise capacity.
PR in IPF patients resulted in significant improvements in grip strength, quadriceps force, maximal expiratory pressure, and maximal inspiratory pressure, as it did in COPD patients. Exercise training in IPF may result in physiological improvements similar to those observed in COPD because peripheral muscle weakness in both IPF and COPD is affected significantly by deconditioning (2, 3). Skeletal muscle weakness caused by deconditioning can be improved by muscle strength and endurance training (24).
In the present study, we observed that PR produced similar improvements in dyspnea, as assessed by the BDI, in IPF and COPD patients. Dyspnea is an independent prognostic factor in IPF, and measurement of dyspnea is used as a major outcome in clinical trials (25). Therefore, dyspnea improvement following PR was crucial. We assumed that the benefits following PR in the present study were greater than those in a previous study (26) because most of our subjects had milder dyspnea, with an MRC dyspnea grade of 2 or 3. The BDI is strongly correlated with HRQoL in both IPF and COPD patients. (27, 28). Therefore, improvement in BDI following PR was an important finding in this study.
We observed similar improvement in HRQoL, assessed by the SGRQ, in patients with both IPF and COPD. The SGRQ is used as a disease-specific HRQoL measurement in patients with chronic pulmonary disease including COPD and IPF. The SGRQ score has been validated and used as a major outcome for clinical trials in both IPF and COPD (29, 30). Our group (8) and the others (23) have previously reported that a PR program significantly improved SGRQ scores in IPF patients. These disease-specific HRQoL indicators may represent responsive measurements for evaluating PR efficacy in IPF patients. A key finding of this study was that PR was beneficial for IPF patients because it improved dyspnea and HRQoL.
The present study has several limitations. First, this was a single-center study with a small sample size. Second, we did not evaluate the long-term effects of PR in IPF patients. Further studies are required to examine the long-term effect of PR in earlier stages of IPF.
In conclusion, a PR program can provide comparable improvements in exercise capacity, including endurance time, HRQoL, and dyspnea, in both IPF and COPD patients after 10 weeks of exercise training. A PR program is an important tool for reversing physical deconditioning and improving patient-oriented outcomes in IPF as well as COPD.
Financial support:
This work was supported by JSPS KAKENHI Grant Number 16K16470.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiyama O, Taniguchi H, Kondoh Y, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127(6):2028–33. doi: 10.1378/chest.127.6.2028. [DOI] [PubMed] [Google Scholar]
- 3.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153(3):976–80. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 4.Spruit MA, Singh SJ, Garvey C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 5.Rugbjerg M, Iepsen UW, Jørgensen KJ, Lange P. Effectiveness of pulmonary rehabilitation in COPD with mild symptoms: a systematic review with meta-analyses. Int J Chron Obstruct Pulmon Dis. 2015;10:791–801. doi: 10.2147/COPD.S78607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arizono S, Taniguchi H, Nishiyama O, et al. Improvements in quadriceps force and work efficiency are related to improvements in endurance capacity following pulmonary rehabilitation in COPD patients. Inter Med. 2011;50(21):2533–9. doi: 10.2169/internalmedicine.50.5316. [DOI] [PubMed] [Google Scholar]
- 7.Arizono S, Taniguchi H, Sakamoto K, et al. Endurance time is the most responsive exercise measurement in idiopathic pulmonary fibrosis. Respir Care. 2014;59(7):1108–15. doi: 10.4187/respcare.02674. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13(3):394–9. doi: 10.1111/j.1440-1843.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 9.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax. 2008;63(6):549–54. doi: 10.1136/thx.2007.088070. [DOI] [PubMed] [Google Scholar]
- 10.Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014;(10):CD006322. doi: 10.1002/14651858.CD006322.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Kozu R, Senjyu H, Jenkins SC, Mukae H, Sakamoto N, Kohno S. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration. 2011;81(3):196–205. doi: 10.1159/000315475. [DOI] [PubMed] [Google Scholar]
- 12.Holland AE, Hill CJ, Glaspole I, Goh N, McDonald CF. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med. 2012;106(3):429–35. doi: 10.1016/j.rmed.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Jackson RM, Gómez-Marín OW, Ramos CF, et al. Exercise limitation in IPF patients: a randomized trial of pulmonary rehabilitation. Lung. 2014;192(3):367–76. doi: 10.1007/s00408-014-9566-9. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med. 2003;167(2):211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A, Izumi T. The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. Am J Respir Cri Care Med. 2000;161(6):1897–901. doi: 10.1164/ajrccm.161.6.9905045. [DOI] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–24. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crapo RO, Hankinson JL, Irvin C, et al. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 20.Hanamoto S, Ohsuji T, Tsuyuguchi I, Kawabata S, Kimura K. Prediction formulas for pulmonary function tests expressed in linear and exponential form for healthy Japanese adults. Nihon Kyobu Shikkan Gakkai Zasshi. 1992;30:2051–60. [PubMed] [Google Scholar]
- 21.Watanabe F, Taniguchi H, Sakamoto K, et al. Quadriceps weakness contributes to exercise capacity in nonspecific interstitial pneumonia. Respir Med. 2013;107(4):622–8. doi: 10.1016/j.rmed.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. Statistical power analysis for the behavioural sciences. [Google Scholar]
- 23.Vainshelboim B, Oliveira J, Yehoshua L, et al. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration. 2014;88(5):378–88. doi: 10.1159/000367899. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea SD, Taylor NF, Paratz J. Peripheral muscle strength training in COPD. A systematic review. Chest. 2004;126(3):903–14. doi: 10.1378/chest.126.3.903. [DOI] [PubMed] [Google Scholar]
- 25.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 26.Kozu R, Jenkins S, Senjyu H. Evaluation of activity limitation in patients with idiopathic pulmonary fibrosis grouped according to medical research council dyspnea grade. Arch Phys Med Rehabil. 2014;95(5):950–5. doi: 10.1016/j.apmr.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 Pt 1):785–90. doi: 10.1164/ajrccm.157.3.9703055. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama O, Taniguchi H, Kondoh Y, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor. Respir Med. 2005;99(4):408–14. doi: 10.1016/j.rmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Richeldi L, du Bois RM, Raghu G, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 30.Qaseem A, Wilt TJ, Weinberger SE, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]