Abstract
Background: Upper respiratory tract (URT) involvement in sarcoidosis may be refractory to corticosteroids and immunosuppressants. Whether TNF-antagonists are efficient and safe in such phenotype is unknown. Methods: STAT is a French national drug registry including patients presenting sarcoidosis treated with TNF alpha antagonists. All cases of biopsy-proven sinonasal and laryngeal sarcoidosis were extracted and retrospectively analyzed from July 2014 to July 2015. Results: Twelve patients presenting biopsy-proven sarcoidosis with URT involvement were included in the STAT registry. Infliximab appeared effective in decreasing URT symptoms, as assessed by a significant decrease of the e-POST (extra-pulmonary Physician Organ Severity Tool) (1.5 [0-2] vs 5 [1.5-5], p=0.03) and a corticosteroids-sparing effect (7.5mg per day [5-10] vs 17.5 mg per day [7.5-20], p=0.04) at the end of follow-up. Conclusions: TNF-antagonists may be an efficient treatment of refractory URT manifestations and should be discussed when prolonged or high dosages of corticosteroids despite immunosuppressive therapy are required. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 343-351)
Keywords: refractory sarcoidosis, TNF antagonists, upper respiratory tract
Introduction
Sarcoidosis is a systemic disorder of unknown origin, characterized by the presence of granuloma in different organs (1). All the organs may virtually be affected, but the frequency and the impairment are variable. Apart intrathoracic lymphadenopathy and lung involvement, the most frequent sarcoidosis localizations include the eyes, skin, and liver, with a prevalence of 10-25% in most studies (1).
Approximately 10-15% of the patients have symptomatic specific otolaryngological involvement at the the larynx (0.5-1.4%), major salivary glands (5-10%), and nose and paranasal sinus level (1-4%) (2). While major salivary gland involvement most often follows a benign course, sinonasal and laryngeal sarcoidosis are usually severe, associated with other severe manifestations, with a particularly longstanding course and represent a therapeutic challenge. Moreover, life-threatening complications may be seen in laryngeal sarcoidosis (3).
Corticosteroids are the cornerstone of systemic sarcoidosis management. However, numerous patients develop a corticosteroid-dependence disease despite an adequate corticosteroid-sparing immunosuppressive regimen (4). TNF-alpha antagonists have been widely used for the treatment of corticosteroid-refractory sarcoidosis, since TNF-alpha play a critical role in granuloma formation (5). While the efficacy of these drugs on extra-pulmonary sarcoidosis is supported by several controlled and open-label studies (6-8), there is little evidence for its benefit in the treatment of upper respiratory tract involvement (URT). To date, most literature reports include single or a few cases (9-11).
The STAT (Sarcoidosis treated with TNF AnTagonist) registry includes all patients with a biopsy-proven sarcoidosis or a neurosarcoidosis treated by TNF-alpha antagonists (infliximab and adalimumab). We present here a subgroup analysis of patients presenting with URT-involvement (sinonasal or laryngeal) who were included in this nationwide study.
Patients and methods
Descriptive study
STAT is a French national drug registry of patients with biopsy-proven sarcoidosis treated with TNF antagonists. We e-mailed pneumology and internal medicine departments affiliated the Groupe Sarcoïdose Francophone (GSF) and Société Nationale Française de Médecine Interne (SNFMI) networks, to gather data on sarcoidosis patients who had received at least one TNF antagonist infusion. The registration period extended from July 2014 to July 2015.
To be included, patients had confirmed sarcoidosis (12). The subset of patients having histologic evidence of non-caseating granuloma on the URT tissues was extracted from the registry. Inclusion criteria comprised the exclusion of other causes of URT granulomatosis supported by research of fungus (using Grocott stain) and mycobacteria, and the absence of clinical evidence for another disorder associated with URT granulomatosis such as granulomatosis with polyangiitis, aspergillosis, syphilis and lepromatous leprosy.
According to the current French Legislation (Loi Huriet-Sérusclat 88-1138 https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000508831&dateTexte=20160501), declaration to research ethics board is not needed for observational studies when disease management is unchanged. The authors observed a strict accordance to the Helsinki Declaration guidelines.
Data collection
The participating physicians collected: i) personal data: sex, age, geographic origin, time of diagnosis, comorbidities, ii) disease phenotype: pulmonary and extra thoracic data, iii) treatment data: prior treatments, TNF antagonists type, time of treatment initiation and discontinuation, concomitant treatments, and iv) adverse events (AE) data: time, type, and severity.
URT involvement was defined by a suggestive symptomatology (crusting, epistaxis, nasal obstruction…), tomography abnormalities, or laryngeal infiltration observed during laryngoscopy.
The main outcome was a standardized URT assessment using the URT category of the extra pulmonary Physician Organ Severity Tool (e-POST), which examined the state of extra pulmonary involvement on a scale from 0 (not affected) to 6 (very severely affected) (13).
Patients had a clinical evaluation at treatment initiation, after 3, 6, 12 months (M0, M3, M6, M12), and if so, when treatment was discontinued.
The secondary outcomes included corticosteroid sparing (by comparing prednisone dosage at baseline and at end-point) and treatment safety. An adverse event was considered serious when it led to treatment discontinuation, hospitalization or death.
Statistical analyses
All the analyses were performed using R statistical software, version 3.0.2 (http://www.R-project.org). Categorical variables are expressed as numbers (percentages), continuous variables as means (standard deviations) or medians (interquartile range, IQR) and were compared using non-parametric tests (Wilcoxon comparison test for matched data for continuous variables), and Fisher or chi-square tests for categorical variables. Results are expressed as odds ratios (OR) with their 95% confidence intervals. A p-value inferior to .05 was considered for statistical significance.
Results
Patient characteristics
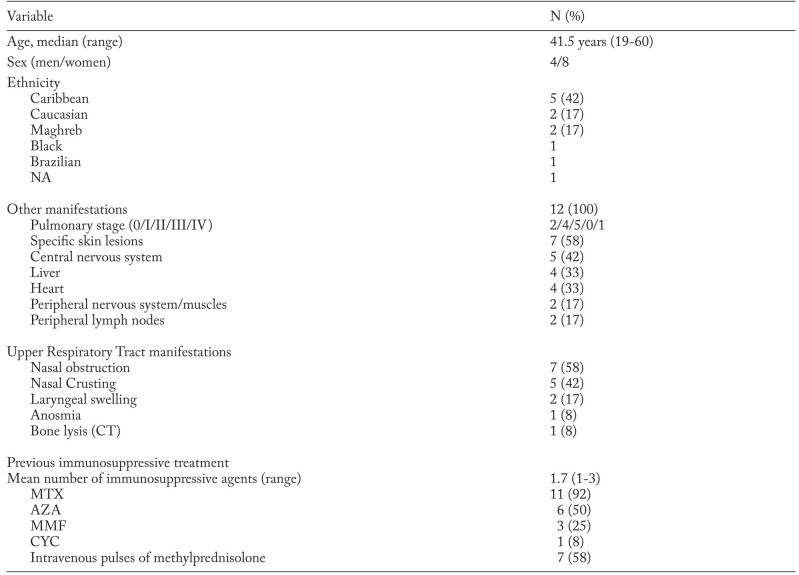
Among the 132 patients listed in the STAT registry, 12 had biopsy-proven URT-involvement. Two of these patients were previously published (9). Main characteristics at inclusion are presented in Table 1. There was a majority of women (67%) and a high proportion of patients of French Caribbean origin (42%). At initiation of anti-TNF therapy, the median age was 41.5 years and the median duration of sarcoidosis was 87 months (range: 21-228).
Table 1.
Main characteristics of the 12 patients with URT sarcoidosis
Ten patients presented with sinonasal involvement. Clinical symptoms included nasal obstruction (n=7), crusting (n=5), and epistaxis (n=1). A thickening of sinus mucosa was seen on tomography in 5 cases (9 CT) and bone lysis in one case. Endoscopic examination of nasal mucosa was consistently abnormal. Nasal biopsy displayed non caseating granuloma in every case. Two patients presented with laryngeal involvement (dysphonia) with endoscopic lesions and evidence of non caseating granulomas (Figure 1).
Fig. 1.
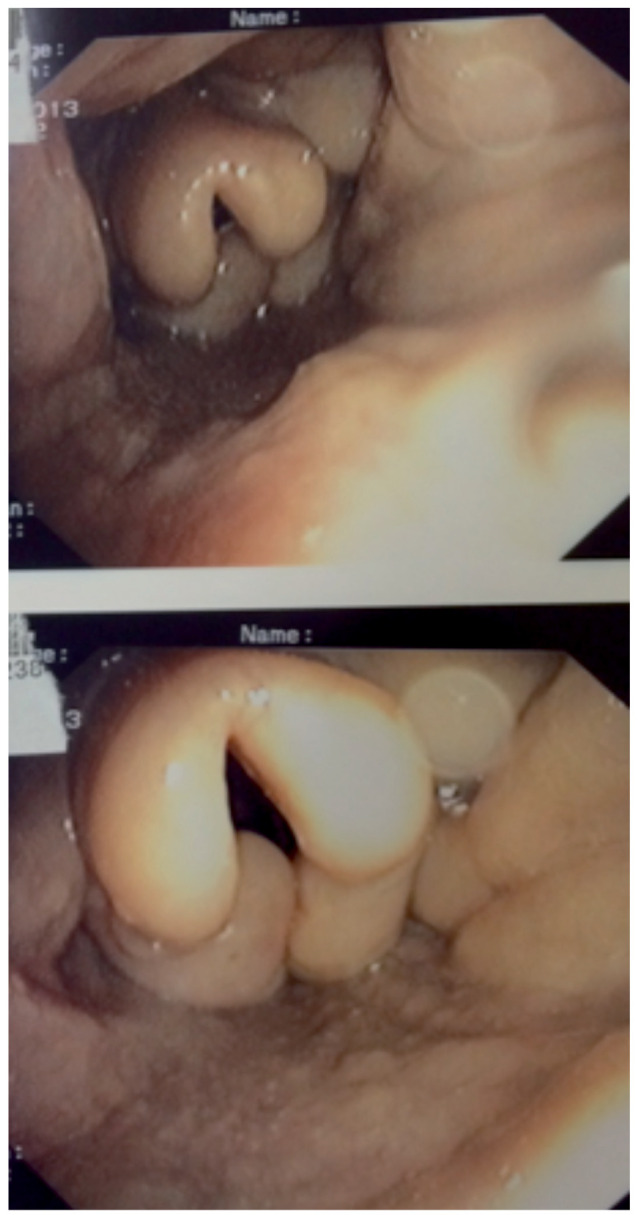
Sarcoidosis with laryngeal involvement
All patients had extra-URT involvement, two thirds of which were considered severe. The median number of localizations was 4.5 [3.75-6]. The most frequent extra-URT localizations were: chest (n=12), Central Nervous System (CNS) (n=5, with 2 cases of diabetes insipidus) and heart (n=4). Skin lesions were notably frequent with lupus pernio associated with sinonasal involvement in 6 patients (50%).
All patients had been given oral corticosteroids and at least one immunosuppressive drug before TNF antagonists. The median treatment durations were: 55 months for methotrexate (MTX) (n=11), 4.8 months for azathioprine (AZA) (n=6), and 186 months for mycophenolate mofetil (MMF) (n=3). One patient had received 18 cyclophosphamide intravenous infusions before TNF antagonists. The clinical indications for biologic therapy are shown in Table 2.
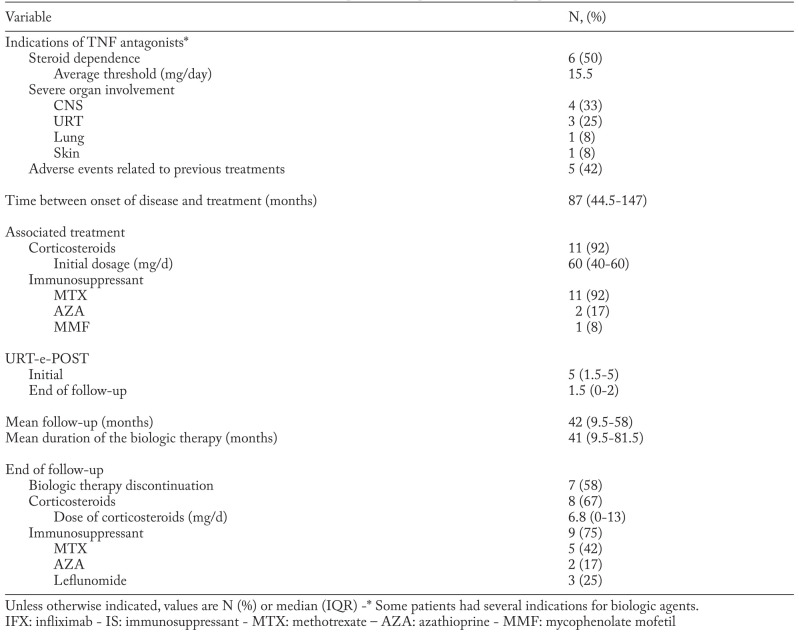
Table 2.
Clinical indications and treatment by biologic therapy of the 12 patients with biopsy-proven URT sarcoidosis
The use of TNF-antagonists was justified by various and sometimes combined reasons: severe disease (n=9), corticosteroid dependence (n=6, with a median of 15 mg per day or prednisone) and/or side effects of corticosteroid therapy (n=5; 3 diabetes mellitus, 1 obesity, 1 induced osteoporosis). The main indication for anti-TNF therapies are summarized in Table 2. Anti-TNF therapy was always used as a third-line treatment, in absence of response to second-line immunosuppressive therapies.
The localizations of severe disease were neurologic involvement (n=4: pituitary involvement=2, MRI white matter hyperintensities and leptomeningitis=2), URT manifestations (n=3: chronic nasal obstruction and rhinitis=2, dysphonia=1), lupus pernio (n=1) and lung involvement (n=1). In 3 cases, TNF-inhibition was justified only by corticosteroid dependency.
In all cases (n=12), infliximab (IFX) was used for TNF-inhibition, with a 5 mg/kg dose in 11 cases, and a 7 mg/kg dose in the remaining patient.
IFX intravenous infusions were given at weeks 0, 2 and 6, then every 4-8 weeks. No patient switched for another TNF antagonist. In association with anti-TNF therapy, 11 patients (92%) received corticosteroids while all patients received an immunosuppressant (IS) either MTX (n=11) or AZA (n=1)).
Clinical response
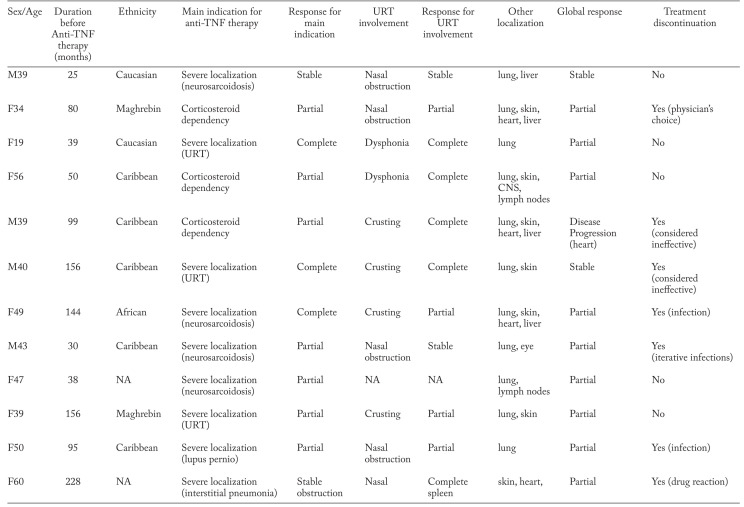
Mean follow-up duration was 42 [9,8-58] months. The response regarding the severe localization at the end of the follow-up was either complete (n=3, 25%), partial (n=4, 33.3%) or stable (2, 16.7%) (Table 3). The response regarding the URT involvement at the end of the follow-up was either complete (n=5, 41.7%), partial (n=4, 33.3%), stable (n=2, 16.7%) and not available in one case. The median URT e-POST 1.5 [0-2] at the end of the follow-up was significantly lower than at baseline (5 [1.5-5], p=0.03). Both patients with initial laryngeal involvement completely recovered after TNF inhibition.
Table 3.
Main indications for anti-TNF therapy and clinical response
The median oral corticosteroid intake which was 17.5 mg per day [7.5-20] at treatment initiation, significantly decreased at the end of follow-up (7.5 [5.00-10.00], p=0.04). Both patients with initial laryngeal involvement completely recovered after TNF inhibition.
Follow-up
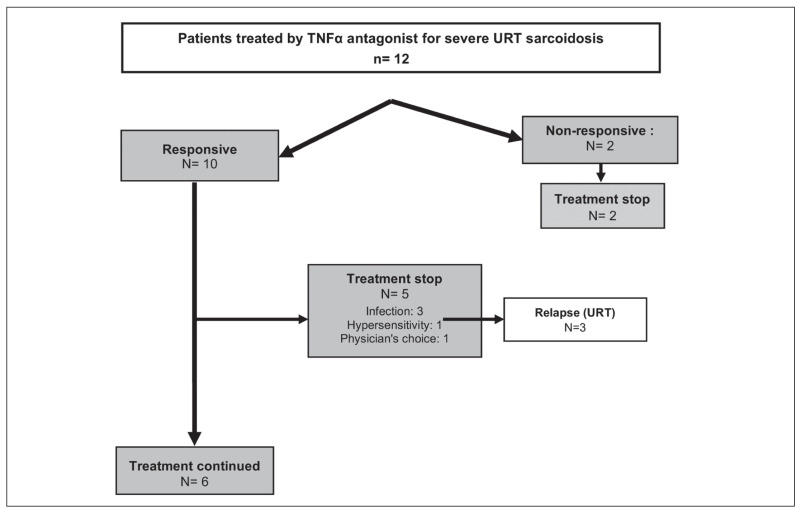
The outcomes of the first line anti-TNF therapy are summarized in Figure 2. The mean duration of anti-TNF treatment was 41 months [9.5-81.5]. It was considered ineffective in two patients (patients #5 and #6) and stopped after 3 and 8 months respectively. The treatment was also interrupted in 5 cases: 1 patient presented a severe hypersensitivity reaction (fever, shivering) and did not relapse after treatment discontinuation (patient #12); 3 patients presented infections (patient #7, #8 and #11); the physicians chose to discontinue the treatment considering the disease in remission in the remaining case (patient #12). Relapses were observed in 3 cases (patients #2, #8 and #11).
Fig. 2.
Outcome of the first-line TNF-antagonists therapy
Adverse effects
Adverse effects were observed in 8 cases (67%), which required anti-TNF permanent interruption in 3 patients (patients #7, #8 and #12). Infections were the main adverse effect of anti-TNF drugs; 6 patients presented infections during the anti-TNF therapy (17 events in total). Four infections requested hospitalization. The patient #8 underwent temporary interruptions due to iterative infections but presented a relapse of the disease at each occurrence. Drug hypersensitivity reactions occurred in one case. No lethal adverse effect was observed during follow-up.
Discussion
Sinonasal and laryngeal manifestations may be severe, associated to other severe localizations and are often difficult to treat. Our series concern cases with URT for which anti-TNF were given following absence of efficacy or bad tolerance with corticosteroids and immunosuppressive drugs. All our cases had histologic evidence of sarcoidosis URT involvement and had multistystemic involvement including particularly frequent skin, CNS and heart involvement. Main results of the study were: (i) complete response in two cases with laryngeal involvement; (ii) 41.7% complete response in sinonasal localizations; (iii) URT e-POST was improved; (iii) a significant sparing of corticosteroid was made possible; (iv) despite severe adverse effects were scarce, infections, were relatively frequent; (v) after obtaining a response, a continuation of anti-TNF treatment was necessary, and (vi) relapse of sarcoidosis under treatment were not observed.
Sinonasal and laryngeal sarcoidosis are unusual localizations and there are no randomized studies focusing on the treatment of URT involvement (9). These two localizations are considered to be resistant manifestations of sarcoidosis and represent a therapeutic challenge (14). Aubart et al. showed that systemic therapy is always required for sinonasal sarcoidosis, often with high corticosteroid dosage and associated immunosuppressive drugs (70% of the patients) (15). This was confirmed in a more recent series of 12 laryngeal sarcoidosis that required a high corticosteroid dosage and a long-term treatment (3). This statement encourages corticosteroid-sparing strategies for the management of URT sarcoidosis. Furthermore, several authors suggested an association between sinonasal manifestations and severe extrapulmonary localizations such as lupus pernio (15, 16), and CNS involvement (17). Consistent with these data, lupus pernio was present in 6/12, and CNS involvement in 5/12 of our cases. All these elements call for an active management of sarcoidosis sinonasal manifestations.
To our knowledge, the present study describes the largest published cohort of biopsy-proven URT sarcoidosis treated with TNF-alpha antagonists.
Infliximab proved to be effective for the treatment of URT involvement with a significant reduction of the e-POST score URT subset. We also report a full recovery in 2 cases of laryngeal involvement. The successful treatment by infliximab occurred after the failure of classical first-line drugs, including methotrexate and azathioprine in all cases. In a recent paper, Baughman et al summarized our current knowledge of TNF-alpha blockade effects in the management of sarcoidosis (18). All the anti-TNF agents are not equally effective in cases of refractory sarcoidosis. While subcutaneous adalimumab (however at higher doses than in rheumatoid arthritis) seems to be as effective as intravenous infliximab, etanercept, which acts as a decoy TNF-receptor, may not have much effect on granuloma formation.
The largest reported experience with infliximab is a multicenter randomized, placebo-controlled trial of 138 patients (19). Although the severity of extrapulmonary organ involvement, as measured by the e-POST, decreased significantly for the combined infliximab groups compared to the placebo, no meaningful conclusion could be drawn for individual organs, such as URT involvement. Cases of sarcoidosis with URT-involvement treated with anti-TNF agents are anecdotic in the literature: in a 10-case-large series of infliximab-treated sarcoidosis, Doty et al. reported one patient with URT involvement responding successfully to TNF antagonization(11). In a previous study, 3 out of 4 patients with otolaryngological sarcoidosis also benefited from infliximab (10). In these two studies, URT-involvement was not biopsy-proven. Hermet et al. were the first to publish a series of 3 cases with biopsy-proven sarcoidosis involving head and neck. Two out of these three cases are included in the present report. The last patient presented with trismus that revealed myostitis of the pterygoid muscle. Infliximab was found effective in treating the myositis but the patient still developed myocardial sarcoidosis after seven infusions.
Overall our data and these previous studies support the use of infliximab as a second-line therapy in case of refractory URT symptoms. The TNF-alpha inhibitors also permitted to significantly reduce the oral corticosteroid intake. This result is of great interest in the management of sarcoidosis with URT involvement, where corticosteroid-based therapy is often prescribed on a long-term scale.
In line with previous studies on sarcoidosis, infections were the most frequent adverse events, observed in 8 of our cases and causing treatment discontinuation in 3 cases. Infliximab was used in combination with first-line immunosuppressive drugs (mainly corticosteroids and methotrexate) in every case. This association of immunosuppressive drugs is more likely causative of opportunistic infections than the TNF-alpha blockade itself (10). No mycobacterial infection was observed in our cases, thanks to clear guidelines on latent mycobacterial infection screening before the introduction of TNF-alpha antagonist. In their systematic review on the efficacy and safety of TNF antagonists in sarcoidosis, Maneiro et al. reported mean weighted rates of infections and serious infections and malignancy of 22.1, and 5.9 per 100 patient-years, respectively, which is greater than in approved indications (i.e rheumatoid arthritis) (20). Chapelon-Abric et al. recently reported infections in 44% of 16 patients with severe and refractory sarcoidosis, mostly observed in men and with long-term use of corticosteroids and immunosuppressants (21). However, the proportion of patients with adverse effects was similar across the treatment groups in a controlled trial comparing infliximab and placebo, while no or a low rate of severe infections was reported in several case-series (22-24). These discordant results might be explained in our patient series by a long-term and high-dose immunosuppression, in relation with the severity of the disease. Contrary to some previous studies, here, none of our patients developed paradoxical granulomatous reactions, demyelinating lesions or neoplasia.
Besides the side effects, one important issue for responders is the risk of relapse during treatment and/or after IFX withdrawal. Moreover, how long the TNF-blockade should be maintained is not known. Vorselaars et al. and Panselinas et al. reported 62 and 86% of relapses after IFX discontinuation in their series, with a median time of 11 and 3 months respectively (23, 25). Here, 3 patients experienced relapses, after a mean duration of 15 months. Longer treatment durations might be needed but expose patients to high risks of AE.
The present study has several limitations. First is due to the heterogeneity in the management of sarcoidosis between the different centers. The criteria that led to anti-TNF therapy differed for each case, mainly dependent of the physician in charge. The second important limitation is the retrospective way of measuring the response to the treatment: the item of the e-POST score are subjective. Nevertheless, this study reports the largest series of patients presenting biopsy-proven sarcoidosis of the URT treated with anti-TNF drugs. The low number of patients that are currently treated with anti-TNF alpha does not permit to design large scale prospective study. The last limitation is the lack of data regarding the nature of the infections that occurred during the treatment, and sometimes that led to treatment discontinuation. However, although the infections were frequent in our series, they only led to treatment discontinuation in 2 of the cases. We suspect that the other events were benign sufficient events, that the physicians judged to pursue the therapy.
In conclusion, our report of 12 patients suggests that infliximab may be useful in cases of progressive URT sarcoidosis refractory to corticosteroids and immunosuppressive drugs. Relapses are frequent and seem to respond after resuming anti-TNF therapy.
References
- 1.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P-Y, Müller-Quernheim J. Sarcoidosis. Lancet. 2014 Mar 29;383(9923):1155–67. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Mrówka-Kata K, Kata D, Lange D, Namysłowski G, Czecior E, Banert K. Sarcoidosis and its otolaryngological implications. Eur Arch Otorhinolaryngol. 2010 Oct;267(10):1507–14. doi: 10.1007/s00405-010-1331-y. [DOI] [PubMed] [Google Scholar]
- 3.Duchemann B, Lavolé A, Naccache J-M, Nunes H, Benzakin S, Lefevre M, et al. Laryngeal sarcoidosis: a case-control study. Sarcoidosis vasculitis and diffuse lung disease. 2014;31(3):227–34. [PubMed] [Google Scholar]
- 4.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011 Mar 1;183(5):573–81. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broos CE, van Nimwegen M, Hoogsteden HC, Hendriks RW, Kool M, van den Blink B. Granuloma Formation in Pulmonary Sarcoidosis. Front Immunol [Internet] 2013 Dec 10 [cited 2016 Mar 19]:4. doi: 10.3389/fimmu.2013.00437. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3857538/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014 Nov;44(5):1296–307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- 7.Pariser RJ, Paul J, Hirano S, Torosky C, Smith M. A double-blind, randomized, placebo-controlled trial of adalimumab in the treatment of cutaneous sarcoidosis. J Am Acad Dermatol. 2013 May;68(5):765–73. doi: 10.1016/j.jaad.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 8.Loza MJ, Brodmerkel C, Du Bois RM, Judson MA, Costabel U, Drent M, et al. Inflammatory profile and response to anti-tumor necrosis factor therapy in patients with chronic pulmonary sarcoidosis. Clin Vaccine Immunol. 2011 Jun;18(6):931–9. doi: 10.1128/CVI.00337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermet M, le Guenno G, Rieu V, Philippe P, Ruivard M. Sarcoïdose nasosinusienne et cervicale traitée par infliximab: trois observations et revue de la littérature. La Revue de Médecine Interne. 2012 Jan;33(1):46–9. doi: 10.1016/j.revmed.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Jounieaux F, Chapelon C, Valeyre D, Israel Biet D, Cottin V, Tazi A, et al. Infliximab treatment for chronic sarcoidosis-a case series. Rev Mal Respir. 2010 Sep;27(7):685–92. doi: 10.1016/j.rmr.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest. 2005 Mar;127(3):1064–71. doi: 10.1378/chest.127.3.1064. [DOI] [PubMed] [Google Scholar]
- 12.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999 Aug;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 13.Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008 Jun;31(6):1189–96. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 14.Sharma OP. Sarcoidosis of the upper respiratory tract. Selected cases emphasizing diagnostic and therapeutic difficulties. Sarcoidosis Vasc Diffuse Lung Dis. 2002 Oct;19(3):227–33. [PubMed] [Google Scholar]
- 15.Aubart FC, Ouayoun M, Brauner M, Attali P, Kambouchner M, Valeyre D, et al. Sinonasal involvement in sarcoidosis: a case-control study of 20 patients. Medicine (Baltimore) 2006 Nov;85(6):365–71. doi: 10.1097/01.md.0000236955.79966.07. [DOI] [PubMed] [Google Scholar]
- 16.Neville E, Mills RG, Jash DK, Mackinnon DM, Carstairs LS, James DG. Sarcoidosis of the upper respiratory tract and its association with lupus pernio. Thorax. 1976 Dec;31(6):660–4. doi: 10.1136/thx.31.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazziotti S, Gaeta M, Blandino A, Vinci S, Pandolfo I. Perineural spread in a case of sinonasal sarcoidosis: case report. AJNR Am J Neuroradiol. 2001 Jul;22(6):1207–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Baughman RP, Lower EE. Treatment of Sarcoidosis. Clinical Reviews in Allergy & Immunology. 2015 Aug;49(1):79–92. doi: 10.1007/s12016-015-8492-9. [DOI] [PubMed] [Google Scholar]
- 19.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab Therapy in Patients with Chronic Sarcoidosis and Pulmonary Involvement. American Journal of Respiratory and Critical Care Medicine. 2006 Oct;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 20.Maneiro JR, Salgado E, Gomez-Reino JJ, Carmona L. BIOBADASER Study Group. Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthritis Rheum. 2012 Aug;42(1):89–103. doi: 10.1016/j.semarthrit.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Chapelon-Abric C, Saadoun D, Biard L, Sene D, Resche-Rigon M, Hervier B, et al. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol. 2015 Aug;33(4):509–15. [PubMed] [Google Scholar]
- 22.Wijnen PA, Cremers JP, Nelemans PJ, Erckens RJ, Hoitsma E, Jansen TL, et al. Association of the TNF-α G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur Respir J. 2014 Jun;43(6):1730–9. doi: 10.1183/09031936.00169413. [DOI] [PubMed] [Google Scholar]
- 23.Vorselaars ADM, Crommelin HA, Deneer VHM, Meek B, Claessen AME, Keijsers RGM, et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J. 2015 Jul;46(1):175–85. doi: 10.1183/09031936.00227014. [DOI] [PubMed] [Google Scholar]
- 24.Sweiss NJ, Noth I, Mirsaeidi M, Zhang W, Naureckas ET, Hogarth DK, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG/World Association of Sarcoidosis and Other Granulomatous Disorders. 2014;31(1):46. [PMC free article] [PubMed] [Google Scholar]
- 25.Panselinas E, Rodgers JK, Judson MA. Clinical outcomes in sarcoidosis after cessation of infliximab treatment. Respirology. 2009 May;14(4):522–8. doi: 10.1111/j.1440-1843.2009.01518.x. [DOI] [PubMed] [Google Scholar]