Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) and sarcoidosis are recognized causes of pulmonary hypertension according to the World Health Organization classification scheme. This case series describes seven patients with sarcoidosis with a mean age of 61 who developed pulmonary hypertension. They were found to have CTEPH, diagnosed by either CT pulmonary angiography or a lung ventilation perfusion scan. They all underwent confirmatory right heart catheterization showing elevated mean pulmonary artery pressures (mean of 42 mmHg – normal less than 25 mmHg). Sarcoidosis has been previously shown to be associated with increased rates of venous thromboembolic disease. In these cases, patients with sarcoidosis later developed CTEPH and this may be another mechanism in which sarcoidosis can lead to pulmonary hypertension. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 352-355)
Keywords: sarcoidosis, CTEPH
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) develops due to pulmonary emboli (PE) that become organized fibrous scars and in time, lead to small vessel thrombosis and plexiform lesions (1-3). Pulmonary hypertension (PH) and right ventricular (RV) dysfunction can develop and in severe cases, result in death (1). After an acute PE, CTEPH occurs in up to 9% of patients at 2 years but it remains unclear why only some individuals develop this complication (1, 2 ,4). Risk factors have been identified however, including chronic inflammation (3-5). This is supported by the elevated levels of inflammatory biomarkers including C-reactive protein (CRP), interleukin (IL)-1, IL-2, IL-4 and IL-8 in CTEPH patients (3, 4).
Sarcoidosis is an idiopathic granulomatous disease characterized by a CD4+ Th1 cell-mediated immune response affecting nearly any organ. The Th-1 cells secrete pro-inflammatory markers including IL-2, interferon-g and augment TNF-a production by macrophages and perpetuate a local cellular immune response (6). Like CTEPH, sarcoidosis is a recognized cause for PH. The mechanisms leading to PH are multi-modal, including compression of pulmonary arteries by adenopathy, cardiomyopathy, pulmonary fibrosis and hypoxemia (7).
A pro-inflammatory state is a common link between sarcoidosis and CTEPH and may underlie an unrecognized clinical association. Based on our experience, as described in the following cases, the co-occurrence of these two diseases may be under reported. To our knowledge, these are the first reported cases of CTEPH occurring in patients with sarcoidosis.
Methods and Results
A retrospective review was approved by the Rush University Institutional Review Board (15100907-IRB01). Informed consent was waived. Cases diagnosed with both CTEPH and sarcoidosis were identified.
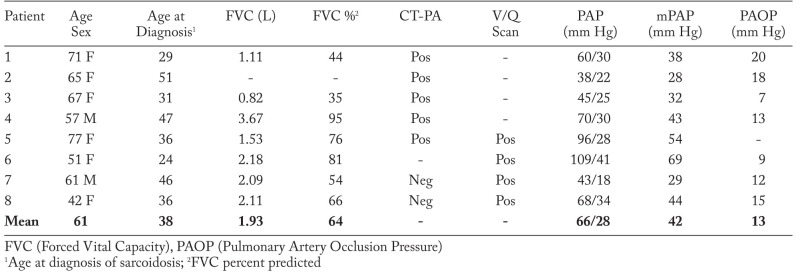
A total of 8 patients are included in this series (Table 1). They are all African American, 6 are female and the mean age is 61 (42-77). Five patients were current or former smokers. All had pulmonary sarcoidosis based on chest CT, diagnosed by transbronchial biopsy (7 patients) or on clinical grounds alone (1 patient). The mean age at diagnosis was 38 (24-51). All patients were treated with prednisone and 5 received methotrexate. One refractory patient received prednisone, methotrexate, azathioprine, leflunomide and infliximab at different times.
Table 1.
Patients diagnosed with sarcoidosis and chronic thromboembolic pulmonary hypertension. Relevant clinical data is included
All 8 patients were diagnosed with CTEPH after carrying a diagnosis of sarcoidosis for 6 to 40 years. 5 patients had CT pulmonary angiography (CTPA) showing filling defects, decreased arterial opacification, wall-adherent thrombi or intra-arterial webs consistent with CTEPH. 4 patients had lung ventilation/perfusion (V/Q) scans showing unmatched perfusion defects consistent with PE. Technique for V/Q scans included ventilation imaging performed with 7.8 mCi inhaled xenon-133.
Pulmonary perfusion imaging was then performed with 1.1 mCi Tc-99m-MAA, administered by intravenous injection. All patients underwent right heart catheterization confirming PH. Mean pulmonary artery pressure (mPAP) was 42 mmHg (28-69 mmHg), mean pulmonary artery occlusion pressure (PAOP) was 13.2 (normal mPAP <25 mm Hg and PAOP <15 mm Hg) mmHg (7-20 mmHg) and mean cardiac output was 4.89 L/min (3.9-5.5 L/min – normal 4 to 6 L/min). All patients were anticoagulated with warfarin. Riociguat, a soluble guanylate cyclase stimulator, was started on 2 patients. Two patients had also been placed on a phosphodiesterase-5 inhibitor during their course. 7 patients were screened for antiphospholipid antibody (APA), which was negative. 2 patients had further screening for hypercoagulable states, which was also unrevealing. One patient had a known history of cancer from which the patient died. No other patients have expired as of this review. No patients qualified for pulmonary thromboendarterectomy due to distal involvement of pulmonary arteries, limiting co-morbidities or patient preference.
Discussion
These cases raise the possibility of an association between CTEPH and sarcoidosis leading to PH. An increased incidence of PE has been reported in patients with sarcoidosis. In the Oxford Record Linkage Study, it was shown that the risk of PE was higher in sarcoidosis patients (relative risk of 2.0, 95% CI of 1.1-3.4) compared with a reference cohort (8). In a study of United States decedents, sarcoidosis patients had a significantly higher risk of PE (2.54%) compared with controls (1.13%), independent of age, gender and race (9). A smaller single-center review of sarcoidosis patients found a PE incidence of 6.2%, higher than historical controls (10). Potential mechanisms are speculative but may include a pro-inflammatory state, hypercoagulability and pro-coagulant effects of immunosuppressive medications.
It is suspected that chronic inflammatory states lead to venous thromboemboli (VTE) based on observational studies. In disorders such as inflammatory bowel disease and vasculitis, there is an increased frequency of VTE as well as CTEPH. CRP is a sensitive inflammatory marker and in the ARIC study, its increase above the 90th percentile was associated with a 76% increase in the risk for VTE (11).
Increases in inflammatory biomarkers including IL-1, IL-2, IL-10 and factor VIII are also associated with both VTE and CTEPH (11). Sarcoidosis, similarly induces an inflammatory response with increases in IL-2, IFN-g and TNF-a. Neutrophilic and macrophage infiltration and activation has been shown to produce a pro-coagulant effect (6). It is possible that inflammation due to sarcoidosis increases the risk for VTE and the subsequent development of CTEPH.
A higher frequency of APA has been observed in patients with both sarcoidosis and CTEPH. A serological study of 55 patients with sarcoidosis showed that 38% had positive IgG or IgM for APA while only 7% of healthy controls were positive (12). Similarly, in CTEPH patients, elevated levels of APA have been demonstrated (3). Both CTEPH and sarcoidosis involve immune dysregulation and may be associated with APA positivity by unknown mechanisms.
Glucocorticoids have recently received attention in augmenting VTE risk. A large Denmark study showed a 3-fold elevation in VTE risk in patients taking glucocorticoids after controlling for disease severity. The risk was higher if patients had taken glucocorticoids recently and at higher doses (14). In the surgical population, the NSQIP database was used to show an increased risk for VTE (OR of 1.54) in patients who had taken preoperative glucocorticoids (15). These findings raise the possibility that the use of glucocorticoids, as with sarcoidosis, may be associated with VTE.
CTEPH may be yet another unrecognized way in which sarcoidosis patients develop PH. In patients with sarcoidosis associated PH, evaluation for CTEPH is prudent, since specific therapy such as riociguat and pulmonary thromboendarterectomy may be offered, providing a substantial benefit or potential cure. However a limitation of VQ scanning and CTPA in the setting of granulomatous lung disease could be two separate factors. Typical findings of webbing of the pulmonary vasculature, intimal irregularities with abrupt narrowing and post stenotic dilatation could be effect of granulomatous disease from sarcoidosis within the pulmonary vasculature or from interstitial lung granulomatous inflammation leading to such perfusion changes. This could suggest an epiphenomenon between sarcoidosis and CTEPH, true causality of sarcoidosis leading to CTEPH or a misdiagnosis of CTEPH with extensive sarcoidosis. This case series is mainly hypothesis generating and larger observational cohort studies are needed to confirm a positive association given the biological link between CTEPH and sarcoidosis.
Centers treating pulmonary hypertension have large populations of sarcoidosis patients and ruling out chronic thromboembolic disease is of utmost importance as it is the only cause of pulmonary artery hypertension which has a surgical cure with pulmonary thromboendarterectemy (PTE) at few selected centers or medication specific for nonsurgical candidates (riociguat.) If patients have concomitant sarcoidosis with CTEPH, it is paramount to identify this population as traditional therapies for PAH may not be effective and there maybe a need for chronic anticoagulation. Clinicians rely on the V/Q scan over CT scan for diagnosing CTEPH. There was some discordance between V/Q scan positivity for CTEPH versus CT scan which is expected in diagnosis of CTEPH and V/Q scan is still considered superior to CT. If patients are considered surgical candidates for PTE, then pulmonary angiogram is typically the next step to evaluate the specific anatomy of pulmonary artery thromboses. Given most patients with arcoidosis are unlikely candidates for PTE, most patients did not undergo pulmonary angiography. With the overlap we describe here, we must either acknowledge the coexistence of both diseases based on pathophysiology or look for other non interventional modes for ruling out CTEPH with parenchymal lung disease from sarcoidosis with improved specificity over V/Q scan.
Abbreviations List
- CTEPH
chronic thromboembolic pulmonary hypertension
- mPAP
mean pulmonary artery pressure
- PA
pulmonary artery
- PE
pulmonary embolism
- PH
pulmonary hypertension
- RV
right ventricle
- APA
antiphospholipid antibodies
- CRP
c-reactive protein
- IL
interleukin
- CTPA
computed tomography pulmonary angiography
- V/Q
ventilation and perfusion scan
- VTE
venous thromboemboli
- PTE
pulmonary thromboendaretecemy
Acknowledgements
No funding was required. Dr. Robert Baughman has a current grant from Bayer evaluating Riociguat in sarcoidosis associated pulmonary hypertension. No other disclosures to report.
References
- 1.Lang I. Chronic Thromboembolic Pulmonary Hypertension: a Distinct Disease Entity. Eur Respir Rev. 2015;24:246–252. doi: 10.1183/16000617.00001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeper MM, Madani MM, Nakanishi N, Meyer B, Cebotari S, Rubin LJ. Chronic Thromboembolic Pulmonary Hypertension. Lancet Respir Med. 2014;2:573–82. doi: 10.1016/S2213-2600(14)70089-X. http://dx.doi.org/10.1016/S2213-2600(14)70089-X . [DOI] [PubMed] [Google Scholar]
- 3.Kim NH, Lang IM. Risk Factors for Chronic Thromboembolic Pulmonary Hypertension. Eur Respir Rev. 2012;21(123):27–31. doi: 10.1183/09059180.00009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Van der Hulle T, Den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Reviews. 2014;28:221–226. doi: 10.1016/j.blre.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Jais X, Ioos V, Jardim C, et al. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax. 2005;60:1031–1034. doi: 10.1136/thx.2004.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Guzman E, Farver C, Parambil J, Culver DA. Pulmonary Hypertension Caused by Sarcoidosis. Clin Chest Med. 2008;29(3):549. doi: 10.1016/j.ccm.2008.03.010. doi:10.1016/j.ccm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawshaw AP, Wotton CJ, Yeates DGR, Goldacre MJ, Ho L- Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax. 2011;66:447–448. doi: 10.1136/thx.2010.134429. [DOI] [PubMed] [Google Scholar]
- 9.Swigris JJ, Olson AL, Huie TJ, et al. Increased Risk of Pulmonary Embolism Among US Decedents with Sarcoidosis from 1988 to 2007. Chest. 2011;140(5):1261–1266. doi: 10.1378/chest.11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vorselaars ADM, Snijder RJ, Grutters JC. Increased Number of Pulmonary Embolisms in Sarcoidosis Patients. Chest. 2012:826–827. doi: 10.1378/chest.11-2514. DOI: 10.1378/chest.11-2514. [DOI] [PubMed] [Google Scholar]
- 11.Goldhaber SZ, Bounameaux H. Pulmonary Embolism and Deep Vein Thrombosis. Lancet. 2012;379:1835–46. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 12.Ina Y, Takada K, Yamamoto M, Sato T, Ito S, Sato S. Antiphospholipid Antibodies, a Prognostic Factor in Sarcoidosis. Chest. 1994;105:1179–83. doi: 10.1378/chest.105.4.1179. [DOI] [PubMed] [Google Scholar]
- 13.Pathak R, Khanal R, Aryal MR, et al. Sarcoidosis and Antiphospholipid Syndrome a Systematic Review of Cases. N Am J Med Sci. 2015;7(9):379–383. doi: 10.4103/1947-2714.166213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of Glucocorticoids and Risk of Venous Thromboembolism. A Nationwide Population-Based Case-Control Study. JAMA Intern Med. 2013;173(9):743–752. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- 15.Kantar RS, Haddad AG, Tamim H, Jamali F, Taher AT. Venous thromboembolism and preoperative steroid use: analysis of the NSQIP database to evaluate risk in surgical patients. Eur J Intern Med. 2015;26(7):528–33. doi: 10.1016/j.ejim.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins D, Mayer E, Screaton N, Madani M. State-of-the-Art Chronic Thromboembolic Pulmonary Hypertension Diagnosis and Management. Eur Respir Rev. 2012;21(123):32–39. doi: 10.1183/09059180.00009211. [DOI] [PMC free article] [PubMed] [Google Scholar]