Abstract
Background: Pirfenidone is an antifibrotic drug used for the treatment of idiopathic pulmonary fibrosis. Objectives: The aim of this study was to evaluate the efficacy of pirfenidone in patients with chronic hypersensitivity pneumonitis (HP). Methods: Twenty-three patients with chronic HP treated with pirfenidone were enrolled in this study based on a retrospective medical record review. Results: The change in vital capacity (VC) in the 6 months after the start of treatment (-152±56.1 ml) was significantly improved compared with that in the 6 months before treatment (-292±77.8 ml, p=0.047). No patients discontinued the treatment with adverse events. Conclusions: These results demonstrate that pirfenidone may reduce the decline of VC in patients with chronic HP without eliciting significant adverse events. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 139-142)
Keywords: pirfenidone, chronic hypersensitivity pneumonitis, bird fancier’s lung
Introduction
Hypersensitive pneumonitis (HP) is an allergic lung disease caused by hypersensitivity to inhaled organic dusts. HP presents in acute and chronic clinical forms, the latter of which is thought to be influenced by persistent or recurrent exposure to a causative antigen. The physical findings and radiological and pathological abnormalities characteristic of chronic HP have similarities to those seen in idiopathic pulmonary fibrosis (IPF), and both diseases share a poor prognosis (1). Avoidance of exposure to a causative antigen is the most important step in management of HP, however in some cases pulmonary fibrosis progresses in spite of elimination of antigen exposure. Therefore, corticosteroid treatment are often used in many patients with chronic HP based on expert opinion. Though effective in relieving symptoms, long-term outcome of corticosteroid treatment has not been validated by randomized control study (2).
Pirfenidone is an antifibrotic agent used for the treatment of IPF. In the phase 3 trial, pirfenidone reduced the decline in vital capacity (VC) at week 52 and improved progression-free survival (3). Pirfenidone also reduced disease progression in the Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis (ASCEND) study, as reflected by lung function, exercise tolerance, and progression-free survival (4). Several reports have shown that pirfenidone may be an effective therapy for fibrotic interstitial lung diseases (ILD) other than IPF, such as clinically amyopathic dermatomyositis and scleroderma (5, 6). Yet, in spite of these reports of favorable effects with pirfenidone, the treatment has not conferred sufficient effects in patients with fibrotic ILD other than IPF. Moreover, very little is known about the effect of pirfenidone for patients with chronic HP. In this study, we evaluated the efficacy and feasibility of pirfenidone in patients with chronic HP.
Methods
Subjects
This study was a retrospective review and analysis of medical records. The study subjects were 23 patients with chronic bird-related HP who received pirfenidone at our hospital from January 2009 through March 2014. The diagnostic criteria for chronic bird-related HP included [1] history of avian contact, [2] antibodies and/or lymphocyte proliferation to avian antigens, [3] reproduction of symptoms by an environmental provocation or laboratory-controlled inhalation of avian antigens, either [4] evidence of pulmonary fibrosis with or without granulomas on histopathological analysis, or [5] honeycombing on computed tomography (CT) scans, [6] progressive deterioration of a restrictive impairment on pulmonary function over 1 year, and [7] respiratory symptoms related to HP of 6 months duration (7). The decision to treat the patients with pirfenidone is based on case conferences. This study conformed to the Declaration of Helsinki and was approved by the ethical committee on human research of Tokyo Medical and Dental University (No. 1820).
Statistical analyses
All statistical analyses were performed with the Statistical Package for Social Sciences (version 21.0, IBM, Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant. Comparisons between groups were performed using the paired t-test.
Results
Background and characteristics of patients
The background and characteristics of the patients are summarized in Table 1. Most of the patients were male (73.9%). Approximately 60% of the patients had received prior treatment with corticosteroid, at a mean dose of 15.6 mg. The average VC percentage predicted was 52.9%, lower than that reported from prior clinical trials (3, 4).
Table 1.
Patients characteristics

Therapeutic responses
Fourteen patients were able to continue pirfenidone for over 12 months, while 9 patients had to discontinue therapy before 12 months was reached. Four of the 9 patients who discontinued were transferred to other hospitals and the other 5 died from progressive disease. The median treatment period was 16 months and the median survival time from therapy initiation was 22 months (Figure 1).
Fig. 1.
Kaplan-Meier distribution of overall survival time
Efficacy before and after pirfenidone therapy
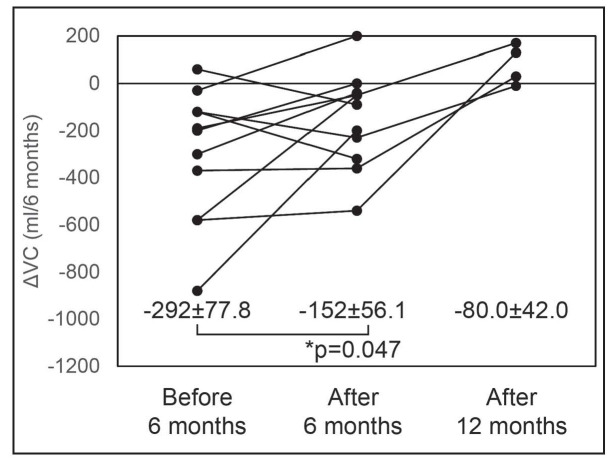
Respiratory function tests could be evaluated in 12 patients over the 6 months and 4 patients over the 12 months after the start of therapy. The change in VC was -292±77.8 ml (mean±SEM) over the 6 months before start of therapy and -152±56.1 ml over the 6 months after the start of therapy (p=0.047) (Figure 2). The pirfenidone therapy reduced the decline in VC.
Fig. 2.
Change in VC over the 6 months before the start of therapy and the 6 months after the start therapy.
Data are presented as mean±SEM
Adverse events
Five patients (21.7%) respectively experienced anorexia, nausea, chest pain, liver injury, and heartburn as adverse events, however none of the patients had to discontinue treatment as a consequence of adverse events. None of the 23 patients showed signs of photosensitivity during the treatment period.
Discussion
This is the first report to evaluate the use of pirfenidone in patients with chronic HP. Pirfenidone has been recommended as one of the standard therapies for IPF under an official guideline (8) and significantly attenuated the degree of VC decline in patients with IPF (3). There is little data, however, on the efficacy of pirfenidone for fibrotic ILD other than IPF. In this study, pirfenidone has been shown to reduce the decline in VC in chronic HP patients. Pirfenidone was well tolerated in this study, with an adverse event profile similar to that reported from phase 3 trials in IPF patients (3, 4).
To our knowledge, patients with chronic HP often develop progressive fibrosis and pathologic features of the disease may mimic IPF in advanced stage (9). Study of bleomycin-induced pulmonary fibrosis in mice suggested that pirfenidone exerts its antifibrotic effect through regulation of lung interferon-gamma, basic fibroblast growth factor and transforming growth factor (TGF) beta level (10). TGF beta is known as a key molecule in the pathogenesis of chronic HP. Bone marrow–derived fibrocytes enhance the expression of profibrotic molecules in lung fibroblasts of patients with chronic HP, which are mediated by TGF beta signaling (11).
This study was based on retrospective medical record review in single hospital, which made it difficult to rule out bias related to the patient selection and some of the patients had to be excluded from the evaluation of efficacy because we were unable to find the appropriate data of pulmonary function tests or physical examination. Placebo controlled multicenter trials are strongly needed to further evaluate the treatment of chronic HP with pirfenidone. However, we conclude that pirfenidone is well tolerated and has become a treatment option for patients with chronic HP.
References
- 1.Perez-Padilla R, Salas J, Chapela R, et al. Mortality in Mexican patients with chronic pigeon breeder’s lung compared with those with usual interstitial pneumonia. Am Rev Respir Dis. 1993;148:49–53. doi: 10.1164/ajrccm/148.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 4.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 5.Li T, Guo L, Chen Z, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep. 2016;6:33226. doi: 10.1038/srep33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura Y, Saito T, Fujita K, et al. Clinical experience with pirfenidone in five patients with scleroderma-related interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:235–238. [PubMed] [Google Scholar]
- 7.Yoshizawa Y, Ohtani Y, Hayakawa H, et al. Chronic hypersensitivity pneumonitis in Japan: a nationwide epidemiologic survey. J Allergy Clin Immunol. 1999;103:315–320. doi: 10.1016/s0091-6749(99)70507-5. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192:e3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 9.Churg A, Muller NL, Flint J, et al. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30:201–208. doi: 10.1097/01.pas.0000184806.38037.3c. [DOI] [PubMed] [Google Scholar]
- 10.Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–408. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Garcia de Alba C, Buendia-Roldan I, Salgado A, et al. Fibrocytes contribute to inflammation and fibrosis in chronic hypersensitivity pneumonitis through paracrine effects. Am J Respir Crit Care Med. 2015;191:427–436. doi: 10.1164/rccm.201407-1334OC. [DOI] [PubMed] [Google Scholar]