Abstract
Background: A hallmark of sarcoidosis is lung disease. However, the prognosis and long-term outcome of pulmonary sarcoidosis are not well-defined due to the limited number of studies with long duration of follow-up. Objectives: This study was undertaken to characterize the course of pulmonary sarcoidosis in a population-based cohort. Methods: A population-based cohort of 311 incident cases of pulmonary sarcoidosis among Olmsted County, Minnesota residents in 1976-2013 were identified. Medical records of the confirmed cases were reviewed from diagnosis to last follow-up. Data on stage of pulmonary sarcoidosis at diagnosis, serial pulmonary function tests, requirement of oxygen therapy and treatment were abstracted. The cumulative incidence of chronic respiratory impairment (defined as forced vital capacity of <50%, diffuse capacity for carbon monoxide of <40% or requirement to use oxygen supplementation) adjusted for the competing risk of death was estimated. Cox models were used to assess the association of stage of pulmonary sarcoidosis and treatment on the development of chronic respiratory impairment. Results: 25 patients developed chronic respiratory impairment which corresponded to a 10-year event rate of 4.4% (95% confidence interval [CI], 1.9.-6.9). Stage of pulmonary sarcoidosis at diagnosis was a strong predictor for chronic respiratory impairment with hazard ratio compared with stage I of 5.29 (95% CI, 1.65-16.96) for stage II and 8.36 (95% CI, 26.3-26.52) for stage III and IV. Use of glucocorticoids and immunosuppressive agents was associated with a significantly increased risk of chronic respiratory impairment. Conclusion: Patients with pulmonary sarcoidosis have a good pulmonary prognosis with a low incidence of chronic respiratory impairment. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 123-128)
Keywords: sarcoidosis, outcome, pulmonary function test
Introduction
Sarcoidosis is an immune-mediated disorder characterized by granulomatous inflammation. The etiology of sarcoidosis is unknown but it is believed to involve an interplay between environmental and genetic factors (1). It is estimated that approximately 70,000 new cases of sarcoidosis are diagnosed in the United States each year with significant regional variation (2, 3).
Sarcoidosis can virtually affect any organ, with lungs and intra-thoracic lymph nodes being the most commonly affected sites. Pulmonary sarcoidosis is usually categorized into stage I to IV based on radiographic changes (4). However, the prognosis and long-term outcome of pulmonary sarcoidosis are not well-defined due to the limited number of studies with long duration of follow-up. Moreover, most of the previous studies investigating the outcome of pulmonary sarcoidosis put an emphasis on the radiologic progression/resolution which may not reflect the functional outcome (5-8). In the current study, pulmonary function tests (PFTs) of residents of Olmsted County, Minnesota (MN) who were newly diagnosed with sarcoidosis between 1976 and 2013 were examined to characterize the natural history of the disease. Baseline characteristics at diagnosis of the patients were also analyzed for association with the outcome.
Methods
Participants and study design
This retrospective cohort study utilized the resource of the Rochester Epidemiology Project (REP) medical record-linkage system which provided comprehensive access to medical records from all health care providers, including the Mayo Clinic, the Olmsted Medical Center and their affiliated hospitals, local nursing homes and the few private practitioners for all residents of Olmsted County, MN seeking medical care for over six decades. The potential of this record-linkage system for epidemiologic study has previously been described (9).
Potential cases of sarcoidosis were identified from the database using diagnosis codes related to sarcoid, sarcoidosis, and contextual noncaseating granuloma. Medical records of these potential cases were individually reviewed. Inclusion required physician diagnosis supported by presence of non-caseating granuloma on biopsy, radiologic features of intrathoracic sarcoidosis, compatible clinical presentation and exclusion of other granulomatous diseases such as tuberculosis and fungal infection. The only exception to the requirement of histopathology was stage I pulmonary sarcoidosis that required only symmetric bilateral hilar adenopathy on thoracic imaging without any other known causes. Isolated granulomatous disease of a specific organ without intra-thoracic disease was not included. Prevalent cases (i.e., cases with sarcoidosis prior to residency in Olmsted County) were excluded. This approach allows identification of virtually all clinically recognized cases of sarcoidosis in the community.
The medical records of the confirmed cases were then further reviewed. Data on demographic characteristics, presence of extra-thoracic involvement by sarcoidosis, radiographic staging at diagnosis, PFTs at baseline and during follow-up, treatment and the requirement of oxygen supplementation therapy were collected. Only those with at least one PFT done during follow-up or within 2 years prior to diagnosis date were included. Inclusion of PFT was extended up to 2 years prior to diagnosis because some patients had PFT prior to the diagnosis of sarcoidosis as a part of their initial evaluation. The PFT that was closest to diagnosis date was used as the baseline PFT. Results of all PFTs were recorded in percent predicted values. These included total lung capacity (TLC), force vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and diffusing capacity for carbon monoxide (DLCO). The DLCO results were corrected for hemoglobin level when appropriate. The primary outcome of this study was development of chronic respiratory impairment, defined as the presence of at least one of the following DLCO <40%, FVC <50% or the requirement to use chronic oxygen supplementation (10). Follow-up was continued until death, migration from Olmsted County or January 1, 2016. This study was approved by the Mayo Clinic and the Olmsted Medical Center Institutional Review Boards (Mayo Clinic IRB 14-008651, Olmsted Medical Center IRB 012-OMC-15).
Statistical analysis
Descriptive statistics (percentages, mean, etc.) were used to summarize the characteristics of the cohort. The cumulative incidence of chronic respiratory impairment adjusted for the competing risk of death was estimated (11). These methods are similar to the Kaplan-Meier method with censoring of patients who are still alive at last follow-up. However, patients who die before experiencing chronic respiratory impairment are appropriately accounted for to avoid overestimation of the rate of occurrence of the events of interest, which can occur if such subjects are simply censored at death. Cox models were used to examine the associations between stage of pulmonary sarcoidosis at diagnosis, treatment and chronic respiratory impairment. Treatment exposures were modeled using time-dependent covariates that modeled patients as unexposed until the time when they started treatment and exposed thereafter. These analyses were adjusted for sex, age and calendar year of sarcoidosis diagnosis. Changes in predicted values of TLC, FVC and DLCO over time were estimated using general linear models with random subject effects to account for multiple PFT measures per subject. A P-value of less than 0.05 was considered statistically significant for all analyses. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, United States) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
335 incidental cases of pulmonary sarcoidosis were identified. 24 cases did not have any PFTs within the period of interest. Therefore, a total of 311 cases of incident pulmonary sarcoidosis (mean age 45.6 years, 50% female and 90% Caucasian) who had at least one PFT in the period from 2 years prior to sarcoidosis diagnosis to last follow-up were included. 121 patients (39%) also had extra-thoracic disease. The majority of patients had stage I disease (54%) followed by stage II (29%), III (15%) and IV (2%). The median length of follow-up was 13.8 years. The average number of PFTs per patient was 3.9. The average duration between the first and the last PFT was 6.1 years.
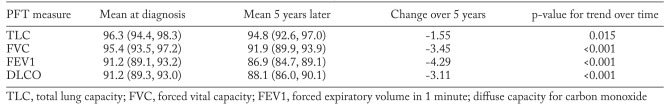
The means of PFT measures at baseline in this cohort were 97.1%, 95.3%, 91.4% and 91.1% of predicted values for TLC, FVC, FEV1 and DLCO, respectively. The median time from diagnosis date to baseline PFT was 0 days with interquartile range of – 7 days to 7 days. Over the course of follow-up, there was a decline in the mean values of each measure. At 5 years, the mean percentage predicted for TLC, FVC, FEV1 and DLCO decline by 1.6%, 3.5%, 4.3% and 3.1%, respectively. All of these changes were statistically significant. The estimated means of PFT measures at baseline and at 5 years after follow-up based on generalized linear models adjusted for age, sex and calendar year of sarcoidosis diagnosis are shown in table 1.
Table 1.
Trends in pulmonary function test measures over time among 311 patients with pulmonary sarcoidosis
Chronic respiratory impairment developed in 25 patients, which corresponded to a 10-year event rate of 4.4% (95% confidence interval [CI], 1.9-6.9). The cumulative incidence of chronic respiratory impairment according to time since sarcoidosis diagnosis is shown in figure 1. Stage of pulmonary sarcoidosis at diagnosis was a strong predictor of chronic respiratory impairment. The hazard ratio (HR) for chronic respiratory impairment of patients with stage II pulmonary sarcoidosis compared with stage I disease was 5.29 (95% CI, 1.65-16.96) while the HR for chronic respiratory impairment of patients with stage III and IV pulmonary sarcoidosis compared with stage I disease was 8.36 (95% CI, 26.3-26.52).
Fig. 1.
Cumulative incidence of chronic respiratory impairment among 311 patients with pulmonary sarcoidosis
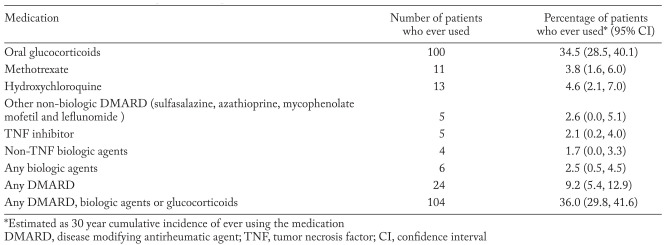
Less than half of the patients received glucocorticoids or disease modifying treatment for their sarcoid disease. Oral glucocorticoids were prescribed for 100 patients at some point during follow-up while 24 patients were exposed to non-biologic disease modifying antirheumatic agents (DMARDs) and 6 patients received biologic agents. The number and percentage of patients exposed to oral glucocorticoids, individual non-biologic DMARDs and biologic agents are described in table 2.
Table 2.
Medication use in 311 patients with pulmonary sarcoidosis
Use of glucocorticoids was associated with a significantly increased risk of chronic respiratory impairment (HR 4.60; 95% CI, 1.94-10.88 adjusted for age, sex and calendar year of sarcoidosis diagnosis). Use of non-biologic DMARDs was also associated with an increased risk of chronic respiratory impairment (HR 5.13; 95% CI, 1.47-17.86), but this risk was attenuated and no longer reached statistical significance after adjustment for glucocorticoid use (HR 2.79; 95% CI, 0.76-10.26) due to the small number of patients who were exposed to non-biologic DMARDs. Analysis of the association between use of biologic agents and chronic respiratory impairment could not be conducted due to a low number of patients exposed to biologic agents (6 patients). Additional adjustment for stage attenuated the association between glucocorticoids and chronic respiratory impairment, but it remained significant (3.50; 95% CI, 1.46-8.40). Similarly, the association between non-biologic DMARDs and chronic respiratory impairment was attenuated after additional adjustment for stage (HR 2.44; 95% CI, 0.63-9.48).
Discussion
The current study is the first population-based study using comprehensive individual medical record review to investigate the long-term outcome of patients with pulmonary sarcoidosis. Overall, the outcome of patients in this cohort was good with a 10-year chronic respiratory impairment rate of less than 5%. Pulmonary function of the patients at baseline was generally preserved as the means of all PFT measures were more than 90% of the predicted values. Moreover, on average, these patients lost only about 3.5% of FVC and 3.1% of DLCO by 5 years after sarcoidosis diagnosis. It should be noted that these changes were statistically significant but the absolute difference was small. Thus, the clinical impact of these changes in pulmonary function parameters is probably minimal.
The results of the current study are in line with previous studies that have suggested a good prognosis among patients with sarcoidosis. Low rates of development of respiratory impairment have been noted by other investigators as well. A single-center study that followed 479 patients with sarcoidosis for 7 years found that 13 patients (3%) died from respiratory impairment (12), similar to a Swedish cohort in which 1% of deaths were ascribed to respiratory impairment over 15 years of follow-up (5). Another study from Japan that followed 337 patients for over 10 years found that 18 patients (5%) developed respiratory impairment (13).
Preserved pulmonary function has been previously noted in referral-based studies as well. The prospective study from the ACCESS research group found that approximately 80% of patients with sarcoidosis had either stable or improved FVC and FEV1 after 2 years of follow-up (14). Another single-center cohort found gradual improvement of PFT measures up to 10 years of follow-up (15). The mean FEV1 and FVC improved by 7% and 6% at 5 years, respectively. However, it should be noted that the ethnic component of the cohort was different from the current study (the majority of patients in that study were African-American) and their PFT measures at diagnosis were lower compared with the current cohort (FEV1 84.1% and FVC 88.6%).
Stage of pulmonary sarcoidosis at diagnosis was a strong predictor of chronic respiratory impairment in this cohort. This finding is consistent with studies on radiographic progression of pulmonary sarcoidosis as spontaneous resolution of radiographic abnormality is seen in 60%-80% of patients with stage I pulmonary sarcoidosis while spontaneous resolution was observed less frequently, approximately 30%-50%, among those with stage II and III disease (5-8, 16).
Not unexpectedly, the use of glucocorticoids and immunosuppressive agents was a significantly associated with chronic respiratory impairment, as patients with more severe disease are more likely to be identified by clinicians for treatment (17). It is unlikely that treatment with glucocorticoids had a deteriorative effect on pulmonary function as randomized controlled trials have demonstrated a modest but significant beneficial effect of oral glucocorticoids among patients with stage II and III pulmonary sarcoidosis (16-19). A better understanding of the indications and strategies for glucocorticoid therapy in pulmonary sarcoidosis is needed.
The major strength of this study is that it is a population-based study with a long duration of follow-up. The REP medical record-linkage database allows capture of nearly all the clinically recognized cases of sarcoidosis in the population, which minimizes the likelihood of referral bias, a common concern for hospital-based studies. The long duration of follow-up ensures that the events of interest occurring long after initial diagnosis are also captured. The diagnosis of sarcoidosis was verified by individual medical record review using pre-specified diagnostic criteria which minimized the likelihood of misclassification.
The major limitations are those inherent to the retrospective nature of the study. Clinical information were not systematically obtained and recorded. Thus, some of the pertinent data might not be available. Pulmonary function testing was obtained at physician discretion without a standard protocol, which could introduce elements of detection and selection bias. It is possible that patients with more severe disease were more likely to have had more PFT evaluations over the long period of follow-up. Generalizability of the results to other populations is another potential limitation as the clinical phenotype and epidemiology of sarcoidosis varies between ethnic groups (2, 3) and the population of Olmsted County is predominately of northern European ancestry. Moreover, the Olmsted County population has a higher proportion of workers in the healthcare industry who may have a pattern of healthcare utilization that is different from other populations.
Conclusion
Patients with pulmonary sarcoidosis have a good pulmonary prognosis with a low incidence of chronic respiratory impairment and preserved pulmonary function during follow-up. Patients with more advanced stages of pulmonary sarcoidosis at diagnosis were much more likely to develop chronic respiratory impairment.
Acknowledgement
Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Yee AM. Sarcoidosis: Rheumatology perspective. Best Pract Res Clin Rheumatol. 2016;30:334–56. doi: 10.1016/j.berh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Ianuzzi MC. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 3.Dumas O, Abramovitz L, Wiley AS, Cozier YC, Canargo CA., Jr Epidemiology of sarcoidosis in a prospective cohort of U.S. women. Ann Am Thorac Soc. 2016;13(1):67–71. doi: 10.1513/AnnalsATS.201508-568BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc. 2013;10:362–70. doi: 10.1513/AnnalsATS.201303-069FR. [DOI] [PubMed] [Google Scholar]
- 5.Hillerdal G, Nou E, Osterman K, Schmekel B. Sarcoidosis: Epidemiology and prognosis, A 15-year European study. Am Rev Respir Dis. 1984;130:29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. II. Course and prognosis. Sarcoidosis. 1990;7:113–8. [PubMed] [Google Scholar]
- 7.Pietinalho A, Ohmichi M, Lofroos AB, Hiraga Y, Selroos The prognosis of pulmonary sarcoidosis in Finlnad and Hokkaido, Japan. A comparative five-year of biopsy-proven cases. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:158–66. [PubMed] [Google Scholar]
- 8.Nagai S, Handa T, Ito Y, Ohta K, Tamaya M, Izumi T. Outcome of sarcoidosis. Clin Chest Med. 2008;29:565–74. doi: 10.1016/j.ccm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a century of medical records linkage in a U.S. population. Mayo Clin Proc. (3rd) 2012;87:1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014 - An update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of impairment probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Wignet DB, Bowen EH, Lower EE. Predicting respiratory impairment in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:154–8. [PubMed] [Google Scholar]
- 13.Nagai S, Shigematsu M, Hamamda K, Izumi T. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293–8. doi: 10.1097/00063198-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:204–11. [PubMed] [Google Scholar]
- 15.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–27. [PubMed] [Google Scholar]
- 16.Pietinalho A, Tukiainen P, Haahtela T, Selroos O. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24–31. doi: 10.1378/chest.121.1.24. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandraiah V, Aronow W, Chandy D. Pulmonary sarcoidosis: an update. Postgrad Med. 2017;129:149–58. doi: 10.1080/00325481.2017.1251818. [DOI] [PubMed] [Google Scholar]
- 18.Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: A systematic review. JAMA. 2002;287:1301–7. doi: 10.1001/jama.287.10.1301. [DOI] [PubMed] [Google Scholar]
- 19.Zaki MH, Lyons HA, Leilop L, Huang CT. Corticosteroid therapy in sarcoidosis: a 5 year controlled follow-up study. N Y State J Med. 1987;87:496–9. [PubMed] [Google Scholar]