Abstract
Background: Recent studies have suggested that patients with idiopathic pulmonary fibrosis (IPF) may have a higher risk of venous thromboembolism (VTE) compared to general population even though the results were inconsistent. Objective: To investigate the risk of VTE among patients with IPF. Methods: Comprehensive literature review using MEDLINE and EMBASE database were performed to identify studies that compared the risk of VTE among patients with IPF to general population. Effect estimates from each study were combined together using random effect model, generic inverse variance method of DerSimonian and Laird. Results: Out of 510 retrieved articles, 5 studies met the inclusion criteria and were included in the meta-analysis. A significant risk of VTE in patients with IPF was observed with the pooled risk ratio of 2.11 (95% confidence interval, 1.28-3.48). The heterogeneity was moderate with I2 of 64%. Conclusion: An approximately 2-fold increased risk of VTE among patients with IPF was observed in this meta-analysis. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 109-114)
Keywords: idiopathic pulmonary fibrosis, venous thromboembolism, thrombosis, deep vein thrombosis, pulmonary embolism
Search Strategy
Database: Ovid MEDLINE
interstitial lung disease.mp. or exp Lung Diseases, Interstitial/
pulmonary fibrosis.mp. or exp Pulmonary Fibrosis/
or/1-2
exp Thromboembolism/
Thromboembolism.mp.
exp Venous Thrombosis/
venous thrombosis.mp.
exp Pulmonary Embolism/
pulmonary embolism.mp.
or/4-9
3 and 10
Database: EMBASE
idiopathic pulmonary fibrosis.mp. or exp fibrosing alveolitis/
fibrosing alveolitis.mp.
pulmonary embolism.mp. or exp lung embolism/
deep vein thrombosis.mp. or exp deep vein thrombosis/
venous thromboembolism.mp. or exp venous thromboembolism/
exp thromboembolism/ or thromboembolism.mp.
or/3-6
or/1-2
7 and 8
Introduction
Idiopathic pulmonary fibrosis (IPF) is a form of chronic progressive fibrosing interstitial lung disease characterized by the histopathologic pattern of usual interstitial pneumonia. Patients with IPF usually present with progressive respiratory symptoms with periods of acute exacerbations, resulting in significant morbidity and mortality. Prognosis of patients with IPF is poor with median survival of only two to five years (1). The exact etiology is still unknown despite extensive research effort. There is no medication with proven efficacy for IPF and current standard treatment focuses mainly on symptom relief although lung transplantation could be an option for selected patients (2).
Venous thromboembolism (VTE), which consists of deep venous thrombosis (DVT) and pulmonary embolism (PE), is one of the common medical problems with approximately 900,000 new and recurrent cases diagnosed every year in the United States (3). Traditional risk factors of VTE include cancer, trauma, surgery, immobilization hospitalization and use of certain medications (4, 5). More recently, chronic inflammation has been recognized as an independent risk factor for VTE as increased incidence of VTE has been observed in several chronic inflammatory conditions, such as rheumatoid arthritis, psoriasis, systemic vasculitis and inflammatory myositis (6-9).
Patients with IPF may be at an elevated risk of VTE as well due to their increased systemic inflammatory burden. In addition, patients with advanced respiratory symptoms are also likely to have limited mobility, resulting in venous stasis that can predispose to thromboembolism. In fact, several epidemiologic studies have suggested an association between IPF and VTE although the results are inconsistent (10-14). To further investigate this possible association, we performed a systematic review and meta-analysis of studies that compared the VTE risk in patients with IPF to subjects without IPF.
Method
Search strategy
Both investigators independently searched published articles in MEDLINE and EMBASE database from inception to February 2017 using the search terms for idiopathic pulmonary fibrosis and venous thromboembolism as described in online supplementary data without any language restriction. References of selected articles were also manually searched for additional studies.
Inclusion criteria
Studies were eligible for this meta-analysis if they met these inclusion criteria: 1) Cohort (either prospective or retrospective), case-control study or cross sectional study published as original study to evaluate the association between IPF and VTE, 2) odds ratios (OR), relative risk (RR), hazard ratio (HR), and standardized incidence ratio (SIR) with 95% confidence intervals (CI) or sufficient raw data to calculate these ratios were provided, and 3) subjects without IPF were used as comparators in cohort and cross-sectional study while subjects without VTE were used as controls in case-control study.
Study eligibility was independently evaluated by the two investigators. Any disagreement was resolved by mutual consensus. The quality of each study was appraised using the Newcastle-Ottawa quality scale (15). This scale assesses each study in three domains including 1) the representativeness of the subjects, 2) the comparability between the study groups, and 3) ascertainment of the exposure of interest for case-control study and the outcome of interest for cohort study. The modified version of Newcastle-Ottawa scale as described by Herzog et al. was used for cross-sectional study (16).
Review process and data extraction
The two study investigators independently reviewed the titles and abstracts of all retrieved articles. Articles that clearly did not fulfill the inclusion criteria were excluded. Only potentially relevant articles underwent full-text review to determine the eligibility. A standardized data collection form was used to extract the following information from the included studies: first author’s name, year of publication, year of study, country where the study was conducted, study design, source of population, number of subjects, baseline characteristics of the subjects, methods used to identify IPF and VTE, and effect estimates. This data extraction process was also performed by both investigators to ensure the accuracy.
Statistical analysis
All statistical analyses were performed using Review Manager 5.3 software from the Cochrane Collaboration (London, UK). The pooled RR of VTE in IPF patients in comparison to subjects without IPF was calculated using generic inverse method of DerSimonian and Laird (17). Random effect model was used given the high likelihood of between-study variance due to the difference in underlying population and methodology. As the outcome of interest was relatively uncommon, the ORs of cross-sectional study and case-control study were used as an estimate to pool with the RR from cohort study. Cochran’s Q-test, which is supplemented by I2 statistic, was used to evaluate the statistical heterogeneity. This I2 statistic quantifies the proportion of the total variation across studies, that is, due to true heterogeneity rather than chance. A value of I2 of 0% to 25% represents insignificant heterogeneity, more than 25% but ≤50% represents low heterogeneity, more than 50% but ≤75% represents moderate heterogeneity, and more than 75% represents high heterogeneity(18).
Results
The initial search yielded 510 articles, all of which underwent title and abstract review. The majority of them were excluded at this step as they were case report, letter to editor, review article or interventional study which clearly did not fulfill our inclusion criteria. A total of 15 studies underwent full-length article review and 10 of them were excluded at they did not include patients with IPF or did not report the outcome of interest. Therefore, a total of five studies met our inclusion criteria (four cohort studies and one cross-sectional study (10-14)) and were included in the meta- analysis. Baseline characteristics of the included studies are summarized in table 1.
Table 1.
Main characteristics of the studies included in the meta-analysis. USA indicates United States of America; UK, United Kingdom; N/A, Not available; IPF, idiopathic pulmonary fibrosis
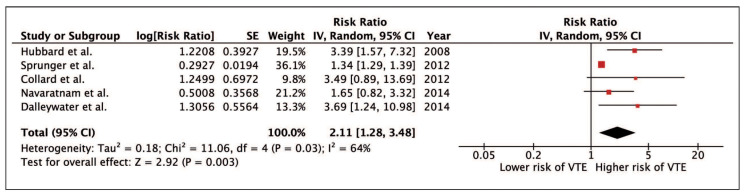
Our meta-analysis revealed a significantly increased risk of VTE among patients with IPF with the pooled RR of 2.11 (95% CI, 1.28-3.48). The heterogeneity was moderate with I2 of 64%. Figure 1 demonstrates the forest plot of this study
Fig. 1.
Evaluation for publication bias
The funnel plot is shown in figure 2. It is symmetric and does not suggest the presence of publication bias in favor of positive study.
Fig. 2.
Sensitivity analysis
Since the statistical heterogeneity was not low in this meta-analysis, a sensitivity analysis was performed by excluding one study at a time to investigate the effect of each study on the overall heterogeneity. Interestingly, exclusion of the study by Sprunger et al. (12), the only cross-sectional study, dramatically reduced I2 to 0%. The pooled effect estimate from this sensitivity analysis remained essentially unchanged (RR 2.58; 95% CI, 1.66-4.02).
Discussion
This is the first meta-analysis to demonstrate a significantly increased risk of VTE among patients with IPF. The risk is increased by approximately 2-fold. The exact mechanism behind the increased risk is not known but several possible explanations have been proposed.
First, the higher inflammatory burden in patients with IPF could be responsible for the increased tendency for blood clot. The underlying mechanisms of inflammation-induced thrombosis include up-regulation of coagulation factors and down-regulation of anticoagulants/fibrinolysis by inflammatory cytokines as well as injury to endothelial cells by free radicals and oxidative stress. Further evidence to support that inflammation plays an important role in the development of VTE is that VTE is observed more frequently when the inflammatory disease is active (19, 20).
Second, VTE and IPF may share a common origin. Thrombin, a key enzyme in coagulation cascade, could be the link as it is also an inducer of fibrogenic cytokines (21) and has been found in increased concentration in bronchoalveolar lavage from patients with fibrotic lung disease (22). In fact, a recent study has suggested that recombinant human thrombomodulin, which could form a reversible complex with thrombin to inactivate coagulation cascade, is effective for treatment of acute exacerbation of IPF (23).
Third, it is also possible that the increased risk is simply due to immobility as most patients with IPF have respiratory symptoms and reduced exercise capacity (24).
Fourth, the increased risk could be due to exposure to glucocorticoids, the medication often used to treat acute exacerbation of IPF. In vitro studies have demonstrated that glucocorticoids increase levels of coagulation factors and fibrinogen (25, 26). In addition, a dose-response relationship between exposure to glucocorticoids and incidence of VTE has been demonstrated by a recent population-based study (27).
Although the literature review process was rigorous and the included studies were of high quality, this meta-analysis has some limitations. Therefore, the interpretation of the results needs to be performed with caution. First, most of the included studies were medical registry-based studies, with the exception for the study by Navaratnam et al. (14), which were inherently at risk of inaccurate coding for both IPF and VTE. As a result, the completeness of case/event identification and the accuracy of diagnosis were limited. Second, statistical heterogeneity was not low in this study. Interestingly, the I2 dropped dramatically to 0% with the sensitivity analysis that excluded the study by Sprunger et al. (12). We suspect that the difference in study design was responsible for the between-study heterogeneity as the study by Sprunger et al. was the only cross-sectional study. Third, this is a meta-analysis of observational studies that can only demonstrate an association but cannot confirm causality. It is possible that confounders that were not adjusted in the primary studies, rather than IPF itself, are accountable for the increased risk of VTE. Last, surveillance bias may also play a role. It is possible that patients with IPF may have more medical examinations because of their chronic illness. Also, they may have more imaging studies of the thorax due to their respiratory symptoms.
Conclusion
An approximately 2-fold increased risk of VTE among patients with IPF was observed in this meta-analysis.
References
- 1.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2011;183(4):431–40. doi: 10.1164/rccm.201006-0894CI. Epub 2010/10/12. doi: 10.1164/rccm.201006-0894CI. PubMed PMID: 20935110. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2015;192(2):e3–19. doi: 10.1164/rccm.201506-1063ST. Epub 2015/07/16. doi: 10.1164/rccm.201506-1063ST. PubMed PMID: 26177183. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA. The epidemiology of venous thromboembolism in the community. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(3):370–2. doi: 10.1161/ATVBAHA.108.162545. Epub 2008/02/26. doi: 10.1161/atvbaha.108.162545. PubMed PMID: 18296591; PubMed Central PMCID: PMCPMC2873781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(2):199–205. doi: 10.3324/haematol.10516. Epub 2007/02/14. PubMed PMID: 17296569. [DOI] [PubMed] [Google Scholar]
- 5.Ungprasert P, Srivali N, Wijarnpreecha K, Charoenpong P, Knight EL. Non-steroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford, England) 2015;54(4):736–42. doi: 10.1093/rheumatology/keu408. Epub 2014/09/26. doi: 10.1093/rheumatology/keu408. PubMed PMID: 25252703. [DOI] [PubMed] [Google Scholar]
- 6.Ungprasert P, Sanguankeo A. Risk of venous thromboembolism in patients with idiopathic inflammatory myositis: a systematic review and meta-analysis. Rheumatology international. 2014;34(10):1455–8. doi: 10.1007/s00296-014-3023-1. Epub 2014/04/22. doi: 10.1007/s00296-014-3023-1. PubMed PMID: 24748492. [DOI] [PubMed] [Google Scholar]
- 7.Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: a systematic review and meta-analysis. QJM : monthly journal of the Association of Physicians. 2014;107(10):793–7. doi: 10.1093/qjmed/hcu073. Epub 2014/04/10. doi: 10.1093/qjmed/hcu073. PubMed PMID: 24713224. [DOI] [PubMed] [Google Scholar]
- 8.Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL. Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clinical rheumatology. 2014;33(3):297–304. doi: 10.1007/s10067-014-2492-7. Epub 2014/01/16. doi: 10.1007/s10067-014-2492-7. PubMed PMID: 24424839. [DOI] [PubMed] [Google Scholar]
- 9.Tomasson G, Monach PA, Merkel PA. Thromboembolic disease in vasculitis. Current opinion in rheumatology. 2009;21(1):41–6. doi: 10.1097/BOR.0b013e32831de4e7. Epub 2008/12/17. doi: 10.1097/BOR.0b013e32831de4e7. PubMed PMID: 19077717; PubMed Central PMCID: PMCPMC3204384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. American journal of respiratory and critical care medicine. 2008;178(12):1257–61. doi: 10.1164/rccm.200805-725OC. Epub 2008/08/30. doi: 10.1164/rccm.200805-725OC. PubMed PMID: 18755924. [DOI] [PubMed] [Google Scholar]
- 11.Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. Journal of medical economics. 2012;15(5):829–35. doi: 10.3111/13696998.2012.680553. Epub 2012/03/30. doi: 10.3111/13696998.2012.680553. PubMed PMID: 22455577. [DOI] [PubMed] [Google Scholar]
- 12.Sprunger DB, Olson AL, Huie TJ, Fernandez-Perez ER, Fischer A, Solomon JJ, et al. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. The European respiratory journal. 2012;39(1):125–32. doi: 10.1183/09031936.00041411. Epub 2011/07/09. doi: 10.1183/09031936.00041411. PubMed PMID: 21737559; PubMed Central PMCID: PMCPMC3757572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalleywater W, Powell HA, Fogarty AW, Hubbard RB, Navaratnam V. Venous thromboembolism in people with idiopathic pulmonary fibrosis: a population-based study. The European respiratory journal. 2014;44(6):1714–5. doi: 10.1183/09031936.00099614. Epub 2014/10/18. doi: 10.1183/09031936.00099614. PubMed PMID: 25323238. [DOI] [PubMed] [Google Scholar]
- 14.Navaratnam V, Fogarty AW, McKeever T, Thompson N, Jenkins G, Johnson SR, et al. Presence of a prothrombotic state in people with idiopathic pulmonary fibrosis: a population-based case-control study. Thorax. 2014;69(3):207–15. doi: 10.1136/thoraxjnl-2013-203740. Epub 2013/09/05. doi: 10.1136/thoraxjnl-2013-203740. PubMed PMID: 24002055. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. Epub 2010/07/24. doi: 10.1007/s10654-010-9491-z. PubMed PMID: 20652370. [DOI] [PubMed] [Google Scholar]
- 16.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes. A systematic review. BMC public health. 2013;13:154. doi: 10.1186/1471-2458-13-154. Epub 2013/02/21. doi: 10.1186/1471-2458-13-154. PubMed PMID: 23421987; PubMed Central PMCID: PMCPMC3602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. Epub 1986/09/01. PubMed PMID: 3802833. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. Epub 2003/09/06. doi: 10.1136/bmj.327.7414.557. PubMed PMID: 12958120; PubMed Central PMCID: PMCPMC192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stassen PM, Derks RP, Kallenberg CG, Stegeman CA. Venous thromboembolism in ANCA-associated vasculitis--incidence and risk factors. Rheumatology (Oxford, England) 2008;47(4):530–4. doi: 10.1093/rheumatology/ken035. Epub 2008/03/22. doi: 10.1093/rheumatology/ken035. PubMed PMID: 18356178. [DOI] [PubMed] [Google Scholar]
- 20.Weidner S, Hafezi-Rachti S, Rupprecht HD. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis and rheumatism. 2006;55(1):146–9. doi: 10.1002/art.21704. Epub 2006/02/08. doi: 10.1002/art.21704. PubMed PMID: 16463427. [DOI] [PubMed] [Google Scholar]
- 21.King CS, Nathan SD. Idiopathic pulmonary fibrosis: effects and optimal management of comorbidities. The Lancet Respiratory medicine. 2017;5(1):72–84. doi: 10.1016/S2213-2600(16)30222-3. Epub 2016/09/08. doi: 10.1016/s2213-2600(16)30222-3. PubMed PMID: 27599614. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Rodriguez NA, Cambrey AD, Harrison NK, Chambers RC, Gray AJ, Southcott AM, et al. Role of thrombin in pulmonary fibrosis. Lancet (London, England) 1995;346(8982):1071–3. doi: 10.1016/s0140-6736(95)91744-6. Epub 1995/10/21. PubMed PMID: 7564789. [DOI] [PubMed] [Google Scholar]
- 23.Isshiki T, Sakamoto S, Kinoshita A, Sugino K, Kurosaki A, Homma S. Recombinant human soluble thrombomodulin treatment for acute exacerbation of idiopathic pulmonary fibrosis: a retrospective study. Respiration; international review of thoracic diseases. 2015;89(3):201–7. doi: 10.1159/000369828. Epub 2015/02/11. doi: 10.1159/000369828. PubMed PMID: 25659984. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127(6):2028–33. doi: 10.1378/chest.127.6.2028. Epub 2005/06/11. doi: 10.1378/chest.127.6.2028. PubMed PMID: 15947316. [DOI] [PubMed] [Google Scholar]
- 25.Squizzato A, Gerdes VE, Ageno W, Buller HR. The coagulation system in endocrine disorders: a narrative review. Internal and emergency medicine. 2007;2(2):76–83. doi: 10.1007/s11739-007-0026-X. Epub 2007/07/28. doi: 10.1007/s11739-007-0026-X. PubMed PMID: 17657422. [DOI] [PubMed] [Google Scholar]
- 26.van Zaane B, Nur E, Squizzato A, Gerdes VE, Buller HR, Dekkers OM, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. Journal of thrombosis and haemostasis: JTH. 2010;8(11):2483–93. doi: 10.1111/j.1538-7836.2010.04034.x. Epub 2010/08/26. doi: 10.1111/j.1538-7836.2010.04034.x. PubMed PMID: 20735729. [DOI] [PubMed] [Google Scholar]
- 27.Johannesdottir SA, Horvath-Puho E, Dekkers OM, Cannegieter SC, Jorgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA internal medicine. 2013;173(9):743–52. doi: 10.1001/jamainternmed.2013.122. Epub 2013/04/03. doi: 10.1001/jamainternmed.2013.122. PubMed PMID: 23546607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy
Database: Ovid MEDLINE
interstitial lung disease.mp. or exp Lung Diseases, Interstitial/
pulmonary fibrosis.mp. or exp Pulmonary Fibrosis/
or/1-2
exp Thromboembolism/
Thromboembolism.mp.
exp Venous Thrombosis/
venous thrombosis.mp.
exp Pulmonary Embolism/
pulmonary embolism.mp.
or/4-9
3 and 10
Database: EMBASE
idiopathic pulmonary fibrosis.mp. or exp fibrosing alveolitis/
fibrosing alveolitis.mp.
pulmonary embolism.mp. or exp lung embolism/
deep vein thrombosis.mp. or exp deep vein thrombosis/
venous thromboembolism.mp. or exp venous thromboembolism/
exp thromboembolism/ or thromboembolism.mp.
or/3-6
or/1-2
7 and 8